Abstract
This study sought to construct and validate a prognostic nomogram for predicting cause-specific survival (CSS) in patients with nasal cavity squamous cell carcinoma (NCSCC). We conducted a retrospective analysis of clinical data from NCSCC patients registered in the SEER database between 2007 and 2015. Statistical analyses were performed to assess CSS rates and identify prognostic factors. The study cohort comprised 580 NCSCC patients, with CSS probabilities of 89.1%, 74.8%, and 63.6% at 1, 3, and 5 years, respectively. Multivariate Cox regression analysis identified age, American Joint Committee on Cancer (AJCC) stage, and radiotherapy administration as independent prognostic factors significantly associated with CSS. The accuracy of the prediction was evaluated using the C-index and calibration curve. Decision curve analysis (DCA) was utilized to compare the nomogram with the AJCC stage system in order to assess its superiority. We developed and validated a predictive model for 1-, 3-, and 5-year CSS in NCSCC based on a large retrospective cohort. The nomogram demonstrates clinical utility in guiding individualized treatment strategies and patient management.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinoma (HNSCC) represents the seventh most prevalent malignancy worldwide, with an estimated annual incidence of 890,000 new cases, constituting 4.5% of global cancer diagnoses, and accounting for 450,000 deaths, which corresponds to 4.6% of total cancer-related mortality1. This neoplasm exhibits a predilection for elderly male populations. Nasal cavity squamous cell carcinoma (NCSCC), a relatively uncommon subtype within the head and neck region, demonstrates an incidence rate of approximately 0.148 cases per 100,000 individuals2. Established etiological factors for NCSCC include tobacco smoking and human papillomavirus (HPV) infection, as evidenced by multiple studies3,4. The limited epidemiological data on nasal cavity malignancies, compounded by the historical classification of nasal cavity and sinonasal cancers as a unified entity5,6,7,8 has resulted in a paucity of large-scale studies specifically examining survival outcomes in NCSCC. Malignancies of the nasal cavity demonstrate considerable histological diversity compared to other head and neck subsites. Nasal cavity malignancies encompass a diverse spectrum of histopathological subtypes, including but not limited to melanoma, adenocarcinoma, salivary gland-type carcinomas, olfactory neuroblastoma, and sinonasal undifferentiated carcinoma. However, epidemiological evidence consistently demonstrates that squamous cell carcinoma (SCC) represents the predominant histological variant, accounting for the majority of cases3. The distinct pathological subtypes exhibit significant variations in epidemiological prevalence, therapeutic approaches, and prognostic trajectories. By restricting analytical investigations to a specific pathological subtype, researchers can effectively minimize confounding variables, thereby enhancing the precision of study outcomes and improving their clinical applicability.
As SCC represents the predominant histological subtype among nasal cavity malignancies, accounting for the majority of cases9 this study aims to analyze cancer-specific survival (CSS) and prognostic factors in NCSCC patients using survival data from the SEER database. A predictive model will be developed to visualize clinical data, providing actionable insights for clinical decision-making.
Methods
Clinical data
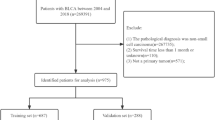
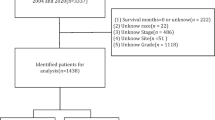
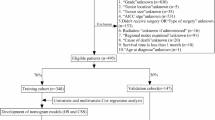
In this study, data on NCSCC patients were downloaded using SEER Stat 8.4.5 software from the database “Incidence - SEER Research Data, 17 Registries, Nov 2023 Sub (2000–2021) - Linked To County Attributes - Time Dependent (1990–2022) Income/Rurality, 1969–2022 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2024, based on the November 2023 submission.” The specific workflow is illustrated in Fig. 1.
Variable selection
The study analyzed 16 demographic and clinical variables. Considering the predominantly middle-aged and elderly population of NCSCC patients, age stratification was established using the cohort’s median age of 60 years, dividing participants into non-elderly (< 60 years) and elderly (≥ 60 years) groups. Demographic variables included sex, race, and marital status. Clinical characteristics comprised tumor differentiation grade (well-differentiated-I, moderately-differentiated-II, poorly-differentiated-III, and undifferentiated-IV), tumor size (categorized into three tiers at 2-cm intervals), surgical intervention, radiotherapy, chemotherapy, systemic therapy, American Joint Committee on Cancer (AJCC) stage (6th edition), SEER combined stage (SCS), T-stage (early [T1-T2] vs. advanced [T3-T4]), N-stage (localized [N0-N1] vs. extensive [N3-N4] nodal involvement), and M-stage. The dataset was randomly divided into training and validation cohorts in a 7:3 ratio.
Statistical analysis
Statistical analyses were performed using R software (version 4.43). Categorical variables in this study will be described as frequency (percentage). Comparative analyses between the training and validation cohorts were conducted using chi-square tests. The development of the prognostic model in the training cohort followed a three-stage analytical approach: initially, potential predictors were screened through univariable Cox regression analysis, with variables demonstrating a significance level of p < 0.10 proceeding to subsequent analysis. These selected variables were then subjected to LASSO regression for feature selection and dimensionality reduction. Finally, the variables retained from the LASSO regression were incorporated into a multivariable Cox proportional hazards model, which served as the foundation for constructing the nomogram and generating the corresponding forest plot. Model validation encompassed the assessment of discrimination capability through Harrell’s concordance index (C-index) and evaluation of calibration accuracy using bootstrap-corrected calibration curves (1,000 iterations). The clinical utility of the nomogram was compared against the AJCC staging system through decision curve analysis (DCA). In this study, a P-value < 0.05 in the multivariate COX analysis was considered statistically significant.
Results
Clinical and demographic characteristics
The demographic and clinical characteristics of 580 patients with NCSCC, encompassing age, sex, race, tumor grade, SCS, surgical intervention, radiotherapy, and chemotherapy status, are comprehensively detailed in Table 1, illustrating their respective distribution patterns. The P-values for all variables between the training cohort and validation cohort were greater than 0.05, indicating no significant difference between the two cohorts. CSS analysis demonstrated that the cohort exhibited survival probabilities of 89.1% at 1 year, 74.8% at 3 years, and 63.6% at 5 years.
Univariable Cox regression analysis
Univariable Cox regression analysis identified the following variables with p-values < 0.10: tumor differentiation grade, AJCC stage, T stage, N stage, M stage, surgical intervention, radiotherapy, tumor size, race, age, marital status, and SCS. Comprehensive results are presented in Table 2.
LASSO regression analysis
The variable selection process of LASSO regression is shown in Fig. 2. As illustrated in Fig. 2A, as the penalty coefficient (λ) increases, the compression of coefficient estimates for independent variables intensifies, accompanied by a gradual reduction in the number of variables until they are compressed to zero. Figure 2B displays the relationship between the number of independent variables and log(λ). The two dashed lines represent λ(min) and λ(1se), respectively. λ(min) corresponds to the optimal coefficient when the mean squared error is minimized, selecting 8 variables, while λ(1se) represents the best coefficient within one standard error of the mean squared error, selecting 1 variable. Considering the practicality of clinical prediction models, we ultimately adopted the 8 most potential variables selected by λ(min) for inclusion in the prediction model: age, race, surgery, radiation, N-stage, AJCC stage, tumor size, and marital status.
Multivariable cox regression analysis and nomogram construction
The screened variables were incorporated into multivariate Cox regression analysis, which identified AJCC Stage, Radiation, and Age as independent factors influencing CSS in NCSCC patients. The first row of the nomogram displays scores corresponding to each variable, and the cumulative total score from the nomogram can predict 1-, 3-, and 5-year CSS for individual patients. See Fig. 3 for details.
Validation and clinical utility assessment of the prediction model
The model was validated using the C-index and calibration curves. As shown in Fig. 4, the C-indices for predicting 1-, 3-, and 5-year CSS with the nomogram were all > 0.70, indicating the strong discriminatory ability of the nomogram. Additionally, calibration curves were employed to assess model calibration (Fig. 5). In the training cohort, the calibration curve for 1-year CSS clustered around the ideal curve, while the 3- and 5-year calibration curves nearly overlapped with the ideal curve. When the predictive coefficients from the Cox regression analysis in the training set were applied to the validation set, the calibration curves for the validation cohort also aligned closely with the ideal curve, demonstrating excellent model consistency. To evaluate the clinical utility of the model, decision curve analysis (DCA) was performed (Fig. 6). The horizontal purple line represents the net benefit of no treatment, while the blue diagonal line denotes the net benefit of the treatment strategy. The results revealed that the new model provided a higher overall net benefit compared to the AJCC staging system, with a broader probability threshold range for survival. This suggests that the new model offers greater clinical utility and potential net benefit for patients.
Discussion
Malignancies of the head and neck region represent a heterogeneous group of neoplasms characterized by their complex oncological nature, originating from diverse anatomical subsites. Among these, nasal cavity malignancies constitute a distinct clinical entity that is relatively uncommon in otolaryngological practice, as evidenced by epidemiological studies2. The incidence of head and neck malignancies has shown a sustained upward trend in multiple countries in recent years1. However, to date, no studies have utilized multi-institutional data or large-scale national databases to statistically evaluate demographic characteristics and survival outcomes in NCSCC. Furthermore, most previously published studies have grouped nasal cavity malignancies with paranasal sinus malignancies, resulting in a lack of large-sample survival analyses specifically focused on nasal cavity malignancies8,10. While nasal cavity malignancies exhibit histologically heterogeneous subtypes, SCC remains the most prevalent histological type11. Therefore, leveraging the SEER database, we aimed to develop a prognostic nomogram through comprehensive analysis of patients’ demographic and clinicopathological characteristics, to provide actionable insights for clinicians and researchers in related fields. This study further benchmarked the novel model against the conventional AJCC staging system to ascertain its potential superiority in prognostic stratification.
Multivariate Cox regression analysis revealed that among demographic characteristics, age—as anticipated—emerged as a significant independent prognostic factor for NCSCC6. In contrast, race, sex, and marital status did not demonstrate statistically significant prognostic value in this cohort.
In this study, systemic therapy, chemotherapy, surgical intervention, and lymphadenectomy demonstrated no significant prognostic impact on NCSCC. Systemic therapy, primarily chemotherapy, serves as an alternative treatment modality, typically reserved for advanced-stage patients. It may be integrated with surgery and radiotherapy or used as a standalone approach in advanced disease12. Furthermore, chemotherapy alone showed no observable survival benefit in NCSCC. A retrospective analysis by Sarah Nicole Hamilton involving 159 NCSCC patients found no association between concurrent chemotherapy and local recurrence or overall survival (OS). However, the limited sample size of chemotherapy-treated patients (likely representing high-risk subgroups) challenges the generalizability of these conclusions13. The potential prognostic benefits of chemotherapy in NCSCC should not be categorically dismissed. In a retrospective analysis of 21 sinonasal and nasal cavity malignancies by Chan-Young Ock et al., patients receiving neoadjuvant chemotherapy achieved significant T-stage downstaging and increased feasibility of eye-preserving salvage surgery10. Furthermore, a meta-analysis by Ruth S. Goh et al. demonstrated that induction chemotherapy combined with definitive treatment significantly improved OS (HR = 0.56, 95% CI [0.36–0.86], p = 0.009), suggesting that rational integration of chemotherapy into multimodal treatment strategies may confer survival benefits14.
Lymphadenectomy was not significantly associated with prognostic outcomes in NCSCC (OR = 1.64; 95% CI: 0.97–2.75; P = 0.063). This observation may be attributable to the early-stage predominance of nasal cavity malignancies, which exhibit low rates of cervical lymph node metastasis3,7. In a retrospective analysis of 39 NCSCC patients by Christoph Becker et al., only 1 of 310 dissected lymph nodes harbored metastatic disease3. Similarly, our cohort revealed that 93% of cases were classified as AJCC N0-N1. Although surgical resection remains the mainstay of treatment for nasal cavity malignancies3,6,12 no significant survival benefit was observed in this analysis. However, clinical evidence indicates that radiotherapy as a monotherapy often demonstrates limited efficacy in controlling lymph node metastasis in NCSCC, whereas neck dissection has proven to be an effective intervention for regional lymph node metastasis15. Surgical resection remains the primary therapeutic modality for NCSCC management16. The subsequent administration of adjuvant radiotherapy and chemotherapy in postoperative NCSCC patients may potentially confound the assessment of therapeutic benefits specifically attributable to surgical intervention and lymph node dissection. While current data have not demonstrated statistically significant differences in outcomes between lymph node dissection and surgical treatment modalities, the establishment of definitive conclusions necessitates the implementation of large-scale, well-designed prospective clinical trials for comprehensive validation.
Radiotherapy emerged as an independent prognostic factor for NCSCC in this study. Early survival analyses of NCSCC suggested generally poorer outcomes with radiotherapy17. However, researchers at MD Anderson Cancer Center reported outcomes in a cohort of 68 nasal cavity carcinoma patients, where 32 (47%) underwent definitive radiotherapy. The 5-year local control rate reached 81.1%, with comparable outcomes between definitive radiotherapy and postoperative adjuvant radiotherapy (P = 0.10). No significant difference in 5-year survival was observed between postoperative radiotherapy and definitive radiotherapy alone18. These findings suggest that optimized radiotherapy techniques with precise dose delivery can achieve therapeutic equivalence to surgery-based multimodal approaches, particularly for early-stage NCSCC14. Although radiotherapy carries inherent toxicities, proton beam therapy has demonstrated reduced toxicity profiles without compromising oncologic outcomes19. Due to the clinical rarity of malignant nasal squamous cell carcinoma, there is currently no unified treatment protocol20. Various treatment approaches have been reported, with most evidence derived from small case series, thus the optimal treatment regimen remains unknown19,21,22. Based on the findings of this study and previous research, we recommend that early-stage tumors may be treated with surgical resection alone. However, for advanced tumors, surgery combined with radiotherapy is essential, and chemotherapy should be considered on an individual basis13. This aligns with the guidelines of the National Comprehensive Cancer Network13.
In tumor staging, AJCC staging demonstrated significant prognostic relevance in NCSCC, primarily attributed to its incorporation of tumor invasion extent and distant metastasis status—critical prognostic determinants in squamous cell carcinoma2,6. Reported median overall survival (OS) for NCSCC with distant metastasis is 14.3 months23. Intriguingly, our study failed to identify distant metastasis as an independent prognostic factor, which seemingly contradicts previous findings and clinical consensus. Given the rarity of distant metastasis in nasal cavity squamous cell carcinoma2,3 we hypothesize this discrepancy may reflect insufficient statistical power from the limited subsample size (n = 4, 1% of the training cohort). Additionally, tumor differentiation grade showed no prognostic significance in our analysis, aligning with prior studies reporting no robust association between histological differentiation and survival outcomes in NCSCC13. Multivariable analysis revealed a non-significant trend toward worse prognosis with increasing tumor size (P > 0.05), consistent with existing literature13.
Following the construction of the nomogram and consideration of identified prognostic factors, we conducted a comprehensive evaluation of the model, which is essential for validating any clinical prediction model prior to real-world implementation. Overall, the model demonstrated a C-index > 0.7, indicating robust discriminatory ability. Furthermore, calibration curves confirmed excellent agreement between predicted and observed outcomes. An increasing number of researchers now employ DCA to assess the net clinical benefit of therapeutic interventions. Our results revealed that the novel model provided superior overall net benefit compared to the AJCC staging system across a broader range of probability thresholds for survival, suggesting its enhanced clinical utility in optimizing patient management and facilitating better clinical decision-making.
This study has several limitations. First, the retrospective design utilizing data from the SEER database may introduce information bias due to inherent constraints in data completeness and documentation variability. Furthermore, this prognostic model does not incorporate critical factors such as HPV status, smoking history, or alcohol status, which may significantly influence disease progression and treatment response. This omission potentially compromises the model’s predictive accuracy. Consequently, prospective cohort studies are warranted to enhance prognostic precision in future research. Third, while internal validation demonstrated robust performance, external validation across independent multicenter cohorts remains essential to confirm generalizability and clinical applicability. Moreover, limited sample sizes in specific subgroups (notably those with distant metastasis) may compromise the statistical power for robust risk stratification in corresponding clinical scenarios. Finally, as a retrospective investigation, this study carries inherent limitations including undocumented treatment protocols and incomplete patient background profiles, potentially limiting the accuracy of prognostic evaluations.
In conclusion, we developed the first nomogram for predicting 1-, 3-, and 5-year CSS in NCSCC patients using a large retrospective cohort. This tool integrates key demographic and clinicopathological variables with rigorous validation, confirming its discriminative power (C-index > 0.7) and clinical utility (superior net benefit vs. AJCC staging). The model provides clinicians with a practical and intuitive framework for personalized risk stratification and therapeutic decision-making. Future efforts will focus on external validation and the incorporation of emerging biomarkers to enhance prognostic precision.
Data availability
The datasets analyzed in this study are available in the SEER database repository: https://seer.cancer.gov/.
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249 (2021).
Unsal, A. A. et al. Squamous cell carcinoma of the nasal cavity: A population-based analysis. Laryngoscope 126 (3), 560–565 (2016).
Becker, C., Kayser, G. & Pfeiffer, J. Squamous cell cancer of the nasal cavity: new insights and implications for diagnosis and treatment. Head Neck. 38 (Suppl 1), E2112–2117 (2016).
Chowdhury, N. et al. Outcomes of HPV-related nasal squamous cell carcinoma. Laryngoscope 127 (7), 1600–1603 (2017).
Scurry, W. C. Jr. et al. Regional recurrence of squamous cell carcinoma of the nasal cavity: a systematic review and meta-analysis. Arch. Otolaryngol. Head Neck Surg. 133 (8), 796–800 (2007).
Bhattacharyya, N. Cancer of the nasal cavity: survival and factors influencing prognosis. Arch. Otolaryngol. Head Neck Surg. 128 (9), 1079–1083 (2002).
Dale, O. T. et al. Squamous cell carcinoma of the nasal cavity: A descriptive analysis of cases from the head and neck 5000 study. Clin. Otolaryngol. 44 (6), 961–967 (2019).
Sharma, R. K. et al. Conditional and overall Disease-Specific survival in patients with paranasal sinus and nasal cavity cancer: improved outcomes in the endoscopic era. Am. J. Rhinol Allergy. 36 (1), 57–64 (2022).
Sharma, R., Sahni, D., Uppal, K., Gupta, R. & Singla, G. A clinicopathological study of masses of nasal cavity paranasal sinuses and nasopharynx. Int. J. Otorhinolaryngol. Head Neck Surg. (2017).
Ock, C. Y. et al. Induction chemotherapy in head and neck squamous cell carcinoma of the paranasal sinus and nasal cavity: a role in organ preservation. Korean J. Intern. Med. 31 (3), 570–578 (2016).
Kumari, S., Pandey, S., Verma, M., Rana, A. K. & Kumari, S. Clinicopathological challenges in tumors of the nasal cavity and paranasal sinuses: our experience. Cureus, 14(9), e29128 (2022).
Jakimovska, F., Stojkovski, I. & Kjosevska, E. Nasal cavity and paranasal sinus cancer: diagnosis and treatment. Curr. Oncol. Rep. 26 (9), 1057–1069 (2024).
Hamilton, S. N. et al. Population-based Long-term outcomes for squamous cell carcinoma of the nasal cavity. Am. J. Clin. Oncol. 46 (5), 199–205 (2023).
Goh, R. S. & Keng, C. G. H. A Meta-Analysis on the impact of induction chemotherapy on survival outcomes for sinonasal squamous cell carcinoma. J. Rhinol. 32 (1), 10–16 (2025).
Lill, C. et al. The role of elective neck dissection in T1 and T2 nasal cavity squamous cell carcinomas. Eur. Arch. Otorhinolaryngol. 280 (4), 1875–1883 (2023).
Schneider, T. A., Gryn, O. & Lutz, M. A case of squamous cell carcinoma of the nasal cavity treated with total rhinectomy. Cureus 14 (3), e23576 (2022).
Jang, N. Y. et al. Definitive radiotherapy with or without chemotherapy for T3-4N0 squamous cell carcinoma of the maxillary sinus and nasal cavity. Jpn J. Clin. Oncol. 40 (6), 542–548 (2010).
Allen, M. W. et al. Long-term radiotherapy outcomes for nasal cavity and septal cancers. Int. J. Radiat. Oncol. Biol. Phys. 71 (2), 401–406 (2008).
Mimica, X. et al. Organ preservation for patients with anterior mucosal squamous cell carcinoma of the nasal cavity: Rhinectomy-free survival in those refusing surgery. Head Neck. 41 (8), 2741–2747 (2019).
Issa, K. et al. Nasal cavity squamous cell carcinoma: factors associated with treatment outcomes and potential organ preservation. Am. J. Rhinol Allergy. 37 (1), 35–42 (2023).
Nishiya, Y. et al. Endoscopic surgery for squamous cell carcinoma in the nasal cavity and ethmoid sinus: A retrospective observational study. Auris Nasus Larynx. 51 (6), 996–1002 (2024).
Qian, Y. et al. Pembrolizumab with chemotherapy for patients with recurrent or metastatic nasal cavity and paranasal sinus squamous cell carcinoma: A prospective phase Ll study. Clin. Cancer Res. 31 (9), 1636–1643 (2025).
Qian, Y. et al. Pembrolizumab with chemotherapy for patients with recurrent or metastatic nasal cavity and paranasal sinus squamous cell carcinoma: A prospective phase Ll study. Clin Cancer Res. (2025).
Acknowledgements
We express our sincere gratitude to Ms. Hongxiang Qu, M.S., from the School of Mathematics and Statistics, Hunan Normal University, for her expert guidance and valuable advice on the statistical analysis in this study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Qw.Z., M.S., and S.Oy.: Jointly completed data organization, manuscript draft-ing, and statistical data analysis. B. L.: Research supervision, paper review, and revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The data utilized in this study were obtained from the publicly available SEER database and contained no personally identifiable information. Therefore, this research was exempt from ethics committee approval in accordance with regulations governing de-identified public datasets.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, Q., Sheng, M., Ouyang, S. et al. Development and validation of a prognostic model for nasal cavity squamous cell carcinoma based on the SEER database. Sci Rep 15, 26939 (2025). https://doi.org/10.1038/s41598-025-10355-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10355-w