Abstract
As an important part of blast hole filling in blasting engineering, rapid setting taphole clay material has been paid attention by many scholars. Although curing time is an important factor affecting the mechanical properties of quick setting taphole clay materials, scholars have little research on quick setting taphole clay materials. This study investigated the influence of curing time on the mechanical properties of rapid-setting taphole clay materials, analyzed the variation pattern of mechanical strength during the increase of curing time, and employed techniques such as XRD and SEM-EDS to study the impact of curing time on the crystalline phase transformation, microstructure, and element distribution of taphole clay materials. The results revealed that with the increase of curing time, all mechanical strengths of the taphole clay materials increased significantly, with a faster growth rate within the first 6 h and stabilization around 24 h. The main hydration products of the taphole clay materials are calcium carbonate and ettringite, whose contents increase with curing time, promoting the hydration process and enhancing mechanical strength. The internal structure of the material becomes denser with increasing curing time, resulting in decreased porosity, specific surface area, the average pore size and total pore volume, and the formation of a dense spatial structure, which explains the trend of strength change. This study is of great significance for selecting optimal plugging materials and curing times for mines in China to achieve efficient blasting operations.
Similar content being viewed by others
Introduction
At present, metal mines both domestically and internationally can be mainly classified into two categories: open-pit mines and underground mines. Regardless of the type, blasting mining is the predominant method employed, which involves drilling blastholes in the ore body, followed by charging explosives. To optimize the blasting effect, after explosives are loaded, specific materials are utilized to stem the blastholes. Common stemming materials include sand, clay mixtures, rock dust, and specially formulated taphole clay products. Once these materials are inserted into the blastholes, the energy from the explosive detonation is harnessed to fracture the rock, thereby separating the ore from the surrounding rock mass1. The efficiency of explosive energy utilization directly impacts the quality of the blasting effect, subsequently exerting profound influences on various factors such as mining costs, safety, and efficiency2. Among numerous methods to enhance explosive energy utilization, blasthole stemming (achieved through rational selection and application of taphole clay materials) stands out as a crucial and effective approach. Superior blasthole stemming quality (dependent on the proper use of high-quality taphole clay materials) can extend the duration of blast-generated gases acting within the hole, thereby generating fractures over a larger area that subsequently expand, coalesce, and cut through the rock. It can also ensure complete reaction of the explosives within the blasthole, reduce specific explosive consumption, and improve the blasting effect3. The selection of appropriate taphole clay stemming materials and ensuring their stemming quality play a pivotal role in enhancing overall blasting efficiency. This not only effectively mitigates explosive energy dissipation but also allows blast-generated gases to act for a longer duration within the hole through physical obstruction, concentrating the blasting energy more intensively on the rock and thereby enhancing the blasting effect4,5.
The core of blasthole stemming lies in the selection and application of taphole clay stemming materials. Different taphole clay materials possess distinct physicochemical properties that directly influence the quality of blasthole stemming and, consequently, have a profound impact on the blasting effect6,7. In recent years, scholars have shifted their focus towards the research of rapid and efficient hole-sealing materials, exploring the optimal material ratios. The choice of the main material of high water rapid-setting material is based on sulfur-aluminate cement8. In the formulation of high water rapid-setting materials, auxiliary materials predominantly consist of gypsum and lime, complemented by the addition of a small amount of suspending agents and early strength agents9. Sometimes small amounts of alkaline materials or fly ash slag are added, etc10. Devenda et al.11 posited that stemming (or borehole blocking) could substantially reduce energy loss during an explosion and asserted that sand was the most effective material for borehole stemming, capable of effectively containing the high-pressure stress generated by blasting. Qiu et al.12 through experimental comparison of three water-soil composite stemming schemes, found that the best blasting effect was achieved when water was added to the upper section of the borehole and soil was placed beneath the water. Shi et al.13 argued that water-sealed blasting could significantly reduce the vibration response of adjacent structures and proposed a construction method for vibration-attenuating water-sealed blasting. Wang et al.14, in response to the issues of low efficiency, poor quality, and high labor intensity associated with taphole clay plugging operations at construction sites, developed a new type of taphole clay material with fluidity that could be used for pouring and plugging boreholes.
Strengthen the research on the mechanical strength characteristics of rapid-setting materials, establish a unified and comprehensive theoretical system, and provide a more scientific and rational basis for the selection and application of stemming materials in mine blasting operations15,16,17. In addition, scholars have also carried out some investigations on the mechanical strength of rapid-setting materials18,19. Tang et al.20 developed a new type of packing material that is convenient to transport, has a short setting time, and exhibits high hole-sealing efficiency. The uniaxial compressive strength of this new packing material decreases with an increase in the water-cement ratio and is significantly higher than that of taphole clay materials. Cao et al.21 used loess as the base material and incorporated cement and additive slurry to formulate grouting materials. They conducted single-factor and orthogonal experiments to analyze the influencing factors on the gel time and uniaxial compressive strength of the materials, ultimately determining the optimal formulation. Feng et al.22 conducted uniaxial compressive strength as well as triaxial compression tests at different curing times under normal curing for high water materials with 91%~97% water volume. It was pointed out that the strength of high water rapid-setting materials under uniaxial compression accounted for 66%~90% of the final strength at 7 d, with obvious early strength characteristics, and the strength at all curing times increased by about 25% on average when compressed in three directions. Zhang et al.23 further found that the peak and residual intensity were increased when the water temperature increased within 25 °C to 40 °C, and the lower the water-cement ratio, the greater the strength increase. However, Liu et al.24 did not find the phenomenon of strength retrogression in the test for the effect of curing temperature. In summary, the existing research on the effect of curing time on the strength of rapid-setting materials is relatively small, and the conclusions are different, and there is no uniform conclusion. There are differences in the selection of main and auxiliary materials among different researchers, and no unified conclusion has been reached yet regarding the research on the mechanical strength characteristics of rapid-setting materials. Therefore, conducting in-depth research on stemming materials and exploring higher-quality, more efficient stemming materials and their reasonable proportions are of great practical significance for improving mine blasting effects, reducing mining costs, ensuring mining safety, and enhancing mining efficiency.
Existing research primarily focuses on macroscopic mechanical properties, with relatively little analysis on the microscopic mechanisms of taphole clay. Relevant studies mainly concentrate on rapid-setting materials such as cement and mortar25. Zhang et al.26 investigated the effects of accelerators and flocculants on the properties of cement-based grouts. The results indicated that the additives promoted the formation of needle-rod-shaped ettringite (AFt), enhancing the integrity and stability of the solidified samples. Gu et al.27 conducted detailed studies on the macroscopic and microscopic characteristics and cementation mechanisms of cement-bonded calcareous sand, exploring the strength changes of cemented samples at different curing times when the cement content was 5%, 7.5%, and 10%. Wan et al.28 analyzed the effects of curing times and cement content on the macroscopic properties and microscopic structural characteristics of cement-bonded calcareous sand in a seawater environment. Luo et al.29 studied the mechanical properties of nano-SiO2-modified cement-bonded calcareous sand based on macroscopic and microscopic testing. In summary, although there has been some exploration in the field of taphole clay materials, numerous issues remain to be addressed urgently.
Based on this, this study explores the influence of curing time on the mechanical properties of rapid-setting materials, and analyzes the variation of each mechanical strength in the process of curing time increase. Using XRD, SEM-EDS and other analytical means, this study investigates the effect of curing time on the crystallization transition, microstructure and elemental distribution of taphole clay materials, revealing the intrinsic connection between strength growth and microstructure evolution. This study is of great significance for choosing better stemming materials and curing time periods in blasting operations in China’s mines, so as to realize the convenient, safe, reliable and efficient blasting operation.
Rapid-setting mechanism of rapid-setting taphole clay
Rapid-setting taphole clay is a new type of taphole clay material with high solid water content, which is based on the generation mechanism of high water rapid-setting materials and obtained through formula experiments according to the requirements of taphole clay stemming. It is mainly formed by hydration reaction of main material of sulfoaluminate cement and auxiliary material of dihydrate gypsum and quicklime. The main body of the structure is ettringite. Therefore, the whole hydration reaction mechanism is based on the formation mechanism of ettringite to a certain extent24.
Rapid-setting taphole clay main and auxiliary materials through the hydration reaction to generate solidification is a complex process. In the process of using the main material and auxiliary materials are first prepared into a slurry, and then mix the two when using, after mixing the reaction to generate the solidified body. From the material composition analysis, the main substances involved in the reaction are \({C_4}{A_3}\overline {S}\), \(\beta - C2S\), calcium sulfate and calcium oxide, and its hydration process and products determine the main mechanical properties of the solid. When the main and auxiliary materials slurry mixing, the main substances in the liquid phase environment will quickly react to generate ettringite precipitation, when the system is sufficient reactants, the reaction will give priority to all generated ettringite, and analyze the main substances before and after the reaction changes. The reaction equation is as follows.
When there is not enough CaO in the system, the product will not be all ettringite, but will instead form a partial aluminum gel.
When the CaSO4 content in the system is insufficient, the reaction will no longer produce ettringite but low-sulfur hydrated calcium thioaluminate (AFm) and aluminum gel. The generated ettringite will also decompose to form low-sulfur hydrated calcium thioaluminate30.
When calcium carbonate is added to the formula of rapid-setting taphole clay, it will prevent the transformation of high sulfur type salt to low sulfur type salt in the solution, which may lead to the absence of AFm in the product. So a certain proportion of calcium carbonate can be added to the formula of rapid-setting taphole clay instead of calcium oxide, which prevents the generation of AFm and improves the coagulation time of the taphole clay.
In addition to \({C_4}{A_3}\overline S\), another major component in sulfoaluminate cement, hydrates to produce calcium silicate gel (C-S-H). The C/S in this gel is related to the concentration of calcium oxide in the liquid phase. When the concentration of calcium oxide is low, type I hydrated calcium silicate is generated, with a C/S between 0.5 and 1.5. Therefore, for every 1 mol of \(\beta - C2S\) hydrated to form 1 mol of C-S-H gel, 1 mol of calcium hydroxide is also generated31. The precipitated calcium hydroxide will continue to react with aluminum gel and calcium sulfate to form ettringite. The reaction equation is as follows.
Therefore, the formation of C-S-H can be accelerated by promoting the hydration reaction of \(\beta - C2S\). Thus, the pores between the structural skeleton formed by ettringite are filled with cementing matters, and the structure of the solid body is made denser.
In the above, chemical equations are used to describe the changes of substances in the formation process of ettringite from the perspective of reaction mechanism. However, in reality, the change of substances in the formation process of rapid-setting taphole clay is more complicated than the theoretical description. This is because in the actual hydration reaction, on the one hand, with the deepening of hydration reaction, the consistency of slurry will significantly increase, resulting in the migration of ions in the slurry system becoming more and more difficult. On the other hand, due to the generation of ettringite crystals will be adsorbed on the surface of solid particles which have not yet been involved in the reaction, hindering its further participation in the reaction. Therefore, the hydration process of rapid-setting taphole clay will be constrained by the hydration diffusion rate. The hydration process of rapid-setting taphole clay can be roughly divided into the following four stages according to the state change32.
-
(1)
Hydration induction period.
At this stage of hydration induction, the main and auxiliary grout are just mixed and stirred. The ions contained in it quickly dispersed into the mixed slurry and began to react to form part of the ettringite unit cells. This stage lasted for a short time, the hydration heat release was not obvious and the viscosity of the slurry almost did not change.
-
(2)
Hydration acceleration period.
When the mixed slurry enters the stage of accelerated hydration, the rate of hydration reaction starts to increase dramatically. Ettringite unit cells formed earlier start to develop and are constantly accompanied by the formation of new ettringite unit cells. In this process, because the slurry system is not ideally uniformly distributed, there will be a small amount of other products such as gel generation. The continuously formed and developed ettringite unit cells will be connected with each other to form a ettringite skeleton, which will fix the gel and free water in it, so that the viscosity of the slurry will increase rapidly to complete the initial coagulation and begin to harden continuously. The whole process of accelerated hydration will release a large amount of heat of hydration, so that the surface temperature of rapid-setting taphole clay is significantly higher than the ambient temperature.
-
(3)
Hydration deceleration period.
When the rapid-setting taphole clay hydration rate reached the peak, began to enter the stage of hydration deceleration period. At this time, the system in the ettringite unit cell generation is significantly reduced, began to generate a large number of other substances such as gel, hydration exothermic slowed down significantly. The surface temperature of the gradually hardened rapid-setting taphole clay consolidated body began to decrease.
-
(4)
Hydration stabilization period.
In this stage, the hydration reaction rate is further reduced, and the hydration process has basically ended. The surface temperature of the slurry gradually decreases until it is the same as the ambient temperature.
Experimental plan
Experimental material
The material formation includes two parts: main and auxiliary materials (hereinafter referred to as A and B materials). When the main and auxiliary material slurries are mixed, the substances in them react in a liquid phase environment to form the main structure with ettringite as the backbone. Part of the free water and gel filled with it, so as to complete the transformation from slurry to solid. A material is composed of sulfoaluminate cement and compound retarding and dispersing agent. B material consists of other auxiliary materials such as lime, gypsum and composite rapid-setting agent, A and B microscopic morphology is shown in Fig. 1. The picture of material A is a large number of granular materials with different shapes. The surface of the particles presents complex textures and bulges, with porous structure after crystallization or sintering. The picture of material B shows that the particle size is different, the shape is irregular, and the particle heterogeneity is significant. In order to equalize the A, B composition, improve the suspension performance of the B material, the B material is sometimes also added to a certain suspension of dispersant agent.
Retarder is one of the key links in the development of ultra-high water materials. Efficient retarding agents not only simplify the filling process, but importantly, the fired materials in water need to be rapidly put in a stable state by retarding agents to avoid premature hydration with water. The role of rapid-setting agent is to promote their rapid hydration, so as to achieve the purpose of rapid hardening and early strength. And with a lot of water, conventional admixtures can’t be as effective as they should be. Finding composite admixtures with good compatibility should be the goal of the test. In the stage of retarding and rapid-setting, suitable suspending and dispersing agents, so that the solid material can be more uniformly and stably suspended in the liquid. The material can be uniformly and stably hydrated to form a homogeneous hydration hardening body.
The composition of sulfoaluminate cement clinker used in this experiment is shown in Table 1.The main component of material A is sulphoaluminate cement clinker. The main chemical components are Cao and Al2O3, accounting for 44.8% and 27.63% respectively. The main mineral components are 3CaO·3Al₂O₃·CaSO₄ and 2CaO·SiO₂, accounting for 52.86% and 37.31% respectively. The main components of material B are gypsum, lime, calcium carbonate, bentonite and admixtures. In the preliminary formula of material B, gypsum: Lime: calcium carbonate: bentonite = 7:2:3:3, gypsum used in material B is gypsum (calcium sulfate dihydrate), and the amount of admixture in material a and material B is calculated according to the percentage of the total weight of the main and auxiliary materials.When making taphole clay, the mass specific gravity of material a and material B is 1:1, and the cement mesh shall be grinded and sieved to more than 200 mesh.
The role of rapid-setting taphole clay auxiliary additives is to make the main and auxiliary materials of the two slurries can be quickly solidified after mixing. Therefore, in this part of the test, first, select different types of rapid-setting agent may be applicable. And through the test to determine the type of rapid-setting agent and the amount of additive, so that the rapid-setting taphole clay main and auxiliary slurry can be mixed in a short period of time to complete the rapid solidification (the initial solidification time of < 3 min, the final solidification time of about 30 min). And then optimize and adjust the proportion of auxiliary materials of rapid-setting taphole clay. Finally, the test was conducted to observe whether the addition of rapid-setting agent would affect the flow state of the auxiliary slurry.
Preparation process
In order to ensure the accuracy of the test data, the following specific test operation steps are set up. The single pulp preparation process consists of weighing and mixing the material using an analytical electronic balance, taking tap water from the measuring cylinder, adding it to the mixing pot and wetting it, and then slowly adding the material to the pot and placing it on the mixing equipment. With low gears stir into container in about 10 min, finally clean mixing pot and impeller. Then mix the slurry and add B and A slurry to the mixing container according to the test requirements. Mix by hand for 30 s to combine the two thoroughly. Then pour the mixture into the test block box and divide it into three parts, record the start time of standing and observe the state of the test block.
In the preparation process of taphole clay, the core function of accelerator additives for rapid-setting taphole clay is to facilitate rapid solidification after the mixing of the main and auxiliary materials in the slurry. This ensures that the mixture of the main and auxiliary slurries for rapid-setting taphole clay completes the solidification process within an extremely short time. At various stages of retarded and rapid setting, appropriate suspending agents and dispersants are crucial, as they ensure the uniform and stable suspension of solid materials in the liquid, promoting uniform and stable hydration reactions of the materials, thereby forming a homogeneous hydrated and hardened body. Specifically:
(1) Determination of initial setting time: After mixing the main and auxiliary slurries, allow them to stand still. When the container is tilted to a 45-degree angle, if the rapid-setting taphole clay no longer flows, it is recorded as the completion of initial setting.
(2) Measurement of final setting time: After mixing, pour a portion of the slurry into a test mold placed on a glass plate. Gently tamp and vibrate it several times with a small knife, then scrape off the excess slurry. After leveling the surface, quickly place the test mold and base plate on a Vicat apparatus. During testing, bring the bottom of the test needle with an annular attachment into contact with the surface of the slurry, tighten the screw for 1 to 2 s, and then quickly release it. When the distance the test needle sinks into the slurry is less than 0.5 millimeters, i.e., when the annular attachment cannot leave a mark on the surface of the slurry, it is recorded as the completion of final setting.
Test method
(1) Uniaxial compression test and splitting test: The instrument used is WDW microcomputer-controlled electro-hydraulic servo testing machine, supplemented by XTDIC three-dimensional whole strain deformation measurement and analysis system. The acquisition speed of the high-speed camera is 5000 frames/s. The loading rate is 0.01 kN/s through the pressure loading control mode, and the test loading and measurement system is shown in Fig. 2.
(2) XRD and XRF: The dried specimens were ground into powder form, and the test data were processed and analyzed using XRD analysis software. X-ray diffractometer (XRD, Bruker D8 Advance) was used with Cu Kα (λ = 1.5406), voltage 45 KV, current 40 mA, scanning range 5°–80°, step length 0.02°, scanning rate 2°/min. XRF analyzers are employed to determine the types and content ranges of elements.
(3) SEM: The samples of different curing times were observed by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS). The samples were observed by scanning electron microscopy (SEM, FEI Quanta 250) at an accelerating voltage of 15 kV and a working distance of 10 mm. The surface of the samples was gold-sprayed to enhance conductivity.
(4) Mercury injection test scheme: The pore structure parameters were determined by mercury injection method (MIP) to analyze the pore structure changes of taphole clay at different curing times. Mercury injection instrument is used to measure pore structure parameters of materials.
Experimental results and analysis
Mechanical strength
Figure 3 shows the stress-strain curves of the test blocks at different curing times. The key parameters, such as the magnitude of peak stress and the rate of ascent, are different. According to the Fig. 3, the stress-strain curve can be roughly divided into four stages: pore fracture compaction stage, elastic deformation to microelastic fracture stable development stage, plastic yield to unstable fracture development stage, and strain softening stage after fracture. After the peak compressive strength is reached, the curve is basically flat and slowly decreases within a certain range, and the internal structure of the sample is destroyed. The samples basically keep the whole shape, the cracks expand rapidly, cross and combine with each other to form a fracture surface. The compressive strength measured by the test blocks of different curing times is shown in Fig. 3. The strengths of the four curing times are 0.51 MPa, 0.83 MPa, 1.31 MPa and 1.58 MPa, respectively. The compressive strength from 0.5 h to 1 h increased at a faster rate with time, increasing by 0.32 MPa, with a growth rate of 62.75%. The compressive strength from 1 h to 6 h increased the most with time, increasing by 0.48 MPa, with a growth rate of 57.83%. The growth rate from 6 h to 24 h decreased, increasing by 0.27 MPa, with a growth rate of 20.61%. It can be seen from the figure that with the same other conditions, the compressive strength of the test block increases with the increase of curing time. It can be seen that the curing time has a great influence on the compressive strength of the taphole clay. The analysis indicates that in specimens with a shorter curing time, the internal moisture does not evaporate completely, and residual moisture remains in the pores, resulting in a lower degree of stress concentration during the pore compaction stage and a larger strain value corresponding to the peak stress. As the curing time increases, moisture migration and the hydration reaction of the cementitious materials refine the pore structure, hindering the propagation of microcracks and significantly increasing the peak stress of the material.
The change of the test force along with the displacement during the loading process of the splitting experiment is shown in Fig. 4. The shape of the failure curve is basically the same for different curing times. No visible crack was generated at the center of the specimen before the peak load was reached. When the peak load is reached, a clear crack appears and begins to expand rapidly. Subsequently, cracks permeate the whole specimen and the load drops sharply. As can be seen from the figure, there are obvious differences in the test forces of the test blocks at different curing times during failure, and the peak loads at the four curing times (0.5, 1, 6 and 24 h) are 0.25, 0.45, 1.02 and 1.25 kN respectively. The peak load increases with the curing time. It can be seen that with the increase of curing time, the tensile strength of the taphole clay also increases significantly. The measured tensile strength of the test blocks at different curing times is shown in Fig. 4. The tensile strength of the four curing times are 0.033 MPa, 0.059 MPa, 0.13 MPa and 0.16 MPa respectively. It can be seen from the figure that the tensile strength before 6 h increases rapidly with time, and the growth rate from 1 h to 6 h is 1.48%. 6 h to 24 h showed a slower growth rate of 0.17%. It can be concluded that the taphole clay is more sensitive to curing time. In the curing time period from 1 h to 6 h, the strength increases most obviously. The rapid increase in early-stage strength primarily stems from the rapid hydration of cementitious materials, which generates products such as C-S-H gel and ettringite that preferentially fill large pores, forming a dense skeleton. The growth rate of strength slows down in the middle and later stages because the hydration reaction enters the diffusion-controlled phase, with limited room for reducing the overall porosity.
XRD analysis of taphole clay at different curing times
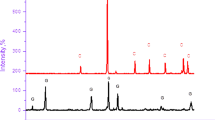
Figure 5 shows the XRD analysis results of 0.5 h and 6 h taphole clay samples respectively. The figure shows the characteristic peaks of taphole clay at different curing times. After adequate hydration, the main products are calcium carbonate and ettringite, followed by calcium clinosilicate, quartz and hatrurite. Ettringite, as the main hydration product of sulfoaluminate cement, has a significant contribution to the strength of taphole clay. Under the same temperature and water-cement ratio, the contents of calcium carbonate and ettringite increased with the increase of curing time, and the peak diffraction value increased significantly. This is consistent with the description in the rapid-setting mechanism, wherein a amount of C-S-H is generated, develops, and forms a skeletal structure during the accelerated hydration period32. Therefore, the content of hydration products produced by different taphole clay curing times is also different. This shows that the curing time can affect the hydration of cement, and the longer the curing time, the more hydration products. At the same time, it also explains that with the increase of curing time, the strength of taphole clay is better.
The diffraction pattern of the sample to be measured is compared with that of the standard sample. According to the intensity and position of the diffraction peak, the content of each phase in the sample to be measured can be calculated. By integrating the intensity and position of XRD diffraction peaks and using XRF detection, the content of each phase in the sample can be obtained. Tables 2 and 3 detail the composition and content of taphole clay at curing times 0.5 h and 6 h. The contents of calcium carbonate, ettringite, quartz, calcium clinosilicate and hatrurite at 0.5 h curing time are 50.4%, 23.4%, 3.1%, 18.9% and 4.2% respectively. The contents of various substances in the 6 h taphole clay are 39.5%, 40.0%, 1.0%, 17.9% and 1.6% respectively. With the curing time increasing from 0.5 h to 6 h, the content of ettringite increased by 16.6% and the content of calcium carbonate decreased by 10.9%. The content of quartz, calcium clinosilicate and hatrurite decreased, but not obviously. Changes in calcium carbonate content reflect its consumption or morphological transformation during the hydration process, indirectly supporting the regulatory role of calcium carbonate on the hydration process as described in the mechanism. It can be seen that the curing time has a great influence on the content of calcium carbonate and ettringite in the hydrated products of the taphole clay, so the curing time is also an important factor affecting the properties of the taphole clay.
Microscopic mechanism analysis of taphole clay at different curing times
SEM of taphole clay samples at different curing times is shown in Fig. 6. The hydration products of the taphole clay cured to 0.5 h are mainly fibrous and honeycomb hydrated calcium silicate and a small amount of needle-like ettringite. Its internal structure is fluffy and rich in holes, which is not conducive to the strength performance of the taphole clay. In the figure, the red, yellow, and orange circles represent honeycomb-like, fibrous, and needle-rod-like structures, respectively. This further confirms the existence and role of high-density C-S-H gel. With the increase of curing curing time, a large amount of ettringite is interspersed in the gap between the hydrated calcium silicate and the particle. The internal pore network structure of the taphole clay is refined, segmented and filled, and the density of the taphole clay is improved. At the curing time of 6 h, a large amount of ettringite is attached to the surface of calcium silicate hydrate in the taphole clay, which strengthens the connection between calcium silicate hydrate and promotes the formation of dense spatial structure. Combined with XRD results, the hydrated calcium silicate produced by taphole clay hydration reaction is mostly high-density C-S-H gel. With the increase of curing time, SEM shows more ettringite, forming denser microscopic results and better strength properties. This further confirms the existence and role of high-density C-S-H gel. This phenomenon is highly consistent with the description in the rapid-setting mechanism, where ettringite and calcium silicate hydrate work synergistically to fill pores and enhance density.
In order to further study the mechanism of increasing compressive strength, flexural strength and splitting tensile strength of taphole clay with curing time. SEM-EDS analysis was performed on sample microblocks of 0.5 h and 6 h curing times, as shown in Figs. 7 and 8. It can be seen from the figure that elements can be evenly distributed in each area of the scan in the element distribution diagram, and no significant difference was found in the scanning results of the two curing times. The highest chemical elements are O and Ca elements, followed by Al, S and C, and Si elements are the least. Therefore, according to XRD, the main components of the taphole clay are calcium silicate (Ca(CO3)) and ettringite (Ca6Al2(SO4)3(OH)12(H2O)26), so the O and Ca elements are highest. At 0.5 h curing time, the contents of O and Ca elements are 51.30% and 27.72% respectively. At 6 h curing time, the contents of O and Ca elements are 47.27% and 28.75% respectively. The content of O element decreased by 17.6%, and the content of Ca element increased by 3.6%. It can be seen that with the growth of curing time, the content of O elements decreases and the content of Ca elements increases, which is mutually confirmed with the results of XRD. The results of scanning electron microscopy (SEM) microscopic analysis are highly consistent with the description of the rapid-setting mechanism. The synergistic effect of ettringite and calcium silicate hydrate, the formation of high-density C-S-H gel, and changes in element distribution and content collectively explain the mechanism by which the strength properties of taphole clay increase with curing time33. SEM-EDS analysis further confirms the distribution and content changes of major elements during the hydration process, providing an important basis for understanding the hydration mechanism of taphole clay and optimizing its performance.
Mercury injection analysis of taphole clay
Mercury injection test was carried out on taphole clay samples of different curing times (0.5, 1, 6, 24 h), and the experimental data were analyzed and processed to obtain the structural characteristics of taphole clay.
Porosity is defined as the percentage of the total apparent volume of the void of the material, calculated as follows.
In the formula: Va is the actual volume of mercury injected into the sample, which can also be called the void volume, ml; Vb is the total volume of the sample, ml.
The porosity of the taphole clay samples under four curing times are shown in Fig. 9. It can be seen from the figure that the porosity of the taphole clay decreases with the increase of curing time. The porosity of the taphole clay at 0.5 h is 27.39%. When the curing time is 1 h, the porosity decreases by 8.18–25.15%. When the curing time reaches 6 h and 24 h, the porosity relative to the curing time of 0.5 h decreases by 16.79% and 20.74% respectively, to 22.79% and 21.71%. The strength of the taphole clay increases gradually with the increase of curing time, mainly because the chemical composition of the raw material and the binder gradually react to form a more stable structure, so that the internal organization of the taphole clay is more compact. At the same time, the particle size composition and particle grading of the raw material enable the fine particles to fill the void between the coarse particles, further reducing the porosity. In addition, the evaporation of water in the raw material also promotes the compact structure of the taphole clay and reduces the porosity. In the initial hardening period of the taphole clay, especially in the first 6 h, the rapid reaction of the binder to the feedstock, the rapid evaporation of water, and the tight arrangement and filling of particles together lead to a significant reduction in porosity during this stage.
The variation of cumulative pore area with pore diameter per unit mass of taphole clay measured by intrusive mercury test is shown in Fig. 10. It can be seen from the figure that the cumulative pore area changes with the pore size at different curing times in a basically consistent trend, showing that the large pore area is small. With the increasing of mercury inlet pressure, mercury gradually invades smaller diameter pores. The pore area mainly depends on the pore diameter of 5 ~ 500 nm. The peak point of the curve represents the specific surface area of the porous material, which is defined as the internal surface area of the pores in the porous material per unit mass (volume). When the pore size is 10 nm, the cumulative pore areas at the four curing times are 24.98, 23.61, 23.21, and 21.53 m2/g, respectively. The specific surface area of the taphole clay at curing time 0.5 h is the largest, and that of 24 h curing time taphole clay is the smallest.
The total pore volume is defined as the total volume of pores per unit mass of the material, that is, the total amount of mercury injected. The total pore volume and specific surface area of taphole clay at different curing times calculated by mercury injection are shown in Fig. 11. The results show that the total pore volume in the taphole clay increases with the increase of curing time. Since the mercury injection method assumes that the pores in the material are all connected cylindrical holes, the pores in the actual material are irregular in shape and there are closed holes, resulting in irregular variation of the specific surface area. The total pore volume and specific surface area of the 0.5 h taphole clay are 1.827 ml/g and 29.08 m2/g. The total pore volume and specific surface area of 1 h are 1.7579 ml/g and 24.568 m2/g respectively, and the total pore volume and specific surface area are reduced by 3.78% and 15.52% respectively. The total pore volume and specific surface area at curing time 6 h are 1.5586 ml/g and 25.37 m2/g. At 24 h curing time, the total pore volume and specific surface area are 1.4372 ml/g and 26.03 m2/g. The total pore volume continues to decrease, while the specific surface area increases slightly. With the hydration reaction of cement, more cement gel fills the pores. This process not only increases the gel-space ratio, but also results in the decrease of the total pore volume of the material. With the increase of curing time and the deepening of cement hydration, the compactness of the taphole clay increases gradually. The increase in compactness means that the internal pores of the material are reduced, that is, the total pore volume is reduced.
The pore size distribution and the average pore size of quick-setting taphole clay at different curing times is shown in Table 4. With the extension of curing time, the average pore size of the material gradually decreased. Specifically, the average pore sizes at curing times of 0.5, 1, 6, and 24 h were 576.2 nm, 549.8 nm, 510.3 nm, and 481.5 nm, respectively, showing an overall reduction of approximately 16.4%. The cumulative pore size distribution curve of the quick-setting taphole clay at different curing times is shown in Fig. 12. In the range of pore size from 1000 nm to 50,000 nm, the curve rose slowly, and the accumulated mercury content in the taphole clay increased with the increase of curing time. The variation trend of accumulated mercury content in taphole clay at different curing times is similar. In the range of pore size from 5 nm to 1000 nm, the slope of the curve increases rapidly, and it can be seen that the pore size of the taphole clay is concentrated in the range of 5 nm to 1000 nm. The cumulative mercury content of taphole clay at 0.5 h and 1 h increased significantly, while the cumulative mercury content of taphole clay at 6 h and 24 h increased relatively little.The pore size of taphole clay is mainly concentrated between 5 nm and 1000 nm. With the increase of curing time, the quantities of gel pores (< 10 nm), transition pores (10–100 nm), capillary pores (100–1000 nm), and macropores (> 1000 nm) in the taphole clay have all decreased, and the change amplitude was more obvious when the curing time increased from 1 h to 6 h. This is consistent with the growth of the strength of the quick-setting taphole clay from 1 h to 6 h, which can be mutually confirmed.
In the cumulative pore diameter distribution curve, the pore diameter corresponding to the slope mutation point is defined as the critical pore diameter, that is, the maximum pore diameter corresponding to the significant increase in the volume of mercury injected. The cement-based material contains pores of different sizes, and the larger pores are connected by smaller pores. The critical aperture is the maximum aperture of each hole connecting the larger pores, which reflects the connectivity of the pores and the curvature of the permeability path23. The critical pore sizes of 0.5, 1, 6 and 24 h taphole clay are 2916.21, 2466.93, 1597.29 and 1311.12 nm, respectively, which gradually decreases with the curing time. In other words, the increase of curing time reduces the connectivity of the pore structure of taphole clay. With the increase of curing time, the hydration reaction of cement inside the quick-setting taphole clay continues, resulting in the change of pore structure. Larger pores may gradually decrease or close due to the filling of hydration products, while smaller pores may become more complex and tortuous due to the growth of hydration products. Therefore, although the total volume of the pores may be reduced, the connectivity between the pores may also be reduced due to the reduction of the pore size and the zigzag of the path. In summary, the critical pore diameter of quick-setting taphole clay decreases with the increase of curing time, which reflects the decrease of pore structure connectivity and the increase of permeability path curvature. This is caused by the continuous hydration reaction of cement and the gradual change of pore structure.
With dV/dlgr as the vertical axis and aperture as the horizontal axis, the differential aperture distribution curves of taphole clay under different curing time were obtained, as shown in Fig. 13. The curve reflects the pore distribution in different pore sizes, and the area surrounded by the curve is the total pore volume of the sample. In the differential aperture distribution curve, the aperture corresponding to the peak value in different aperture ranges is defined as the most feasible aperture in the corresponding aperture range. Its physical meaning is the largest aperture within a certain aperture distribution, namely hole has the highest probability aperture. It can be seen from the analysis of the figure that the maximum availability and pore size of the taphole clay at the curing time of 0.5, 1, 6 and 24 h are 1053.93, 1053.93, 830.37 and 830.37 nm, respectively, distributed in the range of 500 nm to 1500 nm. The most available apertures of 0.5 and 1 h, 6 and 24 h are consistent, respectively. It shows that the number of pores in the taphole clay is more because the curing time is shorter.
Conclusion
This study focused on the performance evolution of quick-setting taphole clay materials under different curing times, taking curing time as a key variable to systematically study its influence on mechanical properties and microstructure. Through XRD, SEM-EDS and other means, this study revealed the intrinsic relationship between mechanical strength growth and material crystallization phase transition and pore structure change, providing theoretical support for the rational selection of plugging materials and construction sequence optimization.
(1) With the increase of curing time, the mechanical strength of taphole clay showed a significant increase trend. The curing time has a great influence on the mechanical properties of taphole clay. The mechanical strength before 6 h increases rapidly with time. The maximum growth rate was observed between 1 h and 6 h, while the growth rate was slower between 6 h and 24 h. About 24 h, the strength is basically stable.
(2) The main hydration products of taphole clay are calcium carbonate and ettringite, and their contents increase with the increase of curing time. This indicates that the curing time promotes the hydration process of the taphole clay, which in turn improves its mechanical strength. With the increase of curing time, the content of ettringite increased significantly, and the content of calcium carbonate decreased.
(3) The material at 0.5 h curing time has fluffy internal structure and abundant holes, which is not conducive to the strength performance of the taphole clay. With the increase of curing curing time, the density of taphole clay is improved. A large amount of ettringite is attached to the surface of hydrated calcium silicate, which promotes the formation of dense spatial structures. With the increase of curing time, the content of O element decreases and the content of Ca element increases, which is consistent with the results of XRD.
(4) The porosity of the taphole clay decreases with increasing curing time, mainly because the chemical composition of the feedstock and the binder gradually react to form a more stable structure. In the initial hardening period, the porosity decreased significantly. The average pore size and specific surface area decreased with curing time, while the total pore volume showed a complicated trend. However, it eventually decreased due to the increase of material density caused by cement hydration reaction, which explained the change trend of strength. The critical pore size increases with curing time, which decreases the connectivity of pore structure. Most of the pore sizes can be in the range of 500 nm ~ 1500 nm, and the number of short pores in the curing time is more.
(5) The current study conclusions still have the following limitations that can be further explored in subsequent research. The study did not clarify the relationship between the formation pattern of hydration products in taphole clay and curing time. Combining mercury intrusion porosimetry with nuclear magnetic resonance analysis to study pore structure evolution could establish a quantitative relationship between pore parameters and hydration product formation. Additionally, the relationship between the formation rate of hydration products and curing time under different temperature and humidity conditions should be supplemented.
Data availability
Some or all data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Li, Y., Shi, X., Liu, B. & Zhao, J. Experimental esearch on easonable length of water stemming. Blast 32 (02), 11–16 (2015).
Leng, Z. et al. Research progress in theory and technology of energy regulation for rock drilling and blasting. Met. Mine. 05, 64–76. https://doi.org/10.19614/j.cnki.jsks.202305006 (2023).
Wang, C. & Han, L. Optimization study on charge structure for blasting in small cross-section hard rock roadways of metal mines. Gold 45 (10), 40–46 (2024).
Tang, W. et al. Progress in research on borehole plugging and automation operations. Min. Technol. 24 (06), 203–210 (2024).
Wang, Y. & Hu, Y. The application research of tamping plug stemming technology in Sanxin gold copper mine. World Nonferrous Met. 18, 214–216 (2023).
Li, P. et al. Research on the flow characteristics of blasthole stemming slurry in open-pit mining. Front. Earth Sci. 12, 1430046 (2024).
Wang, X. & Luo, Y. Research on the influence of binder and asphalt on the performance of anhydrous stemming. Jiangxi Metall. 43 (01), 56–60 (2023).
Zou, C., Guan, Y. & Kang, Y. Application of high water quick setting material in filling mining in Ningdong mining area. Copp. Eng. 03, 28–31 (2022).
Yi, Q. Study on deformation characteristics of surrounding rock in loess cemented fill mining under slope terrain. Hunan Univ. Sci. Technol (2023).
Qiu, J., Yan, Z., Yang, W., Liu, X. & Wu, P. Research progress on dealkalization and activation of red mud and its application in cementitious materials. Appl. Chem. Ind. 53 (06), 1421–1426 (2024).
Uttarwar, M. D. & Devendar, M. Investigation into the effect of stemming on blast performance in underground excavations-a model study. Helix 10 (01), 43–50 (2020).
Qiu, X., Shi, X., Zhang, S., Liu, B. & Zhou, J. Experimental study on the blasting performance of Water-Soil composite stemming in underground mines. Adv. Mater. Sci. Eng. 1, 1–11. https://doi.org/10.1155/2018/3523509 (2018).
Shi, C. et al. Water-Sealed blasting control measures of the metro station undercrossing existing structures in Ultra-Close distances: A case study. Front. Earth Sci. 10, 1–13. https://doi.org/10.3389/feart.2022.848913 (2022).
Wang, F. Research on new material replacement mud sealing technology. Coal Mine Mod. 2, 115–116 (2020).
Jin, L. et al. Study progress and prospect on prevention and control technology of blasting dust and poison in metal mine Stope. Metal Mine. 01, 120–134 (2021).
Zhu, W. et al. Influence of alkaline accelerators on performance of backfill grouting in shield engineering and its Micro-mechanism. Mater. Rep. 38 (19), 44–50 (2024).
Wang, L. et al. Engineering performance and mechanism of Alkali-Activated ground granulated blast furnace Slag-Zeolite powder grouting materials. Appl. Sci. 15 (6), 3345 (2025).
Shi, S., Liu, C., Wu, H. & Chen, K. Study on physical and mechanical properties of modified high water filling material with fly Ash and calcium carbide slag. Mater. Rep. 35 (07), 7027–7032 (2021).
Tu, B. et al. Differential analysis of properties of cement based and mining alkali activated cementitious materials. Met. Mine. 10, 48–56 (2022).
Tang, S., Di, M. & Zhang, H. New sealing materials and packing effect of deep hole pre-splitting blasting in coal seam roofs. Coal Eng. 56 (06), 152–157 (2024).
Cao, X., Wang, Y., Liu, X., Cao, Z. & Wu, B. Performance test and proportioning optimization of loess-based grouting materials in coal mining subsidence area of Northern Shaanxi. Coal Geol. Explor. 48 (03), 8–16 (2020).
Feng, G. Research on the superhigh-water packing material and filling mining technology and their application. China Univ. Min. Technol (2009).
Zhang, Z., Liu, C., Xu, Z., Wu, H. & Guo, B. Experimental study on the effect of water temperature on the mechanical properties of high-water materials. J. Sichuan Univ. Sci. Eng. (Nat Sci. Ed). 32 (06), 60–66 (2019).
Liu, D. Study on hydrating and hardening mechanisms of high-water rapid-setting materia. China Univ. Min. Technol (2015).
Wang, Z. et al. Strength mechanism and electrochemical characterization of cement-bonded calcareous sand in different water environments. Constr. Build. Mater. 389, 131751 (2023).
Zhang, Y., Deng, Z. & Jiu, Y. Effects of accelerators and flocculants on the properties of cement-based grout [C]//Chinese Hydraulic Engineering Society, Xi’an University of Technology. Proc. 2024 China Water Conserv. Acad. Conf. (Vol.6) : 391–398. (2024) (2024). https://doi.org/10.26914/c.cnkihy.2024.070211
Gu, J., Lyu, H., Yang, J. & Zeng, C. Effects of cement content and curing period on geotechnical properties of cement-treated calcareous sands. Transp. Geotech. 33, 100732 (2022).
Wan, Z. H., Dai, G. L., Gong, W. M. & Gao, L. C. Experimental study on micro-erosion mechanism of cement stabilized calcareous sand under seawater environment. Rock. Soil. Mech. 42, 1871–1882 (2021).
Luo, J., Zhou, A. & Li, N. Mechanical properties and microscopic characterization of cement stabilized calcareous sand modified by nano SiO2. Case Stud. Constr. Mater. 17, e01636 (2022).
Neto, J. D. S. A., Angeles, G. & Kirchheim, A. P. Effects of sulfates on the hydration of Portland cement-A review. Constr. Build. Mater. 279, 122428 (2021).
Zhang, J. et al. Influence of alkaline carbonates on the hydration characteristics of β-C2S. Constr. Build. Mater. 296, 123661 (2021).
Fang, K. Structure, activity and reaction mechanism of coal gasification slag and its performance as a composite cementitious material. China University of Mining & Technology, Beijing. (2023). https://doi.org/10.27624/d.cnki.gzkbu.2023.000120
Yuan, B. et al. Optimized reinforcement of granite residual soil using a cement and alkaline solution: A coupling effect. J. Rock. Mech. Geotech. Eng. 17 (1), 509–523 (2025).
Zhang, B. et al. Harnessing iron tailings as supplementary cementitious materials in limestone calcined clay cement (LC3): an innovative approach towards sustainable construction. Constr. Build. Mater. 453, 139111 (2024).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52274107) and Interdisciplinary Research Project for Young Teachers of USTB (FRF-IDRY-GD21-001).
Author information
Authors and Affiliations
Contributions
Xiao Wang: Formal analysis, Investigation, Methodology, Validation, Visualization, Data Curation, Writing original draft, Review & editing. Houyou Zhou: Conceptualization, Supervision, Methodology, Funding acquisition, Project administration. Wenbo Zhao: Investigation, SupervisionQingwen Li: Conceptualization, Investigation, Methodology, Validation, Review &editing.Ya Yin: Writing original draft, Investigation, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Zhou, H., Zhao, Wb. et al. The influence of curing time on mechanical properties and microstructure of taphole clay. Sci Rep 15, 24368 (2025). https://doi.org/10.1038/s41598-025-10663-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10663-1