Abstract
The purpose of this study is to evaluate the effects of substrate surface roughness (35 μm and 40 μm) and preheat temperature (50 °C, 100 °C, 150 °C) on the properties of the FeCrBMnSi coating layer applied to 304 stainless steels using the TWAS (Twin Wire Arc Spray) method. Surface preparation involved sandblasting and preheat treatment, followed by coating application with TWAS, and subsequent characterization using pull-off bonding, hardness, corrosion rate, Light Optical Microscope (LOM), and scanning electron microscopy (SEM) test. The current study’s findings indicate that increasing preheat temperature and surface roughness consistently reduces the percentage of porosity, unmelted material, and coating layer thickness. This enhances the hardness and adhesive strength of the coating layers. The hardness of the coating layer obtained in the present study was improved by 360–439% compared to the uncoated material. The best specimen in this study was found on a substrate with a surface roughness of 40 μm and performed a preheating treatment at a temperature of 150 °C. The thickness of the coating layer for this specimen was 150.58 × 10⁻³ mm, with a porosity and unmelted materials of 7.233%, a hardness of 1114.6 HV, an adhesive strength of 20.29 MPa, and a corrosion rate of 1.0640 × 10⁻² mmpy. However, the corrosion resistance of the coated specimens remains lower than that of the uncoated 304 stainless steel.
Similar content being viewed by others
Introduction
Pumps are essential in numerous industrial applications, especially in flood control and prevention systems. In these systems, pumps are used to redirect rain or excess water that may result in flooding, thereby preventing inundation and reducing the potential negative consequences1,2,3,4. A pump’s impeller is the component responsible for transforming the mechanical energy generated by the motor or engine into the kinetic energy contained within the fluid. The impeller, while rotating, produces a centrifugal force that expels the liquid from the pump and channels it into the pipeline5,6,7,8. The ideal performance of a pump is significantly influenced by the durability of its impeller material, particularly concerning cavitation, corrosion and wear, which often arise from contact with water and the abrasive particles it contains.
Impellers constructed from materials that cannot endure these conditions may diminish pump efficiency, elevate maintenance expenses, and reduce operational lifespan9,10,11,12,13.
Wear in a pump impeller, typically caused by cavitation, fatigue abrasive particles or corrosion, can considerably diminish pump performance, elevate vibration, and, in severe instances, result in pump failure9,13. The presence of sediment particles during the pumping process interferes with the internal fluid dynamics of the pump, as these particles engage with the impeller and casing surfaces. The mechanical stress from these interactions hastens the deterioration of both the impeller and the pump casing. The continuous deterioration of surface integrity leads to heightened hydraulic losses, diminishing the pump’s operational efficiency. As wear advances, the efficiency of transferring energy from the motor to the fluid decreases, resulting in elevated energy consumption and heightened operational expenses. Furthermore, the wear-induced alterations in the geometry of the impeller and casing cause imbalances, potentially resulting in elevated vibration levels. These vibrations intensify the wear process and lead to additional mechanical damage, diminishing the pump’s durability and elevating maintenance demands. Over time, accumulated wear and tear considerably reduces the service life of essential components, especially those subjected to overflow circumstances, consequently impairing the long-term performance and cost-effectiveness of the system14,15,16,17. Cavitation is a serious problem that can result in significant damage to the impeller. Cavitation starts at the blade inlet near the tongue and produces unstable vibrations18. The vapor bubbles that form can cause erosion and damage to the impeller surface, reducing overall pump performance19. Meanwhile, corrosion occurs when the impeller is exposed to corrosive chemicals or water, causing a decrease in efficiency and increasing the risk of leakage or complete failure20,21,22.
An efficient method for enhancing components’ corrosion, wear, erosion, and cavitation resistance is the application of thermally sprayed coatings23,24,25,26. “Thermal spray” denotes several procedures that utilize chemical or electrical energy to melt and push material particles, subsequently depositing them onto a substrate’s surface. The quality of coatings generated by thermal spray methods is intricately connected to the characteristics of the process and the settings employed during application. This technique facilitates the restoration of components without substantially modifying the steel’s microstructure or the part’s geometry while providing the anticipated protective advantages of the new surface from cavitation and erosion23,24,25,26. Thermally sprayed coatings with wear resistance offer a cost-efficient method for reducing cavitation erosion by utilizing the substrate’s structural integrity and improving tribomechanical properties at the surface.
This technology provides an environmentally sustainable solution, as worn components can be renewed by reapplying the coating, hence obviating the necessity for total part replacement27.
Various coating techniques are used extensively in industries, including Twin Wire Arc Spraying (TWAS), high-velocity oxy-fuel (HVOF), cold spray, and atmospheric plasma spraying (APS)28,29,30,31. Among these procedures, HVOF is distinguished by its exceptional resistance to oxidation, abrasion, and corrosion28. At the same time, it necessitates substantial equipment and operational expenses and is hindered by its lack of portability due to the requirement for a controlled environment30. Conversely, cold spray enables coating without elevated temperatures, minimizing the risk of oxidation in heat-sensitive materials29. This technique may result in plastic deformation of the coating layer, diminishing its ductility and strength29,30. On the other hand, APS can spray a range of materials with high deposition rates; nevertheless, it entails more expenses and produces coatings with increased oxide content and porosity relative to HVOF30.
Lencová et al.31 assert that there is no substantial difference in the corrosion protection efficacy of TWAS and HVOF coatings. Their analysis demonstrates that the material expenses for both procedures are essentially identical. Nonetheless, the inefficiency of HVOF regarding material utilization, spraying duration, and energy expenditure leads to substantially elevated overall costs compared to TWAS. Furthermore, on-site portable coating methods, such as TWAS technology, now present feasible alternatives to conventional HVOF spraying, which generally necessitates off-site application in a specialized chamber.
Twin Wire Arc Spray (TWAS) is a thermal spray technology renowned for its economic efficiency32,33. It is widely utilized throughout multiple sectors, including energy, aerospace, automotive, maritime, medical device, and general industry33,34. TWAS is mainly used to offer sacrificial exterior corrosion protection and improve the wear resistance of parts. Moreover, it is esteemed as a surface coating technique owing to its elevated deposition rate, enabling substantial coatings to be created. TWAS has many advantages, including operational simplicity, equipment mobility, and cheap processing costs, rendering it an efficient solution for various industrial applications35,36,37,38.
The Twin Wire Arc Spray (TWAS) procedure involves the placement of two metal wires within a spray nozzle, where a voltage differential is supplied, generating an electric arc that melts the wire ends. The molten metal is subsequently atomized by compressed air and applied to the substrate’s surface. A protective coating is formed on the substrate as the molten metal rapidly solidifies following impact37,39. The TWAS technology has been widely used in wear prevention to improve surface tribological and mechanical qualities38.
The TWAS technique has effectively deposited various coating materials, such as Inconel, nickel alloys, aluminium alloys, aluminium, and steel, onto diverse substrate metals39. In general, the final properties of the coating layer produced by the TWAS method are significantly influenced by the parameter settings, including arc voltage and current, nozzle shape and geometry, standoff distance (SOD), traversal speed, and atomizing gas pressure (compressed air pressure)8,33,39,40,41. Nonetheless, the adhesion strength of the coating layer generated by the TWAS technique has consistently posed difficulties35. This constraint can be mitigated by several ways to enhance the adhesion between the coating and the substrate. A key method involves employing grit or sand blasting on the substrate, resulting in a textured surface that improves mechanical adhesion35,42,43. Alongside surface preparation, thermal treatments such as preheating and post-heating enhance adhesion by mitigating thermal stresses and facilitating superior bonding between the coating and substrate35,36,42,44. Moreover, selecting a suitable bonding layer can augment adhesion strength, establishing stronger interfaces between the substrate and the coating35,42,45.
Related research has examined the deposition of numerous substances and alloys onto substrates using the TWAS method. However, the author’s review of the literature shows that there have been no studies that show how surface roughness and preheating of the substrate affect the properties of NiAl and FeCrBMnSi coating layers made using the TWAS method on 304 stainless steels for pump impellers. Surface preparation is very important in the coating process to improve the adhesion strength between the protective layer and the substrate46. Methods such as sandblasting and substrate preheat treatment help optimize surface texture for better mechanical adhesion47,48. Therefore, this study examines the effects of substrate surface roughness and preheat temperature on the properties of FeCrBMnSi coating layers produced using the Twin Wire Arc Spray (TWAS) method. The results of this study are expected to contribute to improving wear resistance, preventing cavitation in pump impellers, and extending the service life of these components.
Materials and methods
Material
The research utilized a 304 stainless steel plate as the substrate for the pump impeller. Recognized for its exceptional mechanical properties, weldability, ductility, thermal stability, and corrosion resistance, 304 stainless steel is widely utilized in several industrial applications49,50.
The stainless-steel plate (1000 mm x 100 mm x 10 mm) was procured from Tira Austenite Ltd. in Semarang, Indonesia. The substrate material used in the research exhibits a high modulus of elasticity (193 GPa), high thermal conductivity (16.2 W/mK), a coefficient of thermal expansion of 17.2 × 10−⁶/K, a density of 8,000 kg/m³, and a compressive strength of 210 MPa43,44. The NiAl (TAFA 75B) wire served as the bond coat in the coating process, whereas the FeCrBMnSi (TAFA 95MXC) wire was utilized as the top coat. The TAFA 75B and 95MXC wire products, each with a diameter of 1.6 mm, have melting points of 1450 °C and 1204 °C, respectively. Both wire products were sourced from PT Cipta Agung, located in Surabaya, Indonesia. Table 1 presents the chemical compositions of the substrate, bond coat, and top coat materials.
Specimen fabrication and testing
Figure 1 depicts the experimental setup utilized in this study. 304 stainless steel was processed with an Everising S-12 H machine, supplied by Bhinneka Bajanas Ltd. in Semarang, Indonesia, to create substrates measuring 100 mm in length, 100 mm in width, and 10 mm in thickness. The abrasive material utilized in the sandblasting procedure was garnet sand with a particle size of 40–60 mesh, as illustrated in Fig. 2.
The sandblasting process utilized a Norblast Sandblasting machine, model NOB35CE, manufactured by Norexco SA, Ville-la Grande, France. The sandblasting procedure was conducted at a pressure of 8 bar and a stand-off distance of 150 mm. The procedure was regulated to attain a surface roughness of 35 to 40 μm on the substrate material. The substrate’s surface roughness was assessed with an Elcometer 123 Surface Profile Gauge, obtained from INDOMULTIMETER, Banten, Indonesia. Measurements of surface roughness were conducted in accordance with the ISO 8503 standard.
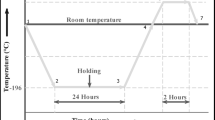
After achieving the required surface roughness, we applied a preheating treatment at temperature variations of 50 °C, 100 °C, and 150 °C (Fig. 3). The preheating procedure utilized a small portable gas torch, while the substrate temperature was assessed with a non-contact infrared thermometer. The substrate material was warmed prior to the coating procedure to minimize surface moisture and improve the substrate’s readiness for the protective coating layer. The adhesion of the protective coating layer was enhanced by preheating treatment, which minimized thermal stress and increased the bond between the substrate and the coating material51,52,53,54,55.
A Miller Delta Weld 602 machine (Miller Electric, Wisconsin, United States of America) applied the coating, with 75B wire as the bond coat and 95MXC wire as the top coat. The machine weighs 174 kg and operates on a three-phase power supply, providing an output of 450 A at 38 V with a 100% duty cycle and 590 A at a 60% duty cycle56. For this investigation, the machine was set up with a stand-off distance of 100 mm, a primary gas pressure of 3 bar, a voltage of 28 V, and a current of 200 A. The parameters were continuously maintained during the bond and topcoat materials deposition.
Several tests were conducted to assess the impact of surface roughness and preheating temperature on the properties of coating layers. These tests included pull-off bonding, hardness, corrosion rate, Light Optical Microscope (LOM), and scanning electron microscopy (SEM). This study also carried out hardness and corrosion rate tests on the substrate material before the coating process. Scanning Electron Microscope (SEM) analysis was conducted utilizing the Phenom ProX Desktop SEM (Thermo Fisher Scientific, Waltham, USA) to examine the surface morphology of the specimens. The SEM pictures were analyzed using ImageJ software to ascertain the proportion of porosity and unmelted materials. This research utilized the grayscale image and histogram within the grayscale range of 0–408,43,57. Furthermore, Light Optical Microscope (LOM) analysis was conducted utilizing the Olympus U-MSSP4 model (Evident, Tokyo, Japan) to acquire cross-sectional images of the coating layer. The specimen preparation adhered to ASTM E3-2007 requirements and the resultant images were processed using ImageJ software to quantify the coating thickness.
The coating’s microhardness was assessed via the Vickers method with a Mitutoyo HM-21 tester (Mitutoyo, Kanagawa, Japan), following the ASTM E384 standard. The test was performed with a 0.5 kgf force applied for 10 s. The adhesion strength between the coating layers and substrate materials was evaluated using a pull-off bonding test with the Positest AT-M Adhesion Tester (DeFelsko, New York, USA), following ASTM D4541-02, with a 20 mm diameter adhesive dolly. Finally, corrosion rate testing was performed utilizing the SUGA Salt Spray Test Instrument (Nihon Denkei Co., Ltd., Tokyo, Japan) following ASTM G102. This study conducted tests for thickness, porosity, hardness, adhesion, and corrosion rate with three repetitions, followed by calculations to ascertain the average values.
Results and discussions
Thickness of the coating layer
Figure 4 presents the results of the LOM testing to examine the cross-sectional area of the coating material and substrate. The coating process utilized in this study yields satisfactory results, with each procedure phase executed effectively. The substrate, bond coat, and top coat are three distinct layers visible in the LOM results, supporting this finding. Each layer demonstrates properties aligned with the specified coating goals. These three layers indicate that the coating process was conducted systematically, with strict compliance to the necessary stages, thus guaranteeing that the quality of the resultant layers aligns with the established standards58.
Figure 5 presents detailed observations to enhance understanding of each specimen’s structural characteristics of the substrate, bond coat, and top coat. This figure thoroughly analyses the layers, highlighting the uniformity and integrity of the coating applied to the specimens.
The TWAS coating structure on specimens with a substrate surface roughness of 35 μm, subjected to preheat treatments at 50 °C, 100 °C, and 150 °C, is illustrated in Fig. 5a and b, and 5c, respectively. Furthermore, Fig. 5d and e, and 5f illustrate the TWAS coating’s structure on specimens with a substrate surface roughness of 40 μm and preheat treatments at 50 °C, 100 °C, and 150 °C.
The thickness of the coating layer generated in this investigation was demonstrated to be affected by the substrate’s surface roughness and the preheat treatment temperature, as depicted in Fig. 6. The thickness of the coating layer in this investigation was determined using calculations utilizing ImageJ software based on pictures acquired from the LOM testing results. In this study, the thickness of the coating layer was tested three repetitions, and the average value was then calculated.
This study’s results indicate that both the substrate’s surface roughness and the preheat treatment temperature significantly influence the thickness of the final coating layer. As the surface roughness of the substrate rises, the thickness of the coating layer reduces. Moreover, elevated preheat temperatures result in a reduction in coating thickness. Specimens with the thinnest coating layer were found at a preheat temperature of 150 °C and a substrate surface roughness of 40 μm. This suggests that elevated surface roughness and high preheat temperatures result in a reduction of coating thickness. Conversely, samples exhibiting the most substantial coating layer were noted at a substrate surface roughness of 35 μm and a preheat temperature of 50 °C.
Under these circumstances, diminished surface roughness and low preheating temperatures facilitate the development of thicker layers.
Research indicates that substrate surface roughness significantly affects the morphology and properties of the resultant coating layer. Bir et al.59 demonstrated that the hydroxyapatite layer thickness on a rough substrate is less than on a smooth surface. This indicates that surface irregularities may impede the formation of a uniform layer. Jantac et al.60 found that surface roughness can enhance the contact area between the substrate and molten particles. Rough surfaces offer increased anchor points for molten particles, facilitating a higher adhesion rate of particles to the substrate. Furthermore, increased surface roughness improves the contact area between the substrate and molten particles, promoting more effective material dispersion. This method allows smaller particles to fill finer splits, decreasing overall coating thickness while ensuring efficient coverage61. Zhu et al.62 noted that reduced surface roughness decreases the porosity of the oxide layer, resulting in the development of denser oxide layers62,63. Decreased porosity and the enhanced density of oxide layers result in thinner coating layers36,44. Fitriyana et al.8 indicated that the thinnest coating thickness was attained in specimens exhibiting minimal porosity and reduced unmelted material, specifically at a stand-off distance of 100 mm and a compressed air pressure of 3 bar.
The final coating layer thickness is typically reduced due to increased preheat temperature64,65. Elevated preheat temperatures can enhance the wetting and adherence of the coating material to the substrate, potentially resulting in a thinner and more uniform layer. This occurrence is because the expedited bonding procedure between the substrate and the coating material at elevated temperatures diminishes the probability of uneven or overly thick layers. In the tungsten carbide coating process utilizing the flame spray coating technique by Winarto et al.65the coating thickness reduces as the preheat temperature rises from 300 °C to 400 °C. The coating thickness at preheats temperatures of 300 °C and 400 °C was measured at 369 μm and 343 μm, respectively.
Porosity and unmelted material of the coating layer
Scanning Electron Microscopy (SEM) testing was performed to examine the morphology of the coating layer structure on each specimen. Figure 7 displays the SEM images of all specimens at a magnification of 2500x. The SEM results for specimens exhibiting a substrate surface roughness of 35 μm, which underwent preheat treatments at temperatures of 50 °C, 100 °C, and 150 °C, are presented in Fig. 7a and b, and 7c, respectively.
Figure 7d and e, and 7f present the SEM results for specimens exhibiting a substrate surface roughness of 40 μm, preheated at temperatures of 50 °C, 100 °C, and 150 °C.
The SEM analysis of all specimens indicates the presence of porosity, unmelted material, and oxides. In this study, the percentage of porosity and unmelted material was quantified using ImageJ software (Fig. 8). Figure 9 shows the effect of substrate surface roughness and preheat treatment temperature on the percentage of porosity and unmelted material in the resulting coating layer. The finding of this study indicates that the surface roughness of the substrate and the preheating temperature significantly affect the porosity and the percentage of unmelted material.
The percentage of porosity and unmelted material decreases with increasing surface roughness and preheating temperature. The lowest porosity and unmelted material were found in the specimens with a surface roughness of 40 μm and a preheating temperature of 150 °C. On the other hand, the specimens with the highest percentage of porosity and unmelted material were seen in the specimens with a preheating temperature of 50 °C and a surface roughness of 35 μm.
Enhancing the preheat temperature has demonstrated an increase in layer density by diminishing the formation of pores. Preheating facilitates the uniform distribution of molten particles and enhances their adhesion to the substrate, minimizing the spaces that lead to porosity. Moreover, increased preheat temperatures improve the adhesion between the layer and the substrate, resulting in a denser structure with reduced porosity66.
At each same preheat temperature, the surface with rougher roughness (40 μm) showed lower porosity compared to the smoother surface (35 μm). The rougher surface seems to provide more contact area for the coating particles, which improves adhesion and reduces the space between particles that can cause pores71. This is in line with research by Ortiz-Fernandez et al.72which showed that preheat increases the union of particles with the substrate and between the particles themselves, reducing the number of unmelted particles. In addition, research by Wang et al.73 also confirmed that an increase in post-sandblasting surface roughness can lead to better bonding between the coating and the substrate, thereby reducing the formation of porosity.
Ashokkumar et al.74 highlight that achieving a minimum porosity level is essential for enhancing the performance properties of coatings, including hardness and corrosion resistance. The study demonstrated that the AA20224/Al2O3 coating on A31B Mg alloy achieved a minimum porosity of 2% under 520 °C temperature, 12 mm stand-off distance, and 22 g/min powder feeding rate in the cold spray process. The ANOVA results indicated that temperature in cold spray coating significantly influenced porosity formation, with stand-off distance and powder feed rate being important. The coating exhibits a considerable presence of unsoftened and partially softened particles at low input temperatures. The cooling process following spraying results in the formation of micro-cracks and pores at the boundaries of unsoftened coating particles, subsequently impacting coating quality.
Hardness of the coating layer
The effect of preheat temperature and surface roughness on the hardness of the resulting coating layer is illustrated in Fig. 10. The findings indicate increased preheat temperature and enhanced substrate surface roughness improve coating hardness. The specimen with a surface roughness of 40 μm and a preheat temperature of 150 °C had the highest hardness value (1114.6 HV). On the other hand, the lowest hardness value of 951.8 HV was discovered in the specimen with a preheat temperature of 50 °C and a surface roughness of 35 μm.
This phenomenon arises from increased surface roughness and preheat temperature on the substrate, resulting in reduced porosity and unmelted material, alongside a reduction in the coating layer thickness. Consequently, the hardness of the coating layer is increased. This study’s findings are confirmed by Prasad et al.75who applied WC–Co–Cr coatings on a 304 Stainless Steel substrate utilizing the HVOF method. Their research indicated that oxygen flow rate, liquefied petroleum gas flow rate, powder feeding rate, stand-off distance, and carrier gas flow rate significantly affect coating performance. Their findings indicated that an increase in oxygen flow rate, fuel flow rate, and powder feeding rate generally reduces porosity, thereby improving the coating adhesion. In contrast, increased stand-off distance typically results in elevated porosity and reduced hardness. With an increase in porosity, there is a corresponding decrease in microhardness.
This results from decreased interfacial bonding between the coating and the substrate caused by increased porosity, ultimately resulting in diminished mechanical properties, including hardness.
A study by Sugiarti et al.76 indicates that reduced particle sizes enhance coating layer density, boosting hardness. Larger particles, conversely, tend to enhance porosity, resulting in diminished hardness of the coating layer. Khandanjou et al.77 assert that increasing particle velocity can elevate the impact pressure of molten droplets on the substrate surface. Increased particle velocities facilitate more uniform distribution and improved contact between the deposited layers, hence augmenting the cooling rate of the molten droplets. This situation facilitates the decrease of porosity and encourages the development of a more compact microstructure in the coating layer. Moreover, an elevated argon gas flow rate has been demonstrated to diminish the porosity of the coating layer and enhance microhardness. At 30, 35, 40, and 45 L/min flow rates, the aluminium coating had average microhardness values of 33 Hv0.025, 35 Hv0.025, 35 Hv0.025, and 36 Hv0.025, respectively. The research by Thirumalaikumarasamy et al.78 indicates that an efficient particle flattening and densification procedure on the deposited layer might diminish porosity and enhance microhardness. The presence of unmelted materials increases porosity and reduces coating hardness due to reduced particle cohesion. Enhanced porosity leads to decreased layer stiffness, thereby decreasing the hardness value.
The increased hardness of the obtained coating layer can be ascribed to preheating’s capacity to decrease residual stress inside the coating layer. High residual stress can lead to micro-cracks or delamination, which impairs the hardness and integrity of the coating79,80. By reducing these stresses, the coating becomes more stable and harder. Residual stresses in the coating process generally occur from many sources, including quenching, peening, and thermal mismatch stresses81,82. Quenching stresses arise as the coating cools quickly, resulting in contraction and inducing tensile stress (+) inside the layer. Peening stresses come from the effect of high-velocity particles, which induce plastic deformation in the substance used for coating and/or substrate, leading to compressive stresses (−). Deposition stresses are the general term for both stresses arising during the deposition stage. Conversely, thermal mismatch stresses arise post-coating during cooling, resulting from discrepancies in the thermal expansion coefficients of the coating and substrate, which induce tension between the two. This form of stress typically manifests after deposition during the cooling process81,82.
Luo et al.83 demonstrate that increased coating thickness increases compressive and tensile residual stresses.
This condition leads to diminished wear resistance in adhesive wear assessments. Zhu et al.84 further suggest that an increase in residual tensile stress decreases hardness and elastic modulus. Their research showed that tensile residual stress in FeCrBSi coatings increased with the distance (in mm) from the substrate. The assessed residual stress in their study varied from 40 MPa to 112 MPa, indicating a distinct upward trend. Increased tensile residual stresses result in larger indentation depth and contact area for the same indentation load, thereby diminishing hardness and elastic modulus. Hardness observations at distances of 0.005 mm, 0.1 mm, and 0.2 mm from the substrate indicated approximately 11.6 GPa, 9.2 GPa, and 4.5 GPa, respectively.
Furthermore, preheat also plays an important role in creating a more uniform and denser microstructure, which is obtained when the melt particles adhere more tightly to the preheated substrate. This stronger bond reduces the porosity of the coating and increases the surface hardness85,86. Furthermore, research by Wang et al.87 found that increasing the preheat temperature contributes to a more uniform distribution of elements in the coating layer. This uniform distribution is important because the elements play a role in determining the mechanical properties, including the hardness of the coating. At higher preheat temperatures, such as 250 °C, the coating shows a more uniform distribution of elements, which directly contributes to higher hardness.
In addition, the study also showed that higher surface roughness (from 35 μm to 40 μm) correlated with an increase in coating hardness at various preheat temperatures. This is due to the increase in contact area between the substrate and the coating material, which improves adhesion and allows for more even heat distribution during the coating process. This improvement not only increases the hardness but also improves the resistance to wear and damage87. In this study, the hardness of the uncoated substrate was measured at 206.8 HV. The hardness of the FeCrBMnSi coating generated in this investigation varied from 951.8 HV to 1114.6 HV. The data verify that the FeCrBMnSi coating enhances the substrate’s surface hardness. The increase in hardness attained ranges from 360 to 439% related to the uncoated specimen. Consequently, the elevation of preheat temperature and substrate surface roughness significantly increases the coating layer’s hardness. This occurs through multiple mechanisms, such as reducing residual stress, developing a denser and more homogeneous microstructure, enhanced contact area and adhesion between the layer and the substrate, and a more uniform elemental distribution within the coating layer. The results of this investigation indicate that the hardness of all post-coating specimens meets the minimum hardness requirement for coated pump impellers, which is 60 Rockwell C or 700 HV8,88.
Adhesive strength of the coating layer
Figure 11 illustrates the influence of preheat temperature and substrate surface roughness on the adhesive strength of the coating layer generated in the current study. The findings demonstrate that increased preheat temperature and substrate surface roughness improved adhesive strength. A reduction in these two factors typically reduces adhesive strength. The highest adhesive strength value of 20.29 MPa was found in the specimen with a surface roughness of 40 μm and a preheat temperature of 150 °C. On the other hand, the lowest adhesive strength value of 15.75 MPa was identified in the specimen with a preheat temperature of 50 °C and a surface roughness of 35 μm.
The current study’s findings indicate that specimens exhibiting the greatest adhesive strength are associated with the lowest percentages of porosity and unmelted material. The specimens with the lowest adhesive strength possess the highest porosity and unmelted material. This study’s findings are consistent with the findings reported by Mohankumar et al.89 who indicated that an increase in porosity percentage leads to a reduction in the bonding area at the interface, thereby decreasing the adhesion strength of the coating. The AA2024/Al2O3 coating was applied to the AZ31B magnesium alloy substrate via the cold spray method, utilizing a powder feeding rate of 22 g/min, a stand-off distance of 12 mm, and a processing temperature of 520 °C, resulting in a coating with an adhesion strength of 70 MPa.
The ANOVA results demonstrated that the powder feeding rate (g/min) significantly influenced the adhesion strength of the coating, with stand-off distance (mm) and processing temperature (°C) following in importance. At reduced powder flow rates, the powder particles inadequately navigate the narrow path, and the enhanced softening of the coating powder leads to obstructions in the nozzle. This leads to inadequate particle adhesion, uneven coating distribution, and a highly porous structure, resulting in suboptimal adhesion properties of the coating.
Irawan et al.44 conducted a study indicating that post-heat treatment enhances the adhesion strength of the coated layer relative to untreated layers. This enhancement arises from the efficacy of the post-heat treatment process in diminishing porosity and the presence of unmelted components, thus fortifying the adhesion between the coated layer and the substrate. Elevated porosity levels can diminish adhesive strength by compromising both mechanical and chemical bonding and establishing pathways for crack propagation that may result in delamination of the coating layer. Decreased porosity leads to a denser and more cohesive layer structure, enhancing adhesive strength. Pores, gaps, or a significant quantity of unmelted components are critical indicators of diminished adhesive strength in the coating layer.
Preheat plays an important role in improving the adhesion of the coating layer to the substrate. By balancing the temperature between the substrate and the melt particles, the preheat ensures that the particles can adhere better to the heated substrate. A higher substrate temperature prevents the melt particles from cooling too quickly on contact, allowing them to fuse better and form a stronger bond72. In addition, preheat also reduces the thermal stress between the substrate and the coating layer. Lower thermal stress reduces the risk of cracks or delamination, which can impair bonding strength. By reducing the temperature difference, the preheat helps maintain the integrity of the coating layer and improves its adhesion. Pang’s (2020) research supports these findings by showing that preheat reduces thermal stress in Plasma-sprayed Mo/8YSZ thermal barrier coatings, thereby improving the bond strength between the substrate and the coating72,86.
In addition to the preheat temperature, the surface roughness of the substrate also plays an important role in improving the adhesion strength of the coating layer. Rougher surfaces have more anchor points, allowing the melt particles to stick more firmly to the substrate. This increases the mechanical adhesion strength as the melt particles are trapped in the indentations and protrusions of the rough surface, creating a stronger bond90. Optimal surface roughness helps to create a better microstructure, allowing the melt particles to spread out and fill small crevices in the substrate.
Thus, the contact and bonding between the particles and the substrate increases, resulting in better adhesion at the micro level and improving the overall bonding strength91.
Corrosion rates of the coating layer
The impact of substrate surface roughness and preheat temperature on the corrosion rate of each specimen in this investigation is shown in Fig. 12. The results indicate that the correlation between these factors and the corrosion rate does not exhibit a linear trend. The maximum corrosion rate, 5.1598 × 10⁻² mmpy, was observed in the specimen exhibiting a surface roughness of 40 μm and subjected to a preheat temperature of 100 °C. In contrast, the minimum corrosion rate of 1.0640 × 10⁻² mmpy was observed in the specimen subjected to a preheat temperature of 150 °C, exhibiting a surface roughness of 40 μm. The corrosion rate of the coated specimens in this study exceeded that of the untreated substrate material, which showed a corrosion rate of 0.3600 × 10⁻² mmpy. This happens because a passive chromium oxide (Cr₂O₃) layer forms on the surface of the 304 stainless steel substrates, making it very resistant to corrosion. The passive layer is a natural barrier, preventing additional interactions between the metal and the corrosive environment92.
Mohankumar et al.93 assert that the coating layer is susceptible to damage when acidity levels rise. At elevated pH levels, the oxides in the AA2024/Al2O3 coating are more prone to dissolution, resulting in an augmented corrosion rate.
Conversely, the AA2024/Al2O3 coating experiences passivation at reduced pH levels, forming a thin oxide layer on the surface that can safeguard the coating from further corrosion. The passivation layer diminishes the corrosion rate by obstructing the flow of ions and molecules into the coating layer92,93.
The present study indicates that although the coating technique enhances mechanical attributes like hardness and adhesive strength, it fails to ensure optimal corrosion protection. This suggests that the coating layers developed in the present study are not yet adequately effective in preserving materials against corrosion damage. Consequently, additional advancement is necessary to improve the equilibrium between increasing mechanical characteristics and corrosion resistance, especially in applications demanding prolonged durability against corrosive conditions. This study confirms the conclusions of Gecu et al.94who asserted that a single Al2O3 layer exhibits the most significant wear resistance, with the lowest corrosion resistance. Meanwhile, the incorporation of TiO2 elevates the wear rate, rendering the coating increasingly susceptible to friction-induced damage. Their findings suggest that the Al2O3 − TiO2 hybrid coating with a 6:1 ratio is optimal for wear and corrosion resistance. This combination effectively diminished wear and corrosion rates on the ISI 304 stainless steel substrate by 83% and 97%, respectively94.
The existence of porosity and unmelted material in the coating layer is responsible for its inferior corrosion resistance compared to the substrate. Fitriyana et al.43 report that porosity and unmelted material significantly influence corrosion resistance more than the chemical composition of the coating material. The existence of porosity and unmelted material permits corrosive agents to infiltrate the coating layer, hence hastening material deterioration and compromising its protective attributes. The test results indicate that porous samples exhibit elevated corrosion rates and generate less stable oxide layers, diminishing total corrosion resistance. Surface treatments preceding coating, such as sandblasting, alongside internal elements inside the coating layer, may influence corrosion resistance95.
Investigation by Mohankumar et al.93 demonstrates that increased porosity promotes the penetration of corrosive agents, hence expediting the corrosion process. Their experimental results indicate that the metallic matrix composite (MMC) with 20% Al2O3 reinforcement exhibits enhanced corrosion resistance relative to other coating materials. The coating created with this composition shows a low porosity value of 0.65%, leading to a significantly decreased corrosion rate. Geng et al.96 demonstrated that sandblasting markedly diminished the corrosion resistance of stainless steel 316 L owing to alterations in surface morphology, the emergence of the α’-martensite phase, and an escalation in dislocation density.
Wang and Li97 discovered that sandblasting induces the creation of a nanocrystalline layer on the surface of stainless steel 304. This layer resulted in a substantial decrease in corrosion resistance when evaluated in a 3.5% NaCl solution.
The corrosion rate test results shown in Fig. 12 reveal that preheat at 150 °C results in excellent corrosion resistance performance over a wide range of surface roughness variations. Increasing the preheat temperature plays an important role in strengthening the adhesion of the coating particles to the substrate, which reduces the risk of delamination or peeling of the coating. This directly reduces the exposure of the substrate to the corrosive environment, reducing the corrosion rate. Preheat also helps to lower porosity in the coating layer by giving the melt particles more time to spread and fill small crevices on the substrate surface, resulting in a denser, low porosity coating. This dense coating inhibits the penetration path for corrosive agents such as water, oxygen, and chloride ions, thereby improving corrosion resistance98.
In addition, preheat removes moisture and contamination from the substrate, which could be the starting point of corrosion, thus further improving the coating’s resistance to corrosive attack99. The surface roughness of the substrate also plays an important role in improving the corrosion resistance of the coating. A rougher surface creates more anchor points for melt particles, increasing the bond strength between the coating and the substrate. This not only reduces the chance of delamination or peeling of the coating but also results in stronger mechanical adhesion, which makes the coating more resistant to corrosion. Optimal surface roughness allows the melt particles to fill small crevices and indentations in the substrate, forming a denser and more uniform coating. With fewer and smaller pores, the penetration path for corrosive agents is reduced, increasing the corrosion protection effectiveness of the coating100. Research by Liu et al.100 supports these findings by showing that coatings heated at 200 °C have the lowest corrosion current density and a smoother and more uniform microstructure, contributing to superior corrosion resistance properties.
Conclusion
This study examines the effect of substrate surface roughness and preheat temperature on the properties of the resultant coating layer. The Miller Delta Weld 602 machine (Miller Electric, Wisconsin, USA) was employed to apply the coating, using NiAl wire as the bonding layer and FeCrBMnSi wire as the upper layer. The coating procedure was executed with the apparatus configured at a standoff distance of 100 mm, a primary gas pressure of 3 bar, a voltage of 28 V, and a current of 200 A.
The study’s findings show that a surface with a roughness of 35 μm produces a greater coating thickness than a roughness of 40 μm at the same temperature. Increasing preheat temperature and surface roughness consistently reduces the percentage of porosity, unmelted material, and coating layer thickness. This enhances the hardness and adhesive strength of the coating layers. The phenomenon arises from increased surface roughness and preheats temperature on the substrate, resulting in reduced porosity and unmelted material, alongside a reduction in the coating layer thickness. Consequently, the hardness and adhesive strength of the coating layer is increased.
In this study, the hardness of the uncoated substrate was measured at 206.8 HV. The hardness of the FeCrBMnSi coating generated in this study varied from 951.8 HV to 1114.6 HV. The data verify that the FeCrBMnSi coating enhances the substrate’s surface hardness. The increase in hardness attained ranges from 360 to 439% related to the uncoated specimen. The best specimen in this study was found on a substrate with a surface roughness of 40 μm and performed a preheating treatment at a temperature of 150 °C. The thickness of the coating layer for this specimen was 150.58 × 10⁻³ mm, with a porosity of 7.233%, an unmelted material content of 1114.6 HV, an adhesive strength of 20.29 MPa, and a corrosion rate of 1.0640 × 10⁻² mmpy. However, the corrosion resistance of the coated specimens remains lower than that of the uncoated 304 stainless steel. The best corrosion rate of 1.0640 × 10⁻² mmpy was observed in the specimen with a substrate surface roughness of 40 μm and subjected to a preheat temperature of 150 °C. Notably, the corrosion rates of all coated specimens were significantly higher than those of the uncoated substrate, which showed a corrosion rate of 0.3600 × 10⁻² mmpy. The study supports SDG Goal 9 (Industry, Innovation, and Infrastructure) by enhancing the efficacy of coating layers, specifically regarding hardness and corrosion resistance, hence extending the longevity of industrial components. Furthermore, it contributes to SDG Goal 12 (Responsible Consumption and Production) by reducing waste and optimizing material usage through more efficient coating techniques, including regulating surface roughness and preheat temperature. This study is also consistent with SDG Goal 13 (Climate Action), as prolonging the lifecycle of materials reduces the necessity for component replacements, thereby reducing the consumption of resources and environmental impact.
Data availability
Data availabilityThe raw data supporting the conclusions of this article will be made available by the corresponding author, without undue reservation.
References
Pramono, S., Roekminiati, S. & Vitianingsih, A. V. Effectiveness of Flood Management Through Pump Houses Based on Geographic Information Systems in the City of Surabayavol. 1 (Atlantis Press SARL, 2023).
Ward, P. J. et al. The need to integrate flood and drought disaster risk reduction strategies. Water Secur. 11, 1–14 (2020).
Kusnan & Suryaman, H. Sustainable flood prevention using interconnection of house pump system. J. Phys. Conf. Ser. 1569, 1–5 (2020).
Hunt, J. D. et al. Role of pumped hydro storage plants for flood control. J. Energy Storage. 104, 1–17 (2024).
Bantelay, D. T., Gebresenbet, G., Admasu, B. T., Tigabu, M. T. & Getie, M. Z. Unveiling the startup characteristics of pump as turbine using computational and experimental techniques. Results Eng. 25, 103955 (2025).
Kadaj, E. & Bosleman, R. Chapter 11 - Energy Recovery Devices in Membrane Desalination Processes. in Surface Production Operations (ed. Gude, V. G. B. T.-R. E. P. D. H.) 415–444 (Butterworth-Heinemann, (2018). https://doi.org/10.1016/B978-0-12-815244-7.00011-8
Stewart, M. 3 - Centrifugal pumps. in Renewable Energy Powered Desalination Handbook (ed. Stewart, M. B. T.-S. P. O.) 61–309 (Gulf Professional Publishing, 2019). https://doi.org/10.1016/B978-0-12-809895-0.00003-X
Fitriyana, D. et al. The effect of compressed air pressure and Stand-off distance on the twin wire Arc spray (TWAS) coating for pump impeller from AISI 304 stainless steel. Springer Proc. Phys. 242, 119–130 (2020).
Pokharel, N., Ghimire, A., Thapa, B. & Thapa, B. S. Wear in centrifugal pumps with causes, effects and remedies: A review. IOP Conf. Ser. Earth Environ. Sci 1037, (2022).
Uralov, B. et al. Influence of hydroabrasive wear of impeller blades on head of centrifugal pump. E3S Web Conf. 365, 1–7 (2023).
Duran-Sarmiento, M. A., García-Rodríguez, L. F., Sandoval-Rodríguez, C. L. & Ascanio-Villabona, J. G. Lengerke-Pérez, O. Performance analysis of PKM 60 peripheral pumps as a consequence of impeller wearing. IOP Conf. Ser. Mater. Sci. Eng. 1253, 1–8 (2022).
Kumar, V., Singh, V., Verma, R., Bansal, A. & Ghosh, G. Cavitation-corrosion analysis of HVOF-sprayed WC-Co-Cr-graphene nanoplatelets coatings with LST pre-treatment. Int. J. Refract. Met. Hard Mater. 120, 1–15 (2024).
Güner, M. & Özbayer, M. M. Wear and its effects in centrifugal pumps. Yuz Yil Univ. J. Agric. Sci. 29, 569–582 (2019).
Shen, Z., Li, R., Han, W., Quan, H. & Guo, R. Erosion wear on impeller of Double-Suction centrifugal pump due to sediment flow. J. Appl. Fluid Mech. 13, 1131–1142 (2020).
Shen, Z., Chu, W., Li, X. & Dong, W. Sediment erosion in the impeller of a double-suction centrifugal pump – A case study of the Jingtai yellow river irrigation project, China. Wear 422–423, 269–279 (2019).
Bandi, S., Banka, J., Kumar, A. & Rai, A. K. Effects of sediment properties on abrasive erosion of a centrifugal pump. Chem. Eng. Sci. 277, 1–16 (2023).
Jiang, Z., Zeng, X. & Li, Y. Study on the Relationship between Wear and Flow Characteristics of a Centrifugal Pump at Different Mass Concentrations. Processes vol. 9 1–19 at (2021). https://doi.org/10.3390/pr9060988
Dong, J. & Li, W. Numerical simulation of centrifugal pump cavitation based on ANSYS. J. Phys. Conf. Ser. 2450, 1–6 (2023).
Long, Y. et al. Research on Cavitation Wake Vortex Structures Near the Impeller Tip of a Water-Jet Pump. Energies vol. 16 1–20 at (2023). https://doi.org/10.3390/en16041576
Mousavi Jarrahi, M., Khajavian, E., Noorbakhsh Nezhad, A. H., Zahrani, M. & Alfantazi, A. E. Elucidating the Electrochemical Corrosion of a Water Pump Impeller in an Industrial Cooling System with Zero Liquid Discharge. Water vol. 17 1–22 at (2025). https://doi.org/10.3390/w17020173
Sinha, M. & Tyagi, R. K. Strength and corrosion analysis in alloy steel and E-glass composite wear ring in automotive engine cooling water pump. Mater. Today Proc. 21, 1474–1478 (2020).
LEE, T. Y. K. and S. H. Combustion and Emission Characteristics of Wood Pyrolysis Oil-Butanol Blended Fuels in a Di Diesel Engine. Int. J. … 13, 293–300 (2012).
Lakkannavar, V. et al. Thermal spray coatings on high-temperature oxidation and corrosion applications – A comprehensive review. Results Surf. Interfaces. 16, 1–12 (2024).
Sadeghi, E., Markocsan, N. & Joshi, S. Advances in Corrosion-Resistant thermal spray coatings for renewable energy power plants. Part I: effect of composition and microstructure. J. Therm. Spray. Technol. 28, 1749–1788 (2019).
Santa, J. F., Espitia, L. A., Blanco, J. A., Romo, S. A. & Toro, A. Slurry and cavitation erosion resistance of thermal spray coatings. Wear 267, 160–167 (2009).
Babu, A., Perumal, G., Arora, H. S. & Grewal, H. S. Enhanced slurry and cavitation erosion resistance of deep cryogenically treated thermal spray coatings for hydroturbine applications. Renew. Energy. 180, 1044–1055 (2021).
Algoburi, A., Ahmed, R. & Kumar, V. Influence of hiping Post-Treatment on the cavitation Erosion in HVOF thermally sprayed WC-NiCrBSi coatings. J. Therm. Spray. Technol. 34, 992–1015 (2025).
Singh, J. Wear performance analysis and characterization of HVOF deposited Ni–20Cr2O3, Ni–30Al2O3, and Al2O3–13TiO2 coatings. Appl. Surf. Sci. Adv. 6, 1–13 (2021).
KARTHIKEYAN, J. 4 - The advantages and disadvantages of the cold spray coating process. in Woodhead Publishing Series in Metals and Surface Engineering (ed. Champagne, V. K. B. T.-T. C. S. M. D. P.) 62–71Woodhead Publishing, (2007). https://doi.org/10.1533/9781845693787.1.62
Gaur, U. P. & Kamari, E. Applications of thermal spray coatings: A review. J. Therm. Spray. Eng. 4, 106–114 (2024).
Lencová, K. et al. Hot corrosion behavior of TWAS and HVOF NiCr-Based coatings in molten salt. Mater. (Basel). 16, 1–14 (2023).
Houdková, Šulcová, P., Lencová, K., Česánek, Z. & Švantner, M. Twin wire Arc sprayed coatings for power industry Applications - Process parameters optimization study. J. Phys. Conf. Ser. 2572, 3–9 (2023).
Wagner, N. Effect of process parameters on twin wire Arc sprayed steel coatings. J. Mater. Eng. Perform. 30, 6650–6655 (2021).
HVTS vs. Twin Wire Arc Spray: Discover Superior Coating Solutions for Heavy Industries. (2025). https://integratedglobal.com/hvts-vs-twin-wire-arc-spray-the-difference/
Nango-Blanco, M. et al. Oxynitrided Ti-6Al-4V coatings deposited by twin wire Arc spray for protection of aluminum Die-Casting molds. J. Therm. Spray. Technol. 2801–2814. https://doi.org/10.1007/s11666-024-01881-6 (2024).
Deni Fajar et al. The effect of Post-Heat treatment on the mechanical properties of fecrbmnsi coatings prepared by twin wire Arc spraying (TWAS) method on pump impeller from 304 stainless steel. J. Adv. Res. Fluid Mech. Therm. Sci. 93, 138–147 (2022).
Bolot, R., Planche, M. P., Liao, H. & Coddet, C. A three-dimensional model of the wire-arc spray process and its experimental validation. J. Mater. Process. Technol. 200, 94–105 (2008).
Tillmann, W., Hagen, L. & Kokalj, D. Embedment of eutectic tungsten carbides in Arc sprayed steel coatings. Surf. Coat. Technol. 331, 153–162 (2017).
DePalma, K., Walluk, M., Martin, L. P. & Sisak, K. Investigation of mechanical properties of twin wire Arc repair of cast Iron components. J. Therm. Spray. Technol. 31, 315–328 (2022).
Horner, A. L., Hall, A. C. & McCloskey, J. F. The Effect of Process Parameters on Twin Wire Arc Spray Pattern Shape. Coatings vol. 5 115–123 at (2015). https://doi.org/10.3390/coatings5020115
Tillmann, W., Rademacher, H. G., Hagen, L. & Abdulgader, M. El barad’ei, M. Spray pattern analysis in TWAS using photogrammetry and digital image correlation. IOP Conf. Ser. Mater. Sci. Eng. 373, 1–8 (2018).
Boulos, M. I., Fauchais, P. L. & Pfender, E. Handbook of Thermal Plasmas (Springer International Publishing, 2023). https://doi.org/10.1007/978-3-030-84936-8
Fitriyana, D. F. et al. Effect of sandblasting on the characterization of 95MXC coating layer on 304 stainless steel prepared by the TWAS (twin wire Arc spray) coating method. Mech. Eng. Soc. Ind. 4, 142–155 (2024).
Irawan, A. P. et al. Influence of Post-Heat treatment on the characteristics of fecrbmnsi coating on stainless steel 304 substrate prepared by twin wire Arc spray (TWAS) method at various Stand-off distance. Jordan J. Mech. Ind. Eng. 18, 327–337 (2024).
Sadki, A., Younes, R., Bradai, M. A. & Mesrati, N. Effect of NiAl bond layer on the wear resistance of an austenitic stainless steel coating obtained by Arc spray process. SAE Int. J. Mater. Manuf. 16, 321–330 (2023).
Bezgodov, A. & Ovchinnikov, I. Surface Preparation as one of the main factors for ensuring the durability of anti-corrosion coatings. Russ J. Transp. Eng. 7, 1–16 (2020).
Li, J., Ma, Z., Man, R., Jia, C. & Zhang, Z. Modeling the Interfacial Bonding Strength of Al-Zn-Si Alloy between Substrates with Linear Surface Roughness and Its Coating. Coatings vol. 13 1–17 at (2023). https://doi.org/10.3390/coatings13060997
Xie, Y. et al. Effect of substrate preheating on adhesive strength of SS 316L cold spray coatings. J. Therm. Spray. Technol. 25, 123–130 (2016).
Amininejad, A., Jamaati, R. & Hosseinipour, S. J. Improvement of strength-ductility balance of SAE 304 stainless steel by asymmetric cross rolling. Mater. Chem. Phys. 256, 1–14 (2020).
Amininejad, A., Jamaati, R. & Hosseinipour, S. J. Achieving superior strength and high ductility in AISI 304 austenitic stainless steel via asymmetric cold rolling. Mater. Sci. Eng. A. 767, 1–11 (2019).
Shi, W. et al. Effect of Substrate Preheating Temperature on the Microstructure and Properties of Laser Cladding Fe/TiC Composite Coating. Lubricants vol. 12 1–13 at (2024). https://doi.org/10.3390/lubricants12060216
S, V. et al. Study on developments in protection coating techniques for steel. Adv. Mater. Sci. Eng. 2022, 1–10 (2022).
Zhang, B. et al. Effect of Pre-Heat-Treatment on the Oxidation Resistance of MCrAlY Coatings: A Review. Coatings vol. 13 1–16 at (2023). https://doi.org/10.3390/coatings13071222
Tang, J., Bai, Y., Wang, J. & Yang, J. Preheating treatment of thermal barrier coatings by supersonic plasma jet. Mater. Lett. 182, 181–184 (2016).
Dahmen, M., Vedder, C., Speckens, M., Stollenwerk, J. & Loosen, P. Influence of surface preheating and pretreatment on the adhesion of laser-melted PEEK to aluminum substrates. Surf. Coat. Technol. 448, 1–11 (2022).
Miller Electric Mfg. CO. Deltaweld Series. (2007). https://www.dutagrahamiller.co.id/wp-content/uploads/2020/09/deltaweld.pdf
Sucharski, G. B., Pukasiewicz, A. G. M., Váz, R. F. & Paredes, R. S. C. Optimization of the deposition parameters of HVOF FeMnCrSi + Ni + B thermally sprayed coatings. Soldag E Insp. 20, 238–252 (2015).
Wang, Y., Hsu, P. & Wu, Y. Thermal radiative properties of zirconium oxide films in the near-infrared wavelengths. Int. J. Appl. Ceram. Technol. 19, 2311–2321 (2022).
Bir, F., Khireddine, H., Ksouri, D., Benhayoune, H. & Maho, A. Characterization of HA/FHA coatings on smooth and rough implant surface by pulsed electrodeposition. Int. J. Appl. Ceram. Technol. 12, E222–E234 (2015).
Jantač, S. et al. Triboelectric charging model for particles with rough surfaces. Adv. Powder Technol. 36, 1–19 (2025).
Kim, H. J., Kim, J. J. & Lee, J. K. Enhancement of the surface roughness by powder spray coating on zirconia substrate. J. Nanosci. Nanotechnol. 19, 6285–6290 (2019).
Zhu, L., Petrova, R. S., Gashinski, J. P. & Yang, Z. The effect of surface roughness on PEO-treated Ti-6Al-4V alloy and corrosion resistance. Surf. Coat. Technol. 325, 22–29 (2017).
Xi, F. et al. Growth mechanism of oxide layer on Ti-6Al-4 V substrate with different surface topographies during the early stage of micro-arc oxidation. Surf. Coat. Technol. 467, 1–12 (2023).
Li, R. et al. Effects of preheating temperatures on the formation of sandwich compression and density distribution in the compressed wood. J. Wood Sci. 64, 751–757 (2018).
Winarto, W., Sofyan, N. & Rooscote, D. Porosity and wear resistance of flame sprayed tungsten carbide coatings. AIP Conf. Proc. 1855, 1–7 (2017).
Lee, H. J. & Ahn, D. G. Investigation of elimination of powder spreading in manufacture of thin and wide preheating beads from Co–Cr alloy powders using a P-ebeam. J. Mater. Res. Technol. 14, 1873–1883 (2021).
Khamsepour, P., Moreau, C. & Dolatabadi, A. Numerical simulation of the effect of particle and substrate preheating on porosity level and residual stress of As-sprayed Ti6Al4V components. J. Therm. Spray. Technol. 31, 70–83 (2022).
Deng, W. et al. Effect of substrate preheating treatment on the microstructure and ultrasonic cavitation erosion behavior of plasma-sprayed YSZ coatings. Ultrason. Sonochem. 46, 1–9 (2018).
Huang, F. et al. The Effect of Preheating Temperature on the Corrosion Resistance and Porosity Defects Development Behaviour of Ni60A Coating. Coatings vol. 14 1–15 at (2024). https://doi.org/10.3390/coatings14060688
Ariati, M., Putra, W. N. & Rachmansyah, R. P. Optimizing the substrate preheating process of high velocity oxygen fuel cobalt-based alloy coating on alloyed and carbon steels mechanical properties. Mater. Sci. Forum. 929 MSF, 142–149 (2018).
Miwa, T., Miya, G. & Kanno, S. Effect of surface roughness on small particle adhesion forces evaluated by atomic force microscopy. Jpn J. Appl. Phys. 59, 1–24 (2020).
Ortiz-Fernandez, R., Imbriglio, S., Chromik, R. & Jodoin, B. The role of substrate preheating on the adhesion strength of Cold-Sprayed soft particles on hard substrates. J. Therm. Spray. Technol. 30, 655–667 (2021).
Wang, J., Ai, C., Yun, X., Chen, Z. & He, B. Effects of 3D Roughness Parameters of Sandblasted Surface on Bond Strength of HVOF Sprayed WC-12Co Coatings. Coatings vol. 12 1–20 at (2022). https://doi.org/10.3390/coatings12101451
Ashokkumar, M., Thirumalaikumarasamy, D., Sonar, T., Vignesh, P. & Deepak, S. Optimization of cold spray coating parameters using RSM for reducing the porosity level of AA2024/Al2O3 coating on AZ31B magnesium alloy. Int. J. Interact. Des. Manuf. 127–141. https://doi.org/10.1007/s12008-023-01597-x (2023).
Prasad, R. V., Rajesh, R., Thirumalaikumarasamy, D., Ashokkumar, M. & Rajakumar, S. Multi response optimization of HVOF process parameters in low carbon steels. Sadhana - Acad. Proc. Eng. Sci. 47, 1–17 (2022).
Sugiarti, E., Desiati, R. D., Zaini, K. A., Hartanto, K. & Prastomo, N. Effect of Co concentration on hardness of NiCo coating layer synthesized by electroplating method. J. Phys. Conf. Ser. 1191, 1–7 (2019).
Khandanjou, S., Ghoranneviss, M. & Saviz, S. The investigation of the microstructure behavior of the spray distances and argon gas flow rates effects on the aluminum coating using self-generated atmospheric plasma spray system. J. Theor. Appl. Phys. 11, 225–234 (2017).
Thirumalaikumarasamy, D., Kamalamoorthy, K. S. & Visvalingam, V. B. Effect of experimental parameters on the micro hardness of plasma sprayed alumina coatings on AZ31B magnesium alloy. J. Magnes Alloy. 3, 237–246 (2015).
van den Berg, N., Xin, H. & Veljkovic, M. Effects of residual stresses on fatigue crack propagation of an orthotropic steel Bridge deck. Mater. Des. 198, 1–19 (2021).
Matejicek, J. & Sampath, S. In situ measurement of residual stresses and elastic moduli in thermal sprayed coatings: part 1: apparatus and analysis. Acta Mater. 51, 863–872 (2003).
Hauer, M., Krebs, S., Kroemmer, W. & Henkel, K. M. Correlation of residual stresses and coating properties in Arc-Sprayed coatings on different substrates for maritime applications. J. Therm. Spray. Technol. 29, 1289–1299 (2020).
Varis, T., Suhonen, T., Laakso, J., Jokipii, M. & Vuoristo, P. Evaluation of residual stresses and their influence on cavitation Erosion resistance of high kinetic HVOF and HVAF-Sprayed WC-CoCr coatings. J. Therm. Spray. Technol. 29, 1365–1381 (2020).
Luo, W., Selvadurai, U. & Tillmann, W. Effect of residual stress on the wear resistance of thermal spray coatings. J. Therm. Spray. Technol. 25, 321–330 (2016).
Zhu, L., Xu, B., Wang, H. & Wang, C. On the evaluation of residual stress and mechanical properties of fecrbsi coatings by nanoindentation. Mater. Sci. Eng. A. 536, 98–102 (2012).
Xiao, Y., Wu, L., Luo, J. & Zhou, L. Mechanical response of thin hard coatings under indentation considering rough surface and residual stress. Diam. Relat. Mater. 108, 1–12 (2020).
Pang, M. et al. Effect of preheating temperature of the substrate on residual stress of Mo/8YSZ functionally gradient thermal barrier coatings prepared by plasma spraying. Surf. Coat. Technol. 385, 1–13 (2020).
Wang, H., Cheng, Y., Yang, J. & Liang, X. Microstructure and properties of Fe based amorphous coatings deposited by laser cladding under different preheating temperatures. J. Non Cryst. Solids. 602, 1–11 (2023).
Lobanoff, V. S. & Ross, R. R. CENTRIFUGAL PUMPS Design & Application Second Edition. Journal of Chemical Information and Modeling vol. 53 (1985).
Mohankumar, A. et al. Cold spray processing of AA2024/Al2O3 coating on magnesium AZ31B alloy: process parameters optimization, microstructure and adhesive strength performance of coating. Int. J. Light Mater. Manuf. 7, 721–737 (2024).
Feng, Y., Pang, X., Feng, K., Feng, Y. & Li, Z. A method for evaluating the crack resistance and predicting the preheating temperature of high hardness coating prepared by laser cladding. Surf. Coat. Technol. 432, 1–12 (2022).
Tan, A. W. Y. et al. Effect of substrate surface roughness on microstructure and mechanical properties of Cold-Sprayed Ti6Al4V coatings on Ti6Al4V substrates. J. Therm. Spray. Technol. 28, 1959–1973 (2019).
Dudziak, T. et al. Characterisation of the austenitic steels super 304H and HR3C after air and steam oxidation. Mater. High. Temp. 39, 206–219 (2022).
Mohankumar, A., Duraisamy, T., Packkirisamy, V. & Sampathkumar, D. Response surface methodology and mayfly optimization for predicting the properties of Cold-Sprayed AA2024/Al2O3 coatings on AZ31B magnesium alloy. J. Mater. Eng. Perform. 33, 13424–13442 (2023).
Gecu, R., Birol, B. & Özcan, M. Improving wear and corrosion protection of AISI 304 stainless steel by Al2O3-TiO2 hybrid coating via sol-gel process. Trans. Inst. Met. Finish. 100, 324–332 (2022).
Ding, L., Torbati-Sarraf, H. & Poursaee, A. The influence of the sandblasting as a surface mechanical attrition treatment on the electrochemical behavior of carbon steel in different pH solutions. Surf. Coat. Technol. 352, 112–119 (2018).
Geng, S., Sun, J. & Guo, L. Effect of sandblasting and subsequent acid pickling and passivation on the microstructure and corrosion behavior of 316L stainless steel. Mater. Des. 88, 1–7 (2015).
Wang, X. Y. & Li, D. Y. Mechanical and electrochemical behavior of nanocrystalline surface of 304 stainless steel. Electrochim. Acta. 47, 3939–3947 (2002).
Tillmann, W., Khalil, O. & Baumann, I. Influence of direct Splat-Affecting parameters on the Splat-Type distribution, porosity, and density of segmentation cracks in Plasma-Sprayed YSZ coatings. J. Therm. Spray. Technol. 30, 1015–1027 (2021).
Zhang, C. et al. Interface improvement of plasma cladded Ni-based coating on copper induced by substrate preheating. Sci. Technol. Weld. Join. 27, 353–360 (2022).
Liu, Y. et al. Effect of a Substrate’s Preheating Temperature on the Microstructure and Properties of Ni-Based Alloy Coatings. Lubricants vol. 12 1–13 at (2024). https://doi.org/10.3390/lubricants12010021
Acknowledgements
The authors thank the Faculty of Engineering, Universitas Negeri Semarang (UNNES), Semarang, Indonesia, for providing the financial support needed to complete this research through the Penelitian Dasar grant in 2025 with contract number 119.14.3/UN37/PPK.11/2025. This project was supported by Ongoing Research Funding program, (ORF-2025-7), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
CRediT authorship contribution statementDeni Fajar Fitriyana and Windy Desti Puspitasari: Methodology, Formal analysis, Conceptualization: Sivasubramanian Palanisamy and Mufti Muhadzdzib: Writing – review & editing, Methodology, Formal analysis; Samsudin Anis, Sarath K S and Januar Parlaungan Siregar: Validation, Formal analysis, Data curation; Tezara Cionita, Aravindhan Alagarsamy and Quanjin Ma: Writing – review & editing, Validation, Investigation, Formal analysis, Conceptualization; Saleh A Al-Farraj and Mansour I Almansour: Administration, Funding, Validation, Investigation. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
This research study did not involve the use of animals and humans.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fitriyana, D.F., Puspitasari, W.D., Palanisamy, S. et al. Impact of surface roughness and preheat temperature on fecrbmnsi coating properties prepared by the twin wire Arc spray method. Sci Rep 15, 24610 (2025). https://doi.org/10.1038/s41598-025-10713-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10713-8