Abstract
Uncontrolled hypertension (HTN) is growing in incidence globally creating a critical need for alternative therapeutic strategies. Directly stimulating the carotid sinus nerve (CSN) is known to potentially reduce blood pressure (BP) but its clinical efficacy has not been consistently demonstrated with existing electrode technologies in humans. We investigated the effect of acute direct CSN stimulation on BP and HR in anesthetized humans using an application-specific multi-contact electrode. Using a novel surgical approach, a custom electrode was implanted around tissue including CSN branches in anesthetized adults. Following functional mapping, presumed baroafferent fibers were identified via response and stimulated. Outcome measures included change in systolic BP (SBP), diastolic BP (DBP), and heart rate (HR) during and after stimulation using multi-level modeling. Secondarily, dose dependency was examined. In 13 subjects, stimulation caused a 24 ± 20 mmHg (mean ± SD, p < 0.01) maximum drop in SBP, with associated drops in DBP (11 ± 9 mmHg, p < 0.01) and HR (10 ± 9 bpm, p < 0.05). In subjects with baseline SBP > 120 mmHg (n = 8), maximum SBP drop was 31 ± 23 mmHg, while subjects with baseline SBP < 120 mmHg (n = 5) exhibited a maximum SBP drop of 11 ± 3 mmHg. In all patients, BP and HR recovered rapidly with stimulus withdrawal. There were results suggesting a positive dose (electrical current) response relationship. Using a novel surgical approach and application specific electrode, direct stimulation of the carotid sinus nerve in anesthetized human subjects caused a marked concurrent drop in BP/HR followed by rapid recovery with stimulation withdrawal. There was an indication of dose dependency.
This study was registered as ClinicalTrials.gov ID NCT06969846, May 9, 2025.
Similar content being viewed by others
Introduction
Hypertension (HTN) is a remarkably prevalent and growing health problem in the US and globally1,2. The Global Burden of Disease study reported that from 1990 to 2019 the number of disability adjusted life years due to high systolic blood pressure (SBP) increased from 154 million to 235 million3. The implications for untreated HTN are vascular disease and end-organ disease in multiple systems including cardiac, renal, retinal and central nervous system4. Thus, HTN is a leading modifiable risk factor for global morbidity and mortality reduction. Anti-hypertensive medications are generally effective, and most compliant patients can achieve the clinical target with acceptable rates of adverse events.
However, there is a growing cohort of patients who exhibit uncontrolled HTN with no durable therapeutic option. Recent studies show the prevalence of clinically relevant resistant HTN to be 10–14% (and growing) among patients with HTN5,6. There is a higher prevalence in certain populations such as obese and older patients7 and given global trends the incidence of uncontrolled HTN is likely to continue growing. Another relevant component of the pathophysiology is the frequent comorbidity of atherosclerosis with resultant stiffening and inflammation of affected vessels often involving the carotid sinus (where the baroreceptors are located)8. Given the scope and nature of this clinical challenge, there is a critical need for alternative therapeutic modalities.
Researchers have been actively pursuing neuromodulation treatments for HTN via the baroreflex for decades9. Because the autonomic nervous system (ANS) exerts strong control over BP, the ANS often plays a critical role in the pathophysiology of drug resistant HTN10. By reducing sympathetic activation and increasing parasympathetic tone, baroafferent stimulation addresses mechanistic elements contributing to cardiovascular disease including HTN comorbid with obstructive sleep apnea11,12. For example, loss of baroreflex sensitivity and the resultant autonomic imbalance have negative prognostic implications in cardiac disease and have been associated with increased cardiac mortality after myocardial infarction13. However, an electrode interface that reproducibly and selectively activates baroafferent fibers via direct CSN stimulation to lower BP in humans has not been shown.
The growing clinical need and recent advances in microelectronics and electrode technology14 have led to a resurgent interest in this therapeutic modality. Recent efforts to translate technology in the treatment of uncontrolled HTN (including carotid sinus stimulation and renal denervation) that leverages advances in understanding of the pathophysiological role of the ANS have shown promise15,16,17,18,19,20. These device-based treatments of HTN reduce BP indirectly, by modulating some aspect of the baroreflex. For example, existing devices seek to restore the baroreflex via endovascular amplification, renal denervation for reduced sympathetic tone, or electrical stimulation of baroreceptors in the carotid body21. The latter neuromodulation approach relies on a neurointerface that we view as suboptimal. A surface electrode in the area of the baroreflex tissue (carotid sinus wall) can only access a limited number of baroreceptors and there is a higher risk of off-target stimulation given the configuration.
We report the results of a novel approach of direct and modest carotid sinus nerve stimulation in anesthetized humans that generates significant and synchronous drops in blood pressure (BP) and heart rate (HR). We hypothesized that direct unilateral carotid sinus nerve (CSN) nerve stimulation—using an application-specific electrode and a uniquely adapted surgical approach to the CSN—would more efficiently interface with baroafferent pathways causing more effective BP reduction and less off-target effect. The surgical approach, the electrode configuration, and electrode placement were unique in the way the electrode array interfaced with terminal branches of the CSN. This location was also optimal as it was readily surgically accessible, eliminating the need for an extensive skull base dissection to isolate the CSN. The goal of this feasibility study was to test the electrode and surgical methodology in anesthetized surgical patients who already had the carotid bifurcation exposed for other therapeutic indications. The primary outcome was the acute relationship between direct CSN stimulation and BP/HR.
Methods
Study design
This was an observational prospective feasibility study of an acute peripheral neuromodulation device. The Louis Stokes Cleveland Veterans Affairs Medical Center Institutional Review Board approved the study, which was performed in accordance with relevant regulations in the United States and the Declaration of Helsinki. Informed consent was obtained from all participants before any study procedures.
Patients were enrolled based on surgical eligibility regardless of a HTN diagnosis. Eligible patients were those scheduled to undergo elective surgical procedure that therapeutically indicated exposure of the carotid bifurcation and vascular sheath (e.g. carotid endarterectomy, neck dissection, or carotid tumor Resection) regardless of a HTN diagnosis. Exclusion criteria included disease states such as a history of stroke, or prior ipsilateral neck surgery or radiation treatment. Cardiovascular disease that precluded enrollment were diagnoses which independently increased surgical and anesthetic risk (American Society for Anesthesia Classification System). Detailed exclusion criteria are in Supplemental Materials.
CSN electrode
The custom electrode developed for CSN neuromodulation was based on the Composite Flat Interface Nerve Electrode (C-FINE)22,23 which has been used in clinical trials for upper and lower extremity applications for more than a decade. The electrode array was specifically designed to encompass nerve tissue and interface with all aspects of the enclosed tissue. It applies a small force causing a flat geometric reconfiguration of enclosed tissue without compromising blood flow, thus preserving neural tissue health. This optimizes selective access to axons for effective activation via embedded multi-contact platinum electrodes. When applied to the mobilized tissue of the CSN, the C-FINE enabled the neuro-interface without having to dissect or surgically isolate individual carotid sinus (baroafferent) fibers. A contact density of one contact every 0.5 mm along the internal surfaces of the C-FINE was used to decrease the distance from one or more of the electrical contacts to baroafferent fibers.
Hemodynamic monitoring
Prior to surgery, arterial lines were placed in all patients (by the anesthesia team) to monitor vitals such as BP and HR. An anesthesia hemodynamic monitoring system (HemoSphere, Edwards Lifesciences, Irvine CA) recorded BP and HR every 2 s; displayed values were used operatively to guide the CSN stimulation protocol. A 20-second update interval was used in which BP and HR values were averaged. After each procedure, BP and HR data were downloaded from the system for post-hoc data analysis.
Anesthesia
Anesthesia was performed under the supervision of the same anesthesiologist for all trial patients. A predetermined protocol was followed to minimize effects on hemodynamic functioning. Full anesthesia details are presented in the Supplemental Materials. During the experimentally relevant dissection and placement of the C-FINE, any extraneous tissue manipulation was avoided, as were vasoactive drugs unless required for patient safety. During CSN stimulation medication administration was kept unchanged and there was a complete absence of surgical manipulation. In general, patients were allowed to spontaneously ventilate during the experimental procedure. However, the anesthesiologist would ventilate patients on an ‘as-needed basis’ as they deemed clinically necessary. This introduced confounding variables with respect to collecting respiratory data. Therefore, given that we could not control those variables we did not record RR or R-R intervals. Stimulation was immediately discontinued if hypotension or bradycardia (SBP < 90 mmHg, HR < 50 bpm) needed to be treated.
Surgical implantation of the stimulating lead
The same two surgeons operated on all subjects. They worked together on 10 of the 14 total subjects and each surgeon worked alone in 2 cases. All subjects were placed in the supine position with the neck in gentle extension, prior to sterilely prepping the surgical field. All experimental interventions were performed under binocular loupe magnification and the dissection was delicately performed using fine tipped instruments.
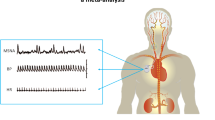
The experimental dissection followed exposure of the vascular sheath of the common carotid artery as it bifurcates into the internal carotid artery (ICA) and external carotid artery (ECA). The anterolateral surface of the carotid bifurcation along with the proximal ICA containing the more elastic and thin-walled dilation (sinus) was identified. The distal CSN was mobilized together with surrounding connective tissue superior to the sinus. The Vagus nerve and sympathetic trunk were excluded from the tissue mobilization. The electrode was passed around the mobilized tissue and folded fully encompassing it (see Fig. 1). Both sides of the electrode were approximated using polypropylene sutures to secure it in place. Irrigation was applied to prevent desiccation, which can compromise the conductive interface. The lead bodies were passed off the field for connection to the stimulator. A surgical retractor in the periphery of the surgical wound was used as a common, distant return electrode (anode) during stimulation.
Schematic representation of C-FINE applied to the CSN. (Left) The C-FINE causes a flat geometric re-configuration of enclosed tissue improving access to select nerve fibers. In the boxed region, note the application of the C-FINE close to the distal terminal of the CSN. Abbreviations: CCA = Common Carotid Artery, ECA = External Carotid Artery, ICA = Internal Carotid Artery.
Stimulation protocol
A three-phase protocol was used for all experimental procedures, which took place over 60 min of operative (Fig. 2). Stimulus was delivered with 100 Hz frequency, 100 µs pulse width (PW), and 1 mA pulse amplitude (PA) during contact mapping. Contact mapping used 20-second stimulus periods applied to sequential C-FINE contacts to identify those interfacing with baroafferent fibers based on direct arterial line measures of BP. Next, a recruitment protocol was used where PA was varied in 1-mA steps on the same C-FINE contact; stimulus was applied at the same frequency and PW for 60 s at each level of PA (1–8 mA per contact maximum). In some patients (when enough operative time was available) a third repetition protocol was used to repeatedly determine the effects of stimulus and post-stimulus recovery by applying stimulus to a previously identified baroafferent interfacing contact, and with PA observed to cause an observed drop in BP. During the repetition protocol, stimulus was applied for 60 s at a time with a 60 s washout period.
Stimulation protocol. Contact selection (A) was followed in all patients. The recruitment protocol (B) was followed in all patients for at least one C-FINE contact. The repetition protocol (C) was followed in some patients when there was adequate operative time. Abbreviations: PA = Pulse Amplitude, PW = Pulse Width.
Protocol deviations were occasionally used to maximize data acquisition in the limited operative time. Most commonly, stimulus was escalated on functioning contacts without allowing stimulation withdrawal during the recruitment protocol study phase, where PA was increased sequentially. Generally, the contact selection protocol took 15 min, and the recruitment protocol took 30–45 min depending on the number of identified interfacing contacts. Stimulus was applied using an external stimulator (ISIS Neurostimulator, Inomed Medizintechnik GmbH, Emmendingen, Germany). In all cases stimulation was halted if SBP dropped below 90 mmHg or HR dropped below 50 bpm.
Data analysis
The primary study endpoint was to determine the maximum drops in SBP, DBP, and HR during stimulation and the corresponding recovery following stimulation withdrawal. The maximum drop was calculated as the maximum change in SBP, DBP, and HR while stimulus was active, relative to the baseline value 30 s before starting stimulus. Maximum recovery was calculated as the largest change in SBP, DBP, and HR within 30 s of ending stimulus. A subgroup analysis was done dividing the responder group between those with resting SBP above and below 120 mmHg. Changes in outcome parameters (BP & HR) were compared using a Student’s [paired two-sample] t-test. Cumulative drops in SBP throughout the entire stimulation period were measured by pooling the baseline SBP values before the stimulation session and the final SBP at the completion of the last stimulation epoch. Values were compared using Student’s [paired, two sample] t-test.
A secondary analysis was performed to determine the generalized changes in SBP, DBP, and HR from stimulation across all stimulation epochs. The model was designed to ascertain the effect of stimulus compared to baseline (pre-stimulus) values for each tested outcome. Secondary data analysis used R statistical software (The R Foundation, R version 4.3.2 source version: “Eye Holes”) and the “rstan” package used to estimate the posterior probability density functions of the parameters24,25. The model used 24 chains, each with 1,000 warmup samples and 3,000 iterations. Further modeling details are contained in the Supplemental Materials.
Results
From November 2020 to August 2022, 18 patients undergoing surgical therapeutic interventions that involved exposing the carotid bifurcation met inclusion criteria and gave informed consent. Four patients were excluded either due to their clinical course or intraoperative bradycardia or hypotension that precluded stimulation. Demographic and procedure-related details of study subjects are presented in Table 1. Of the 14 patients who met inclusion criteria, 8 underwent carotid endarterectomies for internal carotid artery stenosis and 6 selective neck dissection (which included primary cancer excision) in the treatment of head and neck cancer with cervical nodal metastases. In 13 cases, the surgical procedures nominally resulted in unilateral carotid bifurcation and carotid sinus exposure and the disease process did not affect the perivascular sheath tissue in and around the carotid sinus. In one patient, the dissection to address the primary disease was considered likely to have interfered with the terminal CSN (detailed below).
During the indicated therapeutic operation there were no complications in any of the 14 included patients. Throughout the stimulation protocol, there was no evidence of off-target stimulation in any patient—there was an absence of any observable motor stimulation of the larynx, laryngeal strap muscles, trapezius and sternocleidomastoid muscles (supplied by cranial nerve XI), tongue musculature or facial muscles (supplied by cranial nerves XII and VII respectively).
Postoperatively, there were no known complications or adverse events in any patient related to either the study or therapeutic intervention (follow-up of 24–45 months). Specifically, there was no evidence of any post-surgical cardiovascular or cerebrovascular disease (beyond pre-existing conditions). There was no observed or reported manifestations of injury to the Vagus nerve or any of its branches, the facial nerve branches, the accessory or hypoglossal nerve, or the sympathetic trunk. Two patients died of unrelated causes in the follow-up period (Covid and lung cancer).
Primary outcomes
13 of 14 subjects exhibited evidence of baroreflex activation with immediate and synchronous drops in both BP and HR in response to stimulation of at least one C-FINE contact (Fig. 3). In the non-responder (an endarterectomy patient) the extent of atherosclerotic disease which necessitated a more extensive surgical dissection around the bulb (sinus) raised concern about neuropraxia or disruption of the terminal CSN fibers. In responders, stimulation caused a 24 ± 20 mmHg (mean ± SD, p < 0.01) maximum drop in SBP with associated drops in DBP (11 ± 9 mmHg, p < 0.01), and HR (10 ± 9 bpm, p < 0.01). SBP, DBP, and HR all recovered immediately following stimulation withdrawal (see Fig. 4).
Patient 008 blood pressure and heart rate tracing during CSN Stimulation. A small delay can be seen between the application of stimulus and response. Note that different contacts were stimulated in different stimulation epochs which explains the heterogeneity of wave morphology. The stimulation epoch marked by the arrow caused BP to drop enough to trigger a protocol-mandated immediate withdrawal of stimulation.
Maximum blood pressure drops and recoveries per patient. All drops are relative to the baseline value 30 s before stimulation (not the patient resting BP). Recovery is relative to the value achieved during stimulation, occurring within 30 s after stimulation ceased. Note that responses were larger in patients with pre-stimulation baseline SBP > 120mmHg.
Sub-group primary outcomes
In subjects with baseline (pre-stimulation, not resting) SBP > 120 mmHg (n = 8), the average maximum SBP drop was 31 ± 23 mmHg, while subjects with baseline SBP < 120 mmHg (n = 5) exhibited an average maximum SBP drop of 11 ± 3 mmHg (Table 2). DBP and HR drops were significant in both groups (nominal p < 0.05).
The Pearson’s linear correlations between pre-stimulation and with-stimulation BP [drops] were 0.46 (p < 0.001) for both SBP and DBP, while there was a weaker correlation between pre-stimulation and with-stimulation HR (r = 0.12, p = 0.025).
The data also revealed cumulative declines in the SBP throughout the whole stimulation session in 12/13 responders with an average cohort drop from 130.9 to 119.8 mmHg (n = 13, p < 0.05) mmHg (Fig. 5). Dose dependency was also suggested but the experiment design prevented statistical conclusions (Figs. 3 and 5).
Patient 009 blood pressure and heart rate tracing during CSN stimulation. Note the progressive and cumulative drop in SBP across the entire stimulation period—an effect seen in most responders. Note also the different channels used to in each cluster of stimulation. The arrow to the right of the 35-minute marker indicates the administration of phenylephrine (a vasoconstrictor given to support BP) by the anesthesiologist.
Secondary outcomes
Using multilevel modeling, the pooled analysis of all stimulation epochs with washout of 30, 45, and 60 s each showed posterior means and confidence intervals revealing significant effects (see Table 3). The washout is the time allowed to elapse following either stimulation ‘on’ or ‘off.’ A 30 s washout was used to include the most stimulation epochs and subjects (Fig. 6). Pooled analysis showed a significant drop in SBP of −11.2 mmHg (95%CI: −17.0, −5.2), DBP of −6.3 mmHg (95%CI −8. 8, −3.8), and HR −5.8 bpm (95%CI −9.1, −2.5). BP and HR recovered concurrently close to their respective baselines following stimulation withdrawal. Posterior means and confidence intervals of recovery following cessation of stimulation were also calculated (Fig. 7). BP and HR recovery averages following stimulation withdrawal showed no statistically detected differences from pre-stimulation baselines.
Secondary Analysis of Effects of CSN stimulation on SBP, DBP, and HR with 30 s washout. CSN stimulation caused the largest drops in SBP (A) with associated (smaller) drops in diastolic blood pressure (B). HR changes caused by CSN stimulation (C) tracked with SBP & DBP changes. The pooled analysis (D) suggested CSN stimulation, on average, dropped SBP by 11.2 mmHg, DBP by 6.3 mmHg, and HR by 5.8 bpm in this study.
Secondary Analysis of Recovery on SBP, DBP, and HR after CSN stimulation withdrawal using 30 s washouts. Posterior means and confidence intervals per patient were calculated to compare post-stimulation with pre-stimulation levels for SBP, DBP, and HR (A, B,C). Pooled posterior means and confidence intervals including all patients are also presented (D). Cessation of CSN stimulation led to recovery in all metrics.
Discussion
Primary outcomes
The data presented in this feasibility study support the safety and efficacy of direct unilateral CSN stimulation in people to lower BP in an acute anesthetized setting. Modest stimulation produced immediate and significant drops in SBP, DBP, and HR, which were reversed on withdrawal of stimulation. This suggests a robust and direct neural interface between relevant C-FINE contacts and baroafferent fibers of the CSN. Importantly, BP and HR drops in response to stimulation were concurrent as were the post-stimulation rebound of BP and HR across all epochs.
It is noteworthy that in our analysis of maximum BP drops (Fig. 4) the effect was greater when patients started with a baseline [pre-stimulation] BP > 120 mmHg. Furthermore, this correlation, between higher pre-stimulation SBP/DBP levels and larger observed BP drops during stimulation, was present when analyzing all stimulation epochs. Patients who began with lower BP (or a BP < 120mmHg in the analysis of patients’ maximum BP drop) were exposed to a greater risk of physiologically significant hypotension and hyperperfusion. That they did not drop as markedly could possibly be explained by the presence of anatomic and physiological factors. The contralateral CSN and the bilateral aortic depressor nerves were both intact and presumed functioning in these patients and would likely have buffered the drop in BP. In addition, there are other physiologic mechanisms which contribute to an overall homeostatic mechanism protective against hypotension. For instance, the hysteresis of the vagal limb of the arterial baroreflex has been studied and may reflect a protective mechanism against hypotension and hypoperfusion26,27.
There was also suggestion of dose dependency with drops in BP and HR as a function of escalating stimulation amplitude, however, the experiment was not appropriately designed to warrant statistical analysis of dose dependency. The cumulative BP reductions from repeated stimulations might suggest that repeated stimulation influences the BP set point. This was objectively reflected in data because overall values for BP recovery (following cessation of stimulation) were smaller than the values for BP drop during stimulation. Whether this effect would exist in a chronic stimulation setting is yet unknown.
Electrode placement
Compared to a neural interface that targets the baroreceptors in the region of the carotid sinus (as previously used for an implanted therapeutic system20), placement of the C-FINE around the CSN itself offers the potential for direct baroafferent nerve stimulation and therefore more efficient neuromodulation of baroafferent pathways. Also, the high incidence of atherosclerosis (and associated inflammation) in the population of patients suffering from refractory HTN28 has been linked to a reduction in carotid sinus compliance and barosensitivity and therefore compromised function29. This suggests that baroreceptors in the carotid sinus may not be an ideal neuromodulation target. A direct CSN interface strategy has indeed been previously explored30. This study demonstrates that with improved electrode technology it can be done reliably and without causing off-target motor stimulation. For instance, no evidence of any cranial nerve stimulation was noted.
There are two primary surgical challenges with direct stimulation of the CSN. One is the small and fragile anatomy of the CSN (reported average diameter of 0.8 mm in adult humans31). The CSN branches from the glossopharyngeal nerve at the skull base from where it descends along the internal carotid artery. As it approaches the carotid bifurcation the nerve arborizes to the numerous branches supplying the baroreceptors around the carotid sinus as well as the chemoreceptors of the carotid body32. The more superior the dissection (and closer to the skull base) the more risk there is of serious surgical morbidity. By limiting the dissection to the tissue near the carotid bifurcation the procedure is simplified, but it introduces the second challenge wherein the small CSN baroafferent and chemoafferent fibers remain in close anatomic relationship with each other. Differentially stimulating baroafferent fibers is crucial in this application because off-target stimulation (e.g., chemoafferents) can cause undesirable effects.
We hypothesized that a specially designed electrode (C-FINE) could address both problems22. The paradigm was changed for this application given the different neuroanatomy of the CSN (relative to other applications). This necessitated modifications to the size, geometry and contact density of the electrode but the patterned stiffness of the C-FINE electrode design was essentially unchanged in this CSN application. It caused the enclosed tissue to assume a geometric shape that increased surface area (relative to tissue volume) reducing the distance between enclosed nerve fibers and at least one contact in the electrode array33. Importantly, prior studies have shown that when the electrode is secured, the applied pressure does not compromise blood flow to nerve tissue23,34,35. Therefore, we exploited the C-FINE design to separate out and differentially stimulate baroafferent fibers. In this study we employed a monopolar configuration with a distant return electrode (anode) in the periphery of the surgical wound (see Fig. 1) and, as noted, no patient exhibited evidence of any off-target motor activation.
The surgical procedure to gain access and place the electrode was similar to other procedures commonly performed in the neck. The necessary dissection was highly accessible via a cervical incision. Technically, the carotid sinus was easily located, and preoperative ultrasound can be used prior to incision to verify accessibility of the carotid sinus. In all subjects the incision was placed sufficiently low in the neck so that there was little risk to the marginal mandibular branch of the facial nerve and aesthetically placed in a relaxed skin tension line or neck crease. There were no known cranial nerve injuries in this study.
Neuromodulation response
The physiologic response to stimulation—characterized by a simultaneous decrease in BP and HR with stimulation (with activation of specific contacts) and the inverse with withdrawal—can be interpreted as having activated the baroreflex largely to the exclusion of the chemoreflex. In normal humans there is evidence that the activated baroreflex downregulates the chemoreflex and vice versa36. There are, however, other studies indicating a more nuanced relationship37. In our study, we could not formally monitor respiratory rate/effort as a function of stimulation as a result of respiratory hemodynamic and anethesia concerns. Therefore, no firm conclusion can be drawn concerning the degree of chemoreceptor activation. Nonetheless, it is noteworthy that in all responders, the effect of decreased BP and HR were confined to select contacts within the electrode array. In some patients in this study, we observed that stimulation of at least one contact caused an increase in both SBP and respiratory rate (in spontaneously ventilating patients) but given the study design this did not warrant further analysis. These observations suggest the C-FINE was selective of baroafferent and possibly chemoafferent fibers as well.
To investigate the therapeutic potential of CSN stimulation in patients suffering from uncontrolled HTN, long term (chronic) studies are needed with fully implantable systems in a diverse patient population with appropriate indications. Long-term ambulatory testing will be critical to testing electrode function, safety, as well as treatment efficacy and durability. Chronic studies will also help to better understand how this peripheral CSN stimulation modality affects the baroreflex vs. chemoreflex and the interactions between the two.
Study limitations
This was an acute feasibility study with a small sample size. A confounding variable is heterogeneity in this cohort; not all patients had HTN, and some had atherosclerosis, among varying other diseases. The study population lacked racial, gender, and ethnic diversity, in part due to the small number of participants.
The limited time available for stimulation made it difficult to comprehensively follow the stimulation protocol. For example, the dose-response curve between stimulus amplitude and BP or HR drops likely exists but could not be properly defined across all patients. Because patients experienced differing levels of stimulus, and over differing time points, we analyzed only maximum changes induced by CSN stimulation (per patient), and the pooled effects of stimulation (regardless of amplitude) in a mixed effects statistical model to address the experimental heterogeneity. Our results cannot describe the general response to CSN stimulation versus stimulus amplitude, or the cumulative and/or hysteretic effects of repeated cycles of CSN stimulation. We did observe such trends in some individuals, which warrants further investigation, but likely is not feasible in an acute, intraoperative setting with human patients.
One confounding variable was the general endotracheal anesthesia to which all patients in this study were subjected. Many anesthetic agents depress BP, which would be expected to mute the effect of CSN stimulation. Anesthetics, whether inhaled (volatile) or IV, affect the cardiovascular system though, generally, in a dose dependent fashion38. In general, the multimodal anesthetic plan used in this study allowed attenuation of responses to anesthetic medications but even this could not completely prevent a blunting of the true effect of stimulation.
Conclusion
In this acute human study in patients under anesthesia, using a novel electrode and surgical approach we consistently elicited significant and concurrent decreases in BP and HR with direct unilateral CSN stimulation suggesting an effective interface with baroafferents. Both BP and HR recovered simultaneously and immediately upon cessation of stimulation. There were observations of a stimulation-dose dependent relationship though the study was not designed to properly characterize this. The electrode and surgical technique described here could potentially play an important role in device-based therapy for patients who suffer from uncontrolled HTN.
Clinical perspectives and translational outlook
Clinical perspectives
The carotid sinus nerve can be directly stimulated to cause significant, reproducible and reversible decreases in blood pressure concurrent with HR. This result suggests an effective interface with baroafferents. The study was not designed to detect the dose-dependent relationship between CSN stimulus amplitude and BP changes; results represent stimulation of varying pulse amplitude greater than 1 mA. The electrode design and surgical technique described here could potentially play a role in long-term device-based therapy for patients who suffer from uncontrolled HTN.
Translational outlook
Direct stimulation of the CSN to treat uncontrolled HTN has not been implemented in humans due to the fragility, small scale and complexity of the nerve. This study demonstrated that direct stimulation of the baroafferent fibers within the CSN in a human population is indeed feasible using specially designed electrodes that address the anatomic, physiologic and surgical challenges. Due to the rapid and reversible nature of stimulation on BP, future work should include closed-loop approaches to stimulation based on real-time measurements of patient conditions. Future work should also focus on the chronic durability of the effects of direct CSN stimulation, and the selective activation of baroafferent over chemoafferent fibers in a chronically implanted device using the same stimulating electrode.
Data availability
The raw blood pressure recordings, study notes, and extracted/derived datapoints in this study are not openly available and are available from the corresponding author upon reasonable request. Data are located in a data storage at Case Western Reserve University.
Abbreviations
- ANS:
-
Autonomic nervous system
- BP:
-
Blood pressure
- bpm:
-
Beats per minute (unit)
- CCA:
-
Common carotid artery
- CEA:
-
Carotid endarterectomy
- C-FINE:
-
Composite Flat Interface Nerve Electrode
- CI:
-
Confidence interval
- CN IX:
-
Cranial nerve 9
- CNS:
-
Central nervous system
- CSN:
-
Carotid sinus nerve
- DBP:
-
Diastolic blood pressure
- ECA:
-
External carotid artery
- HR:
-
Heart Rate
- HTN:
-
Hypertension
- ICA:
-
Internal carotid artery
- mmHg:
-
millimeters of mercury (unit)
- PA:
-
Pulse amplitude
- PW:
-
Pulse width
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- UADT:
-
Upper aerodigestive tract
References
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71(6), e13–e115 (2018).
Brant, L. C. C. et al. The burden of resistant hypertension across the world. Curr. Hypertens. Rep. 24 (3), 55–66 (2022).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J. Am. Coll. Cardiol. 76 (25), 2982–3021 (2020).
Oparil, S. et al. Nat. Reviews Disease Primers ;4:18014. (2018).
Groenland, E. H. et al. Apparent treatment resistant hypertension and the risk of recurrent cardiovascular events and mortality in patients with established vascular disease. Int. J. Cardiol. 334, 135–141 (2021).
van der Sande, N. G. C. et al. Group on behalf of the S study. Apparent resistant hypertension and the risk of vascular events and mortality in patients with manifest vascular disease. J. Hypertens. 36 (1), 143 (2018).
Noubiap, J. J. et al. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart 105 (2), 98–105 (2019).
Milic, M. et al. A comparison of Pharmacologic and spontaneous baroreflex methods in aging and hypertension. J. Hypertens. 27 (6), 1243–1251 (2009).
Scheffers, I. J. M., Kroon, A. A. & de Leeuw, P. W. Carotid baroreflex activation: past, present, and future. Curr. Hypertens. Rep. 12 (2), 61–66 (2010).
Fernandez, G., Lee, J. A., Liu, L. C. & Gassler, J. P. The baroreflex in hypertension. Curr. Hypertens. Rep. 17 (3), 19 (2015).
Marrone, O. & Bonsignore, M. R. Blood-pressure variability in patients with obstructive sleep apnea: current perspectives. Nat. Sci. Sleep. 10, 229–242 (2018).
Tun, Y. et al. Nocturnal blood pressure during apnoeic and ventilatory periods in patients with obstructive sleep Apnoea. Eur. Respir. J. 14 (6), 1271–1277 (1999).
Rovere, M. T. L., Bigger, J. T., Marcus, F. I., Mortara, A. & Schwartz, P. J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 351 (9101), 478–484 (1998).
Denison, T. & Morrell, M. J. Neuromodulation in 2035: the neurology future forecasting series. Neurology 98 (2), 65–72 (2022).
Bhatt, D. L. et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 trial. Lancet 400 (10361), 1405–1416 (2022).
Rao, A. & Krishnan, N. Update on renal sympathetic denervation for the treatment of hypertension. Curr. Cardiol. Rep. 24 (10), 1261–1271 (2022).
Schmidli, J. et al. Acute Device-Based blood pressure reduction: electrical activation of the carotid baroreflex in patients undergoing elective carotid surgery. Vascular 15 (2), 63–69 (2007).
Wallbach, M. et al. Long-term effects of baroreflex activation therapy: 2-year follow-up data of the BAT Neo system. Clin. Res. Cardiol. 109 (4), 513–522 (2020).
Alnima, T., de Leeuw, P. W. & Kroon, A. A. Baroreflex activation therapy for the treatment of Drug-Resistant hypertension: new developments. Cardiol. Res. Pract. 2012, e587194 (2012).
Bisognano, J. D. et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J. Am. Coll. Cardiol. 58 (7), 765–773 (2011).
Lauder, L., Azizi, M., Kirtane, A. J., Böhm, M. & Mahfoud, F. Device-based therapies for arterial hypertension. Nat. Rev. Cardiol. 17 (10), 614–628 (2020).
Freeberg, M., Stone, M., Tyler, D. & Triolo, R. J. Chronic Response of the Cat Sciatic, Median, and Ulnar Nerves To a Compliant, Composite Flat Interface Nerve Electrode (C-FINE). IEEE Neural Engineering Short Papers 1 (2013).
Stone, M., Tyler, D. & Triolo, R. J. Mechanical Characterization of a Novel Multilayer Nerve Cuff Electrode with Regionally Patterned Stiffness. In: 6th International IEEE/EMBS Conference on Neural Engineering (NER). San Diego, CA. (2013).
Stan Development Team. RStan: the R interface to Stan.
Carpenter, B. et al. Stan: A probabilistic programming Language. J. Stat. Softw. 76, 1–32 (2017).
Low, P. A. & Tomalia, V. A. Orthostatic hypotension: mechanisms, causes, management. J. Clin. Neurol. (Seoul Korea). 11 (3), 220–226 (2015).
Studinger, P., Goldstein, R. & Taylor, J. A. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J. Physiol. 583 (3), 1041–1048 (2007).
Kumbhani, D. J. et al. On behalf of the REACH registry investigators. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur. Heart J. 34 (16), 1204–1214 (2013).
Ziegler, M. G. Atherosclerosis and blood pressure variability. Hypertension 71 (3), 403–405 (2018).
Testerman, R. L., Hagfors, N. R. & Schwartz, S. I. Design and evaluation of nerve stimulating electrodes. Med. Res. Eng. 10 (1), 6–11 (1971).
Toorop, R. J., Scheltinga, M. R., Moll, F. L. & Bleys, R. L. Anatomy of the carotid sinus nerve and surgical implications in carotid sinus syndrome. J. Vasc. Surg. 50 (1), 177–182 (2009).
Kansal, N., Clair, D. G., Jaye, D. A. & Scheiner, A. Carotid baroreceptor stimulation blood pressure response mapped in patients undergoing carotid endarterectomy (C-Map study). Auton. Neurosci. 201, 60–67 (2016).
Dweiri, Y. M., Stone, M. A., Tyler, D. J., McCallum, G. A. & Durand, D. M. Fabrication of high Contact-Density, Flat-Interface nerve electrodes for recording and stimulation applications. J. Vis. Exp. (116), e54388. (2016).
Tyler, D. J. & Durand, D. M. Chronic response of the rat sciatic nerve to the flat interface nerve electrode. Ann. Biomed. Eng. 31, 633–642 (2003).
Schiefer, M. A., Triolo, R. J. & Tyler, D. J. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 16 (2), 195–204 (2008).
Cooper, V. L., Pearson, S. B., Bowker, C. M., Elliott, M. W. & Hainsworth, R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia – a mechanism for promoting hypertension in obstructive sleep Apnoea. J. Physiol. 568 (2), 677–687 (2005).
Halliwill, J. R., Morgan, B. J. & Charkoudian, N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J. Physiol. 552 (1), 295–302 (2003).
Pardo, M. Miller’s Basics of Anesthesia (Elsevier Health Sciences, 2022).
Funding
Barologics, Inc. (Industry), Cleveland VA Medical Research & Education Foundation, VA Office of Research & Development (I50 RX001871; I01 RX003687). Dustin Tyler is supported by a Research Career Scientist Award (IK6 RX003836).
Author information
Authors and Affiliations
Contributions
S.M. and J.B. wrote the main manuscript text prepared the figures. S.M., J.B., D. T., and E. H. contributed to data analysis. All authors contributed to data acquisition. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosures
Barologics has licensed intellectual property from Case Western Reserve University. Dustin Tyler may receive future royalties. Jonathan Baskin became a surgical consultant for Barologics after all data were collected in this study. The remaining authors have nothing to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Majerus, S.J.A., Pinault, G., Tyler, D. et al. Direct carotid sinus nerve stimulation in anesthetized human subjects. Sci Rep 15, 26488 (2025). https://doi.org/10.1038/s41598-025-10091-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10091-1