Abstract
Excessive ammonium nitrogen (NH4+-N) in aquatic environments poses significant challenges for wastewater treatment and microalgae-based bioremediation. This study investigated the effects of phytohormones on enhancing the tolerance of Oocystis borgei to high NH4+-N stress and explored the underlying mechanisms. Growth of O. borgei was significantly inhibited by NH4+-N in a dose-dependent manner, with 500 mg·L−1 NH4+-N selected as the stress concentration. Supplementation with 10−6 M indole-3-acetic acid (IAA), gibberellic acid (GA3), or zeatin (ZT) significantly alleviated growth inhibition under high NH4+-N stress, increasing cell density by 22–26% and specific growth rate by 217–282% compared to the HAN group. No significant differences were observed among the tested phytohormones. Phytohormone treatments mitigated photosynthetic inhibition by enhancing chlorophyll a (Chla) and carotenoid (Car) contents, as well as Fv/Fm and φPSII values. While rbcL gene expression remained suppressed, phytohormones reduced oxidative stress by decreasing malondialdehyde (MDA) content and modulating superoxide dismutase (SOD) and catalase (CAT) activities. Additionally, phytohormones promoted nitrogen metabolism by significantly increasing glutamine synthetase (GS) activity and gene expression. This study provides insights into the protective mechanisms of phytohormones against high NH4+-N stress in O. borgei, offering potential strategies for enhancing algal-based treatment of high-ammonium wastewater.
Similar content being viewed by others
Introduction
Ammonia nitrogen is one of the most pressing pollutants in aquatic environments, posing severe threats to ecosystem health and human well-being due to its high toxicity and widespread presence in surface water systems1,2,3. Ammonia nitrogen in water predominantly exists in two forms: ionized ammonium (NH₄⁺) and unionized ammonia (NH₃). The relative concentrations of these forms are influenced by pH and temperature. At higher temperatures and pH values (e.g., > 30 °C and pH > 8.5), unionized ammonia predominates, while at lower temperatures and pH levels, ionized ammonium is more common3.
In China, ammonium nitrogen contamination is a significant issue across river basins, primarily originating from the discharge of industrial wastewater, agricultural runoff, urban effluents, and leachate from landfills4,5,6. The concentration of total ammonium nitrogen in wastewater varies depending on the source. For example, ammonium concentrations in urban wastewater typically range from 10 to 200 mg·L⁻¹, while in industrial wastewater, concentrations can range from 5 to 1000 mg·L⁻¹7. Agricultural and livestock activities often release even higher levels of ammonium8. In view of the extensive and detrimental nature of ammonium nitrogen pollution9,10,11it is imperative to implement efficient wastewater treatment prior to discharge in order to reduce the ammonia nitrogen concentration.
Several physical and chemical methods are currently employed to address nitrogen pollutants in wastewater12,13. Despite their utility, these conventional methods are hindered by high operational costs, and inefficiency, and significant adverse environmental impacts14,15underscoring the urgent need for more sustainable alternatives. In contrast, biological nitrogen removal (BNR) technologies, driven by microorganisms, are gaining attention for their efficiency, cost-effectiveness, and lack of secondary pollution16,17. Among emerging solutions, microalgae-based nitrogen recovery technologies stand out for their ability to not only remove ammonia nitrogen efficiently but also to recover valuable resources, making them a sustainable and economically viable alternative to conventional methods7,18,19,20.
Microalgae are known to efficiently absorb ammonium nitrogen, typically preferring it over other nitrogen sources. However, at high concentrations or with prolonged exposure, ammonium nitrogen becomes toxic to algal cells, inhibiting their growth7,20,21. Although certain microalgae strains have been screened or acclimatized to tolerate elevated ammonium concentrations for wastewater treatment, these strains generally perform well only in wastewater with ammonium nitrogen concentrations below 500 mg·L⁻¹, or they require dilution or pre-treatment to support growth7,22,23. Ammonium toxicity remains a significant challenge in biological wastewater treatment. Therefore, mitigating ammonium stress in microalgae is critical for improving their pollutant removal efficiency in high-ammonium nitrogen wastewater.
Phytohormones play a vital role in regulating plant growth and enhancing stress resistance. The regulation of physiological and biochemical processes, as well as microalgal-bacterial interactions by phytohormones enhances microalgae’s resistance to various abiotic stresses24,25,26,27. For instance, phytohormones can upregulate antioxidant enzyme activities, improve photosynthetic efficiency, and modulate productivity28,29,30. Several studies have demonstrated that the exogenous application of phytohormones can alleviate nitrogen stress in microalgae cultures, improving their performance31,32,33. Additionally, research suggests that the crosstalk between nitrogen signaling and phytohormones in algae may be influenced by nitrogen-enriched or nitrogen-depleted growth media, which affects the endogenous concentration of these hormones34,35. Recent studies have also highlighted the potential of using phytohormones to improve nutrient and pollutant removal efficiency in wastewater treatment via microalgae36,37,38,39,40.
Oocystis borgei is a green alga isolated from a subtropical aquaculture pond of Litopenaeus vannamei by our laboratory41. Our previous research has demonstrated that O. borgei exhibits stable population dynamics42broad salinity tolerance43self-settling ability44and capacity for preservation at ambient temperature45demonstrating great potential for regulating aquaculture water and promoting shrimp growth and health46. Recent findings reveal that low concentrations (10⁻⁶ M) phytohormones, including indole-3-acetic acid (IAA), zeatin (ZT), and gibberellin A3 (GA3), effectively stimulate its growth and enhance nutrient removal (nitrogen and phosphorus) from seawater aquaculture wastewater37. While O. borgei preferentially utilizes ammonium nitrogen as its primary nitrogen source47the synergistic effects of phytohormones in enhancing its resistance to high ammonium nitrogen stress (HANS) remain underexplored, hindering the full application of this alga in large-scale wastewater treatment. Addressing this gap is crucial for maximizing the effectiveness of microalgae in ammonia nitrogen removal.
This study seeks to investigate how exogenous phytohormones can be leveraged to enhance O. borgei’s tolerance to HANS, ultimately improving its capacity for efficient and sustainable ammonia nitrogen removal. Specifically, low concentrations (10⁻⁶ M) of phytohormones (IAA, ZT, GA3) were supplemented under HANS conditions, and then growth, photosynthetic activity, antioxidant capacity, and nitrogen metabolism were measured to reveal the toxicity of HANS and the influence of phytohormones on the stress resistance of O. borgei. By elucidating these mechanisms, this research will pave the way for enhanced microalgae-based ammonia nitrogen removal systems, offering a sustainable and scalable solution to one of the most critical environmental challenges worldwide.
Materials and methods
Preculture and starvation treatment of O. borgei
The O. borgei strain, supplied by our laboratory, was pre-cultured in 5 L of Zhanshui 107 − 13 medium37 (prepared with autoclaved artificial seawater48) at 25 °C for 14 days under continuous illumination (21 µmol·m⁻²·s⁻¹) in the culture room. Cells were then harvested by centrifugation (5,000 rpm, 10 min), washed with sterile artificial seawater (salinity 30), and starved in N-deficient medium for 48 h. Prior to experiments, cells were resuspended in fresh N-deficient medium at 1.04 × 10⁶ cells·mL⁻¹ (OD₆₈₀ = 0.26).
Experimental design
Dose-response analysis was conducted to study the toxic effect of high ammonium nitrogen on O. borgei. NH4Cl stock solution (105 mg-N L−l) was used to create five different NH4+-N concentrations gradients (50, 200, 500, 800 and 1000 mg-N·L−1). For the control, N-sufficient medium was used. The initial pH of the medium was adjusted to approximately 7.0 using NaOH or HCl, s at this pH NH₄⁺ is the predominant form of ammonia nitrogen1,7.
To investigate the effect of phytohormones on O. borgei under HANS, O. borgei was cultured in a medium containing 500 mg-N L⁻¹, which was selected based on preliminary toxicity experiments. Subsequently, indole-3-acetic acid (IAA), gibberellic acid (GA3), and zeatin (ZT) (supplied by Hefei Bomei Biotechnology Co., Ltd. Hefei, China) were added to the culture at a concentration of 10−6 M, as previously demonstrated to promote the growth of O. borgei37. The control group was inoculated with cells in standard Zhanshui 107 − 13 medium.
Culture conditions and growth indicators measurement
Cultures (200 mL in 250 mL flasks) were maintained in biological triplicates and then incubated in a light incubator (PRX-350 C, Ningbo Prant Instrument Co., Ltd., Ningbo, China) at 25 °C with a light intensity of 45 µmol·m⁻²·s⁻¹ and a 12 h/12 h light/dark photocycle for 7 days, with manual shaking four times daily. Growth kinetics were monitored through daily optical density measurements (OD₆₈₀) and specific growth rate calculations37 over the 7-day experimental period.
Photosynthetic performance analysis
Comprehensive photosynthetic analyses were conducted at two critical timepoints (Days 4 and 7). The concentrations of photosynthetic pigments, including chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids (Car), were determined using the hot-ethanol extraction method followed by spectrophotometric analysis (UV-1900i, Shimadzu, Japan). The quantification was performed according to established equations (Eqs. 1–3)49. Chlorophyll fluorescence parameters, including the actual photochemical efficiency (φPSII) of photosystem II (PSII) and the maximum quantum yield of PSII (Fv/Fm), were measured with a pulse-modulated fluorometer (FMS-2, Hansatech Instruments, Pentney, UK) with 594 nm amber modulation beam according to established protocols44. For the Fv/Fm measurement, samples were incubated in darkness at room temperature for 20 min before measurement. The photosynthesis-related gene ribulose-bisphosphate carboxylase (rbcL) was quantified via real-time quantitative PCR (qPCR). Total RNA extraction, cDNA synthesis and qPCR reaction were carried out according to the previous research methods50. Ribosomal protein S27 (RPS27) was employed as the internal reference gene to quantify the relative expression levels of the target genes (Table 1). The fold-change in gene expression was calculated using the comparative cycle threshold (2−ΔΔCt) method51.
where Vt is the extract volume (mL), n is the dilution factor, Vq is the sample volume (mL), and C is the cell density of O. borgei (cells·mL−1). Each absorbance value was corrected by subtracting the absorbance value at 750 nm.
Oxidative stress, antioxidant enzyme activities evaluation
10 mL of microalgae samples at the same sampling intervals were collected for evaluation of oxidative stress. Lipid peroxidation was evaluated by measuring malondialdehyde (MDA) content via thiobarbituric acid (TBA) assay. Antioxidant enzyme activities, including superoxide dismutase (SOD) and catalase (CAT), were measured according to the water-soluble tetrazolium (WST-1) method and ammonium molybdate method respectively. The assays followed the protocols provided by commercial kits (Nanjing Jiancheng, Nanjing, China).
Nitrogen metabolism assessment
Nitrogen metabolism was investigated through both enzymatic and molecular approaches. Glutamine synthetase (GS) activity was measured colorimetrically, while transcriptional abundance of the nitrogen metabolism-related gene glutamine synthetase (GS) was quantified via real-time quantitative PCR (qPCR) as described above.
Statistical analysis
Experimental data are presented as the mean ± standard deviation (SD), with n = 3. Data analysis was performed using GraphPad Prism 9. One-way ANOVA followed by Tukey’s multiple comparisons test was employed to assess significant differences among different treatments at the same time. Statistically significant differences are denoted by different letters (p < 0.05). For comparisons between different time points within the same treatment, unpaired t-tests were applied, and significant differences are marked with asterisks (* p ≤ 0.05, ** p ≤ 0.01).
Results
Effect of high ammonium nitrogen stress on the growth of O. borgei
A dose-response analysis was conducted to determine the NH₄⁺-N concentration that induces stress in O. borgei. The results showed that NH₄⁺-N significantly inhibited O. borgei growth in a dose-dependent manner, with higher concentrations leading to more pronounced inhibition (Fig. 1). Specifically, after 7 days of exposure to NH₄⁺-N at concentrations of 50, 200, 500, 800, and 1000 mg·L⁻¹, cell density decreased by 7.77%, 12.98%, 27.70%, 32.08%, and 35.38%, respectively, compared to the control group (p ≤ 0.01). At lower NH₄⁺-N concentrations (50 and 200 mg·L⁻¹), cell density decreased compared to the control but positive growth trends were still observed (Fig. 1A). However, at 500 mg·L⁻¹ and above, cell density significantly decreased after 4 days of cultivation, resulting in negative growth (p ≤ 0.01), which persisted throughout the experiment (Fig. 1B). Based on these findings, 500 mg·L⁻¹ NH₄⁺-N was selected as the stress concentration for subsequent experiments.
Effect of different concentrations of ammonium nitrogen on the growth of O. borgei. (A) Cell density; (B) Daily specific growth rate. Experimental data are presented as mean ± standard deviation (SD), with n = 3. Data analysis was performed using GraphPad Prism 9 with one-way ANOVA followed by Tukey’s multiple comparisons test. Statistically significant differences among treatments at the same time are denoted by different letters (p ≤ 0.05).
Effects of phytohormones on the growth of O. borgei under high ammonium nitrogen stress
The effects of phytohormones on O. borgei growth under HAN stress were evaluated by measuring cell density and specific growth rate (Fig. 2). The addition of 10⁻⁶ M IAA, GA3, and ZT significantly increased cell density compared to the HAN group (p ≤ 0.05), with the differences becoming highly significant after 4 days (p ≤ 0.01). After 7 days, cell densities in the IAA, GA₃, and ZT groups were 1.15, 1.12, and 1.16 times higher than those in the HAN group (p ≤ 0.01), respectively (Fig. 2A). The 7-day average specific growth rates for the IAA, GA3, and ZT groups were 0.027, 0.023, and 0.028 d⁻¹, respectively, significantly higher than the 0.007 d⁻¹ observed in the HAN group (p ≤ 0.001) (Fig. 2B). No significant differences were found among the three phytohormone treatments. These results demonstrate that phytohormones effectively alleviate the inhibitory effects of high NH₄⁺-N stress on O. borgei growth, highlighting their potential as growth regulators under stressful conditions. The 4th day emerged as a critical analytical timepoint, marked by negative growth in the HAN-treated O. borgei (Fig. 1) and significant cell density enhancement through IAA, GA3, and ZT supplementation (Fig. 2). So, physiological, biochemical, and molecular analyses were consequently conducted on days 4 and 7.
Effects of phytohormones on the growth of O. borgei under HANS. (A) Cell density; (B) Average specific growth rate over 7 d. HAN. High ammonium-nitrogen (500 mg-N L−1); HAN + IAA. High ammonium nitrogen with 10−6 M IAA; HAN + GA3. High ammonium nitrogen with 10−6 M GA3; HAN + ZT: High ammonium nitrogen with 10−6 M ZT. Experimental data are presented as mean ± standard deviation (SD), with n = 3. Data analysis was performed using GraphPad Prism 9 with one-way ANOVA followed by Tukey’s multiple comparisons test. Statistically significant differences among treatments at the same time are denoted by different letters (p ≤ 0.05).
Phytohormone-mediated enhancement of stress tolerance in O. borgei
Alleviation of photosynthetic inhibition
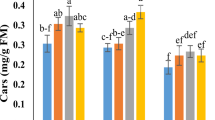
To evaluate the impact of HAN stress on the photosynthetic activity of O. borgei, we measured the contents of Chla, Chlb, and Car (Fig. 3A-C). The contents of Chla and Car in the control group were significantly increased with time during cultivation (p ≤ 0.05). However, exposure to HAN stress caused a significant decline in Chla and Car contents. After 4 days, Chla and Car contents were reduced to 69.48% (p ≤ 0.0001) and 52.51% (p ≤ 0.001) of control levels, respectively, with no further significant decrease observed by day 7. In contrast, Chlb content remained relatively stable (p > 0.05). The supplementation of phytohormones (10⁻⁶ M IAA, GA₃, and ZT) effectively mitigated the decrease in Chla and Car contents. By day 4, Chla and Car levels in treated groups were 1.19–1.33 times (p ≤ 0.05) and 1.48–1.54 times (p ≤ 0.01) those of the HAN group, respectively. However, no significant differences were found among the three phytohormones (p > 0.05), and the alleviating effect of phytohormones on HAN stress did not exhibit a time-dependent pattern.
Effects of phytohormones on photosynthetic metabolism of O. borgei under HANS. (A) Chla content; (B) Chlb content; (C) Carotenoid content; (D) Fv/Fm; (E) φPSII; (F) relative expression level of rbcL. HAN. High ammonium-nitrogen (500 mg-N L−1); HAN + IAA. High ammonium nitrogen with 10−6 M IAA; HAN + GA3. High ammonium nitrogen with 10−6 M GA3; HAN + ZT: High ammonium nitrogen with 10−6 M ZT. Experimental data are presented as mean ± standard deviation (SD), with n = 3. Data analysis was performed using GraphPad Prism 9. One-way ANOVA followed by Tukey’s multiple comparisons test was employed to assess significant differences among different treatments at the same time. Statistically significant differences are denoted by different letters (p ≤ 0.05). For comparisons between different time points within the same treatment, unpaired t-tests were applied, and significant differences are marked with asterisks (* p ≤ 0.05, ** p ≤ 0.01).
Additionally, we measured two chlorophyll fluorescence parameters (Fig. 3D and E) to assess the effects of phytohormones on photosynthetic activity. While no statistically significant differences were observed, the Fv/Fm and φPSII values in the control group gradually increased over time during cultivation (p > 0.05). In contrast, exposure to HAN stress resulted in a pronounced decline in both Fv/Fm and φPSII, with a significant downward trend observed over time. By day 7, these parameters had decreased to 59.3% (p ≤ 0.0001) and 41.6% (p ≤ 0.0001) compared to the control. Phytohormone treatment significantly alleviated photoinhibition under HAN stress. After 4 days, Fv/Fm under all phytohormone treatments and φPSII under ZT treatment recovered to levels that were not significantly different from the control (p > 0.05). Moreover, Fv/Fm and φPSII remained significantly higher than those in the HAN group after 7 days (p ≤ 0.01), despite a partial reduction in the alleviating effect.
We also examined the expression of the rbcL gene (Fig. 3F), which encodes the large subunit of Rubisco. HAN stress significantly reduced rbcL expression to 3.78% (p ≤ 0.0001) and 4.75% (p ≤ 0.0001) of control levels after 4 and 7 days, respectively. Phytohormone treatment resulted in a moderate increase in rbcL expression, though differences compared to the HAN group were not significant (p > 0.05), indicating a mild enhancement of rbcL expression under stress conditions. In conclusion, 10⁻⁶ M IAA, GA₃, and ZT enhanced the photosynthetic activity of O. borgei by increasing photosynthetic pigment content and chlorophyll fluorescence parameters, suggesting their potential as important factors in promoting resistance to HANS.
Reducing lipid peroxidation and alleviating oxidative stress damage
To explore the effects of HAN stress and phytohormones on oxidative stress and antioxidant systems in O. borgei, we measured malondialdehyde (MDA) content and the activities of catalase (CAT) and superoxide dismutase (SOD) (Fig. 4). Under HAN stress, MDA content, a marker of lipid peroxidation, significantly increased to 2.53 times the control level after 4 days (p ≤ 0.0001), then decreased to 1.89 times by day 7 (p ≤ 0.001) (Fig. 4A). This suggests an initial surge in oxidative stress followed by a partial recovery. SOD and CAT activities, key antioxidants, also increased sharply to 1.75 times (p ≤ 0.0001) and 1.79 times (p ≤ 0.001) the control levels at day 4, then slightly decreased to 1.43 times (p ≤ 0.001) and 1.68 times (p ≤ 0.01) by day 7 (Fig. 4B and C), indicating a persistent oxidative challenge. Phytohormone treatment significantly mitigated these oxidative stress indicators. At day 4, the increase in MDA content was substantially reduced in all phytohormone-treated groups compared to the HAN group. By day 7, MDA content in the HAN + IAA group decreased significantly (p ≤ 0.05) and approached control levels (Fig. 4A). Similarly, SOD activity in the HAN + GA3 and HAN + ZT groups and CAT activity in all phytohormone treatments returned to levels indistinguishable from the control group (p > 0.05) (Fig. 4B and C), indicating that these phytohormones effectively regulated antioxidant enzyme activities and reduced lipid peroxidation.
Effects of phytohormones on lipid peroxidation and antioxidant enzyme activities in O. borgei under HANS. (A) MDA content; (B) SOD activities; (C) CAT activities. HAN. High ammonium-nitrogen (500 mg-N L−1); HAN + IAA. High ammonium nitrogen with 10−6 M IAA; HAN + GA3. High ammonium nitrogen with 10−6 M GA3; HAN + ZT: High ammonium nitrogen with 10−6 M ZT. Experimental data are presented as mean ± standard deviation (SD), with n = 3. Data analysis was performed using GraphPad Prism 9. One-way ANOVA followed by Tukey’s multiple comparisons test was employed to assess significant differences among different treatments at the same time. Statistically significant differences are denoted by different letters (p ≤ 0.05). For comparisons between different time points within the same treatment, unpaired t-tests were applied, and significant differences are marked with asterisks (* p ≤ 0.05).
Promoting nitrogen metabolism in O. borgei
To investigate the effects of HAN stress and phytohormones on nitrogen metabolism in O. borgei, we measured GS enzyme activity and GS gene expression levels (Fig. 5). Under HAN stress, there was a significant decrease in GS activity and gene expression over the 7-day period. At 4 days GS activity reduced to 23.91% of the control level (p ≤ 0.0001) and by 7 days, it further decreased to 42.86% (p ≤ 0.01) (Fig. 5 A). Similarly, GS gene expression was significantly reduced to 2.5% at 4 days and slightly increased to 4.22% at 7 days (p ≤ 0.0001) (Fig. 5B), indicating severe and persistent inhibition of nitrogen metabolism by HAN stress. Phytohormone treatments significantly enhanced nitrogen metabolism under HAN stress. At 4 days, GS activity increased significantly in all treated groups compared to the HAN group (p ≤ 0.001), with IAA, GA3, and ZT enhancing activity to 2.64, 2.91, and 2.73 times that of the HAN group, respectively. (Fig. 5 A). GS gene expression also rose significantly, with ZT showing the most pronounced effect at 22.40 times the HAN group level (p ≤ 0.001), followed by GA3 and IAA (Fig. 5B). Furthermore, the promoting effect of phytohormones on the GS activity and GS gene expression persisted until day 7 (p > 0.05). However, there were no significant differences among the three phytohormone treatment groups.
Effects of phytohormones on nitrogen metabolism in O. borgei under HANS. (A) Content of GS; (B) Relative expression level of GS; HAN. High ammonium-nitrogen (500 mg-N L−1); HAN + IAA. High ammonium nitrogen with 10−6 M IAA; HAN + GA3. High ammonium nitrogen with 10−6 M GA3; HAN + ZT: High ammonium nitrogen with 10−6 M ZT. Experimental data are presented as mean ± standard deviation (SD), with n = 3. Data analysis was conducted using GraphPad Prism 9. One-way ANOVA followed by Tukey’s multiple comparisons test was employed to assess significant differences among treatments at the same time. Statistically significant differences are denoted by different letters (p ≤ 0.05). For comparisons between different time points within the same treatment, unpaired t-tests were applied, and significant differences are marked with asterisks (* p ≤ 0.05).
Discussion
Effect of ammonium stress on growth
Nitrogen is a vital element for the growth, development, and reproduction of microalgae, as it plays a key role in the formation of in proteins, nucleic acids, chlorophyll, and other vital substances within these organisms52,53. Among various inorganic nitrogen sources, NH₄⁺-N is generally preferred by microalgae. However, when present in high concentrations, NH₄⁺-N can inhibit growth7,54. Additionally, the degree to which NH₄⁺-N affects microalgae growth varies significantly depending on the species55,56strain57 and cultivation conditions20. Metin et al. (2024) investigated the impact of varying NH₄⁺-N concentrations on the growth of four microalgae species: Chlorella vulgaris, Chlorella minutissima, Chlamydomonas reinhardtii, and Arthrospira platensis. Their findings indicated that C. vulgaris exhibited the highest resistance to ammonium toxicity, while A. platensis experienced significant growth inhibition. Notably, none of these species were able to thrive when NH₄⁺-N concentrations exceeded 1000 mg L−1 21. In our study, we observed that NH₄⁺-N inhibited the growth of O. borgei in a dose-dependent manner. Growth inhibition observed starting from day 4 of cultivation when NH₄⁺-N concentration was ≥ 500 mg L−1 (Fig. 1). This finding consistent with the results of Zhao et al. (2019), who reported that ammonium nitrogen concentrations above 500 mg L−1 were highly toxic to T. cordiformis33. The similarity of these findings across different microalgae species emphasizes the importance of carefully managing ammonium nitrogen levels in microalgae cultivation systems.
Effects of phytohormones on microalgae growth under abiotic stress
Phytohormones have emerged as effective regulators of microalgae growth under abiotic stress conditions, demonstrating significant potential for enhancing stress tolerance and biomass production25,58. In our study, the application of phytohormones (10−6 M IAA, GA3, ZT) alleviated the negative effects of high ammonium stress, resulting in a 12–16% increase in cell density and an 85–131% increase in the specific growth rate (Fig. 2). These findings are consistent with previous studies on other microalgae species under various stress conditions. For instance, exogenous melatonin (MT) and indole-propionic acid (IPA) have been shown to enhance biomass and carbon fixation capacity in Spirulina platensis under low light conditions59. Similarly, the exogenous application of phytohormones such as IAA, ZT, and brassinolide (Br) alleviated oxidative damage and photosynthetic inhibition in T. cordiformis under high ammonia nitrogen stress33. Another study found that supplementing Scenedesmus SDEC-8 and Chlorella SDEC-18 with indole-3-butyric Acid (IBA) and naphthalene acetic acid (NAA) maintained biomass concentration, mitigated nitrogen stress damage, and increased lipid yield in microalgae cells60. Other studies have also observed that the exogenous application of phytohormones like Br, GAs, auxins, cytokinins (CTKs), and MT, or synthetic phytohormones, can effectively promote microalgae growth by mitigating the adverse effects of abiotic stress factors such as heavy metals, high/low temperatures or light, high salinity, and nitrogen deficiency30,31,32,61,62,63. These findings support our results and highlight the broad applicability of phytohormone treatments in enhancing microalgae stress tolerance.
It should be noted that different phytohormones exhibit varying degrees of effectiveness in responding to abiotic stress, primarily due to their distinct characteristics. For instance, auxins and CTKs promote cell division and elongation, GAs enhance overall metabolic activity and stress resistance, while abscisic acid (ABA) regulates stress responses and carbon allocation in microalgae25,39,58,64. This variation highlights the necessity of selecting and optimizing phytohormones when culturing algae and managing stress. However, in our study, the three phytohormones did not show significant differences in cell density of O. borgei under HAN stress, which suggests that cost-effectiveness should be considered before the large-scale application37.
Phytohormones alleviate photosynthetic Inhibition
Photosynthesis is a fundamental process for the growth and development of plants, and abiotic stress primarily reduces photosynthetic efficiency65. Similarly, our study shows that HANS (500 mg L−1 NH₄⁺-N) causes photosynthetic damage in O. borgei. This damage is reflected in reduced photosynthetic pigment content (Chla and Car), decreased chlorophyll fluorescence parameters (Fv/Fm and φPSII), and lowered expression of the photosynthetic gene rbcL (p ≤ 0.01) (Fig. 3).
Under abiotic stress conditions, phytohormones have become critical regulators of photosynthetic efficiency in microalgae. For example, phytohormones such as auxins, CTKs, ABA, Br, and MT have been shown to promote the synthesis of chlorophyll and carotenoids, essential for light absorption and energy transfer25,33,59. This enhancement in pigment content directly contributes to improved photosynthetic efficiency by increasing the light-harvesting capacity66,67. In our study, we observed a significant increase in chlorophyll and carotenoid levels following phytohormone treatment (p ≤ 0.01) (Fig. 3A-C), which indicates enhanced light-harvesting efficiency and energy transfer in O. borgei.
Furthermore, phytohormones can enhance light energy conversion efficiency and the activity of the photosystem reaction centers, promoting photosynthetic metabolic responses in O. borgei. This is evidenced by increased ratios of Fv/Fm and φPSII under stress conditions (Fig. 3D and E). Fv/Fm is an indicator of the energy capture efficiency of PSII, a higher value indicates greater potential for light energy utilization. φPSII reflects the proportion of absorbed light that is used in PSII photochemistry68. Our previous research has also shown that 10−6 M IAA, GA3 and ZT can significantly enhance Fv/Fm and φPSII values in O. borgei under high-salt wastewater conditions37. Similarly, phytohormone supplementation has been shown to increase Fv/Fm in Chlorella sorokiniana under nitrogen-limiting conditions69 and T. cordiformis under HANS33.
The rbcL gene encodes the large subunit of Rubisco, a key enzyme for carbon assimilation, that catalyzes the primary reaction of carbon fixation and is crucial for photosynthesis70. Changes in rbcL expression under stress conditions reflect the extent of photosynthetic impairment32,71. In our study, rbcL expression was significantly inhibited under HANS (p ≤ 0.0001). Despite the improvement in photosynthetic efficiency following phytohormone supplementation, rbcL expression did not recover significantly (Fig. 3F). This discrepancy suggests that enhanced photosynthetic efficiency does not solely depend on the recovery of rbcL expression. Previous studies have shown that phytohormones can partially compensate for the lack of Rubisco activity by protecting essential photosynthetic proteins, increasing the content of photosynthetic pigments, and optimizing the photosynthetic electron transport chain72,73. Moreover, phytohormones can indirectly improve photosynthetic efficiency by activating the antioxidant defense system and reducing ROS-induced damage to the photosynthetic system74. This regulatory mechanism may offset the impact of insufficient rbcL expression on photosynthetic efficiency, explaining the observed improvement in photosynthetic parameters despite the lack of significant recovery in rbcL expression.
Phytohormones mitigate oxidative stress
The redox balance plays a crucial role in innate immunity, a feature universally present across all algal phyla. Under normal growth conditions, microalgae produce ROS, which activate antioxidant systems to maintain intracellular redox balance75. However, when abiotic stress persists over extended periods, ROS production can exceed the capacity of the cell’s antioxidant removal systems, leading to oxidative stress. Excess ROS can react with unsaturated fatty acids in the lipid cell membrane, producing MDA, a byproduct of lipid peroxidation. This disrupts cellular function and can eventually result in cell death74,76.
In response to abiotic stress, microalgae mitigate oxidative damage through both enzymatic and non-enzymatic antioxidant systems. Key antioxidant enzymes, such as SOD and CAT, are essential for microalgal cells and are commonly used as physiological indicators of stress response75. Typically, prolonged exposure to unfavorable conditions prompts microalgae to upregulate antioxidant levels, facilitating adaptation to long-term abiotic stress74,75,76. In this study, we observed that HAN stress induced lipid peroxidation and oxidative stress in O. borgei, with significantly elevated levels of MDA, SOD, and CAT compared to the control group (p ≤ 0.001). In contrast, HAN stress on T. cordiformis caused damage to its enzymatic antioxidant system33suggesting that O. borgei exhibits stronger redox regulation capabilities.
Previous reports have indicated that plant hormone signaling is closely linked to ROS regulation30,77. Exogenous phytohormones can act as antioxidants, regulating the antioxidant system to prevent ROS accumulation, and thereby limiting or alleviating oxidative damage caused by abiotic stress39,74,78. In our study, phytohormones significantly enhanced carotenoid content under HAN stress early in the treatment period (p ≤ 0.5) (Fig. 3C), while MDA, SOD, and CAT levels were significantly reduced throughout the entire culture period (p ≤ 0.05) (Fig. 4). Carotenoids, as non-enzymatic antioxidants, scavenge free radicals, particularly superoxide anions and hydrogen peroxide radicals79. Under abiotic stress, the promotion of carotenoid synthesis via exogenous phytohormones is a a strategy employed by O. borgei to combat ROS toxicity, reducing lipid peroxidation caused by HAN and alleviating pressure on the enzymatic antioxidant system. Similarly, exogenous auxins, CTKs, and MT help maintain redox balance in microalgae by regulating antioxidant systems or related gene expression under environmental stress73,78,80.
This study also found that different phytohormones varied in their effectiveness at alleviating oxidative stress caused by HAN. All three plant hormones positively regulated CAT activity, with IAA showing the most significant effect in reducing lipid peroxidation damage. Although IAA led to higher SOD activity, the SOD activity under GA3 and ZT treatments did not differ significantly from the control group. A similar phenomenon was observed in the alleviation of lead toxicity in Acutodesmus obliquus by phytohormones, where IAA resulted in higher SOD activity, while tZ (trans-zeatin) showed higher CAT activity73. The differences in the effects of phytohormones on antioxidant enzyme regulation are likely result from their distinct roles in cellular signaling and metabolic pathways39.
Phytohormones enhance nitrogen metabolism
The role of phytohormones in regulating nitrogen metabolism under environmental stress has been extensively studied in higher plants28,81yet research on how phytohormones alleviate environmental stress in microalgae, particularly concerning nitrogen metabolism remains limited33. This study contributes valuable insights into this area, focusing on the effects of phytohormones on nitrogen metabolism in O. borgei under HAN stress. During nitrogen metabolism, GS is a critical enzyme not only for the assimilation of NH4+ but also for plant growth and development82. Under HAN stress, we observed a significant downregulation in GS enzyme activity and near-complete suppression of GS expression (p ≤ 0.0001) (Fig. 5), indicating that nitrogen metabolism in O. borgei is impaired. This dysfunction in nitrogen metabolism is a primary factor contributing to the inhibitory effects of 500 mg L−1 NH4+-N on microalgal growth. Importantly, our results show that the phytohormones IAA, GA3, and ZT significantly increased GS activity (p ≤ 0.001) and upregulated GS expression (p ≤ 0.05) in O. borgei under HAN stress (Fig. 5). These findings are consistent with recent studies on other microalgal species. For example, Zhao et al. (2019) reported that Br enhanced nitrogen assimilation in T. cordiformis under high ammonia nitrogen stress by increasing GS activity and gene expression33.
Enhancing nitrogen metabolism is a potential strategy for mitigating damage caused by external stresses. GS plays a central role in nitrogen assimilation and amino acid biosynthesis. Its enhanced activity facilitates more efficient ammonium incorporation and detoxification, which is particularly critical under HAN stress in this study. Moreover, improved nitrogen metabolism might contribute to the synthesis of stress-protective compounds such as proline and glycine betaine, further enhancing the overall stress resistance of microalgae83,84. By promoting GS activity and gene expression, phytohormones may enable more efficient nitrogen assimilation and utilization, thereby mitigating the toxic effects of high ammonium concentrations. This enhanced nitrogen metabolism likely plays a key role in the observed improvements in growth and stress tolerance in O. borgei under high ammonium conditions.
Study limitations and future directions
While this study advances understanding of phytohormone-mediated enhancement in O. borgei tolerance to high ammonium stress, it has limitations including a sole focus on O. borgei monocultures (neglecting microbial community influences), incomplete elucidation of the underlying molecular mechanisms, lack of validation under realistic wastewater conditions, and investigation of only a limited subset of phytohormones. Future research should therefore focus on characterizing microbial community dynamics and O. borgei-bacteria interactions, employing transcriptomic and metabolomic analyses to identify key regulatory genes and pathways, conducting pilot-scale or field trials using actual wastewater, and exploring a broader spectrum of phytohormones for efficacy. Addressing these aspects will yield a more comprehensive understanding and advance the development of efficient microalgae-based wastewater treatment technologies.
Conclusion
This study demonstrates that NH4+-N significantly inhibits O. borgei growth in a dose-dependent manner, with 500 mg L−1 NH4+-N (HAN) causing growth inhibition and physiological metabolic disturbances. Phytohormones (10−6 M IAA, GA3, and ZT) significantly enhanced the tolerance of O. borgei to HAN stress. Specifically, these phytohormones increased pigment content, optimized electron transport, and enhanced photosynthetic efficiency. They also mitigated oxidative damage by regulating antioxidant defense mechanisms and boosted nitrogen metabolism by promoting GS activity and gene expression. As a result, these treatments resulted in a 22–26% increase in cell density and a 217–282% increase in the specific growth rate. These findings highlight the potential of phytohormones as effective tools for enhancing microalgal tolerance to NH₄⁺-N stress for high ammonium-nitrogen wastewater treatment. For practical implementation, we propose optimizing hormone delivery protocols (e.g., timing, dosage, and combinatorial ratios) in cultivation systems and conducting species-specific trials to evaluate the broader applicability of this approach in microalgae-based wastewater remediation and biomass production.
Data availability
The authors will make the raw data supporting the conclusions of this article available without undue reservation. The data is available from the corresponding author on reasonable request. (Ning Zhang: zning496@163.com)
References
Edwards, T. M. et al. Ammonia and aquatic ecosystems – A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 907, 167911. https://doi.org/10.1016/j.scitotenv.2023.167911 (2024).
Yan, F. et al. Ecological risk evaluation of ammonia nitrogen pollution in China based on the ecological grey water footprint model. J. Environ. Manage. 347, 119087. https://doi.org/10.1016/j.jenvman.2023.119087 (2023).
Yan, Z. G. et al. Neglect of temperature and pH impact leads to underestimation of seasonal ecological risk of ammonia in Chinese surface freshwaters. J. Chem., 3051398, (2019). https://doi.org/10.1155/2019/3051398 (2019).
Liu, X. et al. A systematic review on aquaculture wastewater: pollutants, impacts, and treatment technology. Environ. Res. 262, 119793. https://doi.org/10.1016/j.envres.2024.119793 (2024).
Xiang, S. et al. New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters. World J. Microbiol. Biotechnol. 36, 144. https://doi.org/10.1007/s11274-020-02921-3 (2020).
Wang, J. et al. Treatment of wastewater containing high concentrations of ammonia nitrogen using ion exchange resins. Asia-Pacific J. Chem. Eng. 17, e2679. https://doi.org/10.1002/apj.2688 (2021).
Salbitani, G. & Carfagna, S. Ammonium utilization in microalgae: a sustainable method for wastewater treatment. Sustainability 13, 956. https://doi.org/10.3390/su13020956 (2021).
Sun, Y. et al. Synergistic treatment of digested wastewater with high ammonia nitrogen concentration using straw and microalgae. Separations 412 https://doi.org/10.1016/j.biortech.2024.131406 (2024). (2024).
Akinnawo, S. O. & Eutrophication Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 12, 100733. https://doi.org/10.1016/j.envc.2023.100733 (2023).
Zhang, T. X. et al. A review of the toxic effects of ammonia on invertebrates in aquatic environments. Environ. Pollut. 336, 122374. https://doi.org/10.1016/j.envpol.2023.122374 (2023).
Wyer, K. E. et al. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manage. 323, 116285. https://doi.org/10.1016/j.jenvman.2022.116285 (2022).
Zhang, C. et al. China’s wastewater treatment: status quo and sustainability perspectives. J. Water Process. Eng. 53, 103708. https://doi.org/10.1016/j.jwpe.2023.103708 (2023).
Meng, X. et al. Resource and energy utilization of swine wastewater treatment: recent progress and future directions. Separations 10, 591. https://doi.org/10.3390/separations10120591 (2023).
Farghali, M. et al. Strategies for ammonia recovery from wastewater: a review. Environ. Chem. Lett. 22, 2699–2751. https://doi.org/10.1007/s10311-024-01768-6 (2024).
Ahmed, M. et al. Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. Npj Clean. Water. 5, 12. https://doi.org/10.1038/s41545-022-00154-5 (2022).
Ronan, E. et al. Recent advancements in the biological treatment of high strength ammonia wastewater. World J. Microbiol. Biotechnol. 37, 158. https://doi.org/10.1007/s11274-021-03124-0 (2021).
Liu, N. et al. Emerging high-ammonia nitrogen wastewater remediation by biological treatment and photocatalysis techniques. Sci. Total Environ. 875, 162603. https://doi.org/10.1016/j.scitotenv.2023.162603 (2023).
Ali, S. S. et al. Microalgae-mediated bioremediation: current trends and opportunities-a review. Arch. Microbiol. 206, 343. https://doi.org/10.1007/s00203-024-04052-x (2024).
Chen, H. & Wang, Q. Microalgae-based nitrogen bioremediation. Algal Res. 46, 101775. https://doi.org/10.1016/j.algal.2019.101775 (2020).
Li, X. et al. Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour Technol. 273, 368–376. https://doi.org/10.1016/j.biortech.2018.11.042 (2019).
Metin, U. & Altınbaş, M. Evaluating ammonia toxicity and growth kinetics of four different microalgae species. Microorganisms 12, 1542. https://doi.org/10.3390/microorganisms12081542 (2024).
Paskuliakova, A. et al. Phycoremediation of landfill leachate with chlorophytes: phosphate a limiting factor on ammonia nitrogen removal. Water Res. 99, 180–187. https://doi.org/10.1016/j.watres.2016.04.029 (2016).
He, P. et al. Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour Technol. 129, 177–181. https://doi.org/10.1016/j.biortech.2012.10.162 (2013).
Yang, Z. Y. et al. Efficient microalgal lipid production driven by salt stress and phytohormones synergistically. Bioresour Technol. 367, 128270. https://doi.org/10.1016/j.biortech.2022.128270 (2023).
Wang, C. Q. et al. The active phytohormone in microalgae: the characteristics, efficient detection, and their adversity resistance applications. Molecules 27, 46. https://doi.org/10.3390/molecules27010046 (2022).
Patidar, S. K. Metabolic interactions between microalgae and bacteria: multifunctional ecological interplay and environmental applications. Algal Res. 86, 103904. https://doi.org/10.1016/j.algal.2025.103904 (2025).
Zhang, B. et al. Microalgal-bacterial consortia: from interspecies interactions to biotechnological applications. Renew. Sust. Energ. Rev. 118, 109563. https://doi.org/10.1016/j.rser.2019.109563 (2020).
Waadt, R. et al. Plant hormone regulation of abiotic stress responses. Plant. Stress. 23, 680–694. https://doi.org/10.1038/s41580-022-00479-6 (2022).
Zhu, Y. et al. The role of hormones in plant stress: the old and new players. Plant. Stress. 13, 100552. https://doi.org/10.1016/j.stress.2024.100552 (2024).
Zhao, Y. et al. Melatonin, a phytohormone for enhancing the accumulation of high-value metabolites and stress tolerance in microalgae: applications, mechanisms, and challenges. Bioresour Technol. 393, 103708. https://doi.org/10.1016/j.biortech.2023.130093 (2024).
Renuka, N. et al. Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Convers. Manag. 140, 14–23. https://doi.org/10.1016/j.enconman.2017.02.065 (2017).
Giridhar Babu, A. et al. Cultivation of an Indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res. 23, 178–185. https://doi.org/10.1016/j.algal.2017.02.004 (2017).
Zhao, P. et al. The alleviative effect of exogenous phytohormones on the growth, physiology and gene expression of Tetraselmis cordiformis under high ammonia-nitrogen stress. Bioresour Technol. 282, 339–347. https://doi.org/10.1016/j.biortech.2019.03.031 (2019).
Chokshi, K. et al. Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol. Biofuels. 10, 60. https://doi.org/10.1186/s13068-017-0747-7 (2017).
Wu, S. et al. Regulatory mechanisms of oxidative species and phytohormones in marine microalgae Isochrysis zhangjiangensis under nitrogen deficiency. Algal Res. 17, 321–329. https://doi.org/10.1016/j.algal.2016.05.025 (2016).
Chai, W. et al. Efficient nutrient and antibiotics removal from aquaculture wastewater using different microalgae-based systems by agricultural multi-phytohormone induction. Algal Res. 82, 103659. https://doi.org/10.1016/j.algal.2024.103659 (2020).
Liu, Y. et al. Phytohormone supplementation for nutrient removal from mariculture wastewater by Oocystis borgei in sequential batch operation. Water 16, 552. https://doi.org/10.3390/w16040552 (2024).
Kong, W. et al. Application of indole-3-acetic acid in microalgae cultivation to improve the feasibility of simultaneously purifying wastewater, fixing CO₂ and producing fatty acids under hg stress. J. Clean. Prod. 358, 132028. https://doi.org/10.1016/j.jclepro.2022.132028 (2022).
Qiu, J. et al. Phytohormones as a novel strategy for promoting phytoremediation in microalgae: progress and prospects. Algal Res. 373, 102560. https://doi.org/10.1016/10.1016/j.jenvman.2024.123593 (2025).
Zhang, J. L. et al. Regulation effects of indoleacetic acid on lipid production and nutrient removal of Chlorella pyrenoidosa in seawater-containing wastewater. Algal Res. 248, 120864. https://doi.org/10.1016/j.watres.2023.120864 (2024).
Huang, X. A study on dominant phytoplankton species in high-level Prawn ponds and its formation cause. J. Guangdong Ocean. Univ. 22, 8–12. https://doi.org/10.3969/j.issn.1009-5470.2002.04.006 (2002).
Huang, X. et al. Directive breeding of microalgae and effects of it on environment factors in Prawn pond. J. Guangdong Ocean. Univ. 25, 25–31. https://doi.org/10.3969/j.issn.1673-9159.2005.06.006 (2005).
Ren, J. J. et al. Effects of salinity on chlorophyll fluorescence parameters and cloning and characterization of PsbA gene in Oocystis Borgei. J. Guangdong Ocean. Univ. 40, 30–39. https://doi.org/10.3969/j.issn.1673-9159.2020.03.005 (2020).
Xie, L. et al. Effects of sodium nitrate on growth, biochemical components and sedimentation of Oocystis borgei. J. Guangdong Ocean. Univ. 40, 48–55. https://doi.org/10.3969/j.issn.1673-9159.2020.03.007 (2020).
Zhang, N. et al. Transcriptome analysis revealed the advantages of room temperature preservation of concentrated Oocystis borgei cultures for use in aquaculture. Int. J. Mol. Sci. 24 (22), 16225. https://doi.org/10.3390/ijms242216225 (2023).
Chen, H. et al. Effects of Oocystis borgei culture on growth performance, antioxidant capacity and intestinal microflora of Litopenaeus vannamei. J. Guangdong Ocean. Univ. 43, 67–74. https://doi.org/10.3969/j.issn.1673-9159.2023.03.009 (2023).
Liu, M. et al. Study on the uptake of dissolved nitrogen by Oocystis borgei in Prawn (Litopenaeus vannamei) aquaculture ponds and establishment of uptake model. Aquacult. Int. 28, 1445–1458. https://doi.org/10.1007/s10499-020-00534-z (2020).
Kester, D. R. et al. Preparation of artificial seawater. Limnol. Oceanogr. 12, 176–179. https://doi.org/10.4319/lo.1967.12.1.0176 (1967).
Lichtenthaler, H. K. & Wellburn, A. R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochim. Biophys. Acta. 11, 591–592. https://doi.org/10.1042/bst0110591 (1983).
Zhang, N. et al. Identification of stable reference genes for qPCR analysis of gene expression in Oocystis borgei under various abiotic conditions. Algal Res. 86, 103899. https://doi.org/10.1016/10.1038/s41598-019-50035-0 (2025).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR the 2(-∆∆C(T)) method. Methods, 25, 402–408, (2002). https://doi.org/10.1006/meth.2001.1262
Emanuel, S. L. et al. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant. Sci. 6 https://doi.org/10.3389/fpls.2015.00899 (2015).
Kumar, A. & Bera, S. Revisiting nitrogen utilization in algae: A review on the process of regulation and assimilation. Bioresour Technol. Rep. 12, 100584. https://doi.org/10.1016/j.biteb.2020.100584 (2020).
Mandal, S. et al. Functional divergence in nitrogen uptake rates explains diversity–productivity relationship in microalgal communities. Algal Res. 9, e02228. https://doi.org/10.1002/ecs2.2228 (2018).
Gutierrez, J. et al. Ammonia Inhibition in oleaginous microalgae. Algal Res. 19, 123–127. https://doi.org/10.1016/j.algal.2016.07.016 (2016).
Farahin, A. W. et al. Tolerance of Tetraselmis tetrathele to high ammonium nitrogen and its effect on growth rate, carotenoid, and fatty acids productivity. Algal Res. 9, 568776. https://doi.org/10.3389/fbioe.2021.568776 (2021).
Wang, J. et al. Ammonium nitrogen tolerant Chlorella strain screening and its damaging effects on photosynthesis. Algal Res. 9 https://doi.org/10.3389/fmicb.2018.03250 (2019).
Zhao, Y. et al. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Bioresour Technol. 274, 549–556. https://doi.org/10.1016/j.biortech.2018.12.030 (2019).
Li, P. et al. Phytohormones enhanced carbon fixation and biomass production in CO2 absorption-microalgae conversion system under light stress. Algal Res. 308, 133047. https://doi.org/10.1016/j.energy.2024.133047 (2024).
Ze et al. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour Technol. 264, 522–528. https://doi.org/10.1016/j.biortech.2018.07.017 (2018).
Stirk, W. et al. Endogenous brassinosteroids in microalgae exposed to salt and low temperature stress. Physiol. Plant. 163, 273–279. https://doi.org/10.1080/09670262.2018.1441447 (2018).
Arroussi, H. E. et al. Improvement of the potential of Dunaliella tertiolecta as a source of biodiesel by auxin treatment coupled to salt stress. Bioresour Technol. 184, 15–19. https://doi.org/10.1016/j.renene.2014.12.010 (2015).
Nguyen, H. N. et al. The roles of phytohormones in metal stress regulation in microalgae. J. Appl. Phycol. 32, 3817–3829. https://doi.org/10.1007/s10811-020-02271-5 (2020).
Shah, S. et al. Exploration of the phytohormone regulation of energy storage compound accumulation in microalgae. Algal Res. 66 https://doi.org/10.1002/fes3.418 (2022).
Sharma, A. et al. Photosynthetic response of plants under different abiotic stresses: A review. Photosynthetica 39, 509–531. https://doi.org/10.1007/s00344-019-10018-x (2020).
Wang, G. et al. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant. Physiol. 273, 153669. https://doi.org/10.1016/j.jplph.2022.153669 (2022).
Tamaki, S. et al. Illuminating the diversity of carotenoids in microalgal eyespots and phototaxis. Plant. Signal. Behav. 18, 2257348. https://doi.org/10.1080/15592324.2023.2257348 (2023).
Solovchenko, A. et al. Chlorophyll fluorescence as a valuable multitool for microalgal biotechnology. Algal Res. 14, 973–983. https://doi.org/10.1007/s12551-022-00951-9 (2022).
Guldhe, A. et al. Effect of phytohormones from different classes on gene expression of Chlorella sorokiniana under nitrogen limitation for enhanced biomass and lipid production. Bioresour Technol. 40, 101518. https://doi.org/10.1016/j.algal.2019.101518 (2019).
Salesse-Smith, C. E. et al. Increasing Rubisco as a simple means to enhance photosynthesis and productivity now without Lowering nitrogen use efficiency. New. Phytol. 243, 951–965. https://doi.org/10.1111/nph.20298 (2025).
Jaskulak, M. et al. Antioxidative enzymes and expression of RbcL gene as tools to monitor heavy metal-related stress in plants. Plant. Physiol. Biochem. 218, 71–78. https://doi.org/10.1016/j.jenvman.2018.04.052 (2018).
Gu, L. Optimizing the electron transport chain to sustainably improve photosynthesis. Plant. Physiol. 193, 2398–2412. https://doi.org/10.1093/plphys/kiad490 (2023).
Piotrowska-Niczyporuk, A. et al. Exogenously applied auxins and cytokinins ameliorate lead toxicity by inducing antioxidant defence system in green Alga Acutodesmus obliquus. Ecotoxicol. Environ. Saf. 152, 105–112. https://doi.org/10.1016/j.plaphy.2018.09.038 (2018).
Zhu, J. et al. Mitigation of oxidative stress damage caused by abiotic stress to improve biomass yield of microalgae: A review. Bioresour Technol. 896, 165200. https://doi.org/10.1016/j.scitotenv.2023.165200 (2023).
Rezayian, M. et al. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 6, 1309–1313. https://doi.org/10.1016/j.toxrep.2019.10.001 (2019).
Gauthier, M. R. et al. Microalgae under environmental stress as a source of antioxidants. Algal Res. 52, 102104. https://doi.org/10.1016/j.algal.2020.102104 (2020).
Singh, T. et al. The hidden harmony: exploring ROS-phytohormone nexus for shaping plant root architecture in response to environmental cues. Plant. Signal. Behav. 206, 108273. https://doi.org/10.1016/j.plaphy.2023.108273 (2024).
Ding, W. et al. Melatonin: A multifunctional molecule that triggers defense responses against high light and nitrogen starvation stress in Haematococcus pluvialis. J. Agric. Food Chem. 66, 7701–7711. https://doi.org/10.1021/acs.jafc.8b02178 (2018).
Mavrommatis, A. et al. Microalgae as a sustainable source of antioxidants in animal nutrition, health and livestock development. Sustainability 12, 1882. https://doi.org/10.3390/antiox12101882 (2023).
Zhao, Y. et al. Integration of physiological and metabolomic profiles to elucidate the regulatory mechanisms underlying the stimulatory effect of melatonin on Astaxanthin and lipids coproduction in Haematococcus pluvialis under inductive stress conditions. Bioresour Technol. 319, 124150. https://doi.org/10.1016/j.biortech.2020.124150 (2021).
Yang, P. et al. Effects of brassinosteroids on photosynthetic performance and nitrogen metabolism in pepper seedlings under chilling stress. Front. Plant. Sci. 9, 839. https://doi.org/10.3390/agronomy9120839 (2019).
Valderrama-Martín, J. M. et al. A revised view on the evolution of glutamine synthetase isoenzymes in plants. Plant. J. 110, 946–960. https://doi.org/10.1101/2021.11.08.467771 (2022).
Fal, S. Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Heliyon 8, e08811. https://doi.org/10.1016/j.heliyon.2022.e08811 (2022).
Song, X. et al. Lipidomics analysis of microalgal lipid production and heavy metal adsorption under glycine betaine-mediated alleviation of low-temperature stress. J. Hazard. Mater. 480, 135831. https://doi.org/10.1016/j.jhazmat.2024.135831 (2024).
Funding
This research was financially supported by the National Natural Science Foundation of China (32102796).
Author information
Authors and Affiliations
Contributions
Xinyue Song: Formal analysis; Investigation; Methodology; Software; Visualization; Writing-original draft. Yang Liu: Methodology; Validation; Writing-original draft. Chengcheng Deng: Methodology; Validation; Writing-original draft. Zhongdian Dong: Software; Visualization; Writing-review & editing. Zhangxi Hu: Data curation, Writing-review & editing. Xianghu Huang: Conceptualization; Resources; Writing-review. Changling Li: Resources, Writing – original draft. Ning Zhang: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, X., Liu, Y., Deng, C. et al. Effects and mechanisms of phytohormones in enhancing the resistance of Oocystis borgei to high ammonium nitrogen stress. Sci Rep 15, 24604 (2025). https://doi.org/10.1038/s41598-025-10398-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10398-z