Abstract
Shorter telomere length (TL) and postoperative delirium (POD) are associated with aging and inflammation. We hypothesized that shorter TL may predict POD development. This pilot study investigated whether preoperative TL can predict POD occurrence. This single-center, prospective, observational study included 50 patients aged > 65 years scheduled for postoperative intensive care unit stay ≥ 2 days. Patients with Intensive Care Delirium Screening Checklist scores ≥ 4 were categorized into the POD group. Multivariable logistic regression analyses evaluated preoperative TL as a predictor of POD. Ten patients developed POD (POD group) while 40 did not (non-POD group). Preoperative TL showed no significant difference between groups (POD vs. non-POD: 296,502 vs. 327,884 RLU/µg DNA, p = 0.104). However, multivariable analyses revealed that preoperative TL ≥ 309,110 RLU/µg DNA significantly associated with decreased POD risk after adjusting for age (aOR: 0.132; 95% CI: 0.022–0.799; p = 0.047) and preoperative MMSE score (aOR: 0.153; 95% CI: 0.028–0.851; p = 0.032). Shorter preoperative TL was associated with POD development after adjusting for age and preoperative cognitive function. Future studies with larger sample sizes are needed to confirm these associations.
Similar content being viewed by others
Introduction
Postoperative delirium (POD) is a common complication in older adults, marked by an acute and fluctuating disturbance in attention and cognition following surgery. POD has been reported to be related to adverse outcomes such as increased mortality rate, prolonged hospitalization, and higher healthcare cost1,2,3. Postoperative delirium is typically temporary and can be reversed in the majority of cases. However, it may lead to dementia, which is a chronic and progressive loss of previously acquired cognitive ability4 that can negatively affect the quality of life of patients and their families after surgery. Thus, it is important to predict the risk of POD before surgery and use focused care to prevent it in patients at risk.
Older adults are more likely to develop POD than young patients5,6, but the mechanism underlying POD development has not yet been elucidated. We previously reported that a higher preoperative neutrophil-to-lymphocyte ratio, which indicates preoperative chronic systemic inflammation, is associated with POD development7,8. Additionally, we also reported that intraoperative frontal relative ratios of the α-power of electroencephalograms were associated with the development of POD9. Thus, preoperative chronic inflammation and decreased intraoperative frontal relative ratios of the α-power of electroencephalograms may be one of the predictors for the development of POD known as “vulnerable brain”10. “Vulnerable brain” represents a pathophysiological condition where cumulative effects of aging, chronic inflammation, and neurodegeneration reduce brain reserve capacity, increasing the risk of postoperative cognitive complications including POD11. Similar to these, other preoperative factors may contribute to the “vulnerable brain”. Identifying such factors is not only useful for predicting POD preoperatively but also for elucidating the pathology of POD.
Telomeres are chromatin-based structures located at the ends of chromosomes12. The role of telomeres is to prevent the ends of chromosomes from being recognized as double-stranded breaks and protect them from degradation13. Telomere length (TL) varies across individuals and decreases by 30–50 bp during cell division14. Oxidative stress promotes aging and has also been reported to be involved in telomere shortening15. Shorter telomeres are linked with age-related disorders16, and inflammation has been reported to cause telomere shortening17. Thus, shorter telomeres may predict POD development, given that both aging and inflammation are associated with POD. However, no studies have investigated the relationship between theTL and POD.
We hypothesized that a shorter preoperative TL is linked to an increased risk of POD in the present study. This pilot study aimed to determine whether preoperative TL could predict the development of POD. In this study we measured leukocyte TL, because a previous study has shown that leukocyte TL correlates with TL in other tissues and reflects overall physiological aging18. Additionally, leukocyte TL measurement offers practical advantages including non-invasive sampling and standardized methodologies, making it suitable for perioperative research settings.
Methods
Study population
All patients were enrolled between January 31, 2023, and October 6, 2023. We included patients older than 65 years who underwent surgery and were expected to stay in the intensive care unit (ICU) for 2 days or more after surgery at Hirosaki University Hospital. We excluded patients who had undergone cardiopulmonary bypass surgery. We also excluded those with preoperative delirium, cognitive impairment (previous diagnosis of cognitive impairment or preoperative Mini-Mental State Examination [MMSE] score of < 24), psychiatric disorders, alcohol addiction, liver cirrhosis (Child Pugh class ≥ B), heart failure (The New York Heart Association classification ≥ 3), and hematologic disorder. Patients who used opioids, those who refused to participate in this study, and those with missing values were also excluded.
Ethical approval
This single-center, prospective observational study received approval from the Ethics Committee of Hirosaki University Graduate School of Medicine (2022-061). Verbal and written informed consent were obtained from all participants. The study was registered in the Japan Registry of Clinical Trials (jRCT1020220041; principal investigator: Satoshi Uchida, https://jrct.niph.go.jp/latest-detail/jRCT1020220041) prior to patient enrollment, with the registration date of January 30, 2023. All research methods were performed in accordance with Declaration of Helsinki. This manuscript adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Study protocol
The MMSE score was used to measure preoperative cognitive function one business day earlier. As mentioned above, patients with MMSE scores less than 24 were excluded from this study. On the day of surgery, blood samples were collected for the measurement of leukocyte TL in the operating room before anesthesia induction. The patients were transferred to the ICU after surgery. During the ICU stay, the patients were assessed for POD.
POD assessment
We used the Intensive Care Delirium Screening Checklist (ICDSC) to diagnose delirium. The maximum ICDSC score was 8 points, and the minimum score was 0. Higher ICDSC scores are associated with more severe POD symptoms. In this study, we defined POD as an ICDSC score of ≥ 4. Intensivists or ICU nurses familiar with the ICDSC but not involved in the study scored the ICDSC during ICU admission. Patients with an ICDSC score of 4 at one or more time points were assigned to the POD group. Patients with ICDSC scores of ≤ 3 were categorized into the non-POD group.
Anesthesia and ICU care
All surgeries were performed under general anesthesia, with or without epidural anesthesia, and standard monitoring. General anesthesia was induced and maintained using a combination of propofol, remimazolam, ketamine, remifentanil, fentanyl, morphine, and rocuronium. The depth of anesthesia was regulated with the bispectral index, ensuring values remained between 40 and 60. Following surgery, patients were transferred to the intensive care unit (ICU) while intubated. Continuous intravenous infusions of propofol, dexmedetomidine, and/or fentanyl were administered to maintain Richmond Agitation-Sedation Scale (RASS) scores between − 2 and 0 during mechanical ventilation.A spontaneous breathing trial (SBT) was performed at the discretion of an intensive care physician. Patients who passed the SBT were extubated after confirming awakening upon the discontinuation of anesthetics. After extubation, patients were observed during the postoperative course with an RASS score of −1 to 1 in the ICU. Patients were discharged from the ICU once hemodynamic and respiratory stability had been confirmed.
Study endpoint
The primary outcome of this study was the occurrence of POD during ICU stay, and we determined whether preoperative TL could predict the development of POD.
TL measurement
We performed modified phenol-chloroform genomic DNA extraction from whole blood samples within 72 h of collection. This processing timeframe is consistent with established protocols for TL measurement, as previous studies have demonstrated acceptable DNA stability for telomere assays when samples are processed within 72–96 h of collection19. A hybridization protection assay (HPA) was used to measure the TL20. The HPA is suitable for large-scale assessments using a 96-well luminometer. The telomere HPA probe was mixed with 5 µg of nondenatured genomic DNA and incubated at 60 °C for 20 min. Each sample was given hybridization buffer, and it was once more incubated for ten minutes at 60 °C. Luminescence of the plates was measured using a luminometer. The coefficient of variation for the TL was less than 10%.
Data collection
The following patient characteristics were collected from the electronic medical records of our hospital: sex, age, body mass index, medical history, final academic background, American Society of Anesthesiologists Physical Status, diagnosis, academic history, and surgical procedures. We collected the following perioperative data from anesthesia records and our hospital electronic medical records: preoperative and postoperative laboratory data including the white blood cell count, neutrophil percentage, hemoglobin concentration; hematocrit; platelet count; blood urea nitrogen, creatinine, aspartate transferase, alanine transferase, and C-reactive protein concentrations; durations of surgery and anesthesia; intraoperative use of anesthetics, opioids, and inotropes; intraoperative crystalloid use; colloid fluid administration; blood transfusion; intraoperative blood loss and urine output; postoperative use of anesthetics, opioids and inotropes; Numeric Rating Scale, RASS, and ICSDC scores; durations of ICU stay and hospital stay; and duration of mechanical ventilation.
Statistical analysis
We set the sample size to 50 patients based on recommendations that approximately 12 participants per group are needed for pilot studies21 and anticipated POD incidence of 24%22, which would provide approximately 12 patients with POD for this pilot study.
Patient characteristics were presented as medians (25th–75th percentiles) for continuous variables and as numbers (percentages) for categorical variables. Differences between the POD and non-POD groups were evaluated using Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Multivariable logistic regression analyses were conducted to assess whether preoperative TL could predict POD development, adjusting for potential confounders. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutoff values of preoperative TL for predicting POD in the multivariable logistic regression models. In general, at least 10 events per predictor variable are recommended for a reliable multivariable logistic regression model. However, a recent simulation study suggested that five to nine events per predictor variable might be sufficient23. In the present study, given the number of events (10 patients in the POD group), we included two variables in each model, with one variable assigned for every five events to ensure statistical reliability and avoid overfitting. Preoperative TL was forced into all models as an explanatory variable during multivariable logistic regression analyses. As aging and preoperative cognitive impairment are associated with the development of POD5,6,24, age and preoperative MMSE scores were included in multivariable logistic regression models 1 and 2, respectively. We also conducted ROC curve analysis to estimate the optimal cutoff values of age and preoperative MMSE score for predicting the development of POD and TL. Multicollinearity among variables was assessed using the variance inflation factor (VIF). Discrimination was evaluated by calculating the area under the curve (AUC). Results are presented as adjusted odds ratios (ORs) with their corresponding 95% confidence intervals (CIs). All data analyses were performed using EZR ver. 1.37 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). A P-value of < 0.05 was considered statistically significant for all tests.
Results
Patient characteristics
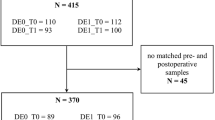
Of the 105 patients, 50 were included in this study (Fig. 1). Of the 50 patients, 10 were in the POD group, and 40 were in the non-POD group. The patient characteristics are shown in Table 1.
Perioperative data
The perioperative data for the patients are shown in Table 2. There were no significant differences in perioperative data between the two groups except for maximum ICDSC score (POD group vs. non-POD group; 4.00 [4.00, 4.00] vs. 1.00 [0.00, 1.25], p < 0.001).
Predictive value of telomere length
The preoperative TL did not differ significantly between the groups (POD group vs. non-POD group: 296,501.75 RLU/µg DNA [265,038, 308,665] vs. 327883.60 [283,672–365,401], p = 0.104) (Fig. 2). The ROC curves revealed that the cut-off value of preoperative TL, age, and preoperative MMSE score for predicting the development of POD were 309,110 RLU/µg DNA, 76 years, and 26 points, respectively (Table 3). Multivariable logistic regression analyses showed that preoperative TL of ≥ 309,110 RLU/µg DNA was significantly associated with a decreased risk of POD development after adjusting for age (aOR: 0.132; 95% CI: 0.022–0.799; p = 0.047) (Table 4, Model 1) and preoperative MMSE score (aOR, 0.153; 95% CI, 0.028–0.851; p = 0.032) (Table 4, Model 2). Age of ≥ 76 years was significantly associated with an increased risk of POD development (aOR: 0.5360; 95% CI: 1.030–28.00; p = 0.028) (Table 4, Model 1). All VIF values were < 5, indicating no significant multicollinearity among the variables.
Discussions
This pilot study investigated the potential relationship between preoperative TL and POD development. While univariate analysis showed no significant difference in preoperative TL between POD and non-POD groups (p = 0.104), likely due to the small sample size, multivariable logistic regression analyses showed that preoperative TL ≥ 309,110 RLU/µg DNA was significantly associated with decreased POD risk after adjusting for age or preoperative MMSE score.
The incidence of delirium is higher in older adults5,6, and it has become a common postoperative complication with the increased aging of populations. Additionally, minimally invasive surgeries such as robot-assisted surgery and transcatheter cardiovascular surgery are being developed. Thus, older adults with several preexisting conditions who were previously ineligible for surgery can now undergo surgery. This may have led to an increase in the number of patients with POD.
A previous animal experiment showed that cognitive impairment and increased hippocampal inflammatory cytokine concentrations were observed in older rats but not in younger rats after abdominal surgery25. Moreover, this study also showed that the release of proinflammatory cytokines after peripheral immune stimulation with lipopolysaccharide was higher in microglia isolated from aged hippocampi than in those isolated from young hippocampi. Thus, neuroinflammation caused by surgery-induced systemic inflammation, a potential cause of POD26, is more likely to develop in older than in younger patients. This may explain why older patients are more likely to develop POD. There was no significant difference between the ages of the POD and non-POD groups in this study, but multivariable analysis showed that ages of ≥ 76 years were significantly associated with the increased risk of POD.
The association between TL and cognitive vulnerability has been well documented in previous studies. Research in patients with mild cognitive impairment has shown that shorter TL are associated with faster progression to dementia27, which is consistent with our findings. A recent meta-analysis demonstrated that longer telomeres were associated with better global cognitive function and greater brain volume, particularly in elderly populations28. Additionally, a large-scale cohort study has reported that shorter TL are linked to impaired processing speed, a cognitive domain that is essential for maintaining attention and executive function29.
To the best of our knowledge, this pilot study is the first to show that a shorter TL is associated with the development of POD. Previous clinical studies have shown that TL shortening is involved in age-related diseases and inflammation15,16, and the results of this study may corroborate this. Additionally, an in vitro study showed that inflammation and oxidative stress play significant roles in accelerating TL shortening in cell cultures30. In summary, a shorter TL may also be a predictor of POD, given that it is a biomarker of the combined effects of inflammation, oxidative stress, and aging. The patients with shorter TL are more likely to have a “vulnerable brain”.
In this study, conventional inflammatory biomarkers such as white blood cell count, neutrophil percentage and C-reactive protein were not significantly different between the two groups. This absence of significant inflammatory differences could indicate that acute-phase inflammatory responses do not fully capture the biological processes underlying POD. Indeed, some previous studies have shown that preoperative C-reactive protein does not have a significant association with the development of POD31,32. Similarly, the relationship between TL and conventional inflammatory biomarkers has also shown mixed results. While some reports demonstrated associations between shorter TL and elevated inflammatory markers such as CRP and IL-633,34, others showed inconsistent results or no significant associations depending on populations and disease conditions35,36. These inconsistencies may be explained by the fact that conventional markers primarily reflect acute-phase inflammation, whereas TL represents cumulative inflammatory burden and chronic oxidative stress accumulated over an individual’s lifespan37,38. Therefore, preoperative TL assessment may provide insights into the cumulative impact of inflammatory stress over time, representing a more integrated assessment of cellular aging than conventional markers that reflect current inflammatory status. Our findings suggest that preoperative TL assessment could identify patients at higher risk for POD, which may enable targeted preventive interventions such as early mobilization protocols39 and optimized perioperative medication management40. Further studies are warranted to comprehensively compare the predictive value of TL versus conventional inflammatory markers for the development of POD, and to elucidate whether TL serves as a more robust indicator of cumulative inflammatory stress contributing to the “vulnerable brain”.
The present study had several limitations. First, because this was a pilot study, the sample size was small. Thus, there was no significant difference in TL between the two groups. Additionally, both multivariable logistic regression analyses showed that shorter TL was associated with the development of POD, but this result should be treated carefully given the limited statistical power and potential for Type II error. Additionally, age and preoperative MMSE scores were adjusted separately and not simultaneously in Models 1 and 2, respectively. Thus, if we include three variables–preoperative TL, age, and preoperative MMSE–in a multivariable logistic regression analysis according to the relaxed rule23, the main study needs 75 patients, assuming that the incidence of POD is 20%. Second, this study included patients who underwent three types of surgery: off-pump coronary artery bypass, esophagectomy, and free-flap reconstruction of the head and neck. These surgeries are relatively highly invasive and associated with a high incidence of POD. The types of surgeries performed for the two groups were also not significantly different. However, the degree of surgical invasiveness, postoperative course, and patient backgrounds were different, which may have affected the results. Thus, we focused on specific surgeries in our main study. As the present study showed that the incidence of POD was the highest in patients who underwent esophagectomy in our institution, focusing on patients who underwent esophagectomy may be the best option to reduce the sample size in the main study. Third, we only followed patients during their ICU stay, and we were not able to evaluate POD after discharge from the ICU. The median ICU stay for the POD and non-POD groups were both 4 days. As patients typically develop POD between postoperative day 1 and 1 week after surgery41, POD developed after discharge from the ICU may have been missed. We needed to follow up with the patients after discharge from the ICU in the main study. To address these limitations, our future main study will focus on esophageal cancer patients with adequate sample size (n = 75) and longer postoperative follow-up beyond ICU discharge.
In conclusion, while there was no significant difference in preoperative TL between POD and non-POD groups in univariate analysis, this pilot study showed that shorter preoperative TL was associated with increased POD risk after adjusting for age or preoperative cognitive function. While these preliminary findings are hypothesis-generating and should be interpreted cautiously due to the small sample size, they suggest that TL may serve as a potential biomarker for identifying patients at higher risk of POD. Future studies with larger sample sizes are needed to confirm these associations and determine their clinical significance.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Raats, J. W., van Eijsden, W. A., Crolla, R. M., Steyerberg, E. W. & van der Laan, L. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One. 10, e0136071 (2015).
Kirfel, A. et al. Postoperative delirium is an independent factor influencing the length of stay of elderly patients in the intensive care unit and in hospital. J. Anesth. 36, 341–348 (2022).
Brown, C. H. et al. The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann. Thorac. Surg. 101, 1663–1669 (2016).
Fong, T. G. & Inouye, S. K. The inter-relationship between delirium and dementia: the importance of delirium prevention. Nat. Rev. Neurol. 18, 579–596 (2022).
Inouye, S. K., Westendorp, R. G. & Saczynski, J. S. Delirium in elderly people. Lancet 383, 911–922 (2014).
Sadeghirad, B. et al. Perioperative factors associated with postoperative delirium in patients undergoing noncardiac surgery: an individual patient data meta-analysis. JAMA Netw. Open. 6, e2337239 (2023).
Oyama, T. et al. Higher neutrophil-to-lymphocyte ratio, mean platelet volume, and platelet distribution width are associated with postoperative delirium in patients undergoing esophagectomy: a retrospective observational study. J. Anesth. 36, 58–67 (2022).
Kinoshita, H. et al. Availability of preoperative neutrophil-lymphocyte ratio to predict postoperative delirium after head and neck free-flap reconstruction: a retrospective study. PLoS One. 16, e0254654 (2021).
Kinoshita, H. et al. The perioperative frontal relative ratio of the alpha power of electroencephalography for predicting postoperative delirium after highly invasive surgery: a prospective observational study. Anesth. Analg. 137, 1279–1288 (2023).
Shao, Y. R. et al. Low frontal alpha power is associated with the propensity for burst suppression: an electroencephalogram phenotype for a vulnerable brain. Anesth. Analg. 131, 1529–1539 (2020).
Fong, T. G., Vasunilashorn, S. M., Libermann, T., Marcantonio, E. R. & Inouye, S. K. Delirium and alzheimer disease: a proposed model for shared pathophysiology. Int. J. Geriatr. Psychiatry. 34, 781–789 (2019).
Slijepcevic, P., Hande, M. P., Bouffler, S. D., Lansdorp, P. & Bryant, P. E. Telomere length, chromatin structure and chromosome fusigenic potential. Chromosoma 106, 413–421 (1997).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Huffman, K. E., Levene, S. D., Tesmer, V. M., Shay, J. W. & Wright, W. E. Telomere shortening is proportional to the size of the G-rich telomeric 3’-overhang. J. Biol. Chem. 275, 19719–19722 (2000).
Kawanishi, S. & Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N Y Acad. Sci. 1019, 278–284 (2004).
Rossiello, F., Jurk, D. & Passos, J. F. D’Adda Di fagagna, F. Telomere dysfunction in ageing and age-related Diseases. Nat. Cell. Biol. 24, 135–147 (2022).
Wang, S. M. et al. Shortening of telomere length May be associated with inflammatory cytokine levels in patients with bipolar disorder. J. Affect. Disord. 365, 155–161 (2024).
Daniali, L. et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 4, 1597 (2013).
Zanet, D. L. et al. Blood and dried blood spot telomere length measurement by qPCR: assay considerations. PLoS One. 8, e57787 (2013).
Wai, K. M. et al. Telomere length and arterial stiffness reflected by brachial-ankle pulse wave velocity: a population-based cross-sectional study. J. Pers. Med. 11, 1278 (2021).
Julious, S. A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 4, 287–291 (2005).
Daiello, L. A. et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology 131, 477–491 (2019).
Vittinghoff, E. & McCulloch, C. E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 165, 710–718 (2007).
Weiss, Y. et al. Preoperative cognitive impairment and postoperative delirium in elderly surgical patients: a retrospective large cohort study (The CIPOD Study). Ann. Surg. 278, 59–64 (2023).
Kawano, T. et al. Impact of preoperative environmental enrichment on prevention of development of cognitive impairment following abdominal surgery in a rat model. Anesthesiology 123, 160–170 (2015).
Hirota, K. Preoperative management and postoperative delirium: the possibility of neuroprehabilitation using virtual reality. J. Anesth. 34, 1–4 (2020).
Koh, S. H. et al. Telomere shortening reflecting physical aging is associated with cognitive decline and dementia conversion in mild cognitive impairment due to alzheimer’s disease. Aging (Albany NY). 12, 4407–4423 (2020).
Gampawar, P., Schmidt, R. & Schmidt, H. Telomere length and brain aging: a systematic review and meta-analysis. Ageing Res. Rev. 80, 101679 (2022).
Mlakar, V. et al. Telomere biology and its maintenance in schizophrenia spectrum disorders: exploring links to cognition. Schizophr Res. 272, 89–95 (2024).
Houben, J. M., Moonen, H. J., van Schooten, F. J. & Hageman, G. J. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol. Med. 44, 235–246 (2008).
Sun, Y., Peng, H. P. & Wu, T. T. Postoperative C-reactive protein predicts postoperative delirium in colorectal cancer following surgery. Clin. Interv Aging. 18, 559–570 (2023).
Lemstra, A. W., Kalisvaart, K. J., Vreeswijk, R., van Gool, W. A. & Eikelenboom, P. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int. J. Geriatr. Psychiatry. 23, 943–948 (2008).
Masi, S. et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol. Med. 50, 730–735 (2011).
O’Donovan, A. et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the health, aging and body composition study. PLoS One. 6, e19687 (2011).
Mazidi, M., Katsiki, N., Mikhailidis, D. P. & Banach, M. Serum anti-inflammatory and inflammatory markers have no causal impact on telomere length: a Mendelian randomization study. Arch. Med. Sci. 17, 1228–1235 (2021).
Sater, M. S., AlDehaini, D. M. B., Malalla, Z. H. A., Ali, M. E. & Giha, H. A. A perceived dissociation between systemic chronic inflammation, age, and the telomere/telomerase system in type 2 diabetes. Biomedicines 13, 531 (2025).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (2002).
Jurk, D. et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 5, 4172 (2014).
Nydahl, P. et al. Early mobilisation for prevention and treatment of delirium in critically ill patients: systematic review and meta-analysis. Intensive Crit. Care Nurs. 74, 103334 (2023).
Qin, C., Jiang, Y., Lin, C., Li, A. & Liu, J. Perioperative Dexmedetomidine administration to prevent delirium in adults after non-cardiac surgery: a systematic review and meta-analysis. J. Clin. Anesth. 73, 110308 (2021).
Liu, X. et al. Emergence delirium and postoperative delirium associated with high plasma NfL and GFAP: an observational study. Front. Med. (Lausanne). 10, 1107369 (2023).
Acknowledgements
This study was supported by the Karoji Memorial Fund for Medical Research. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
AI collected data, performed statistical analysis, and drafted the manuscript. DT designed the study, collected data, performed statistical analysis, and drafted the manuscript. HK collected data and drafted the manuscript. SU designed the study, collected data, and drafted the manuscript. OM designed the study, collected data, analyzed telomere length, and drafted the manuscript. MS designed the study, collected data, analyzed telomere length, and drafted the manuscript. KN collected data and drafted the manuscript. YN collected data and drafted the manuscript. TK collected data and extensively revised the manuscript. KH designed the study, collected data, and extensively revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ishii, A., Takekawa, D., Kinoshita, H. et al. Relationship between telomere length and postoperative delirium: a single center prospective observational pilot study. Sci Rep 15, 24390 (2025). https://doi.org/10.1038/s41598-025-10288-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10288-4