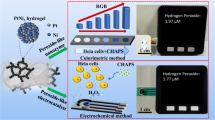
Abstract
Nowadays, electrochemical biosensors have gained extensive consensus to detect a wide variety of analytes such as hydrogen peroxide (H2O2), glucose, dopamine, uric acid, and so on. However, the detection of H2O2 is more and more important because H2O2 plays a vital role in our daily life. Hence, a new nonenzymatic H2O2 biosensor was developed by decorating NiO octahedrons on the 3-dimensional graphene hydrogel (3DGH). The NiO octahedrons were prepared using a mesoporous silica SBA-15 as a hard template. Then, different amounts of NiO octahedrons self-assembled with 3DGH during hydrothermal method. The performance of different prepared nanostructures as H2O2 biosensors electrodes was determined by the cyclic voltammetry and chronoamperometry tests. Various physicochemical methods such as FE-SEM, HR-TEM, XRD, TGA and Raman were utilized to characterize the morphology and structure properties of the as-prepared samples. The electrochemical sensing of H2O2 for the proposed biosensor electrodes are investigated, and the 3DGH/NiO nanocomposite electrode with NiO content of 25% displays high sensitivity with wide linear range and low detection limit, as well as good selectivity, reproducibility and long-term stability. Finally, the analytical utilities of the 3DGH/NiO25 proposed electrode were conducted to detect H2O2 in real products of milk samples.
Similar content being viewed by others
Introduction
In recent years, electrochemical biosensors (EBS) have garnered significant attention within the scientific community and industry, emerging as a pivotal technology in various analytical fields. This surge in interest is primarily attributed to their remarkable combination of cost-effectiveness, operational simplicity, and high sensitivity, which collectively address many of the challenges faced by conventional analytical methods1,2,3,4. The growing necessity for precise and efficient detection of a diverse range of analytes, including but not limited to Hydrogen Peroxide, Glucose, Dopamine, Ascorbic acid, and Uric acid, underscores the escalating significance of Electrochemical Biosensors (EBS) in both current and future analytical landscapes. This rising demand, coupled with the critical role these analytes play in maintaining human health and environmental safety, strongly suggests that EBS will assume an even more pivotal position in forthcoming scientific and technological advancements. Among these analytes, Hydrogen Peroxide (H2O2) plays an extremely important role in wide range of different processes such as a water disinfectant to replace chlorine or chlorine dioxide, food manufacturing, mining industry, bleaching of wood pulp and many other processes5,6,7,8. In addition, H2O2 has direct effect on our health due to the high content of H2O2 accumulation in cells would afford severe health risk of cell damage, including Alzheimer’s disease, cardiovascular disease, cancer and neurodegeneration disease etc9,10,11,12. Although enzyme-based biosensors have been investigated by many preceding efforts, the practical drawbacks including high cost, complicated fabrication and lack of stability could be restricted its commercial application13,14,15. Considering the critical importance of hydrogen peroxide (H2O2) detection in various fields, there is an urgent need to develop advanced materials with exceptional electrocatalytic properties. These materials are crucial for enhancing the sensitivity and stability of nonenzymatic H2O2 electrochemical biosensors, thereby improving their overall performance and reliability. In the pursuit of developing high-performance materials, transition metal oxides (TMOs) have emerged as particularly promising candidates. TMOs such as NiO, MnO2, Co3O4, and Fe2O3, among others, have garnered significant attention from researchers and industry experts over the past decade. This surge in interest can be attributed to their unique and advantageous properties, which make them exceptionally well-suited for a wide range of applications, with a particular emphasis on electrochemical detection16,17,18,19. One of the most common transition metal oxides is Nickle oxide (NiO). It has an excellent applied performance in many applications including lithium and sodium ion batteries20,21,22, supercapacitors23,24,25, gas sensors26 and solar cells27 due to its low toxicity, facile preparation, natural abundance and good electrochemical activities. More recently, different morphologies of NiO have received a lot of attention as electrochemical biosensors materials for detection of various analytes. For example, Fang et al. have modified MWCNT with nickel hexacyanoferrate nanoparticles for amperometric determination of uric acid28. Using graphene oxide film as template, Zhang and Liu fabricated NiO nanosheets for detection of glucose sensing29. Also, NiO nanofibers were prepared via electrospinning technique to modify glassy carbon electrode containing graphene oxide for improving performance nonenzymatic glucose biosensor30. Therefore, most literature confirmed that the integration among metal oxide and carbonaceous materials could greatly improve the performance of electrochemical biosensors by reinforcing the electron transport, ion diffusion and accessibility. Among of carbonaceous materials which have been used as supported materials, graphene has drawn huge attention due to its marvelous electronic and mechanical characteristics31,32,33. However, 2D graphene suffer from agglomeration and restacking from the strong interlayer interactions that can reduce the specific surface area and the number of electrochemically active sites34,35. Thus, 3D graphene hydrogel has attracted great attention recently in different analytical electrochemistry field, owing to its excellent physicochemical properties including large surface area, high intrinsic electrical conductivity, superior controllable pore size distribution and porosity36,37,38,39,40. For instance, Chen et al. prepared 3D porous prussian blue in graphene aerogels by reduction of graphene oxide and FeCl3 for detection of H2O2 sensor with a linear range (0.005–4 mM). In this case, L-ascorbic acid and ferricyanide were selected as a reducing agent and prussian blue source, respectively41. Yin and his coworkers synthesized Ni3N nanoparticles/3D graphene aerogel using hydrothermal reaction and then calcining under NH3 atmosphere. The composite was used for detection of glucose and H2O2 molecules42. Recently, Elsayed et al. introduced a biosensor for COVID-19 detection, combining graphene metasurfaces with gold, silver, and GST materials, optimized through COMSOL Multiphysics simulation in the infrared regime for high sensitivity and precision43. Hence, numerous previous studies demonstrated that metal oxides, hydroxides and nitrides decorated graphene could greatly enhance the electrochemical performance of various electrochemical biosensor applications. Inspired by the good properties of nickel oxide as an efficient electrocatalyst and graphene as supported materials, we have successfully prepared exemplary 3DGH/NiO octahedrons for nonenzymatic H2O2 detection applications. To our knowledge, there is a notable gap in the existing literature regarding the utilization of 3D graphene hydrogel as a sensing electrode material for the detection of H2O2. This presents an exciting opportunity for novel research in the field of biosensing technology. Considering this, our study aims to address this gap and explore the potential of graphene-based materials for H2O2 detection (Fig. 1). As a first step in our innovative approach, we focused on the synthesis of NiO octahedrons using nickel nitrate hexahydrate as nitrate source, ethanol as solvent and a mesoporous silica SBA-15 as a hard template. Second, different amount of NiO octahedrons ranged from 5 to 35% wt were decorated 3DGH via hydrothermal method. X-ray diffraction (XRD), thermogravimetric Analysis (TGA), field emission scanning electron microscopy (FE-SEM), high resolution transmission electron microscope (HR-TEM), Raman spectroscopy and electrochemical tests were applied to characterize the structural, morphological and electrochemical properties of the as-prepared samples. As a result, the optimized 3DGH/NiO25 nanocomposite exhibited a superior electrochemical performance toward H2O2 sensing with a good sensitivity (117.26 µA mM−1 cm−2), wide linear range (10 µM–33.58 mM) and low detection limit (5.3 µM).
Schematic shows the fabrication of 3DGH/NiO nanostructures. The 3D visualization was created using Blender software (version 3.6, https://www.blender.org/).
Materials and methods
Chemicals and reagents
Graphite powder used for the synthesis of graphene oxide (GO) was purchased from Daejung Chemical Reagent Co (South Korea). Silicon dioxide (SiO2), Nickel (II) nitrate hexahydrate (Ni(NO3)2.6H2O) and sodium hydroxide pellets (NaOH) were obtained from Sigma-Aldrich Chemical Co. (USA). D-Glucose, Uric acid (UA), Dopamine hydrochloride (DA) and L-ascorbic acid (AA) were purchased from Tokyo chemical industry Co. Ltd from Bioshop Canada Inc. Ethyl alcohol (EtOH), H2O2 (38.5 wt %), Urea, NaNO2, NaCl, Na2HPO4, KH2PO4 and KCl were purchased from Samchun Pure Co. Ltd (South Korea). All reagents purchased are of analytical grade and used as received without any further purification. Phosphate buffer solution (PBS, 0.1 M, pH 7.4) was freshly prepared in ultrapure water purified using a Millipore-Q system.
Preparation of nickel oxide (NiO) octahedrons
In a typical procedure, 10 mg of silica were dissolved into 100 ml of anhydrous ethanol (EtOH) containing 10 mg of nickel nitrate hexahydrate (Ni(NO3)2.6H2O) and stirred for 24 h at room temperature (RT). Then, the mixed solution was dried at 80 °C for 48 h. The powder was ground well and rinsed following the same procedure one more time. Subsequently, the dry product was transferred to a muffle furnace and calcinated at 550 °C for 3 h at a heating rate of 2 °C min−1. To remove the silica template, the final product was treated two times with 2 M NaOH at 60 °C and washed with ethanol and water repeatedly, and then dried in a vacuum oven at 70 °C for 12 h.
Self-assembly of the 3D graphene hydrogel/nickel oxide octahedrons
First, 48 mg of graphene oxide (GO), which was synthesized by the oxidation of graphite powder according to a modified Hummers method as described previously,44,45 was dispersed in 32 mL of deionized water containing 12 mg of NiO octahedrons using bath-sonicated for 2 h and followed by prop-sonication for 1.5 h. After that, the mixture was transferred to a 45 mL Teflon-lined autoclave, and kept at 180 °C for 12 h. After natural cooling to room temperature, the product named as 3DGH/NiO25 was then washed numerous times by deionized water and finally dried by freeze drying. The identical procedure has been repeated for the formation of 3DGH/NiO5 (2.5 mg of NiO), 3DGH/NiO15 (7.5 mg of NiO) and 3DGH/NiO35 (17 mg of NiO).
Spectroscopic characterization
The morphology of obtained as-fabricated samples was investigated through field emission scanning electron microscopy (FE-SEM, Carl Zeiss SUPRA 40VP, Germany) and high-resolution transmission electron microscopy (HR-TEM, H-7650 Hitachi Ltd, Japan). For investigating the crystallinity and phase structure of the samples, X-ray diffraction (XRD) observations were recorded using X-ray diffractometer (Rigaku, Japan) with high-intensity monochromatic Cu-Kα radiation as an incident beam (λ = 1.54 Å) over a Bragg’s angle range from 10° to 90°. Thermal stability and weight ratio of the prepared samples was analyzed using Q50 TGA device (TA instruments, USA). In addition, Raman spectroscopy measurements were recorded by a Nanofinder 30 (Tokyo Instruments Co., Japan).
Electrochemical characterization
Electrochemical measurements were performed on a ZIVE SP1 electrochemical workstation (WonATech Co. Ltd. Seoul, Korea) with a three-electrode system, which consisted of the modified glassy carbon electrode (GCE) with a geometric surface area of 0.0707 cm2 (3 mm diameter) as working electrode, silver/silver chloride (Ag/AgCl, saturated KCl) as the reference electrode and platinum (Pt) wire as the counter electrode. The modified working electrode was fabricated by dispersion 2 mg of as-synthesized material into 400 µl of isopropanol in the presence of 20 μL of Nafion solution (5 wt %) under sonication condition to obtain homogeneous solution. Then, 15 µl of the prepared sample was coated on a bare glassy carbon electrode. Finally, the fabricated electrode was dried at 60 °C for 30 min. Electrochemical impedance spectroscopy (EIS) was carried out in 5.0 mM K3Fe[CN]6 as redox probe in 0.1 M KCl aqueous solution. However, cyclic voltammetry and chronoamperometry measurements were carried out in 10 mL of 0.1 M PBS (pH 7.4) solution without or with presence hydrogen peroxide and purged with high purity nitrogen for 10 min prior to each measurement. All electrochemical experiments were conducted at room temperature (25 ± 2 °C).
Results and discussion
Physicochemical characterization
The crystal structure and the composition of the as-prepared samples were carried out by X-ray diffraction technique. As most clearly shown in Fig. 2a, an obvious single peak at 2θ = 25° (002) was observed for 3DGH, attributed to a few layers of obtained graphene hydrogel with fewer residual oxygen containing groups 46. In addition, the obtained XRD spectra has NiO main peaks at 2θ = 37.4°, 43.6°, 62.9°, 75.4°and 79.7° can be perfectly assigned to the (111), (200), (220), (311) and (222) crystal planes of the face centered cubic (FCC) NiO, respectively, which is in exact agreement with the (47–1049) standard card from JCPDS47,48 (Figure S2). Furthermore, different sharp XRD diffraction peaks of NiO can be observed in 3DGH/NiO25 nanocomposite, indicating the good synergistic of 3DGH and NiO which additionally confirms the NiO maintain its initial crystal structure during fabrication of 3DGH via hydrothermal process (Table S1). To get the properties of thermal stability and weight ratio of 3DGH, NiO and 3DGH/NiO25 nanostructures, thermogravimetric analysis (TGA) was conducted at air environment with a heating rate of 10 °C/min in the temperature range of 25–700 °C, as can be found in Fig. 2b. Evidently, it is noted that the weight loss value of NiO is negligible, meaning a whole dissolution of residual precursors and the high crystallinity degree of the as-prepared NiO sample. However, the TGA curve of 3DGH divides mainly into two stages: first stage, the weight loss in the temperature range of 25–200 °C is ascribed to the removal of the interlayered adsorbed water molecules; second stage, the eventual weight loss has been occurred after 200 °C is ascribed to the complete degradation and decomposition of the 3DGH sample. For the 3DGH/NiO25 nanocomposite, the initial weight loss is estimated to be 9.4 wt% owing to the removal of the interlayered adsorbed water molecules in the temperature range of 25–200 °C. The major weight loss beyond 450 °C can be attributed to the complete decomposition of 3DGH from the 3DGH/NiO25 nanocomposite. These results indicated that the as-synthesized NiO sample possesses a superior chemical stability, which is greatly, improved the thermal stability of the 3DGH/NiO25 nanocomposite.
The morphological characterization of 3DGH, NiO, and 3DGH/NiO25 has been clearly characterized by using FE-SEM and displayed in Fig. 3. The structure of 3DGH in Fig. 3a shows a three-dimensional (3D) architecture with the presence of mesopores and macropores. Figure 3b shows the FE-SEM image of nickel oxide nanostructure. It is clear that the obtained NiO displays octahedrons-like structure which is had a good uniform size with an average edge length varies from 300 to 400 nm. More importantly, there are a lot of nanopores on the surface of NiO octahedrons, which might be attributed to the etching process of silicon dioxide from as-synthesized NiO octahedrons products using a hot aqueous sodium hydroxide. Furthermore, the particle size distribution indicates that most particles fall within the 300–400 nm range (Fig. 3c). EDS analysis was conducted to determine the percent of elements in the NiO octahedrons As shown in Fig. 3d, the weight percent of Nickel (Ni) and Oxygen (O) are 78.67, and 21.33%, respectively. Figure 3e, f display the low and high magnification FE-SEM images of the 3DGH/NiO25. It can be clearly seen that the NiO octahedrons exhibits a well-decorated 3D graphene hydrogel and the graphene clearly embedded the NiO among its sheets and pores, forming a unique 3DGH/NiO25 hybrid nanostructure. It suggests that the nanopores on the surface of NiO octahedrons plays an important role in increasing the specific surface area and form stronger interfacial functionalization48. In addition, the FE-SEM images in Figure S1 of 3DGH/NiO5, 3DGH/NiO15 and 3DGH/NiO35, respectively, confirms the NiO octahedrons have been clearly decorated the 3DGH.
Transmission electron microscope (TEM) technique was performed to depict the internal structure of the 3DGH/NiO25. As shown in Fig. 4a, it can be clearly noted that there are dark octahedrons spots on the 3D graphene hydrogel, indicating the NiO octahedrons samples were successfully maintained its morphology after self-assembly with the 3D graphene hydrogel. Figure 4b shows the TEM image at high magnification, it can be seen that an average edge length of the octahedrons is 300 nm and the NiO octahedrons seems to be encapsulated by 3D graphene hydrogel. Moreover, EDS analysis was conducted to determine the percent of elements in the 3DGH/NiO25 nanocomposite. As shown in Fig. 4c, the weight percent of Carbon (C), Oxygen (O) and Nickel (Ni) are 68.33, 10.98, and 20.69%, respectively. The presence and distribution of C, O, and Ni elements in the 3DGH/NiO25 nanocomposite were confirmed by STEM elemental mapping (Fig. 4d). The results of selected area showed that the mapped blue, purple, and green zones assigned to C, O, and Ni elements, respectively. On the other hand, the amount of oxygen was too much in the NiO octahedrons, whereas the 3D graphene hydrogel had lower oxygen content, which was in good agreement with XRD patterns. Furthermore, the crystallinity and microstructure of these materials was further analyzed by Raman spectroscopy analysis. As can be seen in Fig. 5, 3DGH and 3DGH/NiO25 nanocomposite clearly exhibits well-documented two characteristic peaks centered at 1349 cm−1 and 1582 cm−1 which were indicative of the D and G band, respectively. The first band (D) is related to the structural defects and disorders from the vibrations of sp3 carbon atoms and the next band (G) is related to the vibration of sp2 hybridized carbon atoms in the graphitic 2D hexagonal lattice 49.
Raman spectra of NiO octahedrons displays two obvious peaks, the first band at 500 cm−1 is assigned to the first-order longitudinal optical (LO), while the second band at 1090 cm−1 is due to 2LO phonon modes 50,51. In addition, a peak at 500 cm−1 is clearly observed in 3DGH/NiO25 Raman spectrum, which provides an evidence of the NiO octahedrons has been existed in the new nanocomposite. The peak ratio of the D and G band (ID/IG) has been testified to investigate the ordered and disordered the crystallized structures of carbonaceous materials 52. The intensity ratio (ID/IG) of the 3DGH/NiO25 (0.94) was significant higher than that in the 3DGH (0.91). This reveal that the defects and disordered graphite increases after 3DGH is decorated by NiO.
Electrochemical measurements
Electrochemical impedance spectroscopy
To ascertain the origin of the conductivity properties of as-prepared samples, we used an equivalent circuit to simulate the electrochemical impedance spectroscopy (EIS) in aqueous solution consisting of 5 mM [Fe(CN)6]−3/−4 and 0.1 M KCl as electrolyte with a frequency range from 1 Hz to 1 MHz at 5 mV amplitude. In the high frequency zone, a semicircle arc section of the Nyquist plots represents a diffusion resistance rate of electrolytes through electrode interface. The EIS spectra (Fig. 6a) were fitted using a Randles equivalent circuit. The extracted Rct values confirm the enhanced charge transfer properties of the 3DGH-modified electrodes. Notably, the 3DGH/GCE and 3DGH/NiO25/GCE electrodes showed the lowest Rct values, reflecting their superior electron transfer kinetics and catalytic efficiency toward H₂O₂ reduction. As shown in Fig. 6a, it is clear that the 3DGH/NiO25 electrode exhibits the smallest charge transfer resistance (Rct ~ 98 Ω) as compared to the bare GC electrode (Rct ~ 140 Ω), 3DGH electrode (Rct ~ 129 Ω) and NiO electrode (Rct ~ 152 Ω), revealing the superior electron transfer process of the fabricated 3DGH/NiO25 electrode. Moreover, the Wargburg resistance (Wd) reflects the capacitive behavior of the developed electrodes at low frequency zone via the straight line section53,54,55.
Obviously, the straight line of the 3DGH/NiO25 electrode are much closer to the Y axis compared to the other prepared electrodes, suggesting that the 3DGH/NiO25 electrode has very small ion diffusion resistance with the ideal capacitive behavior56. That means the 3DGH/NiO25 nanocomposite has unique conductivity features for the electrochemical biosensing application.
Electrocatalytic properties toward H2O2 reduction
In order to evaluate the electrocatalytic activity of as-fabricated electrodes on the detection of H2O2, cyclic voltammetry (CV) were conducted in N2-saturated 0.1 M PBS (pH = 7.4) in the absence and presence of 5 mM H2O2 at a scan rate of 50 mV s−1 using a conventional three-electrode system including Ag/AgCl and a platinum wire as the reference and the counter electrodes, respectively. As shown in Fig. 6b, there were no peaks on all working prepared electrodes in 0.1 M PBS for the absence of H2O2. After injecting 5 mM H2O2 into 0.1 M PBS as shown in Fig. 6b, although the bare GCE shows a minor cathodic wave at − 0.35 V upon H₂O₂ addition, the response is minimal and lacks a well-defined peak. This indicates poor electrocatalytic activity, in contrast to the strong and sharp reduction observed with the 3DGH/NiO25/GCE. Among four 3DGH/NiO nanocomposites, the 3DGH/NiO25 shows the highest current reduction response than other composites (Figure S3). The superior electrochemical catalytic activity of 3DGH/NiO25/GCE towards H2O2 reduction could be ascribed to the good synergistic between 3DGH and NiO octahedrons which increased the electron transfer rate and led to further improve the detection process of H2O2. Furthermore, the significant enhancement in cathodic current response observed for 3DGH/NiO25/GCE, compared to other electrodes at the same potential, indicates a more favorable electrocatalytic environment for H₂O₂ reduction. The electrochemical reduction of H2O2 on the 3DGH/NiO25/GCE surface likely follows a two-electron transfer pathway as Eq. (1):
The 3D graphene hydrogel provides a high surface area and conductive network that enhances electron mobility and promotes efficient contact between H₂O₂ molecules and the electrode surface57. Additionally, NiO nanoparticles act as active catalytic centers, facilitating electron transfer by undergoing redox transitions58. The reduction of H₂O₂ on the 3DGH/NiO25/GCE is proposed to follow a two-electron pathway forming OH⁻, consistent with previously reported mechanisms on NiO-based58 and graphene-based electrodes57, revealing the synergistic interaction between NiO and 3DGH further enhances the catalytic behavior. The electrochemically active surface area was evaluated by estimating the double-layer capacitance in the non-faradaic region via cyclic voltammetry. The 3DGH/NiO25/GCE exhibited of 44.7 μF/cm2, significantly higher than that of the bare GCE (11.9 μF/cm2), indicating an increased electrochemical surface area. The increased current observed in the presence of H₂O₂ is attributed mainly to faradaic processes rather than purely capacitive behavior. The synergistic integration of NiO with 3DGH facilitates improved charge transfer and enhanced accessibility of active sites, thereby accelerating the H₂O₂ electro-reduction process59,60,61. To further demonstrate excellent electrocatalytic activity of 3DGH/NiO25 nanocomposite for reduction of H2O2, cyclic voltammetry (CV) was carried out in 0.1 M PBS with 5 mM H2O2 at different scan rates ranging from 10 to 125 mV s−1. As can be seen from Fig. 7a, the cathodic peak currents which represent the reduction process of H2O2 on the surface of optimized electrode increased gradually with the increase in scan rate. Furthermore, the cathodic peak potential exhibits a gradual shift toward more negative values. This behavior is characteristic of an irreversible or quasi-reversible electrochemical process, where the electron transfer kinetics are not fast enough to keep up with the increasing scan rate62. This shift is typically attributed to the higher overpotential required to drive the redox process at faster scan rates due to limitations in electron transfer and diffusion rates. Similar trends have been observed in previous studies for non-enzymatic H₂O₂ sensors63,64,65,66. Besides that, the cathodic peak currents were found to increase linearly with the square root of the scan rate (Fig. 7b), which indicates a surface diffusion controlled process67. Additionally, the catalytic activity of 3DGH/NiO25/GCE towards H2O2 was investigated at 50 mV s−1 using different concentration of H2O2. As seen from Fig. 7c, the cathodic peak current increased with increasing H2O2 concentrations and a good linear relationship was found between the current reduction peak and the concentration of H2O2 (Fig. 7d), demonstrating the excellent electrocatalytic activity of 3DGH/NiO25 nanocomposite towards H2O2 reduction.
(a) CV curves of the 3DGH/NiO25/GCE electrode at different scan rates in N2 saturated 0.1 M PBS solution containing 5 mM H2O2. (b) Plot of reduction peaks current vs. square root of scan rates. (c) CV curves of the 3DGH/NiO25/GCE electrode in 0.1 M PBS with different concentrations of H2O2 at a scan rate of 50 mV s−1. (d) Plot of reduction peaks current vs. concentrations of H2O2.
Amperometric H2O2 detection on the 3DGH/NiO25/GCE
Amperometric technique at a fixed potential further corroborates the electrocatalytic activities of electrochemical sensors. Figure 8a exhibited the amperometric current–time responses of the 3DGH/NiO25 with successive step changes of H2O2 concentrations into 0.1 M PBS (pH 7.4) with the solution stirred constantly through static applied potential at − 0.35 V. It can be clearly observed that the current response of the sensor changed rapidly after each addition of H2O2 and reached a steady state in approximately 7 s, indicating a fast electrocatalytic response of our fabricated sensor to H2O2 detection.
(a) Amperometric response of 3DGH/NiO25/GCE on successive additions of H2O2 into 0.1 M PBS at − 0.35 V. Inset shows a blown-up image of the low-concentration region. (b) Calibration curve between H2O2 concentration and amperometric response. (c) Interference test of the 3DGH/NiO25/GCE/GCE electrode in 0.1 M PBS at − 0.35 V with 0.5 mM H2O2 and other interferents including 1 mM of DA, AA, NaNO2, Glucose, Urea, KCl and UA. (d) The corresponding bar diagram of the selectivity to different interfering species.
The calibration curve of 3DGH/NiO25 on consecutive addition of various concentrations of H2O2 with time interval around 50 s is shown in Fig. 8b. The as-fabricated electrode displayed a wide linear detection range from 10 µM to 33.58 mM and the linear regression equation can be described as \({I}_{pa}\left(\mu A\right)=14.623-8.22c (mM)\) with a correlation coefficient of 0.9903. The sensitivity is calculated to be 117.26 µA mM−1 cm−2 and a detection limit of 5.3 µM at a signal-to-noise ratio of 3. As observed in Table 1, the performance sensing of the proposed sensor is comparable and even superior to other previously reported H2O2 sensors with regard to graphene/metal oxides nanocomposites. This may attribute to the admirable properties of the NiO octahedrons, which played the significant part in demonstrating satisfactory detection result with a wide linear detection range and lower limit of detection.
Selectivity, reproducibility, stability and real sample analysis
Furthermore, the selectivity test of electrochemical sensors has become a critical area of focus, extensively employed to verify a sensor’s ability to accurately detect target analytes amid potential interferences. To assess the selectivity test of the 3DGH/NiO25 nanocomposite modified electrode, the amperometric measurements were conducted at applied potential of − 0.35 V (vs. Ag/AgCl) in 0.1 M PBS (pH 7.4) solution with initial addition of 1 mM H2O2 and then 2 mM of each interfering species i.e. dopamine (DA), ascorbic acid (AA), NaNO2, glucose, urea, KCl and uric acid (UA). As clearly shown in Fig. 8c and d, a rapid current signal response was obtained with the addition of 1 mM H2O2. Nevertheless, no remarkable current signal change was observed upon addition of interfering species, which demonstrates the higher selectivity of the 3DGH/NiO25 developed sensor toward H2O2 molecules compare to the interfering species. Moreover, the reproducibility of this proposed electrochemical sensor was investigated by measuring the current signal response of five similarly modified electrodes in 0.1 M PBS (pH 7.4) containing 1 mM H2O2. The results shown on Fig. 9a revealed that the current signal response of all modified electrodes were obtained almost same values at 1 mM H2O2, with relative standard deviation (RSD) of 4.3% Additionally, the long-term stability was regarded as one of the most significant factors to monitor the performance of the proposed sensor. To evaluate the long-term stability of the modified electrode, the current signal response of reduction peak was measured via cyclic voltammetry every three days for one month. As can be seen in Fig. 9b, the response of modified electrode decreased to 93.5% of its initial value after being stored for one month under ambient conditions. Therefore, the superior analytical results including selectivity, reproducibility and long-term stability of our fabricated sensing electrode based on 3DGH/NiO25 indicate that practically viable of sensor to detect H2O2 in a real study. Therefore, the analytical utilities of the 3DGH/NiO25 proposed electrode were conducted to detect H2O2 in real products of commercial milk samples.
For sample preparation, one hundreds microliter of commercial milk was diluted into 20 ml of 0.1 M PBS (pH 7.4) solution and then analyzed by amperometric technique at − 0.35 V with an appropriate known amounts of H2O2. The recovery and the accuracy values of the as-fabricated electrode were achieved by the standard addition method. The 3DGH/NiO25 nonenzymatic sensor exhibits high values of recovery percentage with the relative standard deviations (RSD%) of lower than 3.32%, as presented in Table S1. These satisfactory results further proved that the as-fabricated 3DGH/NiO25 sensing electrode is a feasible promising candidate for the detection of H2O2 in the environmental life system.
Conclusions
In this study, a novel approach was utilized for synthesizing NiO octahedrons. In addition, the self-assembly of NiO octahedrons decorated 3DGH via a hydrothermal method and applied for the electrochemical detection of H2O2. Compared with pure 3DGH, NiO octahedrons and four nanostructures of 3DGH/NiO, the 3DGH/NiO25 nanostructure exhibited superior sensing material toward H2O2 detection, including high sensitivity (117.26 µA mM−1 cm−2) with wide linear detection range (10 µM-33.58 mM) and low detection limit (5.3 µM) at a signal-to-noise of 3. Therefore, the 3DGH/NiO25 nanocomposite with superior performance has practical values for H2O2 detection in real samples.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen, A. & Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 42, 5425–5438 (2013).
Liu, M., Liu, R. & Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 45, 206–212 (2013).
Gu, N. & Liu, S. Introduction to biosensors. J. Mater. Chem. B 8, 3168–3170. https://doi.org/10.1039/D0TB90051F (2020).
Yassin, M. A. et al. A novel morphology of 3D graphene hydrogel nanotubes for high-performance nonenzymatic hydrogen peroxide sensor. J. Ind. Eng. Chem. 79, 245–254 (2019).
Rahmawati, N. et al. Pulp bleaching by hydrogen peroxide activated with copper 2, 2′-dipyridylamine and 4-aminopyridine complexes. Chem. Eng. J. 112, 167–171 (2005).
Lu, C.-P., Lin, C.-T., Chang, C.-M., Wu, S.-H. & Lo, L.-C. Nitrophenylboronic acids as highly chemoselective probes to detect hydrogen peroxide in foods and agricultural products. J. Agric. Food Chem. 59, 11403–11406 (2011).
Chen, W., Cai, S., Ren, Q.-Q., Wen, W. & Zhao, Y.-D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 137, 49–58 (2012).
Ramachandran, K. et al. MnO2 nanorods grown NGNF nanocomposites for the application of highly sensitive and selective electrochemical detection of hydrogen peroxide. J. Ind. Eng. Chem. 46, 19–27 (2017).
Miller, E. W., Albers, A. E., Pralle, A., Isacoff, E. Y. & Chang, C. J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J. Am. Chem. Soc. 127, 16652–16659 (2005).
Winterbourn, C. C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278 (2008).
Marinho, H. S., Real, C., Cyrne, L., Soares, H. & Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2, 535–562 (2014).
Maji, S. K., Sreejith, S., Mandal, A. K., Ma, X. & Zhao, Y. Immobilizing gold nanoparticles in mesoporous silica covered reduced graphene oxide: A hybrid material for cancer cell detection through hydrogen peroxide sensing. ACS Appl. Mater. Interfaces. 6, 13648–13656 (2014).
Zhang, S. & Zheng, J. Synthesis of single-crystal α-MnO2 nanotubes-loaded Ag@C core–shell matrix and their application for electrochemical sensing of nonenzymatic hydrogen peroxide. Talanta 159, 231–237 (2016).
Ma, B. et al. A sensitive electrochemical nonenzymatic biosensor for the detection of H2O2 released from living cells based on ultrathin concave Ag nanosheets. Biosens. Bioelectron. 106, 29–36 (2018).
Patel, V., Kruse, P. & Selvaganapathy, P. R. Solid state sensors for hydrogen peroxide detection. Biosensors 11, 9 (2020).
Zhang, M., Sheng, Q., Nie, F. & Zheng, J. Synthesis of Cu nanoparticles-loaded Fe3O4@carbon core–shell nanocomposite and its application for electrochemical sensing of hydrogen peroxide. J. Electroanal. Chem. 730, 10–15 (2014).
Zeng, F. et al. Facile construction of Mn3O4-MnO2 hetero-nanorods/graphene nanocomposite for highly sensitive electrochemical detection of hydrogen peroxide. Electrochim. Acta 196, 587–596 (2016).
Pitiphattharabun, S. et al. Reduced graphene oxide/zinc oxide composite as an electrochemical sensor for acetylcholine detection. Sci. Rep. 14, 14224 (2024).
Humayun, H., Begum, B., Bilal, S., Shah, A. U. H. A. & Röse, P. Polyindole embedded nickel/zinc oxide nanocomposites for high-performance energy storage applications. Nanomaterials 13, 618 (2023).
Mollamahale, Y. B. et al. Simple fabrication of porous NiO nanoflowers: Growth mechanism, shape evolution and their application into Li-ion batteries. Int. J. Hydrog. Energy 42, 7202–7211 (2017).
Shi, W., Zhang, Y., Key, J. & Shen, P. K. Three-dimensional graphene sheets with NiO nanobelt outgrowths for enhanced capacity and long term high rate cycling Li-ion battery anode material. J. Power Sources 379, 362–370 (2018).
Zhang, Y. et al. Tailoring NiO nanostructured arrays by sulfate anions for sodium-ion batteries. Small 14, 1800898 (2018).
Lee, K. S., Park, M. S. & Kim, J.-D. Nitrogen doped activated carbon with nickel oxide for high specific capacitance as supercapacitor electrodes. Colloids Surf. A 533, 323–329 (2017).
Sun, H., Ma, Z., Qiu, Y., Liu, H. & Gao, G. G. Ni@NiO nanowires on nickel foam prepared via “acid hungry” strategy: High supercapacitor performance and robust electrocatalysts for water splitting reaction. Small 14, 1800294 (2018).
Saba, S., Alsharari, A. M., Aldaleeli, N. Y., Aljohani, M. M. & Hamdalla, T. A. Structural, electrochemical, and supercapacitor characterization of double metal oxides doped within ZIF-8 composites. Processes 13, 859 (2025).
Li, G. et al. Controllable synthesis of 3D Ni(OH)2 and NiO nanowalls on various substrates for high-performance nanosensors. Small 11, 731–739 (2015).
Kang, J. S. et al. Electrochemically synthesized mesoscopic nickel oxide films as photocathodes for dye-sensitized solar cells. ACS Appl. Energy Mater. 1, 4178–4185. https://doi.org/10.1021/acsaem.8b00834 (2018).
Fang, B. et al. A uric acid sensor based on electrodeposition of nickel hexacyanoferrate nanoparticles on an electrode modified with multi-walled carbon nanotubes. Microchim. Acta 173, 27–32 (2011).
Zhang, H. & Liu, S. Nanoparticles-assembled NiO nanosheets templated by graphene oxide film for highly sensitive non-enzymatic glucose sensing. Sens. Actuators B Chem. 238, 788–794 (2017).
Zhang, Y., Wang, Y., Jia, J. & Wang, J. Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sens. Actuators B Chem. 171, 580–587 (2012).
Liu, F., Piao, Y., Choi, K. S. & Seo, T. S. Fabrication of free-standing graphene composite films as electrochemical biosensors. Carbon 50, 123–133 (2012).
Zheng, D., Hu, H., Liu, X. & Hu, S. Application of graphene in elctrochemical sensing. Curr. Opin. Colloid Interface Sci. 20, 383–405 (2015).
Lewandowska, W. et al. Gold nanoparticle-enhanced graphene immunosensor for rapid detection of gardnerella vaginalis in clinical diagnostics. Chemosensors 13, 111 (2025).
Han, S., Wu, D., Li, S., Zhang, F. & Feng, X. Porous graphene materials for advanced electrochemical energy storage and conversion devices. Adv. Mater. 26, 849–864 (2014).
Fan, X., Chen, X. & Dai, L. 3D graphene based materials for energy storage. Curr. Opin. Colloid Interface Sci. 20, 429–438 (2015).
Dong, X.-C. et al. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 6, 3206–3213 (2012).
Xia, X., Chao, D., Zhang, Y. Q., Shen, Z. X. & Fan, H. J. Three-dimensional graphene and their integrated electrodes. Nano Today 9, 785–807 (2014).
Wang, H. et al. Three dimensional graphene based materials: Synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation. Adv. Coll. Interface. Sci. 221, 41–59 (2015).
Zhu, S. et al. Self-assembled three-dimensional hierarchical graphene hybrid hydrogels with ultrathin β-MnO2 nanobelts for high performance supercapacitors. J. Mater. Chem. A 3, 1540–1548 (2015).
Meng, W. et al. Preparation of composite graphene hydrogels adsorbent with special-shaped ZnO and TiO2. Colloids Surfaces A Physicochem. Eng. Aspects 581, 123783 (2019).
Chen, L., Wang, X., Zhang, X. & Zhang, H. 3D porous and redox-active prussian blue-in-graphene aerogels for highly efficient electrochemical detection of H2O2. J. Mater. Chem. 22, 22090–22096 (2012).
Yin, D., Bo, X., Liu, J. & Guo, L. A novel enzyme-free glucose and H2O2 sensor based on 3D graphene aerogels decorated with Ni3N nanoparticles. Anal. Chim. Acta 1038, 11–20 (2018).
Elsayed, H. A. et al. Graphene metasurfaces biosensor for COVID-19 detection in the infra-red regime. Sci. Rep. 15, 8573 (2025).
Hummers, W. S. Jr. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958).
Wang, H., Robinson, J. T., Li, X. & Dai, H. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 131, 9910–9911 (2009).
Parveen, N., Ansari, S. A., Ansari, M. O. & Cho, M. H. Manganese dioxide nanorods intercalated reduced graphene oxide nanocomposite toward high performance electrochemical supercapacitive electrode materials. J. Colloid Interface Sci. 506, 613–619. https://doi.org/10.1016/j.jcis.2017.07.087 (2017).
Hayat, K., Gondal, M. A., Khaled, M. M. & Ahmed, S. Effect of operational key parameters on photocatalytic degradation of phenol using nano nickel oxide synthesized by sol–gel method. J. Mol. Catal. A Chem. 336, 64–71. https://doi.org/10.1016/j.molcata.2010.12.011 (2011).
Tong, G. et al. Submicrometer-sized NiO octahedra: facile one-pot solid synthesis, formation mechanism, and chemical conversion into Ni octahedra with excellent microwave-absorbing properties. J. Mater. Chem. 22, 17494–17504. https://doi.org/10.1039/C2JM31790G (2012).
Ma, J., Wang, L. & Yu, F. Water-enhanced performance in capacitive deionization for desalination based on graphene gel as electrode material. Electrochim. Acta 263, 40–46. https://doi.org/10.1016/j.electacta.2018.01.041 (2018).
Zhao, B. et al. Monolayer graphene/NiO nanosheets with two-dimension structure for supercapacitors. J. Mater. Chem. 21, 18792–18798 (2011).
Yao, M. et al. Template synthesis and characterization of nanostructured hierarchical mesoporous ribbon-like NiO as high performance electrode material for supercapacitor. Electrochim. Acta 158, 96–104 (2015).
He, H. & Gao, C. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces. 2, 3201–3210 (2010).
Hasnat, M. A. et al. Inverse effects of supporting electrolytes on the electrocatalytic nitrate reduction activities in a Pt| Nafion| Pt–Cu-type reactor assembly. RSC Adv. 6, 11609–11617 (2016).
Balamurugan, T. et al. Real-time tracking and quantification of endogenous hydrogen peroxide production in living cells using graphenated carbon nanotubes supported Prussian blue cubes. Sens. Actuators B Chem. 257, 220–227 (2018).
Rashed, M. A. et al. rGO/ZnO/Nafion nanocomposite as highly sensitive and selective amperometric sensor for detecting nitrite ions (NO2−). J. Taiwan Inst. Chem. Eng. 112, 345–356 (2020).
Yao, S., Xing, L., Dong, Y. & Wu, X. Hierarchical WO3@ MnWO4 core-shell structure for asymmetric supercapacitor with ultrahigh cycling performance at low temperature. J. Colloid Interface Sci. 531, 216–224 (2018).
Shao, Y. et al. Graphene based electrochemical sensors and biosensors: A review. Electroanal Int. J. Devoted Fundam. Pract. Aspects Electroanal. 22, 1027–1036 (2010).
Roushani, M., Abdi, Z., Daneshfar, A. & Salimi, A. Hydrogen peroxide sensor based on riboflavin immobilized at the nickel oxide nanoparticle-modified glassy carbon electrode. J. Appl. Electrochem. 43, 1175–1183 (2013).
Madhura, T. R., Viswanathan, P. & Ramaraj, R. Nanosheet-like manganese ferrite grown on reduced graphene oxide for non-enzymatic electrochemical sensing of hydrogen peroxide. J. Electroanal. Chem. 792, 15–22 (2017).
Li, Y. et al. Hetero-structured MnO-Mn3O4@rGO composites: Synthesis and nonenzymatic detection of H2O2. Mater. Sci. Eng. C 118, 111443 (2021).
Annalakshmi, M., Kumaravel, S., Chen, T.-W., Chen, S.-M. & Lou, B.-S. 3D flower-like NiCo layered double hydroxides: an efficient electrocatalyst for non-enzymatic electrochemical biosensing of hydrogen peroxide in live cells and glucose in biofluids. ACS Appl. Biol. Mater. 4, 3203–3213 (2021).
Bard, A. J., Faulkner, L. R. & White, H. S. Electrochemical methods: Fundamentals and applications (Wiley, 2022).
Zhang, C., Li, L., Ju, J. & Chen, W. Electrochemical sensor based on graphene-supported tin oxide nanoclusters for nonenzymatic detection of hydrogen peroxide. Electrochim. Acta 210, 181–189 (2016).
Cheng, C. et al. 3D network and 2D paper of reduced graphene oxide/Cu2O composite for electrochemical sensing of hydrogen peroxide. Anal. Chem. 90, 1983–1991 (2017).
Amanulla, B. et al. A non-enzymatic amperometric hydrogen peroxide sensor based on iron nanoparticles decorated reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 487, 370–377 (2017).
Guler, M., Turkoglu, V., Bulut, A. & Zahmakiran, M. Electrochemical sensing of hydrogen peroxide using Pd@Ag bimetallic nanoparticles decorated functionalized reduced graphene oxide. Electrochim. Acta 263, 118–126 (2018).
Jin, W., Wu, G. & Chen, A. Sensitive and selective electrochemical detection of chromium (VI) based on gold nanoparticle-decorated titania nanotube arrays. Analyst 139, 235–241 (2014).
Yu, Z., Li, H., Zhang, X., Liu, N. & Zhang, X. NiO/graphene nanocomposite for determination of H2O2 with a low detection limit. Talanta 144, 1–5 (2015).
Li, Q. et al. Mesoporous NiO nanosphere: A sensitive strain sensor for determination of hydrogen peroxide. RSC Adv. 8, 13401–13407 (2018).
Santhosh, C. et al. A non-enzymatic sensor for hydrogen peroxide based on the use of α-Fe2O3 nanoparticles deposited on the surface of NiO nanosheets. Microchim. Acta 184, 3223–3229 (2017).
Liu, M. et al. Three-dimensional carbon foam supported NiO nanosheets as non-enzymatic electrochemical H2O2 sensors. Appl. Surf. Sci. 542, 148699 (2021).
Medhi, A., Giri, M. K. & Mohanta, D. Non-enzymatic approach of H2O2 and glucose sensing using NiO–MoS2-derived electrochemical sensor. Bull. Mater. Sci. 47, 223 (2024).
Carbone, M., Aneggi, E., Figueredo, F. & Susmel, S. NiO-nanoflowers decorating a plastic electrode for the non-enzymatic amperometric detection of H2O2 in milk: Old issue, new challenge. Food Control 132, 108549 (2022).
Zhao, Y. et al. Biosensor based on 3D graphene-supported Fe3O4 quantum dots as biomimetic enzyme for in situ detection of H2O2 released from living cells. Sens. Actuators B Chem. 244, 1037–1044 (2017).
Jiang, B.-B. et al. A non-enzymatic hydrogen peroxide sensor based on a glassy carbon electrode modified with cuprous oxide and nitrogen-doped graphene in a nafion matrix. Microchim. Acta 181, 1463–1470 (2014).
Fan, Y., Yang, X., Yang, C. & Liu, J. Au-TiO2/graphene nanocomposite film for electrochemical sensing of hydrogen peroxide and NADH. Electroanalysis 24, 1334–1339 (2012).
Balamurugan, J., Thanh, T. D., Karthikeyan, G., Kim, N. H. & Lee, J. H. A novel hierarchical 3D N-Co-CNT@ NG nanocomposite electrode for non-enzymatic glucose and hydrogen peroxide sensing applications. Biosens. Bioelectron. 89, 970–977 (2017).
Acknowledgements
The authors extend their appreciation to Ongoing Research Funding program—Research Chairs (ORF-RC-2025-5511), King Saud University, Riyadh, Saudi Arabia. This work was financially supported by The Regional Leading Research Center Program (2019R1A5A8080326) through the National Research Foundation funded by the Ministry of Science and ICT of the Republic of Korea.
Funding
Open access funding provided by University of Pécs. This research was funded by Ongoing Research Funding program—Research Chairs (ORF-RC-2025-5511), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, supervision, methodology, formal analysis, writing—original draft preparation, writing—review and editing, M.A.Y., A.F.A.-H. and H.M.M.; data curation, project administration, investigation, writing—review and editing, C.H.P., C.S.K., A.S. and M.A.M. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yassin, M.A., Abou-Hadid, A.F., Mousa, H.M. et al. Enzymeless electrochemical detection of hydrogen peroxide using NiO octahedron decorated 3D graphene hydrogel. Sci Rep 15, 28293 (2025). https://doi.org/10.1038/s41598-025-10472-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10472-6