Abstract
Both biochar and humic acid adsorb heavy metals, but their combined effect has rarely been reported. In this paper, the adsorption of Cu(II) by single biochar (BS) and biochar with humic acid (BS + HA) in aqueous solution were studied, and the effects of solution pH, initial Cu(II) concentration, temperature and Ca2+ ionic strength on the adsorption of Cu(II) were investigated. The results showed that under the same time, the adsorption of Cu(II) under the BS and BS + HA systems showed an increasing trend with the increase of pH, and the maximum adsorption capacity of the BS + HA system was reached at a pH of about 4, while the BS system alone exhibited a noticeable lag. The increase of the initial concentration of Cu(II) also promoted the adsorption capacity of the two systems, and the adsorption of Cu(II) by the BS and the BS + HA systems was a spontaneous adsorption process, and the elevation of the temperature effectively promoted the adsorption of Cu(II). The addition of Ca2+ could promote the adsorption efficacy of the single BS system, but had little effect on the BS + HA system. Overall, the BS + HA system showed better adsorption than the single BS system, and the addition of humic acid enhanced the adsorption performance of biochar to Cu(II). The results could provide a theoretical basis for the combined action of biochar and humic acid on the remediation of Cu(II) contaminated water.
Similar content being viewed by others
China is one of the world’s largest polluters of sewage. Data from China’s National Bureau of Statistics (NBS) show that China’s wastewater discharge in 2015 was 73.53 billion tonnes, of which total industrial wastewater discharge accounted for 27.2% of the total wastewater discharge, exceeding 20 billion tonnes1. Copper concentrations are generally high in waters of watersheds in Asian countries such as China2. Copper-containing wastewater is one of the most harmful heavy metal-containing wastewaters generated by industries (mining, chemical, printing, metallurgy, electroplating, etc.), of which Cu(II) is considered to be the most toxic3. Cu(II) has been listed as a priori pollutant by the United States Environmental Protection Agency (EPA), which stipulates that its concentration in water should not exceed 1.3 mg L− 1. The World Health Organization (WHO) standard limits the concentration of Cu(II) in drinking water to 2.0 mg L− 1. Once the content of Cu(II) exceed the purification threshold of the environment, it will continue to be enriched in the food chain and eventually endanger human beings4. In order to avoid the pollution caused by the accumulation of Cu(II) in the environment, the control of Cu(II) pollution in the environment is very important5.
Biochar (BC) is carbon particles produced by high-temperature pyrolysis of biomass (including straw, herbaceous plants, energy dwarfs, and forest logging residues) under anaerobic conditions6. BC has a larger surface area, well developed pore structure, and a large number of functional groups on the surface, which have a significant potential on the adsorb of heavy metal ions in water7. Now, BC is widely used for the adsorption and removal of heavy metal pollutants in water8,9,10. The adsorption capacity of BC for heavy metals is influenced by factors such as surface area, pore distribution, pH, cation exchange capacity, elemental composition, and surface functional groups11, yet findings on its impact vary, with some studies even contradicting each other. Cao et al. found that the adsorption ability of BC for Cu(II) is significantly affected by the initial ion concentration and contact time12. Kinnunen et al. noted that while BC adsorbs metals and elevates pH, lime and ash additives do not consistently improve adsorption. At lower pH, adsorption favors Al and Fe, whereas raising pH enhances adsorption for Cd and Zn13. Qing et al. emphasized the critical role of pH, oxygen functional groups, and carbonate ion content in Cu(II) adsorption improvement14. Conversely, there are studies indicating that the addition of biochar to the San Joaquin River soil, regardless of the amount applied, actually reduces its high adsorption capacity for copper15. Furthermore, Qin et al. demonstrated that over time, protonation on the surface of biochar in acidic soils diminishes its ability to decrease the bioavailability of heavy metals to plants16. These findings highlight the inherent uncertainty in the adsorption behavior of biochar in real-world environments when used as a singular system.
Humic acids (HAs) are the most widely distributed natural organic matter in the earth’s ecosystem17. Compared with synthetic chelating agents, humic acid has high reactivity due to its large specific surface area, complex structure and the presence of multiple functional groups (carboxyl, alcohol hydroxyl, phenol hydroxyl), which has an important effect on the toxicity and bioavailability of heavy metals in the environment and regulates their bioavailability18. On the one hand, its complexation and chelation reaction with metal ions, reducing the exchange fraction of heavy metals and increasing the stability of heavy metals19; on the other hand, it is able to increase the bioavailability of heavy metals. For example, Elmongy et al. showed that humic acid can promote the growth of aquatic microorganisms and plants, which further promotes the uptake of heavy metals by plants20. Research has intensified on modifying BC through pretreatments and combining it with organic materials like HAs, which enhance its adsorption capacity for organic and inorganic pollutants. For instance, Madhavi et al. noted in their study that the combination of biochar with humic acid leads to an increase in soil’s cation exchange capacity (CEC)21. Zhang et al. through experimentation, found that activating biochar with humic acid and phosphate significantly enhances the removal efficiency of cadmium (Cd) and lead (Pb), achieving rates of 48.9% and 55%, respectively22. In another study, Liu et al. showed that the integration of humic acid-activated dolomitic phosphate with biochar resulted in even higher removal rates for Pb and Cd, at 76% and 87.2%, respectively23. Furthermore, biochar impregnated with humic acid has an adsorption capacity for copper (Cu) that is five times greater than untreated biochar24.
Despite previous studies exploring the individual mechanisms of biochar25 and humic acid26, key scientific questions remain unresolved regarding the composite system, particularly how factors such as the solution’s pH, initial concentration of Cu(II), temperature, and ionic strength influence heavy metal adsorption characteristics. Consequently, this research employs rapeseed (brassica campestris L.) straw as a feedstock, producing biochar through high-temperature pyrolysis at 600 °C, to investigate Cu(II) adsorption in a biochar-humic acid-aqueous system. The study extensively examines the effects of pH, initial Cu(II) concentration, temperature, and ionic strength, delving into kinetic and isotherm adsorption processes. Its objective is to elucidate the mechanism of biochar’s Cu(II) adsorption in the presence of humic acid, with the aim of providing theoretical underpinnings for the combined strategy of biochar and humic acid in remediating Cu(II)-contaminated water bodies.
Materials and methods
Biochar and HA
Rapeseed straw was collected from the farmland in Lanzhou, Gansu Province, and was washed, dried naturally, crushed and sieved through a 40-mesh sieve, then loaded into a crucible and carbonized in a muffle furnace (HR-F1200, Luoyang Huarong Kiln CO., LTD, China) at 600 °C for 4 h. After the temperature was cooled down to room temperature, the black residue was washed with 1 mol L− 1 HCl repeatedly to remove the ash, and then washed with deionized water to neutral, dried at 80 °C for 12 h, and then milled and sieved through a 100-mesh sieve to obtain the biochar of rapeseed straw (BS) for the experiments. Determination of ash (GB/T 12,496.3-1999), pH (GB/T 12,496.7-1999), elemental composition (vario EL Elemental Analyser), isotropic potential (Acid–Base Potentiometric Titration), specific surface area (ASAP 2020 M Rapid Specific Surface Area/Porosity Analyser) and surface functional groups (Nexus 870 FTIR Infrared Spectrometer) on biochar. The physical and chemical properties of biochar are shown in Tables 1 and 2. Humic acid (FA > 90%) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. with its main components comprising carbon (52% to 65%), hydrogen (5% to 7%), oxygen (25% to 40%), and nitrogen (approximately 3–4%).
Adsorption experiment
0.05 g of biochar and 0.20 g of humic acid were weighed respectively and put in a 50 mL conical flask with 20 mL of Cu(NO3)2 solution at different concentrations (100, 200, 300, 400, 500, 800, and 1,000 mg L− 1). 0.01 mol L− 1 of NaNO3 was also contained in the solution as a balancing electrolyte to provide the necessary ionic strength, and then 0.1 mL of NaN3 (at a concentration of 1 g L− 1) was added to inhibit the growth of microorganisms, and the pH of the solution was adjusted with 1 mol L− 1 NaOH and 1 mol L− 1 HNO3, respectively. The samples were placed into a thermostatic oscillator (Danyangmen Quartz Glass Factory, Jiangsu Province, China) and shaken for 12 h at 150 r min− 1 (Pre-experiments show that equilibrium has been reached at 12 h). After the adsorption was completed, the mixture was centrifuged at 3500 r min− 1 for 30 min. The supernatant was taken and diluted, and the concentration of Cu(II) was determined by flame atomic absorption spectrometry (Varian Spectrum AA110/220, USA) to obtain the adsorption amount versus time curve, and a control adsorption experiment was also conducted without adding humic acid. Each experimental group was set up with three parallel experiments.
Analytical method
The Cu(II) concentration was determined by an atomic absorption spectrophotometer at 324.8 nm, and the flame type was acetylene-air. For each determination, the instrument was required to draw the standard curve automatically under four concentration gradients (0, 1, 3, 5 mg L− 1) with the R2 around 0.9999 to ensure the accuracy of the test results. The amount of Cu(II) adsorbed was obtained by Eq. (1).
where q is the adsorption amount (mg g− 1); c1 and c2 are the mass concentration of Cu in the solution before and after adsorption (mg L− 1), respectively; v is the volume of the solution (mL); and m is the mass of the adsorbent (g).
Results and discussion
Effect of initial solution pH on adsorption
The adsorption of Cu(II) by BS and BS-HA systems was significantly affected by the initial solution pH, and the adsorption of Cu(II) by both systems increased with the increase of initial pH (Fig. 1). The adsorption of Cu(II) by BS increased from 1.68 to 9.83 mg g− 1 and that of BS-HA system increased from 9.38 to 15.55 mg g− 1 when the initial solution pH increased from 2.00 to 6.00.
For biochar, the adsorption of Cu(II) showed a significant increase when the pH ranged from 2.50 to 5.00, and the adsorption leveled off at pH > 5.00. This is due to the fact that at too low a pH, a large amount of H+ of the solution competes with Cu2+ for adsorption. And the surface groups of the adsorbent may be highly protonated27, resulting in the surface charge of the adsorbent becoming positive, which prevents the adsorbent to act with positively charged metal ions28. In addition, the pHpzc of rapeseed straw biochar was 5.08. When the solution pH < pHpzc, the surface of biochar was positively charged, forming electrostatic repulsion with copper ions. With the increase of pH, the negative charge on the surface of the biochar gradually increased and the electrostatic repulsion gradually weakened, which facilitated the diffusion of Cu(II) to the surface of the biochar29. The increase of pH also promoted the formation of hydroxide (e.g., Cu(OH)2, Cu(OH)3−, and Cu(OH)42−), carbonate, and phosphate precipitates30, which increased the adsorption of Cu(II).
The adsorption process of BS-HA system on Cu(II) was divided into two stages: in the first stage, when the pH value increased from 2.00 to 3.50, the adsorption amount increased significantly from 9.38 to 15.20 mg g− 1, in which the H+ on the surface of the HA particles was released, and the H+ on the reactive groups, such as −COOH and −OH, were dissociated, which in turn increased the negative charge and adsorption sites on the surface of HA, and promoted the adsorption strength of Cu2+. The adsorption was in equilibrium in the second stage when the pH value increased from 4.00 to 6.00, and only increased from 15.37 to 15.55 mg g− 1. Yang et al. showed that with increasing pH, HA occupies only some of the surface sites due to weak interactions with the negatively charged surface of the biochar, and some HA will be involved in the adsorption process, which will lead to a strong complexation of the metal ions with the free HA in solution31.
The experimental data reveals that the adsorption behavior of BS (biochar alone) and BS + HA (biochar combined with humic acid) systems towards Cu(II) intensifies with an increase in pH. Notably, the BS + HA composite achieves its maximum adsorption capacity at a pH around 4.0, which is 1.5 pH units lower than the BS system, which peaks at approximately pH 5.5. This pH advantage stems from the activation of functional groups in HA: HA is rich in various oxygen-containing functional groups such as carboxyl groups (-COOH) and phenolic hydroxyl groups (-OH), making it an extremely strong metal complexing agent. At pH ≈ 4, the −COOH in HA are significantly deprotonated (pKa ≈ 3–5)32. The deprotonated −COOH efficiently form stable inner-sphere complexes with Cu(II) (e.g., −COO-Cu⁺ or chelated structures). The strength of this complexation typically far exceeds the ion exchange or electrostatic attraction capabilities of biochar alone, establishing it as the dominant adsorption mechanism. Simultaneously, HA molecules can adsorb onto the biochar surface via hydrophobic interactions, hydrogen bonding, or π-π interactions, forming an organic coating. This not only directly increases the total adsorption sites in the system but may also cover or modify low-affinity sites on the biochar surface33. Concurrently, it exposes HA’s inherent high-affinity functional groups, enabling more effective sites to function efficiently at relatively lower pH (≈4). Additionally, the introduction of HA likely alters the solution chemistry or properties of the biochar-solution interface, reducing the diffusion resistance of Cu(II) into the internal pores of biochar. Alternatively, it may form soluble Cu-HA complexes that subsequently adsorb onto biochar, thereby accelerating overall adsorption kinetics. This mechanism avoids the hysteresis phenomenon observed in the single BS system, where adsorption equilibrium is reached only at higher pH values. In contrast, the BS system relies solely on pH-induced surface charge modification, requiring a higher alkalinity to accumulate sufficient negative charge for effective cation adsorption.
Overall, the addition of HA to the water increased the adsorption capacity of BS for Cu(II), which was mainly attributed to the formation of HA complexes on the surface sites of the biochar. The zeta potential value of the HA-coated biochar undergoes a slight negative shift, resulting in a more negatively-charged surface, and the HA reduces the aggregation of the biochar particles to provide a larger number of adsorption sites, which is more favorable for the Cu (II) electrostatic attraction, enhancing the formation of biochar-HA-Cu surface complexes33. In addition, previous studies have proposed that the abundant −COOH and −OH functional groups in HA can potentially participate in the formation of metal complexes with Cu(II) through ion exchange and hydrogen bonding32. Based on the reported coordination chemistry of humic substances (e.g., the affinity of carboxyl groups for divalent metal ions32), the proposed reaction pathways may include proton displacement and hydroxide bridging:
Effect of initial solution concentration on adsorption
The adsorption of Cu(II) versus initial concentration is shown in Fig. 2. The adsorption of Cu(II) by the BS-HA system (the saturated adsorption capacity is 35.86 mg g− 1) was higher than that by the single BS at the same initial concentration of Cu(II) (the saturated adsorption capacity is 20.94 mg g− 1). This is due to the ability of Cu(II) to undergo coordination, ion exchange and surface adsorption with some humic acids, Meanwhile, humic acid can form an organic binding state with heavy metals, thus reducing the activity and promoting the adsorption of Cu(II) in the BS-HA system34.
In addition, the adsorption of Cu(II) by both BS and BS-HA systems gradually increased with the increase of the initial concentration of Cu(II). At low initial concentration, Cu(II) was mainly adsorbed by the active sites on the surface of the adsorbent. When the initial concentration increases, the potential energy difference (concentration difference) between the solution and the adsorbent increases, and the mass transfer power is also enhanced, Cu(II) will move toward the adsorbent under the effect of potential energy, which will increase the contact probability between Cu(II) and the active sites of the adsorbent35. However, when the concentration increases to a certain level, these active sites are rapidly occupied and saturated, and the adsorption process is mainly dominated by the partitioning effect36.
The isothermal adsorption data were simulated with Langmuir model, Freundlich model and Dubinin-Radushkevich (D-R) model to explain the adsorption mechanism37. Langmuir simulation assumes that adsorption occurs predominantly on the adsorbent as a monomolecular layer and assumes that the energy of adsorption on the surface of the adsorbent is homogeneous, whereas the Freundlich model is an empirical model that mainly describes adsorption in non-uniform system.
Langmuir isotherm equation:
Freundlich isotherm equation:
where qe is the equilibrium adsorption amount (mg g-1); ce is the equilibrium concentration (mg L− 1); qm is the saturated adsorption amount (mg g-1); b is the adsorption equilibrium constant characterizing the energy of adsorption (L mg− 1), the magnitude of which is related to the amount of adsorption; kF and 1/n are Freundlich constants characterizing the affinity coefficient (mg(1-n) Ln g-1) and adsorption strength, respectively. It is generally believed that the larger the kF value, the higher the adsorption capacity, and the smaller the 1/n value, the better its adsorption performance.
D-R equation:
where R is the universal gas constant (8.314 J mol− 1 k− 1); T is the Kelvin temperature (K); k is the constant related to the adsorption energy, and the average free adsorption energy E is calculated by the value of k (Eq. (5)), the adsorption process is dominated by physical adsorption when 1.0 <|E|< 8.0 kJ mol− 1, ion-exchange when 8.0 <|E|< 16 kJ mol− 1, and chemical adsorption when |E|> 16 kJ mol− 1.
The fitting results of adsorption isotherm in BS and BS-HA systems are shown in Table 1. The adsorption isotherm of Cu(II) by BS is consistent with the Langmuir equation (R2 = 0.919); while in the BS-HA system it is more consistent with the Freundlich equation (R2 = 0.990).
In the fitting results using Langmuir isothermal adsorption model (Table 3), the maximum adsorption of Cu(II) by biochar is basically close to the measured value, which indicated that it is a monolayer adsorption and the adsorption location is homogeneous38. The b and qm values of the BS-HA system were greater than those of the single BS system, which indicated that the adsorption capacity of the BS-HA system was stronger and there was a multilayer adsorption phenomenon in the BS-HA system. According to the Freundlich model (Table 3), the kF value of biochar with humic acid became larger, indicating that the addition of humic acid increased the adsorption capacity of biochar, and 1/n ranged between 0.1–1, indicating that adsorption was easier under this condition. The results from the D-R equation (Table 3) shows that the adsorption free energy in both the BS and BS-HA systems is between 8.0 and 16.0 kJ mol− 1, indicating that the adsorption is dominated by ion exchange39.
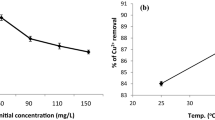
Effect of temperature on adsorption
The results showed that the increase in temperature promoted the adsorption of Cu(II) on BS system (Fig. 3), which was attributed to the fact that the higher temperature elevated the diffusion rate and ionic activity of heavy metal ions, and then facilitated the migration of heavy metal ions to the surface of the adsorbent. While increasing the temperature, the adsorption of Cu(II) by the BS-HA system grew slowly with almost no significant change, and only a slight increasing trend was seen from the experimental data. It indicates that the presence of humic acid can promote the biochar to reach adsorption equilibrium at lower temperature.
To further illustrate the role of temperature, data were fitted through the thermodynamic equation (Eq. (6–8)) to obtain the relevant parameters (Table 4).
where Ko is the thermodynamic equilibrium constant; ΔGθ is the standard Gibbs free energy (kJ mol-1); ΔHθ is the standard molar enthalpy change (kJ mol− 1); ΔSθ is the standard molar entropy change (J mol− 1 K− 1).
Table 4 shows that ΔGθ for Cu(II) adsorption by BS and BS-HA systems at all temperatures was negative, indicating that both adsorption processes proceeded spontaneously, and the higher the temperature, the smaller the value of ΔGθ, indicating that elevated temperatures are favorable for the spontaneous adsorption process40. The positive value of ΔHθ indicates that both chemisorption and ion exchange adsorption in this adsorption process are heat-absorbing reactions, and an increase in temperature is favorable to the adsorption of Cu(II) by the adsorbent. This strongly suggests that chemisorption is the dominant mechanism, such as the formation of strong chemical bonds (ionic bonds, covalent bonds, strong complexation) between Cu(II) and the surface functional groups of biochar or HA functional groups. The entropy value reflects the affinity of the adsorbent for the metal ions and a positive entropy value indicates increasing adsorption disorder, which may be due to structural changes in the adsorbent and adsorbate during the adsorption process41. During the adsorption process, Cu(II) ions transition from a relatively ordered hydrated state (hydration shell) in the aqueous solution to a relatively disordered state on the adsorbent surface. Simultaneously, a large number of bound water molecules are released, leading to an entropy increase. The addition of HA may further influence the entropy change process by providing more diverse binding sites or altering the interfacial structure. Overall, the primary mechanisms by which temperature elevation promotes adsorption are the enhancement of chemisorption (including complexation, ion exchange, and surface precipitation) as well as the entropy-driven effect, which serves as a crucial factor facilitating spontaneous adsorption.
Effect of ionic strength on adsorption
The effect of Ca2+ concentration on the adsorption capacity of Cu(II) on the BS and BS + HA systems are shown in Fig. 4. The adsorption of Cu(II) by BS system showed an increasing trend with increasing Ca2+ concentration. It indicates that the presence of Ca2+ promotes the adsorption capacity of biochar on Cu(II), which may be due to the generation of inner-sphere surface complex between Cu(II) and adsorbent surface functional groups9, and Ca2+ increases the electrostatic and hydrophobic interactions between biochar and Cu(II). It was mentioned that at pH = 5 (< pHpzc), the surface of biochar is partly positively charged and forms electrostatic repulsion with copper ions, whereas studies have shown that when there is electrostatic repulsion between the adsorbent and adsorbate, an increase in the ionic strength is favorable for adsorption42. In addition, the electrostatic repulsion between the adsorbed ions prevents further adsorption, and increasing the ionic strength weakens this electrostatic repulsion, thus favoring adsorption. At this time, the hydrophobicity between the metal ions and the biochar is likely to play an important role in the adsorption43.
In the BS + HA system, the addition of Ca2+ did not have a significant effect on the adsorption capacity of biochar, and this phenomenon was related to the selective adsorption of Cu(II) by humic acid, which can form stable complexes or chelates with Cu(II)44,45, attenuating the displacement of Ca2+. Particular attention was paid to the fact that when the concentration of Ca2+ was increased to 0.5 mmol L− 1, the adsorption of Cu(II) by the BS + HA system was rather smaller than that of the BS system, which may be due to the flocculation and precipitation of humic acid with Ca2+ to form a calcium humate precipitate, which reduces the adsorption of Cu(II) by the BS + HA system44.
Overall, the adsorption of Cu(II) by the BS + HA system was greater than that of the single BS system, which was related to the interactions between humic acid and biochar. Firstly, the biochar could adsorb the components of humic acid with higher molecular weights and higher degree of aromatization, thus forming a composite adsorbent with abundant chemical groups18, which in-creased the adsorption capacity of the BS + HA system. Secondly, Cu(II) was first adsorbed by humic acid, and then humic acid was adsorbed to the surface of biochar33, which increased the adsorption of Cu(II) by biochar. Further, humic acid has surface activity46, and its addition to the solution reduces the surface tension of the solution, which promotes the diffusion of Cu(II) to the biochar and improves the adsorption of Cu(II) by the biochar.
Conclusion
The increase of pH value contributes to the negative charge generation on the surface of biochar and promotes a sustained increase in adsorption, and the strong complexation of free HA and Cu(II) after the addition of HA leads to a rapid equilibrium of adsorption. The potential energy difference (concentration difference) between the solution and adsorbent promoted the adsorption process, but the adsorption was dominated by partitioning in the case of high initial concentration of Cu(II). The adsorption isotherms indicated that the adsorption of Cu(II) by BS was dominated by ion exchange, where the BS system conformed to the Langmuir equation and in the case of the BS-HA system it was more consistent with the Freundlich equation, suggesting that the incorporation of humic acid facilitated the multilayer adsorption. The thermodynamic equations indicated that the adsorption process was a heat-absorbing reaction, and the addition of humic acid could promote the biochar to reach the adsorption equilibrium at lower temperatures. Ca2+ increased the electrostatic and hydrophobic inter-actions between the biochar and Cu(II) and promoted the adsorption capacity of the biochar on Cu(II), but since humic acid could form stable complexes or chelates with Cu(II), the addition of Ca2+ had little effect on the adsorption capacity of the BS + HA system.
This study, through detailed investigation of factors including pH, concentration, temperature, and coexisting ions combined with the mechanistic analysis, clearly elucidates the synergistic enhancement mechanism for Cu(II) adsorption by the combined action of biochar and humic acid, particularly revealing the rapid and efficient adsorption at low pH (attributed to HA’s strong complexation and surface modification) and the unique positive response to coexisting Ca2⁺. This enriches the theoretical foundation concerning heavy metal adsorption by organic–inorganic composites and provides a new perspective for understanding complex environmental interfacial processes. The results confirm that the BS + HA composite system is an efficient Cu(II) adsorbent with adaptability to variations in pH and ionic strength. Compared to single biochar, it exhibits superior performance over a broader pH range (especially at lower pH) and in calcium-containing water bodies, significantly enhancing its application potential and feasibility in the remediation of actual heavy metal-contaminated water bodies (such as acid mine drainage, and calcium-containing groundwater/surface water). Strategies for preparing the composite material (e.g., biochar-loaded HA or pre-mixing) are worth exploring and optimizing in subsequent research. While the proposed adsorption mechanisms are supported by pH-dependent behavior and thermodynamic consistency with organic-metal interaction theories, we acknowledge that direct spectroscopic evidence (e.g., FTIR/XPS) is currently lacking. To address this limitation, we plan to conduct post-adsorption surface characterization using FTIR and XPS in subsequent studies.
Data availability
Data is provided within the supplementary information files.
References
Zhang, C. et al. China’s wastewater treatment: Status quo and sustainability perspectives. J. Water Process Eng. 53, 103708 (2023).
Zhou, Q. et al. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 22, e00925 (2020).
Li, Q. et al. Progress in the treatment of copper(II)-containing wastewater and wastewater treatment systems based on combined technologies: A review. J. Water Process Eng. 58, 104746 (2024).
Thakur, S. et al. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 188, 206 (2016).
Mei, Y. et al. Effect of nitrogen modification on the properties of biochars and their adsorption behavior on Cu2+ removal from wastewater. Environ. Chem. 41(5), 1796–1808 (2022).
Krajcovicova, T. E. et al. Biochar for water pollution control: From sensing to decontamination. Chemosensors 11(7), 394 (2023).
Lin, P. et al. Remediation performance and mechanisms of Cu and Cd contaminated water and soil using Mn/Al-layered double oxide-loaded biochar. J. Environ. Sci. 125, 593–602 (2023).
Lian, F., Wang, Z. Y. & Xing, B. S. Nano-black carbon (biochar) released from pyrogenic carbonaceous matter as a super suspending agent in water/soil environments. biochar, 3, 1–3. (2021)
Chen, Y. et al. The characterization of a novel magnetic biochar derived from sulfate-reducing sludge and its application for aqueous Cr(VI) removal through synergistic effects of adsorption and chemical reduction. Chemosphere 308(1), 136258 (2022).
Xu, L. et al. Biochar application increased ecosystem carbon sequestration capacity in a Moso bamboo forest. For. Ecol. Manag. 475, 118447 (2020).
Negi, M. et al. Clean and green bamboo magic: recent advances in heavy metal removal from water by bamboo adsorbents. Water 17, 454 (2025).
Cao, Q. Y., Huang, Z. H., Liu, S. G. & Wu, Y. P. Potential of Punica granatum biochar to adsorb Cu(II) in soil. Sci. Rep-Uk. 9, 11116 (2019).
Kinnunen, N., Laurén, A., Pumpanen, J., Nieminen, T. M. & Palviainen, M. Biochar capacity to mitigate acidity and adsorb metals-laboratory tests for acid sulfate soil drainage water. Water Air. Soil Poll. 232, 464 (2021).
Qing, M. X. et al. Effective fixation of Cu(II) and Cr(III) in solution by food waste biochar - Innovative and valuable treatment method for municipal solid waste. Fuel 361, 130679 (2024).
Uchimiya M., KLasson K. T., Wartelle L. H. & Lima I. M. Influence of soil properties on heavy metal sequestration by biochar amendment:1. Copper sorption isotherms and the release of cations. Chemosphere 82: 1431–1437. (2011)
Qin, J. H., Wang, X., Ying, J. D. & Lin, C. X. Biochar is not durable for remediation of heavy metal-contaminated soils affected by acid-mine drainage. Toxics 10, 462 (2022).
Zhang, Y., Liu, G., Gao, S., Zhang, Z. & Huang, L. Effect of humic acid on phytoremediation of heavy metal contaminated sediment. J. Hazard. Mater. Adv. 9, 100235 (2023).
Ding, H., Tang, L., Nie, Y. & Ji, H. Characteristics and interactions of heavy metals with humic acid in gold mining area soil at a upstream of a metropolitan drinking water source. J. Geochem. Explor. 200, 266–275 (2019).
Chechevatov, A. I., Miroshnichenko, Y. S., Myasoyedova, T. N., Popov, Y. V. & Yalovega, G. E. Investigations of the capability to heavy metals adsorption humic acids: correlation between structure and absorption properties. In: Parinov, I., Chang, SH., Jani, M. (eds) Advanced Materials. Springer Proceedings in Physics, vol 193. Springer, Cham. (2017)
Elmongy, M. S., Zhou, H., Cao, Y., Liu, B. & Xia, Y. P. The effect of humic acid on endogenous hormone levels and antioxidant enzyme activity during in vitro rooting of evergreen azalea. Sci. Hortic. 227, 234–243 (2018).
Madhavi, P., Sailaja, V., Prakash, T. R. & Hussain, S. A. Characterization of biochar and humic acid and their effect on soil properties in Maize. Int. J. Curr. Microbiol. 6, 449–457 (2017).
Zhang, Z. et al. The synergistic effect of biochar-combined activated phosphate rock treatments in typical vegetables in tropical sandy soil: Results from nutrition supply and the immobilization of toxic metals. Int. J. Env. Res. Pub. He. 19, 6431 (2022).
Liu, W. J., Chai, G. L. & Deng, W. B. A combination of finite mixture distribution model with geo-statistical models to study spatial patterns and hazardous areas of heavy metals in cropland soils of the Guanzhong Plain. Northwest China. Chemosphere 283, 131222 (2021).
Liu, H. et al. Preparation and evaluation of activated carbons from lotus stalk with trimethyl phosphate and tributyl phosphate activation for lead removal. Chem. Eng. J. 228, 425–434 (2013).
Lakshmi, D. et al. Artificial intelligence (AI) applications in adsorption of heavy metals using modified biochar. Sci. Total Environ. 801, 14962 (2021).
Tan, L. Q. et al. Systematic studies on the binding of metal ions in aggregates of humic acid: Aggregation kinet-ics, spectroscopic analyses and MD simulations. Environ. Pollut. 246, 999–1007 (2019).
Hasan, M. M. et al. Sustainable ligand-modified based composite material for the selective and effective cadmium(II) capturing from wastewater. J. Mol. Liq. 37, 121125 (2023).
Awual, M. R., Khraisheh, M., Alharthi, N. H., Luqman, M., Islam, A., Rezaul Karim, M., Rahman, M. M. & Khaleque, M. A. Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem. Eng. J. 34, 118–127. (2018)
Mousavi, S. P. et al. Modeling thermal conductivity of ionic liquids: a comparison between chemical struc-ture and thermodynamic properties-based models. J. Mol. Liq. 32, 114911 (2021).
Hasan, M. N., Salman, M. S., Islam, A., Znad, H. & Hasan, M. M. Sustainable composite sensor material for optical cadmium(II) monitoring and capturing from wastewater. Microchem. J. 16, 105800 (2021).
Yang, S., Hu, J., Chen, C., Shao, D. & Wang, X. Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ. Sci. Technol. 45(8), 3621–3627 (2011).
Zhang, J. S. et al. Removal of Cd2+, Pb2+ and Ni2+ from water by adsorption onto magnetic composites prepared using humic acid from waste biomass. J. Clean Prod. 411, 137237 (2023).
Park, C. M. et al. Influence of solution pH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: Experiment and modeling. J. Ind. Eng. Chem. 48, 186–193 (2017).
Liu, H., Feng, S., Zhang, N., Du, H. & Liu, Y. Removal of Cu(II) ions from aqueous solution by activated carbon impregnated with humic acid. Front. Env. Sci. Eng. 8, 329–336 (2014).
Xiao, Z. et al. O-modified activated carbon fiber electrode efficiently adsorption of Cu (II) in wastewater. Sustainability 15, 10078 (2023).
Zhang, J. X. et al. Adsorption characteristics and mechanism of tetracycline by biochars derived from paper industry sludge. China Environ. Sci. 40, 3821–3828 (2020).
da Silva, M. D. et al. Citrus fruit residues as alternative precursors to developing H2O and CO2 activated carbons and its application for Cu(II) adsorption. Environ. Sci. Pollut. R. 30, 63661–63677 (2023).
Ren, Y. et al. Study on the mechanism of removing Pb (II) and Cd (II) from industrial wastewater by copper based MOF modified with ethylenediamine. Fuel. Process. Technol. 247, 107798 (2023).
Wang, J. & Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Crit. Rev. Env. Sci. Tec. 53(21), 1837–1865 (2023).
Madawala, C. K., Jahinge, T. H. L., Rathnayake, K. T. & Perera, B. A. Adsorption of cadmium (II) from aqueous solutions by coconut dregs residue: Kinetic and thermodynamic studies. Sep. Sci. Technol. 58(11), 1972–1984 (2023).
Ma, P. et al. Green synthesis of Fe/Cu oxides compo-site particles stabilized by pine needle extract and investigation of their adsorption activity for norfloxacin and ofloxacin. J. Disper. Sci. Technol. 42(9), 1350–1367 (2020).
Ohe, K., Oshima, T. & Baba, Y. Effect of ionic strength, temperature on equilibrium and kinetics of arsenic adsorption using magnetite. Kagaku Kogaku Ronbunshu 40(2), 90–97 (2014).
Campinas, M. & Rosa, M. J. The ionic strength effect on microcystin and natural organic matter surrogate adsorption onto PAC. J. Colloid. Interf. Sci. 299(2), 520–529 (2006).
Ma, Y. T., Wang, R., Ma, C. H. & Han, R. P. Adsorption of humic acid onto chitosan polyvinyl alcohol blend membrane from solution and second adsorption toward copper ions. Desalin. Water Treat. 272, 167–182 (2022).
Zhang, Y. B. et al. Interfacial reaction between humic acid and Ca-Montmorillonite: Application in the preparation of a novel pellet binder. Appl. Clay Sci. 180(1), 105177 (2019).
Tan, L. Q. et al. Systematic studies on the binding of metal ions in aggregates of humic acid: Aggregation kinetics, spectroscopic analyses and MD simulations. Environ. Pollut. 246, 999–1007 (2019).
Funding
This research was financially supported by the National Natural Science Foundation of China (51766008).
Author information
Authors and Affiliations
Contributions
B.Z.: funding acquisition, supervision, project administration, resources, methodology; Y.L.: validation, formal analysis, investigation, visualization, writing—original draft; T.S.: data curation, software. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Zhao, B. & Shang, T. Adsorption of copper(II) in biochar-humic acid–water system. Sci Rep 15, 24948 (2025). https://doi.org/10.1038/s41598-025-09880-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09880-5