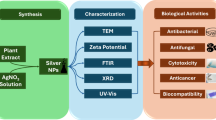
Abstract
Natural plant extracts provide a cost-effective and eco-friendly option for the synthesis of bimetallic nanoparticles, as opposed to traditional chemical or physical methods. This research involved the bio-fabrication of silver-titanium dioxide bimetallic nanoparticles (Ag-TiO2 BNPs) utilizing the leaf extract of Pluchea indica. The Ag-TiO2 BNPs underwent characterization through UV-vis spectroscopy, FTIR, TEM, XRD, and DLS techniques. The UV-Vis spectroscopy results revealed an absorbance peak at 350 nm, which confirms the successful synthesis of Ag-TiO2 BNPs. TEM observations revealed that the average diameter of the Ag-TiO2 BNPs varied between 10 and 60 nm. The assessment of the anticancer, antibacterial, and antioxidant bioactivities of the biosynthesized Ag-TiO2 BNPs was conducted. Results revealed that the IC50 of Ag-TiO2 BNP against Wi-38 normal cell line was 169.6 µg/mL. Moreover, Ag-TiO2 BNPs exhibited anticancer activity against MCF-7 cancerous cell line with an IC50 of 33.5 µg/mL. Furthermore, the produced Ag-TiO2 BNPs exhibited antibacterial properties against a range of pathogenic bacterial strains, with MIC varying from 31.25 to 62.5 µg/mL. Additionally, Ag-TiO2 BNPs showed antioxidant activity with IC50 225 µg/mL. In conclusion, Ag-TiO2 BNPs was successfully biosynthesized using P. indica leaves, where it had anticancer, antibacterial, and antioxidant properties.
Similar content being viewed by others
Introduction
The field of nanotechnology focuses on the regulation of materials at the molecular level. Its phenomenal expansion revealed respective opportunities for advanced scientific studies. It is important to be a supportive leader across various scientific fields specially medical sciences and technology1. A class of nanomaterials known as nanoparticles (NPs), exhibits exceptional properties and has a broad spectrum of scientific and medical applications.
There are multiple approaches to nanomaterial synthesis, such as chemical, physical, and biogenic methods. Nevertheless, traditional chemical and physical techniques often lead to the production of hazardous waste, posing serious environmental concerns and complicating waste disposal efforts2. In contrast, green synthesis has gained prominence as an eco-friendly and cost-effective a different approach for nanoparticle production, offering a safer route with minimal environmental impact3. Among biological sources, plants are considered particularly advantageous for nanoparticle synthesis due to their affordability, absence of toxic chemicals, faster reaction rates, and ability to yield highly stable nanoparticles compared to other biological systems4,5. Various biomolecules found in plants including proteins, enzymes, vitamins, phenolic compounds, tannins, terpenoids, saponins, quinones, tannins, and alkaloids contribute to the downscaling and capping of metal ions throughout nanoparticle formation6.
Moreover, biomolecules present in plant extracts assist in the encapsulation and surface functionalization of nanoparticles, effectively preventing their aggregation during green synthesis4. P. indica L. (Less), belonging to the Asteraceae family, has been traditionally recognized for its medicinal performance. Previous studies have shown that various parts of P. indica possess wound healing activities. For instance, ethanol extracts from the leaves were found to promote oral mucosa cell migration7, while methanol root extracts significantly enhanced wound healing in animal models by accelerating wound contraction, shortening epithelialization time, and promoting collagen deposition8. Extracts from the root, stem, and twigs have also exhibited strong antioxidant and antibacterial effects, likely attributed to the hydrogen or electron-donating capacities of their phenolic constituents9. However, to date, there has been no investigation into the antioxidant and wound healing properties of P. indica branch extract specifically, nor has its major bioactive compound been characterized10.
The field of nanobiotechnology, especially involving silver nanoparticles, offers expansive opportunities for applications across biomedical, environmental, and industrial domains11. Beyond their notable antimicrobial, antioxidant, and antibiofilm capabilities, AgNPs possess unique catalytic, optical, electronic, and energy-related properties that can be strategically exploited12.
In recent years, titanium dioxide (TiO₂) has attained widespread concentration as an environmentally friendly and efficient photocatalyst, attributed to its remarkable optical properties, high chemical stability, and low toxicity13. Titanium dioxide nanoparticles (TiO₂ NPs) are extensively utilized across diverse industries, incorporating cosmetics and pharmaceuticals14, where they contribute to skin protection against ultraviolet radiation and serve in products like sunscreens, paints, plastics, paper, inks, food additives, and toothpaste due to their whitening and opacifying characteristics15. Growing concerns over microbial resistance to antibiotics and metal ions have intensified research into alternative antimicrobial agents, with TiO₂ NPs exhibiting notable antibacterial activity16. Saisruthi et al.17 demonstrated that TiO₂ generates reactive oxygen species upon UV exposure, enhancing its use in antibacterial coatings, wastewater treatment, and even potential anticancer applications. Furthermore, polymer-functionalized TiO₂ NPs showed superior antibacterial effects against Escherichia coli and Staphylococcus aureus compared to unmodified TiO₂18. Althobaiti et al.19 attributed enhanced bactericidal performance to the dispersion of small silver clusters supported on TiO₂ NPs synthesized using ionic liquids. Additionally, multilayered films incorporating TiO₂ NPs as contact-active agents and nanosilver as release-active agents have been fabricated through layer-by-layer assembly, offering multifunctional antibacterial properties20. Shenasa et al.21 explored the bactericidal effects of diamond-like carbon films embedded with TiO₂ NPs, noting that bacterial wall oxidative damage and reduced interfacial energy contributed to increased antibacterial action. Herein, this study aimed to biosynthesize Ag-TiO2 BNPs using P. indica leaf extract for the first time. Furthermore, to evaluate their anticancer, antioxidant, as well as antibacterial activities.
Materials and methods
Chemicals and reagents
All chemicals and reagents employed in this study were of high analytical purity and sourced from reputable suppliers. Titanium nitrate tetrahydrate [Ti(NO₃)₄·4 H₂O] and silver nitrate (AgNO₃), were used as precursors for titanium and silver ions, respectively, and both were obtained from Sigma-Aldrich (Egypt). Distilled water was utilized for the preparation of the aqueous extract and throughout the biosynthesis process. Additionally, the MTT reagent (Sigma-Aldrich, USA) was applied for cytotoxicity and anticancer evaluations by measuring cell viability. Muller Hinton Agar (MHA; Sigma-Aldrich, USA) was employed for antimicrobial susceptibility testing, and DPPH reagent (Sigma-Aldrich, Cairo, Egypt) was utilized to determine antioxidant activity.
For the characterization of the synthesized Ag-TiO₂ BNPs, a UV-Vis (Model: JENWAY 6305, Staffordshire, UK). FTIR Spectrometer (Model: Cary 660 FT-IR, Agilent Technologies, USA). XRD (Model: XRD-6000, Shimadzu Corporation, Japan). TEM (Model: JEM-2100 Plus, JEOL Ltd., Japan) and DLS Apparatus (Model: Zetasizer Nano ZS, Malvern Panalytical Ltd., UK).
Plant collection and extract preparation
Pluchea indica leaves were collected, carefully cleaned, shade-dried, and excellently powdered to a fine consistency. Pluchea indica leaves were assembled from Al-Ayyat region, Giza, Egypt in December 2022. Prof. Dr. Abdou Marie Hamed, from the Botany and Microbiology Dep., Faculty of Science, Al-Azhar University, Cairo, Egypt, identified the plant species used in this research. The plant was kept in Faculty of Science herbarium, Al-Azhar University (Voucher no. 914). All plant-related experimental research and field studies, encompassing the gathering of plant materials, adhered to the necessary institutional, national, and international regulations and laws.
The aqueous leaves extract (LE) was prepared via mixing 5 g of powdered plant material and 100 ml of DW For 20 min, the mixture was then constantly stirred while boiling at 100℃ followed by filtration22.
Synthesis of Ag–TiO2 BNPs
At a uniform molar concentration of 4.0 mM, 100 mL of titanium nitrate tetrahydrate [Ti(NO₃)₄·4 H₂O] and 100 mL of AgNO₃ were separately added to 20 mL of P. indica LE in. The prepared mixture was stirred without interruption and heated to 70 °C on a hotplate. A gradual change in color signaled the formation of Ag–TiO₂ BNPs. The suspension was then centrifuged at 7500 rpm for 10 min23. The obtained pellet was washed several times with DW, then dried at 60 °C until a consistent weight was achieved. The nanoparticles, once dried, were stored in hermetically sealed containers at room temperature for further analysis.
Characterization of the biosynthesized Ag–TiO₂ BNPs
The development of nanoparticles was initially validated by UV–vis, utilizing the distinct SPR absorption. To detect the functional groups engaged in nanoparticle synthesis and stabilization, FTIR spectroscopy was employed. The crystalline nature and morphological characteristics of the synthesized Ag–TiO₂ BNPs were analyzed through XRD and TEM. Additionally, the particle size distribution was assessed using DLS analysis.
Cytotoxicity and anticancer activity
The cytotoxicity of biosynthesized Ag-TiO2 BNPs (1000–7.81 µg/mL) was assessed against Wi-38 normal cell line using MTT assay24. To form a monolayer sheet, cells were injected into a 96-well tissue culture plate and incubated. Decant the growing media and wash the cell monolayer after creation. After that, two-fold dilutions of the material were produced in RPMI medium and evaluated in various wells, with control wells receiving simply maintenance media. After incubation, the plate was checked for cell toxicity. After discarding the medium, a shaking table resuspended the formazan in DMSO. Finally, OD was measured at 560 nm and background subtracted at 620 nm to determine cell amount.
Likewise, its anticancer activity was examined using the same assay in presence of MCF-7 cancerous cell line. The cell viability and inhibition percentages, for both examined cell lines, were estimated using the following Equations:
Antimicrobial activity
The antimicrobial activity of Ag-TiO2 BNPs, Ti (NO3)4·4H2O, and AgNO3 was assessed against S. aureus ATCC 6538, B. cereus ATCC 10,987, K. pneumonia, P. aeruginosa, and E. coli. Each bacterial strain was aseptically inoculated onto Mueller-Hinton agar, and 100 µl of the of 1000 µg/mL was individually introduced into wells (6 mm) and incubated at 4 °C for 2 h. A 2 µg clindamycin disk served as the positive control. Following a 24-hour incubation of plates at 37 °C, the diameter of each inhibitory zone (IZD) was measured and documented25. MIC of Ag-TiO2 BNPs were carried according to method used by El-Didamony et al.26.
Antioxidant activity
DPPH method was used for evaluation the Antioxidant activity of Ag-TiO2 BNPs according to method used by El-Sayed et al.27 with minor modifications. In a 96-well microtiter plate, 100 µL of DPPH reagent was mixed with 100 µL of the sample (1000 − 7.81) µg/mL, then incubated for 30 min at 25 oC and measured at 490 nm. The DPPH scavenging activity was calculated using the following equation:
Results and discussion
Synthesis of Ag–TiO2 BNPs
Bimetallic nanoparticles (BNPs) are nanomaterials that combine two different metals into a single structure, offering unique properties that are often superior to those of individual components28. These nanoparticles exhibit an extensive array of biological and functional activities, making them highly desirable for multiple uses, including antimicrobial, antioxidant, and anticancer activities. Ag-TiO₂ BNPs were synthesized in this study with P. indica LE an eco-friendly method that leverages natural biological systems for nanoparticle fabrication. The combination of silver and titanium in the nanostructure enhances the material’s performance, offering promising biological activity. This segment presents the findings from the synthesis process and evaluates the biological potential of the Ag-TiO₂ BNPs.
The color change observed in the aqueous P. indica LE solution indicated the formation of Ag-TiO₂ BNPs. Initially, the solution was yellow before adding the mixture of silver nitrate and titanium nitrate tetrahydrate. After 30 min of reaction, it had turned dark brown. This color shift is likely owing to the increased SPR effect and the reduction of both metal precursors29. This characteristic is strongly influenced by the composition of the surrounding medium, coupled with the type, shape, size, and composition of the formed nanoparticles30. Thus, our results raised the opportunity that the plant extract serves a function in regulating the stability of the BNPs. Researchers have reported that colloidal silver-titania NPs demonstrates a variety of color tones, including brown and yellow31,32. Also, triterpenes biomolecule and methoxy groups found in AzadirachtaIndica LE may have a significant effect on the reduction and stability of AgNPs33.
Khaled et al.34 investigated the biological applications of Beta vulgaris leaf extract in the biosynthesis of Ag@SeO₂ and Ag@TiO₂ core-shell nanoparticles. In another investigation, Khalil et al.35 explored the biosynthesis of bimetallic Se-Au nanoparticles using P. indica LE. Similarly, watermelon peel was implemented for the biosynthesis of selenium-silver BNPs36. Alshahrani et al.37 employed an aqueous extract of onion peels served as a reducing and stabilizing agent in the synthesis of Ag-onion and Sm₂O₃-onion NPs, and Ag@Sm₂O₃-onion nanocomposites. Additionally, Krishnasamy et al.38 successfully biosynthesized Ag nanoparticles using Allium cepa L. peel extract, evaluating their cytotoxic, antioxidant, and antimicrobial properties. In another study, Abdelmoteleb et al.39 biosynthesize AgNPs from Prosopis glandulosa and Pluchea sericea. Gonfa et al.40 explored the synthesis of AgNPs applying Pluchea ovalis (Pers.) DC. extract and assessed their larvicidal activity against the fall armyworm (Spodoptera frugiperda). In Al Masoudi et al.41 work, environmentally friendly green biosynthesis of TiO2 NPs using leaf extract from Juniperus phoenicea and their characterization were their subjects.
The biosynthesis mechanism begins with dissolving AgNO₃ and Ti(NO₃)₄·4 H₂O in an aqueous solution containing P. indica LE. The plant extract is rich in various biomolecules such as flavonoids, phenols, and terpenoids. These bioactive compounds play a crucial role in the reduction of the metal ions42. Upon adding the metal precursors, these compounds act as electron donors, reducing Ag⁺ to Ag⁰ and Ti⁴⁺ to TiO₂.
The reduction process can be represented as:
Figure 1 shows reduction process, the silver ions are reduced to their metallic state, forming AgNPs. Simultaneously, titanium ions are reduced and form TiO₂, which can either exist as a shell around the silver core or as a part of the core-shell bimetallic structure43. The exact structure depends on the interaction between the silver and titanium com/ponents, which is influenced by the synthesis conditions and the ratio of the metal precursors.
The bioactive molecules found in the P. indica LE are essential to stabilizing the nanoparticles by inhibiting aggregation44. The molecules encase the nanoparticles, creating a protective barrier that stabilizes the particles and inhibits their clumping together. This stabilization is essential for preserving the size of the nanoparticles and improving their distribution in aqueous solutions45. Moreover, the creation of Ag-TiO₂ BNPs results in a significant color shift in the solution, transitioning from yellow to dark brown. The observed color shift can be explained by the SPR effect, a distinctive optical property associated with metallic nanoparticles, particularly those made of silver. The phenomenon observed is due to the synchronized movement of free electrons on the surface of nanoparticles when they interact with light, especially within the visible spectrum46. The interaction between silver and titanium ions in the nanoparticles enhances the SPR effect, giving rise to the observed color change.
Characterization of Ag-TiO2 BNPs
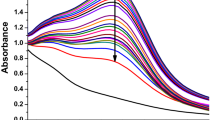
The UV-vis spectra of the combination containing the tested precursors and P. indica LE displayed a noticeable absorption peak at 350 nm (Fig. 2). The absorbance value of the resulting brown solution was measured from 200 to 800 nm, employing a UV-vis spectrophotometer (JENWAY 6305, Staffordshire, UK). Similarly, More et al.47 reported that the UV-Vis data of AgPtNPs confirmed the synthesis of BNPs, as evidenced by an absorption peak at 320 nm. Shkryl et al.48 observed an absorption peak at 409 nm in the UV spectra for Ag-Cu BNPs produced using Lithospermum erythrorhizon callus culture extract. Hashem et al.49 observed an experimental peak in the UV-visible spectrum with an optical density (O.D.) of 0.295 (after 30-fold dilution), corresponding to a wavelength of 380 nm during the watermelon rind-driven biosynthesis of selenium-silver BNPs. Krishnan Sundarrajan and Pottail50 reported that the SPR peak, observed at 552 nm, establishes the formation of Ag@Au BNPs synthesized using the fruit latex extract of Artocarpus heterophyllus in aqueous form.
On another study recorded by Akinola et al.51 the phytofabrication of Ti-Ag NPs by means of Cola nitida extracts that are optimally absorbed between 406 and 434 nm. The different shapes and sizes of NPs, which are influenced by the variation of reducing molecules in each extract, might be the cause of different absorption maxima52. According to Hasanin et al.53, ZnO-CuO NPs were successfully biosynthesized where absorption peaks were detected at 326, 365, and 410 nm that associated with the heterojunction properties of metal oxides. The development of AgNPs from Achillea fragrantissima flower extract was revealed by the findings of Alsayed et al.54, which demonstrated a significant absorption peak centered at 442 nm. The ZnO, TiO2, and BNPs’ maximum spectra were observed at 382 nm, 391 nm, and 387 nm, respectively, under a UV-vis spectrophotometer which validate BNPs synthesis55. Singh et al.56 informed that UV-vis spectroscopy was utilized to verify the synthesis of AgNPs. The SPR absorption bands characteristic for biosynthesis of AgNPs were measured at 432 nm and 444 nm for reactions performed at 25 °C and 60 °C, respectively. Similarly, Rana et al.57 examined the UV-Vis spectra of TiO₂NPs fabricated with Mangifera indica and Azadirachta indica extracts, exhibiting a maximum absorption peak at 370 nm.
FTIR spectrum
As shown in Fig. 3, the FTIR spectrum of Ag-TiO2 BNPs is provided. The P. indica leaf extract, particularly the brown layer, is rich in a range of phytochemicals, including phenolics, flavonoids, and dietary fibers58. These phytochemicals serve a fundamental role in accelerating the Ag-TiO₂ BNPs biosynthesis and stabilization. Distinct peaks at 3698 and 1569 cm⁻¹ were resulting from the deformative vibrations of water molecules. Additional lower peaks recorded at 2522, 2159, 2086, 2040, and 1346 cm⁻¹ were correlated to methyl stretching vibrations59, geminal methyl groups, amide I proteins38, and C = O stretching60. Also, reported peaks at 833, 659, and 532 cm⁻¹ associated with metal-metal or metal-oxide bonds61. These findings could suggest that the existing functional groups may accomplish reducing effects during the biosynthesis of Ag-TiO2 BNPs. Comparing the FTIR analysis of Se–Au BNPs by Khalil et al.62 with the Ag-TiO₂ BNPs in our study, we observed differences in spectral features. The Se–Au BNPs showed bands at 3,247 cm⁻¹ (hydroxyl stretching) and 1,714 cm⁻¹ (carbonyl group, C = O), while our spectra lacked the 1,714 cm⁻¹ peak, instead showing a peak at 1720 cm⁻¹, suggesting carbonyl group involvement in capping Ag-TiO₂ BNPs. Additionally, the Se–Au BNPs exhibited a band at 1,018 cm⁻¹ (C = O = C vibrations), which was absent in our spectra, indicating differences in plant-nanoparticle interactions. Comparing the FTIR spectra of our study (P. indica extract and Ag-TiO₂ BNPs) with the study by More et al.47 (using O. basilicum extract for AgPt nanoparticles), we observed differences in spectral features. The strong band at 1023 cm⁻¹ (C-C or C-O stretching) in their study was absent in our spectra, indicating different plant-nanoparticle interactions. Additionally, the 1720 cm⁻¹ peak suggesting carbonyl group involvement in capping was not observed in our study. Consequently, the FTIR observations confirmed the presence of methyl groups, proteins, flavonoids, amides, phenolics, and indicated the integration of these phytochemical components into the biogenic Ag-TiO2 BNPs.
TEM and DLS analysis
The TEM image clearly revealed the surface topography and particle size of the biosynthesized Ag-TiO₂ BNPs. The Ag-TiO₂ BNPs produced in this study exhibited a spherical shape covering a size range of 10 to 60 nm, with a mean size of 50 nm (Fig. 4A). The spherical nanoparticles produced exhibit minimal scattering and uniform characteristics, rendering them ideal for a variety of applications, covering imaging, catalysis, and drug delivery63. Khalil et al.62 stated the size distribution of Se–Au BNPs, with an average particle diameter of 44.5 nm and a range from 5.00 to 65.3 nm. Using TEM, Selim et al.64 confirmed that the MgO-ZnO nanocomposite synthesized with P. indica leaf extract had stable particles ranging in size from 5 to 35 nm.
Dutta et al.65 reported that the ZnO-Ag BNPs had a size range from 20 to 50 nm. Elkady et al.66 disclosed that CuO-Se BNPs exhibited a spherical shape, with a mean particle size of 30 nm and a size distribution between 5 and 50 nm, synthesized using Lagenaria siceraria leaf extract. Smaller nanoparticles tend to exhibit higher biological reactivity. Akinola et al.67 demonstrated through TEM imaging that biosynthesized Ti-Ag BNPs from Cola nitida were primarily spherical in shape.
The DLS findings are commonly shown as a histogram or intensity-weighted size distribution curve which help in determination of the NPs size distribution in a solution or suspension. DLS provides a measurement of the hydrodynamic size of nanoparticles, which comprises both the particles’ actual size and the enclosing layer of solvent or stabilizing agents, such as proteins or surfactants. This often leads to a larger size being determined when compared to TEM. It offers detailed information about different particle sizes distribution within the tested sample. The DLS results showed Ag-TiO2 BNPs possessing an average particle size 79 nm (Fig. 4B). Dynamic light scattering technique for size analysis the hydrodynamic radius of nanoparticles, taking into account the solvent shell formed by surrounding water molecules. As a result, DLS typically reports larger particle sizes compared to TEM. However, TEM provides more accurate measurements of the true particle size by excluding the effect of the solvent layer. In a similar manner, Kumaravel et al.55 reported ZnO/TiO2 BNPs with a cubic shape and size ranged from 50 to 500 nm. Ahmad et al.68 reported the synthesis of Mn + Cu BNPs using Vinca rosea, which exhibited a narrow size distribution with an average particle size of approximately 136.9 nm. More et al.47 reported that the size of green-synthesized BNPs from Ocimum basilicum (AgPt NPs) was approximately 59 nm, as shown by DLS results. The polydispersity index (PDI) was 0.159, indicating a low level of dispersion and good particle quality. These findings might be due to the interactions amongst the bioorganic capping compounds attached to different NPs. The PDI value in this study was 0.242. Depending on the current findings, the biogenic Ag-TiO2 BNPs showed a moderate mono-size dispersion.
X-ray diffraction analysis
The biogenic Ag-TiO2 BNPs characterization using XRD illustrated in Fig. 5. The exclusive diffraction peaks recognized in the XRD pattern confirm the crystalline arrangement of the assessed Ag-TiO2 BNPs. Obvious peaks were seen at 2θ values of 32.40°, 38.01°, 46.14°, 54.51°, and 77.02°, matching to the (111), (200), (120), (202), and (311) planes, respectively. The peaks signify the face-centered cubic structure and crystalline characteristics of the biosynthesized AgNPs. These are strikingly similar to JCPDS card No. 04-0783, which delineates the cubic structural system of AgNPs69. Singh et al.56 reported diffraction peaks at 2θ values of 38.06°, 44.23°, and 67.43°, which correspond to the (111), (200), and (220) planes, respectively, thereby confirming the crystalline character of AgNPs. The average crystallite size of the biosynthesized AgNPs was determined to be 35 ± 2 nm at 25 °C and 30 ± 3 nm at 60 °C using Scherrer’s equation. Similarly, another study utilizing aqueous Cucumis prophetarum LE for AgNPs synthesis observed peaks associated with the (111), (200), and (311) planes70.
The XRD pattern of the biosynthesized TiO₂ NPs (Fig. 5) displays five distinct diffraction peaks at 2θ values of 27.5°, 38.01°, 44.01°, 54.51°, and 69.70°, corresponding to the (110), (200), (210), (120), and (301) planes, respectively. These peaks are consistent with the tetragonal anatase phase of TiO₂, as confirmed by JCPDS Card No. 21-1272, indicating good crystallinity71. This observation is consistent with TiO₂ NPs synthesized using Kniphofia foliosa root extract, which showed diffraction planes corresponding to the tetragonal crystalline structure72. Furthermore, the inverse relationship between peak broadening and particle size validated the nanoscale dimensions of TiO₂ NPs73.
The Scherrer equation (=/cos) also estimated the average crystalline size of the Ag-TiO₂ BNPs to be approximately 50 nm. The broadening of the peaks supports the nanoscale nature of the synthesized materials, confirming the successful formation of Ag-TiO₂ BNPs with expected nanometric features73.
Cytotoxicity and anticancer activity
The preliminary assessment of the cytotoxic activity of novel substances on normal human cell lines is fundamental for assessing their biosafety for possible human use74. This assay investigates the cytotoxicity of Ag-TiO2 BNPs against the Wi 38 normal cell line using different concentrations, as represented in Fig. 6A&B. The analysis illustrates an IC50 value of 169.6 µg/mL, indicating that Ag-TiO2 BNPs are considered safe for use as the IC50 ≥ 90 µg/mL classifies the tested compound as non-toxic75.
The anticancer effects of Ag-TiO2 BNPs were examined using the cancerous MCF-7 cell line, as demonstrated in Fig. 7. The findings proven a significant antitumor effect, with an IC50 of 33.5 µg/mL. Besides, different safe concentrations of Ag-TiO2 BNPs showed notable cell inhibition rates of 84.2%, 76.0%, 48.0%, 13.0%, and 2.0% at concentrations of 125, 62.5, 31.25, 15.62, and 7.81 µg/mL, respectively. The biogenic Ag-TiO2 BNPs exhibited remarkable efficiency against cancer cells at safe concentrations, suggesting its potential anticancer activity pending further in vivo surveys.
The BNPs have been revealed to exhibit inhibitory effect on a variety of cancerous cell lines at low concentrations, according to various research published in the scientific literature. The biogenic Ag-ZnO BNPs, which were derived from the extract of pomegranate peel, shown potential anticancer action against malignant cell lines CaCO-2 and MCF-7, with an IC50 value of 52.4 and 104.9 µg/mL, respectively76. Moreover, Elsayed et al.77 proven that Ag-ZnO NPs have anticancer activity against HCT-116 and HeLa cancer cell lines. Furthermore, Ag-Au BNPs displayed in vivo antimetastatic activities78. Additionally, Au-Ag BNPs gave activity toward HeLa and DU 145 cell lines79. Besides, Mittal et al.80 recorded that Se-Ag BNPs have antitumor activity on Dalton lymphoma cells with inhibition percentage 80% at a concentration of 50 µg/mL.
Figure 8 shows anticancer mechanism induced by BNPs, showing how their internalization through endocytosis leads to multiple harmful effects within the cell. Upon entering the body, BNPs contribute to the production of reactive oxygen species (ROS), which in turn induce oxidative stress and injury to cellular components. These nanoparticles also target mitochondria, resulting in mitochondrial dysfunction and further ROS production. Additionally, BNPs can penetrate the nucleus and directly cause DNA damage. The combined effects of DNA damage, mitochondrial dysfunction, and elevated ROS levels activate caspase enzymes, ultimately triggering apoptosis, or programmed cell death81,82.
The Ag-TiO2 may be exerting anticancer effect through various synergistic mechanisms. It may be generates ROS upon activation with consequent oxidative stress, damages cancer cell components, and finally induced apoptosis83. Additionally, the existence of silver ions improves antimicrobial properties, lowering the risk of infections that can complicate cancer therapy84. Moreover, the electrochemical interactions between the positively charged metal NPs and the negatively charged cancer cell surfaces may further donate to this cytotoxic effect. Besides, BNPs are capable of disrupting mitochondrial membrane potential, hindering ATP manufacture, initiating mitochondrial-mediated apoptosis, and eventually leading to death of malignant cells. Additionally, these NPs can interrupt signaling pathways correlated to angiogenesis, such as those regulated by vascular endothelial growth factor, efficiently limiting the tumor’s blood supply, and inhibiting its growth85,86.
Antimicrobial activity of biosynthesized Ag-TiO2 BNPs
Antimicrobial activity using Inhibition zone
The antimicrobial activity of Ag-TiO2 BNPs, Ti(NO3)4·4H2O, and AgNO3 against each tested pathogenic bacterial strains using agar well diffusion methods was detailed in Table 1.
The well diffusion assay revealed that Ag-TiO2 BNPs effectively inhibited the growth of all tested bacterial strains, as shown by the varying inhibition zone diameters (IZDs) in Fig. 9. While Ti(NO3)4·4H2O and AgNO3 exhibited differing antimicrobial activities against various microbial strains, each compound demonstrated independent effectiveness. It is noteworthy that the efficacy of their combined application was consistently enhanced, underscoring the significance of comprehending the synergistic interactions between antimicrobial compounds in order to create effective treatment strategies. The results also indicated that Ti(NO3)4·4H2O and AgNO3 have unique antimicrobial properties. The Ag-TiO2 BNPs antimicrobial effect consistently enhanced, as seen with all tested strains except K. pneumonia, suggesting the synergistic interaction between these two metals for most strains. Also, AgNO3 is slightly more active than Ag-TiO2 BNPs.
Silver NPs and traditional antimicrobial agents are used for combating bacterial infections, but they significantly differ in their effectiveness, mechanisms of action, and potential applications. The significant effectiveness of AgNPs towards multidrug-resistant bacteria was recorded. They could act as bactericidal agents even in cases where traditional antimicrobial agents fail due to various resistance mechanisms87. Elsewhere the antimicrobial properties of AgNPs are being explored for wound dressings applications, coatings of medical devices, and adjuvants to improve antimicrobial efficacy. In various studies, AgNPs have proved non-toxicity at therapeutic doses88.
The antibacterial effectiveness of TiO2 NPs influenced by physico-chemical factors, including morphology, particle size, surface modifications, environmental conditions, light activation, and the tested microorganisms. The effectiveness of TiO2 NPs can vary depending on the type of targeted bacteria. The tested Gram-negative bacteria often prove different susceptibility compared to Gram-positive bacteria based on the differences in cell wall structure and composition89.
Silver NPs and TiO2 NPs can act synergistically to improve the antibacterial effectiveness against bacterial infections. Their combined use influences the unique properties of each material, resulting in enhanced antimicrobial action. Incorporation of AgNPs into TiO2 coatings on surfaces associated with enhanced overall antibacterial activity. The presence of AgNPs provides direct antimicrobial activity and increases the efficacy of TiO2’s photocatalytic activity by enhancing charge separation and lowering electron-hole recombination90. This synergy allows for a more robust response against a wider microbial range, including drug-resistant bacterial strains.
The consortium of silver and titanium (Ag-TiO₂ BNPs) demonstrated superior antimicrobial activity compared to either component alone. For instance, inhibition zones against P. aeruginosa (28.3 mm) and S. aureus (21 mm) were significantly larger than those produced by AgNO₃ or Ti(NO₃)₄·4 H₂O individually. Recent studies confirm these findings, reporting that silver-titanium alloy nanoparticles exhibit statistically significant higher inhibitory rates against both E. coli and P. aeruginosa compared to titanium salt alone. The enhanced effect is attributed to the synergistic mechanisms: silver ions disrupt cell membranes and proteins, while titanium dioxide produces ROS, together leading to amplified bacterial cell damage91.
A 2024 comparative study found that Ag-TiO₂ nanoparticles were significantly more effective against P. aeruginosa than TiO₂ nanoparticles, supporting the observed results in this work. Similarly, for E. coli, Ag-TiO₂ nanoparticles outperformed TiO₂, corroborating the higher inhibition zones seen in our data92.
Minimum inhibitory concentration of biosynthesized Ag-TiO2 BNPs
The MIC of Ag-TiO2 BNPs towards various bacterial strains showed important insights into their antimicrobial activity (Table 2). Notably, S. aureus and K. pneumoniae exhibited an MIC of 62.5 µg/mL, while B. cereus, E. coli and P. aeruginosa revealed lower MIC values of 31.25 µg/mL (Fig. 10). This differential susceptibility amongst the tested bacterial strains suggests that the Ag-TiO2 BNPs may be specifically effective against certain Gram-negative bacterial strains.
The findings align with the current literature on silver and titanium NPs antimicrobial properties. Silver NPs are well-documented for their broad-spectrum antimicrobial efficacy, primarily due to their ability to liberate silver ions and generate ROS, which disrupt bacterial cell membrane functions and metabolic processes93. Titanium dioxide NPs, particularly in their anatase form, also show considerable antimicrobial effects when activated by UV light, producing ROS that cause microbial cells damage94. The combination of these two types of NPs may enhance their individual mechanisms of action with consequent improvement of their overall efficacy against a range of pathogenic bacteria.
The observed MIC values suggested more susceptibility of B. cereus, E. coli, and P. aeruginosa to Ag-TiO2 BNPs compared to S. aureus and K. pneumoniae. This could be attributed to differences in NPs targets amongst these bacteria. Also, S. aureus thicker peptidoglycan layer may provide a barrier to NPs penetration. As well, Gram-negative bacteria have a more complex outer membrane structure but often more vulnerable to oxidative stress induced by NPs95. The lower MIC values for the assessed Gram-negative strains indicate a potential application of Ag-TiO2 BNPs in treating infections caused by such pathogens.
Furthermore, the synergistic interaction between silver and titanium NPs can be leveraged for therapeutic applications in clinical settings, principally in the era where antimicrobial resistance. Research has shown that combining different NPs can lead to enhanced antibacterial activity associated with complementary mechanisms96. For example, while silver ions exert a direct bactericidal effect, titanium dioxide can improve this effect through photocatalytic activity. This synergistic interaction improves the antibacterial efficacy and reduces the prospected resistance development, an important concern in modern antimicrobial therapy97.
Mueller Hinton broth resazurin assay showing the specific MIC of Ag-TiO2 BNPs. Raws; A and B: positive and negative control, respectively and C to H: denote the dilution range of the tested BNPs from 500 to15.6 µg/mL, respectively. Columns represent the tested bacterial strains as the following: 1 and 2: E. coli, 3 and 4; B. cereus, 5 and 6: S. aureus, 7 and 8: K. pneumonia, and 9 and 10: P. aeruginosa.
Antioxidant activity of biosynthesized Ag-TiO2 BNPs
Antioxidants are recognized for their therapeutic benefits, including anti-inflammatory, anti-atherosclerotic, antitumor, antiviral, antimutagenic, anticarcinogenic, and antimicrobial effects98. The antioxidant activity of Ag-TiO2 BNPs was evaluated using the DPPH assay over a concentration range of 1000 to 7.81 µg/mL, as illustrated in Fig. 11. The results showed that Ag-TiO2 BNPs possess significant antioxidant activity, with an IC50 value of 225 µg/mL required to scavenge 50% of DPPH radicals. In contrast, ascorbic acid, a well-known antioxidant, demonstrated a significantly lower IC50 value (7.61 µg/mL), underscoring its enhanced antioxidant effectiveness. The findings indicated that although Ag-TiO2 BNPs exhibit encouraging antioxidant properties, their effectiveness is significantly less than that of ascorbic acid. Therefore, a deeper examination of the antioxidant mechanisms of Ag-TiO2 BNPs and its particular active components could yield valuable insights into improving its effectiveness. Herein, these findings contribute to the understanding of the Ag-TiO2 BNPs antioxidant capacity and supporting its probable therapeutic applications in controlling oxidative stress-related disorders.
Conclusion
This study utilized P. indica leaf extract to synthesize Ag-TiO₂ BNPs in an eco-friendly manner. The UV-Vis spectral analysis confirmed the effective synthesis of Ag-TiO₂ BNPs, with a distinct absorbance peak at 350 nm. Synthesized BNPs have a size distribution of 10–60 nm, according to experiments. Biological tests showed promising bioactivities for Ag-TiO₂ BNPs. A targeted anticancer effect was shown in cytotoxicity studies, with an IC₅₀ value of 33.5 µg/mL for MCF-7 breast cancer cells and 169.6 µg/mL for normal Wi-38 cells, confirming biosafety. The Ag-TiO₂ BNPs showed significant antibacterial activity against several pathogenic bacteria strains, with minimum inhibitory doses ranging from 31.25 to 62.5 µg/mL. Antioxidant studies confirmed the therapeutic potential of Ag-TiO₂ BNPs, with an IC₅₀ value of 225 µg/mL in the DPPH radical scavenging experiment. P. indica extract shows promise as a durable and effective reducing agent for Ag-TiO₂ BNP production. Synthesized Ag-TiO₂ BNPs have promising medicinal applications in cancer therapy, infection management, and oxidative stress management. To maximize clinical potential, future research must refine the synthesis process and investigate their therapeutic effects’ molecular mechanisms.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Allan, J. et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 122, 104885. https://doi.org/10.1016/j.yrtph.2021.104885 (2021).
Kumari, S. et al. Biogenic approach for synthesis of nanoparticles via plants for biomedical applications: A review. Mater. Today: Proc. https://doi.org/10.1016/j.matpr.2023.04.242 (2023).
Kirubakaran, D. et al. A comprehensive review on the green synthesis of nanoparticles: advancements in biomedical and environmental applications. Biomedical Mater. Devices. https://doi.org/10.1007/s44174-025-00295-4 (2025).
Pirsaheb, M. et al. Green synthesis of nanomaterials by using plant extracts as reducing and capping agents. Environ. Sci. Pollut. Res. 31 (17), 24768–24787. https://doi.org/10.1007/s11356-024-32983-x (2024).
Soni, V., Raizada, P., Singh, P., Cuong, H. N. & Saini, S. R. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ. Res. 202, 111622. https://doi.org/10.1016/j.envres.2021.111622 (2021).
Acharya, C. et al. Synthesis of metallic nanoparticles using biometabolites: mechanisms and applications. BioMetals 38 (1), 21–54. https://doi.org/10.1007/s10534-024-00642-w (2025).
Ibrahim, S. R., Bagalagel, A. A., Diri, R. M., Noor, A. O., Bakhsh, H. T., & Mohamed, G. A. Phytoconstituents and pharmacological activities of Indian Camphorweed (Pluchea Indica): A multi-potential medicinal plant of nutritional and ethnomedicinal importance. Molecules, 27(8), 2383 (2022).
Kaur, A. et al. Role of plant functional traits in the invasion success: analysis of nine species of Asteraceae. BMC Plant Biol. 24 (1), 784. https://doi.org/10.1186/s12870-024-05498-3 (2024).
Coin, G., Lekutlane, D., Masisi, K., Muzila, M. & Mazimba, O. Grewia flava twig extracts: phytochemical, antioxidant, and antimicrobial evaluations. Bull. Natl. Res. Centre. 48 (1), 75. https://doi.org/10.1186/s42269-024-01234-x (2024).
Chiangnoon, R. et al. Phytochemical analysis, antioxidant, and wound healing activity of Pluchea indica L. (Less) Branch. Extract Nanopart. 27 (3), 635 (2022).
Jangid, H., Singh, S., Kashyap, P., Singh, A. & Kumar, G. Advancing biomedical applications: an in-depth analysis of silver nanoparticles in antimicrobial, anticancer, and wound healing roles. 15–2024 https://doi.org/10.3389/fphar.2024.1438227 (2024).
Husain, S., Nandi, A., Simnani, F. Z., Saha, U., Ghosh, A., Sinha, A. & Verma, S. K. Emerging trends in advanced translational applications of silver nanoparticles: a progressing dawn of nanotechnology. Journal of Functional Biomaterials, 14(1), 47 (2023).
Racovita, A. D. Titanium dioxide: structure. Impact Toxic. 19 (9), 5681 (2022).
Shabbir, S., Kulyar MF-e-A, Bhutta, Z. A., Boruah, P. & Asif, M. Toxicological consequences of titanium dioxide nanoparticles (TiO2NPs) and their jeopardy to human population. BioNanoScience 11 (2), 621–632. https://doi.org/10.1007/s12668-021-00836-3 (2021).
Rashid, M. M., Forte Tavčer, P., & Tomšič, B. Influence of titanium dioxide nanoparticles on human health and the environment. Nanomaterials, 11(9), 2354 (2021).
Fatima, F., Siddiqui, S. & Khan, W. A. Nanoparticles as novel emerging therapeutic antibacterial agents in the antibiotics resistant era. Biol. Trace Elem. Res. 199 (7), 2552–2564. https://doi.org/10.1007/s12011-020-02394-3 (2021).
Saisruthi, V., Kumar, J. A., Rosana, N. T. M., Joseph, K. L. V. & Rubavathy, S. J. J. E. Q. M. Bio-Fabricated TiO2 nanoparticles: A comprehensive insight into its antimicrobial. Anticancer Environ. Appl. 34 (2), e22359 (2024).
Rosli, N. A., Haan, T. Y. & Mahmoudi, E. Current approaches for the exploration of antimicrobial activities of nanoparticles. Sci. Technol. Adv. Mater. 22 (1), 885–907. https://doi.org/10.1080/14686996.2021.1978801 (2021).
Althobaiti, F. et al. New ionic liquid Microemulsion-Mediated synthesis of silver nanoparticles for skin bacterial. Infect. Treatments. 12 (2), 247 (2023).
Mukherjee, S., Maity, S., Patra, D. & Haldar, J. Harnessing Chitosan and its derivatives for innovative antimicrobial biomaterials. In Chitosan for Biomaterials V: Insight into Pharmaceutical Uses (ed. Jayakumar, R.) 63–115 (Springer Nature Switzerland, 2025).
Shenasa, N. et al. Review of carbonaceous nanoparticles for antibacterial uses in various dental infections. Nanotoxicology 19 (2), 180–215. https://doi.org/10.1080/17435390.2025.2454277 (2025).
Hühn, J. et al. Selected standard protocols for the synthesis, phase transfer, and characterization of inorganic colloidal nanoparticles. In Bio-Nano Interfaces 581–758 (Jenny Stanford Publishing, 2024).
Sandhu, S. K., Kumar, S., Raut, J., Singh, M., Kaur, S., Sharma, G. & Kaur, I. P. Systematic development and characterization of novel, high drug-loaded, photostable, curcumin solid lipid nanoparticle hydrogel for wound healing. Antioxidants, 10(5), 725 (2021).
Van de Loosdrecht, A., Beelen, R., Ossenkoppele g, Broekhoven, M. & Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods. 174 (1–2), 311–320 (1994).
Sharaf, M. H. et al. New combination approaches to combat methicillin-resistant Staphylococcus aureus (MRSA). Sci. Rep. 11 (1), 4240 (2021).
El-Didamony, S. E. et al. Melittin alcalase-hydrolysate: a novel chemically characterized multifunctional bioagent; antibacterial, anti-biofilm and anticancer. Front. Microbiol. 15, 1419917 (2024).
El-Sayed, M. H., Alshammari, F. A. & Sharaf, M. H. Antagonistic potentiality of actinomycete-derived extract with anti-biofilm, antioxidant, and cytotoxic capabilities as a natural combating strategy for multidrug-resistant ESKAPE pathogens. J. Microbiol. Biotechnol. 33(1), 61–74. https://doi.org/10.4014/jmb.2211.11026 (2023).
Liu, L. & Corma, A. Bimetallic sites for catalysis: from binuclear metal sites to bimetallic nanoclusters and nanoparticles. Chem. Rev. 123 (8), 4855–4933. https://doi.org/10.1021/acs.chemrev.2c00733 (2023).
Zhao, J., Xue, S., Ji, R., Li, B. & Li, J. Localized surface plasmon resonance for enhanced electrocatalysis. Chem. Soc. Rev. 50 (21), 12070–12097. https://doi.org/10.1039/D1CS00237F (2021).
Baranov, M. V., Kumar, M., Sacanna, S., Thutupalli, S. & van den Bogaart, G. Modulation immune responses part. Size Shape 11. doi: https://doi.org/10.3389/fimmu.2020.607945. (2021).
Abo-Elmagd, R. A., Hamouda, R. A. & Hussein, M. H. Phycotoxicity and catalytic reduction activity of green synthesized Oscillatoria gelatin-capped silver nanoparticles. Sci. Rep. 12 (1), 20378. https://doi.org/10.1038/s41598-022-22976-6 (2022).
Farooq, U. et al. Environmentally sustainable fabrication of palladium nanoparticles from the ethanolic crude extract of oxystelma esculentum towards effective degradation of organic dye. Mater. Today Sustain. 23, 100463. https://doi.org/10.1016/j.mtsust.2023.100463 (2023).
Ramya, J. R. et al. Antimicrobial efficiency against fish pathogens on the green synthesized silver nanoparticles. Microb. Pathog. 193, 106725. https://doi.org/10.1016/j.micpath.2024.106725 (2024).
Elattar, K. M. et al. Multifaceted chemical and bioactive features of Ag@TiO2 and Ag@SeO2 core/shell nanoparticles biosynthesized using Beta vulgaris L. extract. Heliyon 10(7), https://doi.org/10.1016/j.heliyon.2024.e28359 (2024).
Aly Khalil, A. M. et al. Green biosynthesis of bimetallic selenium–gold nanoparticles using Pluchea indica leaves and their biological applications. 11. (2024). https://doi.org/10.3389/fbioe.2023.1294170
Hashemi, Z. et al. In-vitro anticancer and antibacterial activities and comparative of eco-friendly synthesized silver nanoparticles using hull of Pistacia vera and rhizome of Sambucus ebulus extracts. Inorg. Chem. Commun. 154, 110913. https://doi.org/10.1016/j.inoche.2023.110913 (2023).
Alshahrani, A. A., Alqarni, L. S., Alghamdi, M. D., Alotaibi, N. F., Moustafa, S. M. & Nassar, A. M. Phytosynthesis via wasted onion peel extract of samarium oxide/silver core/shell nanoparticles for excellent inhibition of microbes. Heliyon, 10(3), (2024).
Krishnasamy Sekar, R., Sridhar, A., Perumalsamy, B., Manikandan, D. B. & Ramasamy, T. In vitro antioxidant, antipathogenicity and cytotoxicity effect of silver nanoparticles fabricated by onion (Allium Cepa L.) Peel extract. BioNanoScience 10 (1), 235–248. https://doi.org/10.1007/s12668-019-00691-3 (2020).
Abdelmoteleb, A., Gonzalez-Mendoza, D., Valdez-Salas, B., Grimaldo-Juarez, O. & Ceceña-Duran, C. Inhibition of fusarium Solani in Transgenic insect-resistant cotton plants treated with silver nanoparticles from Prosopis glandulosa and Pluchea sericea. Egypt. J. Biol. Pest Control. 28 (1), 4. https://doi.org/10.1186/s41938-017-0005-0 (2018).
Gonfa, Y. H. et al. Essential oil composition of aerial part of Pluchea ovalis (Pers.) DC., silver nanoparticles synthesis, and larvicidal activities against fall armyworm. Sustainability, 14(23), 15785 (2022).
Al Masoudi, L. M., Alqurashi, A. S., Abu Zaid, A. & Hamdi, H. Characterization and biological studies of synthesized titanium dioxide nanoparticles from leaf extract of Juniperus phoenicea (L.) growing in Taif region. Saudi Arabia. 11 (1), 272 (2023).
Roy, A. et al. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. 2022(1), 5445291. (2022).
Singh, R. & Bhateria, R. Core–shell nanostructures: a simplest two-component system with enhanced properties and multiple applications. Environ. Geochem. Health. 43 (7), 2459–2482. https://doi.org/10.1007/s10653-020-00766-1 (2021).
Al-Askar, A. A., Hashem, A. H., Elhussieny, N. I. & Saied, E. Green biosynthesis of zinc oxide nanoparticles using Pluchea indica leaf extract. Antimicrob. Photocatalytic Activities. 28 (12), 4679 (2023).
Li, J., Zengming, W., Hui, Z., Jing, G. & Zheng, A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv. 28 (1), 19–36. https://doi.org/10.1080/10717544.2020.1856224 (2021).
Philip, A. & Kumar, A. R. The performance enhancement of surface plasmon resonance optical sensors using nanomaterials: A review. Coord. Chem. Rev. 458, 214424. https://doi.org/10.1016/j.ccr.2022.214424 (2022).
More, P. R. et al. Antimicrobial applications of green synthesized bimetallic nanoparticles from Ocimum basilicum. 14(11), 2457. (2022).
Shkryl, Y. et al. Biosynthesis and cytotoxic properties of ag, au, and bimetallic nanoparticles synthesized using Lithospermum erythrorhizon. Callus Cult. Extract 22(17), 9305 (2021).
Hashem, A. H. et al. Watermelon rind mediated biosynthesis of bimetallic selenium-silver nanoparticles: characterization, antimicrobial and anticancer activities. Plants, 12(18), 3288 (2023).
Krishnan Sundarrajan, S. & Pottail, L. Green synthesis of bimetallic ag@au nanoparticles with aqueous fruit latex extract of Artocarpus heterophyllus and their synergistic medicinal efficacies. Appl. Nanosci. 11 (3), 971–981. https://doi.org/10.1007/s13204-020-01657-8 (2021).
Akinola, P. O., Lateef, A., Asafa, T. B., Beukes, L. S., Abbas, S. H., & Irshad, H. M. Phytofabrication of titanium-silver alloy nanoparticles (Ti-AgNPs) by Cola nitida for biomedical and catalytic applications. Inorganic Chemistry Communications, 139, 109357 (2022).
Azad, A., Zafar, H., Raza, F., & Sulaiman, M. Factors influencing the green synthesis of metallic nanoparticles using plant extracts: a comprehensive review. Pharmaceutical Fronts, 5(03), e117–e131 (2023).
Hasanin, M. S., Hashem, A. H., Al-Askar, A. A., Haponiuk, J. & Saied, E. A novel nanocomposite based on mycosynthesized bimetallic zinc-copperoxide nanoparticles, nanocellulose and chitosan: characterization, antimicrobial and photocatalytic activities. Electron. J. Biotechnol. 65, 45–55. https://doi.org/10.1016/j.ejbt.2023.05.001 (2023).
Alsayed, M. F. et al. Silver nanoparticles synthesized using aerial part of Achillea fragrantissima and evaluation of their bioactivities. Sci. Rep. 14 (1), 24703. https://doi.org/10.1038/s41598-024-75558-z (2024).
Kumaravel, J., Lalitha, K., Arunthirumeni, M. & Shivakumar, M. S. Mycosynthesis of bimetallic zinc oxide and titanium dioxide nanoparticles for control of Spodoptera frugiperda. Pestic. Biochem. Physiol. 178, 104910. https://doi.org/10.1016/j.pestbp.2021.104910 (2021).
Singh, R., Hano, C., Nath, G. & Sharma, B. Green biosynthesis of silver nanoparticles using leaf extract of Carissa carandas L. and their antioxidant and antimicrobial activity against. Hum. Pathogenic Bacteria. 11 (2), 299 (2021).
Rana, A., Pathak, S., Kumar, K., Kumari, A., Chopra, S., Kumar, M. & Sharma, S. N. Multifaceted properties of TiO 2 nanoparticles synthesized using Mangifera indica and Azadirachta indica plant extracts: antimicrobial, antioxidant, and non-linear optical activity investigation for sustainable agricultural applications. Materials Advances, 5(7), 2767–2784 (2024).
Shabir, I. et al. Nutritional profile, phytochemical compounds, biological activities, and utilisation of onion peel for food applications: A Review. 14 (19), 11958 (2022).
Arulaabaranam, K., Muthu, S., Mani, G., Irfan, A. & Conformational study, F. T. I. R. FT-Raman, solvent effect on UV–Vis, charge transfer and protein–ligand interactions of Methyl-2-pyrazinecarboxylate. J. Mol. Liq. 341, 116934. https://doi.org/10.1016/j.molliq.2021.116934 (2021).
Javed, M. A. et al. Biosynthesis and characterization of silver nanoparticles from Cedrela Toona leaf extracts: an exploration into their antibacterial, anticancer, and antioxidant potential. 13(1). (2024). https://doi.org/10.1515/gps-2023-0248
Fouda, A., Hassan, S. E. D., Eid, A. M., Abdel-Rahman, M. A. & Hamza, M. F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, penicillium crustosum EP-1. Sci. Rep. 12 (1), 11834. https://doi.org/10.1038/s41598-022-15903-2 (2022).
Aly Khalil, A. M. et al. Green biosynthesis of bimetallic selenium–gold nanoparticles using Pluchea indica leaves and their biological applications. 11–2023. (2024). https://doi.org/10.3389/fbioe.2023.1294170
Dolai, J., Mandal, K. & Jana, N. R. Nanoparticle size effects in biomedical applications. ACS Appl. Nano Mater. 4 (7), 6471–6496. https://doi.org/10.1021/acsanm.1c00987 (2021).
Selim, S. et al. Synthesis of novel MgO-ZnO nanocomposite using Pluchea indica leaf extract and study of their biological activities. Bioresources Bioprocess. 12 (1), 33. https://doi.org/10.1186/s40643-025-00848-x (2025).
Dutta, G., kumar Chinnaiyan, S., Sugumaran, A., & Narayanasamy, D. Sustainable bioactivity enhancement of ZnO–Ag nanoparticles in antimicrobial, antibiofilm, lung cancer, and photocatalytic applications. RSC advances, 13(38), 26663–26682 (2023).
Elkady, F. M. et al. Green biosynthesis of bimetallic copper Oxide-Selenium nanoparticles using leaf extract of Lagenaria siceraria: antibacterial, Anti-Virulence activities against Multidrug-Resistant Pseudomonas Aeruginosa. Int. J. Nanomed. 20 (null), 4705–4727. https://doi.org/10.2147/IJN.S497494 (2025).
Akinola, P. O. et al. Phytofabrication of titanium-silver alloy nanoparticles (Ti-AgNPs) by Cola nitida for biomedical and catalytic applications. Inorg. Chem. Commun. 139, 109357. https://doi.org/10.1016/j.inoche.2022.109357 (2022).
Ahmad, M. M., Kotb, H. M., Mushtaq, S., Waheed-Ur-Rehman, M., Maghanga, C. M. & Alam, M. W. Green synthesis of Mn+ Cu bimetallic nanoparticles using Vinca rosea extract and their antioxidant, antibacterial, and catalytic activities. Crystals, 12(1), 72 (2022).
Shumi, G. et al. Biosynthesis of silver nanoparticles functionalized with histidine and phenylalanine amino acids for potential antioxidant and antibacterial activities. ACS Omega. 8 (27), 24371–24386. https://doi.org/10.1021/acsomega.3c01910 (2023).
Hemlata, C., Meena, P. R., Singh, A. P. & Tejavath, K. K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against Cancer cell lines. ACS Omega 5(10), 5520–5528. https://doi.org/10.1021/acsomega.0c00155 (2020).
Kumar, E. A., Kokulnathan, T., Wang, T-J., Kumar, K. M. A. & Chang, Y-H. Coaxially electrospun TiO2@Au core-shell nanofibers for ultrasensitive and reusable SERS detection of Furazolidone. J. Environ. Chem. Eng. 11 (3), 110148. https://doi.org/10.1016/j.jece.2023.110148 (2023).
Bekele, E. T., Gonfa, B. A., Zelekew, O. A., Belay, H. H., & Sabir, F. K. Synthesis of titanium oxide nanoparticles using root extract of Kniphofia foliosa as a template, characterization, and its application on drug resistance bacteria. Journal of Nanomaterials, 2020(1), 2817037 (2020).
Malik, M. A. et al. Biosynthesis of novel Ag-Cu bimetallic nanoparticles from leaf extract of Salvia officinalis and their antibacterial activity. 13 (3), 653 (2023).
Khalil, A. M. A., Abdelaziz, A. M., Khaleil, M. M. & Hashem, A. H. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett. Appl. Microbiol. 72 (3), 263–274. https://doi.org/10.1111/lam.13414 (2021).
Ioset, J-R., Brun, R., Wenzler, T., Kaiser, M. & Yardley, V. Drug screening for kinetoplastids diseases: a training manual for screening in neglected diseases. 74. (2009).
Hashem, A. H. & El-Sayyad, G. S. Antimicrobial and anticancer activities of biosynthesized bimetallic silver-zinc oxide nanoparticles (Ag-ZnO NPs) using pomegranate Peel extract. Biomass Convers. Biorefinery. https://doi.org/10.1007/s13399-023-04126-8 (2023).
Elsayed, K. A. et al. Fabrication of ZnO-Ag bimetallic nanoparticles by laser ablation for anticancer activity. Alexandria Eng. J. 61 (2), 1449–1457. https://doi.org/10.1016/j.aej.2021.06.051 (2022).
Katifelis, H. et al. Ag/Au bimetallic nanoparticles inhibit tumor growth and prevent metastasis in a mouse model. Int. J. Nanomed. 6019–6032. (2020).
Ghosh, N. & Singh, R. In vitro cytotoxicity assay of biogenically synthesized bimetallic nanoparticles. Rasayan J. Chem. 14, 486–492 (2021).
Mittal, A. K., Kumar, S. & Banerjee, U. C. Quercetin and Gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 431, 194–199. https://doi.org/10.1016/j.jcis.2014.06.030 (2014).
Chinnathambi, A. et al. Biofabrication of bimetallic selenium@zinc nanoparticles using Champia parvula aqueous extract: investigation of anticancer activity and its apoptosis induction. Biochem. Biophys. Res. Commun. 733, 150417. https://doi.org/10.1016/j.bbrc.2024.150417 (2024).
Makada, H., Habib, S. & Singh, M. Bimetallic nanoparticles as suitable nanocarriers in cancer therapy. Sci. Afr. 20, e01700. https://doi.org/10.1016/j.sciaf.2023.e01700 (2023).
Juan, C. A. & de la Pérez, J. M. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and Proteins) and induced pathologies. 22(9). (2021). https://doi.org/10.3390/ijms22094642
Abass Sofi, M., Sunitha, S., Ashaq Sofi, M., Khadheer Pasha, S. K. & Choi, D. An overview of antimicrobial and anticancer potential of silver nanoparticles. J. King Saud Univ. - Sci. 34 (2), 101791. https://doi.org/10.1016/j.jksus.2021.101791 (2022).
Alsaab, H. O., Al-Hibs, A. S. & Alzhrani, R. Nanomaterials for antiangiogenic therapies for cancer: A promising tool for personalized medicine. 22(4). (2021). https://doi.org/10.3390/ijms22041631
Tabish, T. A. & Hamblin, M. R. Mitochondria-targeted nanoparticles (mitoNANO): an emerging therapeutic shortcut for cancer. Biomaterials Biosystems. 3, 100023. https://doi.org/10.1016/j.bbiosy.2021.100023 (2021).
Chapa González, C., González García, L. I., Burciaga Jurado, L. G. & Carrillo Castillo, A. Bactericidal activity of silver nanoparticles in drug-resistant bacteria. Brazilian J. Microbiology: [publication Brazilian Soc. Microbiology]. 54 (2), 691–701. https://doi.org/10.1007/s42770-023-00991-7 (2023).
Dove, A. S. et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Front. Microbiol. 13, 1064095. https://doi.org/10.3389/fmicb.2022.1064095 (2022).
Ragheb, N. & Borg, H. Antimicrobial effect of titanium oxide (Tio2) nano particles in completely edentulous patients. A randomized clinical trial. Adv. Dent. J. 3 (4), 173–184 (2021).
Zhang, P., Zhang, Z. & Li, W. Antibacterial TiO2 coating incorporating silver nanoparticles by microarc oxidation and ion implantation. J. Nanomaterials. 2013 (1), 542878 (2013).
Poudel, M. B. & Kim, A. A. Silver nanoparticles decorated TiO2 nanoflakes for antibacterial properties. Inorg. Chem. Commun. 152, 110675 (2023).
Madduluru, S. & Paramasivam, G. Comparative analysis of silver titanium alloy and titanium dioxide nanoparticles for antibacterial activity against Escherichia coli as compared with commercial antibiotic kanamycin. AIP Conference Proceedings. (AIP Publishing, 2023).
Parvekar, P., Palaskar, J., Metgud, S., Maria, R. & Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomaterial Investigations Dentistry. 7 (1), 105–109. https://doi.org/10.1080/26415275.2020.1796674 (2020).
Serov, D. A. & Gritsaeva, A. V. Review of antimicrobial properties of titanium dioxide nanoparticles. 25(19). (2024). https://doi.org/10.3390/ijms251910519
Mohammad, A. M., Fatah, S. K., Majeed, M. H., Mohammed, S. M. & Mohammed, S. M. Enhanced antibacterial activity and structural characterization of ZnO-doped MgO nanocomposites synthesized via sol–gel technique. BioNanoScience. 1–14. (2024).
Ribeiro, A. I., Dias, A. M. & Zille, A. Synergistic effects between metal nanoparticles and commercial antimicrobial agents: A review. ACS Appl. Nano Mater. 5 (3), 3030–3064 (2022).
Thirumoorthy, G. et al. Phytofabricated bimetallic synthesis of silver-copper nanoparticles using Aerva lanata extract to evaluate their potential cytotoxic and antimicrobial activities. Sci. Rep. 14 (1), 1270 (2024).
Elkady, F. M., Badr, B. M., Saied, E., Hashem, A. H., Abdulrahman, M. S., Alkherkhisy, M. M. & Aufy, M. Mycosynthesis of zinc oxide nanoparticles using Mucor racemosus with their antimicrobial, antibiofilm, anticancer and antioxidant activities. Scientific Reports, 15(1), 18772 (2025).
Acknowledgements
The authors really acknowledge the Faculty of Science, Al-Azhar University, Cairo, Egypt for offering the basic research facilities. The authors really acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R357), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors also extend all their respect to Zarqa university-Jordan for partial funding of this research.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R357), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Author contributions: Conceptualization: Ebrahim Saied, Amr H. Hashem; Methodology: All authors; Formulation: Fathy M. Elkady; Writing original draft of Manuscript: Ebrahim Saied, Mohamed H. Moustafa Amr H. Hashem; Review the final version of the manuscript: All authors; All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Selim, S., Badr, B.M., Moustafa, M.H. et al. Green biosynthesis of bimetallic silver titanium dioxide nanoparticles using Pluchea indica with their anticancer, antimicrobial, and antioxidant activities. Sci Rep 15, 26735 (2025). https://doi.org/10.1038/s41598-025-10349-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10349-8