Abstract
The Celestún Lagoon is located on the coast of the Yucatán Peninsula in southern Mexico. The peripheral margins of the lagoon are exposed to a constant influx of water from freshwater springs, where a wide diversity of freshwater fishes occurs. Simultaneously, these freshwater springs are mixing with marine water from the sea and brackish water from the lagoon. Gambusia yucatana is an endemic poeciliid freshwater fish of this coastal region, with no current parasitological records. In December 2023, 24 specimens from two freshwater springs near the Celestún Lagoon were examined for myxozoan infection. Disporic plasmodia containing mature myxospores floating freely within the bile were detected in all fish. The myxospores were ellipsoidal in the valvular and sutural views, measuring 9.5 ± 0.6 μm length and 6.5 ± 0.5 μm width. Two pyriform polar capsules discharging on opposite sides, 3.2 ± 0.4 μm length and 2.3 ± 0.2 μm width, and 5–6 polar filament coils. Polar tubule 31.3 μm long and coiled 5–6 times. The partial sequences of 18S rDNA and 28S rDNA displayed a similarity of ≤ 96.4% and ≤ 85.2% to all other available sequences from the genus Ellipsomyxa. Phylogenetic analyses revealed that the novel species named Ellipsomyxa prima n. sp. is closely related to other congeneric Ellipsomyxa parasites. This study describes the first parasite in G. yucatana and the first Ellipsomyxa species reported in a fish of the order Cyprinodontiformes and the family Poeciliidae.
Similar content being viewed by others
Introduction
Myxozoans (Cnidaria) constitute a group of fish parasites, with more than 2,600 species classified into 64 genera described worldwide1,2,3. A total of 12 nominal myxozoan species belonging to 6 genera have been identified in Mexico2,4. The most recent description was provided by Colunga-Ramírez et al.4, who reported Myxobolus mayarum Colunga-Ramírez, Aguirre-Macedo, Molnár, Székely, Sellyei, Cech, 2025 and Kudoa mayarum Colunga-Ramírez, Aguirre-Macedo, Molnár, Székely, Sellyei, Cech, 2025, parasitizing the cichlid fish Mayaheros urophthalmus (Günther, 1862), an endemic freshwater species of the Yucatán Peninsula, Mexico. Ellipsomyxa papantla Alama-Bermejo, Hernández-Orts, García-Varela, Oceguera-Figueroa, Pecková & Fiala, 2023, infecting Dormitator maculatus (Bloch, 1792), is the sole member of the genus Ellipsomyxa, Køie, 2003, thus far described in Mexico2. The genus Ellipsomyxa is a member of the class Myxozoa (Køie 2003), comprising only 23 species that infect the gallbladder of marine and freshwater fishes globally5,6. However, no species of Ellipsomyxa have been reported in fish belonging to the order Cyprinodontiformes Berg, 1940, including those in the family Poeciliidae Bonaparte, 1831. The family Poeciliidae includes over 40 species of the genus Gambusia Poey, 1854, which are found in freshwater habitats and often occur in salt and brackish water bodies, estuaries, coastal lagoons, or along shores in mangrove areas7,8. Gambusia yucatana, Regan, 1914, is an endemic fish species from the Yucatán Peninsula, Mexico9, where the Celestún Biosphere Reserve is located, and includes the brackish Celestún Coastal Lagoon, known for its extensive ichthyofauna10,11. Gambusia yucatana is of ecotoxicological importance due to its sensibility to petroleum components such as polycyclic aromatic hydrocarbons (PAHs) and pesticides; as a result, it has been proposed as a sentinel organism12,13. Despite its importance, no parasites infecting G. yucatana have been described. In the present study, a myxosporean infection was observed in the gallbladder of G. yucatana collected in the Baldiosera and Ya´xaa freshwater springs adjacent to the Celestún Coastal Lagoon in the Yucatán Peninsula, Mexico. Based on morphological characteristics and molecular analyses of 18S and 28S rDNA sequence data, we describe the first Ellipsomyxa species to infect a poecilid fish and the first parasite reported in G. yucatana.
Materials and methods
Ethical statement
The experimental protocols together with fish handling and sampling were in accordance with the Guidelines for Care and Manipulation of Laboratory Animals of Cinvestav, the Mexican Official norm NOM-062-ZOO-199, and were also approved by the Institutional Animal Care and Use Committee of the Veterinary Medical Research Institute, Budapest, Hungary. All research involving experiments on fish (Gambusia yucatana) was reviewed and approved by the Hungarian National Scientific Ethical Committee on Animal Experimentation under reference number: .PE/EA/00081 − 4/2023. The authors complied with the ARRIVE guidelines (https://arriveguidelines.org).
Sample collection
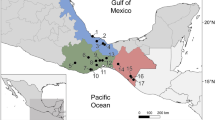
In December 2023, 24 Gambusia yucatana specimens were collected from two freshwater springs at the margin of Celestún Coastal Lagoon, Baldiosera, and Ya´xaa (Table 1). These springs are located within the Celestún Biosphere Reserve in the Yucatán Peninsula, México, in proximity to the brackish water of the Celestún coastal lagoon, and approximately 4.0 km from the sea.
The fish were caught using nets and were transported alive in aerated tanks to the Aquatic Pathology Laboratory at the Centro de Investigación y de Estudios Avanzados, Unidad Mérida (Cinvestav-Mérida), where they were kept in an aerated aquarium. The specimens were examined for myxosporean infections. The mean total length and mean fish body weight were 2.4 ± 0.36 cm (1.6–3.0 cm) and 0.14 ± 0.06 g (0.04–0.26 g). Due to their diminutive fish body size, they were euthanised by brain puncture to avoid mechanical damage to the integrity of the organs. The gills, gastrointestinal tract, gallbladder, muscle, and fins were studied under a stereomicroscope (Olympus SZX16) and excised pieces of fresh tissue were mounted on glass slides, then compressed under a cover glass and observed via light microscopy (Olympus BX53 with a digital camera Olympus DP74). Smear preparations of infected bile, either fresh or Lugol-stained, were used for morphological identification of the parasite. Additionally, samples from four parasitized gallbladders were preserved in molecular-grade 96% ethanol for subsequent molecular analyses.
Morphological identification
Fresh and Lugol-stained bile smears of infected bile were observed under a light microscope. The morphological and morphometric data were determined according to Whipps & Font14 using ImageJ software (http://imagej.nih.gov/ij). All measurements are given in micrometers (µm) expressed as the mean and standard deviation, followed by the range in parentheses.
Molecular characterization
Ethanol-fixed gallbladders with Ellipsomyxa myxospores were rinsed three times in a Tris-HCl buffer (10 mM Tris-HCI, pH 8.5), and then 50 µl nuclease-free water was added. Genomic DNA was isolated from the rinsed gallbladders using the Genomic DNA Mini Kit (Geneaid Biotech Ltd., Taiwan), following the instructions of the manufacturer. The small subunit ribosomal DNA (18S rDNA) and large subunit ribosomal DNA (28S rDNA) were amplified in short overlapping fragments by polymerase chain reaction (PCR) using different combinations of specific myxozoan primers (Table 2). The PCR reactions were performed in a final volume of 25 µl, which contained 1 × DreamTaq buffer (10 ×; Thermo Scientific), 0.2 mM dNTP mix (10 mM; Thermo Scientific), 100 nmol L-1 of each primer, 0.5 U DreamTaq polymerase (5 U; Thermo Scientific), 1 µl of template DNA, and nuclease-free water. The reaction conditions for the 18S rDNA were 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 90 s, with a final extension at 72 °C for 10 min. The reaction conditions for the 28S rDNA were 94ºC for 3 min, followed by 35 cycles of 94ºC for 45 s, 64ºC for 1 min, 72ºC for 1 min 45 s, with a final extension at 72ºC for 7 min. The amplified PCR products were run on a 1% agarose gel with 1× Tris-acetate-EDTA (TAE) buffer, stained with ethidium bromide (0.5 µl/ml) and examined under a UV transilluminator. The appropriate-sized bands (Supplementary Fig. 1) were purified with the DNA Fragment Purification Kit (Invitek, Berlin, Germany). Each PCR product was Sanger sequenced in both forward and reverse directions using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, USA) and run on an ABI PRISM 3100 Genetic Analyser (Applied Biosystems).
Phylogenetic analyses
The sequences were checked and edited according to chromatograms in Chromas v. 2.6.6 software (Technelysium Pty Ltd., Queensland, Australia), assembled, and aligned in MEGA11 software24. To explore the phylogenetic relationships of the Ellipsomyxa in study to other myxozoans, all congeneric sequences available in the GenBank database were downloaded, plus 17 sequences from closely related myxosporean species. In the case of the 28S rDNA, three congeneric sequences available in the GenBank database were added to the analysis, along with 13 additional sequences from closely related myxosporean species. The 18S rDNA Chloromyxum leydigi Mingazzini, 1890 (ON383909) and the 28S rDNA sequence of C. leydigi (FJ417055) were used as outgroups. The 18S rDNA sequences were aligned with MAFFT v. 11 online servers25 using the G-INS-i strategy selected. In turn, 28S rDNA sequences were aligned using ClustalW26 in MEGA11. Poorly aligned positions were eliminated using Gblocks v. 0.91b with less stringent parameters27.
Phylogenetic trees of the 18S rDNA and 28S rDNA sequences were constructed using Maximum Likelihood (ML) and Bayesian inference (BI) under the general time reversible model with a gamma-distributed rate and invariant sites (GTR + G + I) based on the Akaike information criterion (AIC) in MEGA1124. Maximum likelihood analyses were performed using MEGA11, and bootstrap (BS) values were calculated from 1000 replicates. Bayesian Inference analyses were performed in MrBayes v. 3.228, and posterior probabilities (PP) were calculated over 10 million generations with four parallel chains running simultaneously every 1000 generations with the burn-in set at 25%. The ML and BI trees were displayed and annotated in v. MEGA11 and FigTree v. 1.4.429, respectively. The two phylogenetic trees were edited with CorelDRAW Graphics Suite 2019 v. 21.3.0.755 (Corel Corporation, Ottawa, Canada). Genetic distances between the species in this study and other Ellipsomyxa sequences available in GenBank, all 19 ones used above, were calculated with the p-distance model in MEGA1124.
Results
All (n = 24) examined G. yucatana specimens exhibited disporic plasmodia and mature myxospores floating freely within the bile, with morphological characteristics consistent with those of the genus Ellipsomyxa. The morphological and sequence analyses showed that the myxospores described here belong to a novel Ellipsomyxa species. The species description is provided below.
Description
Ellipsomyxa prima n. sp.
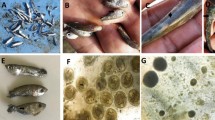
Plasmodia (n = 14): Disporic, subspherical to spherical in shape, measuring 15.5 ± 2.2 (13.0–20.5) µm in length and 14 ± 1.9 (11.8–19.0) µm in width (Figs. 1 and 2). Ellipsoidal plasmodia rarely observed (Fig. 1A).
Ellipsomyxa prima n. sp. plasmodia and mature myxospores in the gallbladder of Gambusia yucatana. (A) Spherical disporic plasmodia (p) containing myxospores (arrows) and immature plasmodia (ip); Ellipsoid plasmodia (*). (B) Myxospores in sutural view showing a sutural line (su). (C) Myxospore showing the polar tubule of a polar capsule coiling 5 − 6 times (white arrow). (D) Lugol stained myxospores in sutural view evidencing the capsular foramina (arrows). (E) Myxospore in sutural view highlighting the sporoplasm (*). (F) Lugol stained myxospore showing one polar tubule extruded (pt). Scale bars = 10 μm.
Spores (n = 42): Myxospores are ellipsoid in frontal and sutural views, measuring 9.51 ± 0.6 (8.3–10.9) µm in length, 6.5 ± 0.5 (5.2–7.6) µm in width, and 4.1 ± 0.5 (3.60–4.9) µm in thickness, with a curved suture line (Fig. 1; Table 3). Two pyriform and equal-sized polar capsules discharging sub-laterally to opposite sides in the sutural view, measuring 3.2 ± 0.4 (2.5–4.5) µm length and 2.3 ± 0.2 (1.7–3.0) µm width. Sporoplasm situated between the polar capsules (Fig. 1E). Polar tubule coiling 5–6 times (Fig. 1C), extruded polar filament (n = 1) 31.39 μm long (Fig. 1F).
Taxonomic summary
Type host: Gambusia yucatana Regan, 1914 (Cyprinodontiformes: Poeciliidae).
Site in host: Coelozoic, in the gallbladder.
Prevalence: 24/24 (100%).
Type locality: Baldiosera and Ya´xaa freshwater springs (20° 87′ 74′ N, 90° 35′ 54′ W) into the Celestún Coastal Lagoon (Biosphere Reserve) (20° 87′, 74′ N, 90° 35′, 54′ W).
Material deposited: Phototypes were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNMPCC-HNHM-PAR-72,085. The DNA samples are available in the Fish Pathology and Parasitology Lab for request. Contact information was added in the data availability section.
Representative sequences: 18S rDNA (1636 bp) and 28S rDNA (2216 bp) were submitted in GenBank (accession numbers: PV385047 and PV428871).
Etymology: The name derives from the Latin “prima”, meaning “first”.
Remarks: The morphological and morphometric features of E. prima n. sp. and their congeners are compared in Table 3. All documented species of Ellipsomyxa, including the novel species in this study, have been reported to infect the gallbladder. Considering the total size and dimensions of E. prima n. sp. myxospore and its polar capsules, it was compared with the following species: Ellipsomyxa ariusi Chandran, Zacharia, Sathianandan & Sanil, 2020, Ellipsomyxa apogoni Heiniger & Adlard, 2014, Ellipsomyxa boleophthalmi Vandana, Poojary, Tripathi, Pavan-Kumar, Pratapa, Sanil & Rajendran, 2021, Ellipsomyxa gordeyi Yurakhno, Ha, & Whipps, 2024, and Ellipsomyxa tucujuensis Ferreira, da Silva, de Carvalho, Bittencourt, Hamoy, Matos & Videira, 2021. Nevertheless, the type host, locality, and some subtle morphological differences should be highlighted for differentiation.
The myxospores of E. prima n. sp. and E. ariusi overlapped in almost all measured dimensions, but the novel species was found to be significantly thinner (4.1 μm vs. 7.7 μm), and have more polar tubules coiled (5–6 vs. 4–5). Furthermore, E. ariusi was found infecting Arius arius (Hamilton, 1822), a marine fish from India. In comparison with E. apogoni, they have similar myxospore dimensions (9.5 × 6.5 μm vs. 10.1 × 6.9 μm), but differ in polar tubule coiling (2–4 vs. 5–6), which is less in E. apogoni. In addition, E. apogoni was collected from the marine fish Apogon doederleini (Jordan & Snyder, 1901) from Australia. Consistent with the E. boleophthalmi, both species occurred in fish inhabiting an estuarine environment, but E. boleophthalmi was found in Boleophthalmus dussumieri (Valenciennes) from India. The dimensions of both latter species are highly similar (9.5 × 6.5 μm vs. 9.8 × 7.2 μm); however, the morphology of the polar capsule is spherical for E. boleophthalmi, and pyriform in E. prima n. sp. It is similar to E. tucujuensis, which has polar capsules with a spherical shape.
The myxospores of E. gordeyi showed the greatest dimensional similarity to myxospores of E. prima n. sp. The absence of data on the thickness and polar tubule coiling prevents their morphological differentiation. The occurrence of E. gordeyi has been reported in different marine fish fish species from various families, including Mugil cephalus (Linnaeus), Planiliza melinoptera (Valenciennes), Planiliza sp. D sensu, Planiliza subviridis (Valenciennes), and Gobiosoma bosc (Lacepède) from Vietnam. Comparing the novel species with the unique Ellipsomyxa species described in Mexico; E. papantla, has been reported infecting D. maculatus in a freshwater environment. Morphologically, the myxospores of E. prima n. sp. are smaller in all dimensions.
However, measurements of E. prima n. sp. myxospores overlapped in almost all spore dimensions with the above mentioned species. The consensus 18S rDNA (1636 bp) and 28S rDNA (2216 bp) sequences of E. prima n. sp. obtained from G. yucatana did not reveal any significant match with myxozoan sequences available in GenBank. In the 18S and 28S rDNA phylogenetic analyses, E. prima n. sp. was not associated closely with any species. In the case of the 18S rDNA phylogeny, E. prima n. sp. is situated in a clade (PP = 0.89) that includes Ellipsomyxa species from freshwater, estuarine and marine habitats as well. The molecular data of this clade were employed to calculate the genetic p-distance and the sequence similarities. In the case of the 28S rDNA, sequences only from three Ellipsomyxa species were available (Table 4).
According to the 18S rDNA sequences, the highest sequence similarity was 96.4% (3.6% of genetic distance) with the of Ellipsomyxa sp. MH212373 (Unpublished) from the fish Toxotes jaculatrix (Pallas) in Terengganu, Malaysia, and 85.2% similarity (14.8% genetic distance) with the 28S rDNA sequence of Ellipsomyxa adlardi Whipps & Font, 2013 from the Gobiosoma bosc (Lacepède) in the estuarine Lake Pontchartrain, Louisiana (USA) (Table 4).
Phylogenetic analyses
Phylogenetic analyses of 18S rDNA and 28S rDNA sequences indicated that E. prima n. sp. is positioned within a clade that includes Ellipsomyxa species found in marine, estuarine, and freshwater fish hosts (Fig. 3). The 18S rDNA phylogeny showed that the Ellipsomyxa divided into two subclades. The smaller one comprises exclusively marine species. The bigger subclade split into two additional distinct lineages, supported only by posterior probability (PP = 1.0): one consisting of a mixture of estuarine, freshwater, and marine species, and the other comprising only freshwater species. The other subclade contains a mixture of marine, estuarine, and freshwater species (E. adlardi, E. ariusi, E. boleophthalmi, E. gobii, E. gordeyi, Ellipsomyxa intravesica Ksepka & Bullard, 2023, Ellipsomyxa mugilis Sitja-Bobadilla & Alvarez-Pellitero, 1993, Ellipsomyxa plagioscioni Zatti, Maia & Adriano, 2020, Ellipsomyxa syngnathi Køie & Karlsbakk, 2009, and Ellipsomyxa sp.), which includes E. prima n. sp. (PP = 0.89). However, E. prima n. sp. did not show any close relationship with any species (Fig. 3A). In the 28S rDNA phylogenetic analysis, E. prima n. sp. is a sister to a clade (PP = 0.88; BS = 99%) comprising the marine species E. gobii and E. gordeyi, and the estuarine E. adlardi (Fig. 3B). Due to the low number of sequences it is not possible to draw conclusions based on the phylogeny of 28S rDNA sequences.
Bayesian inference phylogenetic trees from 18S and 28S rDNA for Ellipsomyxa prima n. sp. and its relatives. GenBank accession numbers are adjacent to the species name. Posterior probabilities (PP) and Bootstrap (BP) values greater than 50% are included at branch nodes. The scale bars represent substitutions per site.
Discussion
Gambusia yucatana is an endemic freshwater fish from Southern Mexico that has been suggested as a suitable sentinel organism for ecotoxicological studies12,13. No parasites have been documented in this fish species to date. In the present study, we found a 100% prevalence of Ellipsomyxa myxospores in the gallbladders of 24 G. yucatana individuals. In most wild fish populations, the prevalence has been documented as lower, while high or full prevalence of myxozoans has been more frequently detected in fish farm settings or laboratory experiments45,46. However, some environmental factors, such as eutrophication, have been associated with high prevalence in wildlife fish populations47,48. It has been suggested that eutrophication may increase the prevalence of infected fish and the infracommunity richness of myxozoans in wildlife hosts47,49. It has been theorized that eutrophication may trigger an increase in invertebrate host populations, with a consequent positive effect on the development and release of infecting actinospores for fish hosts49. The Celestún coastal lagoon has been distinguished due to exhibiting a tendency towards eutrophication, due to often restricted water exchange with the adjacent ocean, leading to the accumulation of nutrients derived from the surrounding watershed50. The 100% prevalence of myxozoans associated with fish host from this lagoon has been reported for M. mayarum infecting M. urophthalmus from Celestún lagoon, and their adjacent Baldiosera freshwater spring was observed4. The specimens of G. yucatana examined in this study were obtained from two freshwater springs adjacent to the Celestún coastal lagoon, Baldiosera and Ya´xaa. The populations of G. yucatana from the Yucatán Peninsula region differ from those in the rest of Mexico, as many stocks in this region inhabit isolated freshwater springs or cenotes8. Future studies with sampling in different geographical regions of Mexico could be useful to verify the distribution of the fish and to make comparisons on the prevalence of E. prima n. sp.
The general morphology of E. prima n. sp. myxospores is consistent with the morphology of other Ellipsomyxa species previously described. Ellipsomyxa gordeyi is the closest species based on morphometric and morphological descriptions; nevertheless, the two species differ regarding the fish host, locality, and genetics. To date, more than 2,600 species of myxosporeans have been reported; however, less than 1% belong to the genus Ellipsomyxa, which was differentiated from Leptotheca Thélohan, 1895 at the beginning of the millennium5,37. Currently, the genus Ellipsomyxa is placed in the family Ceratomyxidae. However, its taxonomic position is questionable, as morphological, molecular, and phylogenetic studies have shown that Ellipsomyxa is closer to the genus Myxidium (family Myxidiidae) than the genus Ceratomyxa18,44. The 18S rDNA sequences of E. prima n. sp. showed moderate genetic sequence similarity with the available sequences reported for the nominal species (≤ 96.4%). In turn, the 28S rDNA sequence showed lower values of similarity (≤ 85.2%) with the only three available sequences of E. adlardi, E. gobii, and E. gordeyi. The genetic similarities should be handled carefully, as the available 28S rDNA sequences are shorter (~ 700 bp) than the obtained sequence of E. prima n. sp. (2216 bp), and it is unknown whether the remaining 28S rDNA sequences of the species above encompass additional variable or conservative regions over those 700 bp analysed in this study; therefore the current data may be insufficient to ensure the accuracy of which species is most genetically similar to E. prima n. sp.
Despite our molecular analyses, which include DNA sequence data of all congeneric Ellipsomyxa, no clear phylogenetic patterns could be observed between species, especially those collected from marine and brackish hosts. The phylogeny shows that Ellipsomyxa parasites have rapidly specialized, complicating the resolution of relationships within the genus. However, the present and previous results5,6,42 indicate that the genus Ellipsomyxa has a monophyletic origin. The molecular data available in the genus are still very limited, and unravelling potential evolutionary patterns requires the identification of additional species and sequences. E. prima n. sp., found in G. yucatana, is a freshwater species that can tolerate brackish water. In the 18S rDNA phylogenetic analysis, it is positioned in a separate clade with Ellipsomyxa species parasitizing freshwater fish (only supported by posterior probability, PP = 1.0). Similar results have been reported by Zatti et al.42 and Ksepka et al.39. In contrast, E. intravesica has been reported in the freshwater host Pangasius macronema Bleeker from Vietnam but phylogenetically associated with Ellipsomyxa species that have been reported with brackish and marine hosts39. Meanwhile, Zatti et al.42 detected the E. plagioscioni parasite in the gallbladder of Plagioscion squamosissimus Heckel, which is mainly a freshwater fish but is commonly found also in brackish water in the Amazonian estuarine environments in South America. Phylogenetic analyses suggested that marine transgressions in the Central Region of South America probably influenced a pathway for the adaptation and rapid radiation of these cnidarian parasites in freshwater environments42. Similarly, the results of our phylogenetic analyses also suggest the potential for the wide distribution of the Ellipsomyxa species in the southern Mexican region. To elucidate this complex phylogenetic question, a larger amount of data on the Ellipsomyxa genus from different water environments in Mexico and other territories will need to be collected.
Conclusion
Based on morphological characterization and molecular data of the 18S rDNA and 28S rDNA sequences, a novel myxozoan parasite, Ellipsomyxa prima n. sp. was described from the Gambusia yucatana endemic fish in Mexico. This new species is the first record of an Ellipsomyxa species reported within the order Cyprinodontiformes, family Poeciliid, and the first detected parasite in G. yucatana. The available information about the genus Ellipsomyxa is still limited. The present study indicates that Ellipsomyxa prima n. sp. does not form monophyletic clade with other Ellipsomyxa species from freshwater environments. Further studies employing molecular and morphological data on novel Ellipsomyxa species from different aquatic environments could clarify the evolutionary context of these parasites.
Data availability
The datasets generated and/or analysed during the current study are available in the GenBank repository, Acc. Number PV385047 (https://www.ncbi.nlm.nih.gov/nuccore/PV385047) (for data request, contact the author Graciela Colunga-Ramírez, graciela.colunga@vmri.hun-ren.hu) and PV428871 (https://www.ncbi.nlm.nih.gov/nuccore/PV428871). Type material is deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNMPCC-HNHM-PAR-72085 is available through the head of the Zoological Department on reasonable request. The DNA sample is available at request (contact: Gábor Cech, cech.gabor@vmri.hun.ren.hu).
References
Lisnerová, M. et al. Correlated evolution of fish host length and parasite spore size: a tale from myxosporeans inhabiting elasmobranchs. Int. J. Parasitol. 52, 97–110 (2022).
Alama-Bermejo, G. et al. Diversity of myxozoans (Cnidaria) infecting Neotropical fishes in Southern Mexico. Sci. Rep. 13, 12106. https://doi.org/10.1038/s41598-023-38482-2 (2023).
Sandberg, T. O. M. et al. Evolution of Myxozoan mitochondrial genomes: insights from Myxobolids. BMC Genom. 25, 388. https://doi.org/10.1186/s12864-024-10254-w (2024).
Colunga-Ramírez, G. E. et al. Two new Myxozoan parasites, Myxobolus Mayarum n. sp. and Kudoa Mayarum n. sp., infecting the Neotropical fish Mayan cichlid, Mayaheros urophthalmus (Günther, 1862) in the Yucatán Peninsula, Mexico. Acta Trop. 262, 107527. https://doi.org/10.1016/j.actatropica.2025.107527 (2025).
Yurakhno, V. M., Ha, V. T. & Whipps, C. M. Phylogenetic analysis of Ellipsomyxa species (Myxosporea) and description of Ellipsomyxa Gordeyi n. Sp. from the gall bladder of mullets (Mugiliformes: Mugilidae) in Nha Trang Bay of the East Sea, Vietnam. Parasitol. Int. 102, 102918. https://doi.org/10.1016/j.parint.2024.102918 (2024).
Pereira, C. M. B. et al. New species of Ellipsomyxa (Bivalvulida: Ceratomyxidae) parasitizing the gallbladder of Ageneiosus ucayalensis (Siluriformes: Auchenipteridae) in the Brazilian Amazon region. Parasit. Int. 106, 103036. https://doi.org/10.1016/j.parint.2025.103036 (2025).
Rivas, L. R. Subgenera and species groups in the poeciliid fish genus Gambusia Poey. Copeia. 331–347 (1963). (1963).
Greenfield, D. W. Review of the Gambusia yucatana complex (Pisces: Poeciliidae) of Mexico and Central America. Copeia 2, 368–378 (1985).
Rodríguez-Fuentes, G., Marín-López, V. & Hernández-Márquez, E. Cholinesterases in Gambusia yucatana: biochemical characterization and its relationship with sex and total length. Bull. Environ. Contam. Toxicol. 97, 776–780 (2016).
Sosa-Medina, T., Vidal-Martínez, V. M. & Aguirre-Macedo, M. L. Metazoan parasites of fishes from the Celestun coastal lagoon, yucatan, Mexico. Zootaxa 4007, 529–544 (2015).
Vega-Cendejas, M. E. Ictiofauna de la Reserva de la Biosfera Celestún, Yucatán: una contribución al conocimiento de su biodiversidad. Anales Inst Biol UNAM Ser Zool. 75, 193–206 (2004).
Rendón-von Osten, J., Ortíz-Arana, A., Guilhermino, L. & Soares, A. M. V. M. In vivo evaluation of three biomarkers in the mosquitofish (Gambusia yucatana) exposed to pesticides. Chemosphere 58, 627–636 (2005).
Aguilar, L. et al. Effects of polycyclic aromatic hydrocarbons on biomarker responses in Gambusia yucatana, an endemic fish from Yucatán Peninsula, Mexico. Environ Sci Pollut Res. 28, 47262–47274 (2021).
Whipps, C. M. & Font, W. F. Interaction of two myxozoan parasites from naked goby Gobiosoma bosc, in Lake Pontchartrain, Louisiana. J Parasitol. 99, 441–447 (2013).
Hallett, S. L. & Diamant, A. Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya Lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the Coast of Queensland, Australia. Dis Aquat Organ. 46, 197–212 (2001).
Diamant, A., Whipps, C. M. & Kent, M. L. A new species of Sphaeromyxa (Myxosporea: sphaeromyxina: Sphaeromyxidae) in devil firefish, Pterois miles (Scorpaenidae), from the Northern red sea: morphology, ultrastructure and phylogeny. J Parasitol. 90, 1434–1442 (2004).
Barta, J. R. et al. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J Parasitol. 8, 262–271 (1997).
Fiala, I. The phylogeny of myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Int J Parasitol. 36, 1521–1534 (2006).
Eszterbauer, E. & Székely, C. Molecular phylogeny of the kidney-parasitic Sphaerospora renicola from common carp (Cyprinus carpio) and Sphaerospora sp. from goldfish (Carassius auratus auratus). Acta Vet Hung. 52, 469–478 (2004).
Whipps, C. M., Adlard, R. D., Bryant, M. S. & Kent, M. L. Two unusual myxozoans, Kudoa quadricornis n. sp. (Mulitvalvulida) from the muscle of goldspotted trevally (Carangoides fulvoguttatus) and Kudoa permulticapsula n. sp. (Multivalvulida) from the muscle of Sp.nish mackerel (Scomberomorus commerson) from the great barrier reef, Australia. J Parasitol. 89, 168–173 (2003).
Bartošová-Sojková, P., Fiala, I., Hypša, V. & Concatenated SSU and LSU rDNA data confirm the main evolutionary trends within myxosporeans (Myxozoa: Myxosporea) and provide an effective tool for their molecular phylogenetics. Mol Phylogenet Evol. 53, 81–93 (2009).
Van der Auwera, G., Chapelle, S. & De Wachter, R. Structure of the large ribosomal subunit RNA of Phytophthora megasperma, and phylogeny of the oomycetes. FEBS Lett. 338, 133–136 (1994).
Whipps, C. M. et al. Phylogeny of the multivalvulidae (Myxozoa: Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol. 3, 618–622 (2004).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38, 3022–3027 (2021).
Katoh, K., Rozewicki, J. & Yamada, K. D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166 (2019).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTALW: improving through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res. 22, 4673–4680 (1994).
Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 17, 540–552 (2000).
Ronquist, F. et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across large model space. Syst Biol. 61, 539–542 (2012).
Rambaut, A. & FigTree V. 1.4.4: Tree Figure Drawing Tool. (2018). http://tree.bio.ed.ac
Chandran, A., Zacharia, P. U., Sathianandan, T. V. & Sanil, N. K. Ellipsomyxa ariusi sp. Nov. (Myxosporea: Ceratomyxidae), a new myxosporean infecting the gallbladder of threadfin sea catfish Arius arius in India. Dis Aquat Org. 142, 8–97 (2020).
Heiniger, H. & Adlard, R. D. Relatedness of novel species of Myxidium Bütschli Zschokkella Auerbach, 1910 and Ellipsomyxa Køie, 2003 (Myxosporea: Bivalvulida) from the gall bladders of marine fishes (Teleostei) from Australian waters. Syst. Parasitol. 87, 47–72 (2014). (1882).
Vandana, V. R. et al. A Nov.l Myxozoan parasite, Ellipsomyxa boleophthalmi sp. Nov. (Myxozoa: Ceratomyxidae) in the brackishwater fish, Boleophthalmus dussumieri valenciennes, 1837 (Perciformes: Gobiidae) from India. Parasitol Res. 120, 1269–1279 (2021).
Ferreira, R. L. et al. Ellipsomyxa tucujuensis n. sp. (Myxozoa: Ceratomyxidae), a parasite of Satanoperca jurupari (Osteichthyes: Cichlidae) from the Brazilian Amazon. Parasitol Int. 83, 102332. https://doi.org/10.1016/j.parint.2021.102332 (2021).
Zatti, S. A. & Et Novel Myxobolus and Ellipsomyxa species (Cnidaria: Myxozoa) parasiting Brachyplatystoma rousseauxii (Siluriformes: Pimelodidae) in the Amazon basin, Brazil. Parasitol Int. 67, 612–621 (2018).
da Silva, D. T. et al. Ellipsomyxa arariensis n. sp. (Myxozoa: Ceratomyxidae), a new myxozoan parasite of Pygocentrus nattereri Kner, 1858 (Teleostei: Characidae) and Pimelodus ornatus Kner, 1858 (Teleostei: Pimelodidae) from Marajó Island, in the Brazilian Amazon region. Parasitol Res. 117, 3537–3545 (2018).
Gunter, N. & Adlard, R. The demise of Leptotheca thélohan, 1895 (Myxozoa: myxosporea: Ceratomyxidae) and assignment of its species to Ceratomyxa thélohan, 1892 (Myxosporea: Ceratomyxidae), Ellipsomyxa Køie, 2003 (Myxosporea: Ceratomyxidae), Myxobolus Bütschli, 1882 and Sphaerospora thélohan, 1892 (Myxosporea: Sphaerosporidae). Syst Parasitol. 75, 81–104 (2010).
Køie, M. Ellipsomyxa gobii gen. Et sp. N. (Myxozoa: Ceratomyxidae) in the common goby Pomatoschistus microps (Teleostei: Gobiidae) from Denmark. Folia Parasit. 50, 269–271 (2003).
Azevedo, C. et al. Fine structure of the plasmodia and myxospore of Ellipsomyxa gobioides n. sp. (Myxozoa) found in the gall bladder of Gobioides broussonnetii (Teleostei: Gobiidae) from the lower Amazon river. J Eukaryot Microbiol. 60, 490–496 (2013).
Ksepka, S. P., Truong, T. N. & Bullard, S. A. A new species of Ellipsomyxa køie, 2003 (Bivalvulida) infecting the gall bladder of Pangasius macronema bleeker (Siluriformes: Pangasiidae) from the Mekong river delta, Vietnam. Syst Parasitol. 100, 647–656 (2023).
Thabet, A., Tlig-Zouari, S., Al Omar, S. Y. & Mansour, L. Molecular and morphological characterization of two species of the genus Ellipsomyxa køie, 2003 (Ceratomyxidae) from the gallbladder of Liza saliens (Risso) off Tunisian Coasts of the mediterranean. Syst Parasitol. 93, 601–611 (2016).
Sitjà-Bobadilla, A. & Alvarez-Pellitero, P. Zschokkella mugilis n. sp. (Myxosporea: Bivalvulida) from mullets (Teleostei: Mugilidae) of Mediterranean waters: light and electron microscopic description. J. Eukaryot. Microbiol. 40, 755–764 (1993).
Zatti, S. A., Maia, A. A. M. & Adriano, E. A. Growing diversity supports radiation of an Ellipsomyxa lineage into the Amazon freshwater: description of two novel species parasitizing fish from Tapajós and Amazon rivers. Acta Trop. 211, 105616. https://doi.org/10.1016/j.actatropica.2020.105616 (2020).
Figueredo, R. T. A., Müller, M. I., Long, P. F. & Adriano, E. A. Myxozoan ceratomyxids infecting the gallbladder of Amazonian ornamental cichlid fish: description of Ellipsomyxa santarenensis n. sp. and report of Ceratomyxa amazonensis in a new host. Diversity 15, 830. https://doi.org/10.3390/d15070830 (2023).
Køie, M. & Karlsbakk, E. Ellipsomyxa syngnathi sp. n. (Myxozoa, Myxosporea) in the pipefish Syngnathus typhle and S. rostellatus (Teleostei, Syngnathidae) from Denmark. Parasitol. Res. 105, 1611–1616 (2009).
Molnar, K. & Kovács-Gayer, É. The pathogenicity and development within the host fish of Myxobolus cyprini Doflein, 1898. Parasitology 90, 549–555 (1985).
Yanagida, T., Sameshima, M., Nasu, H., Yokoyama, H. & Ogawa, K. Temperature effects on the development of Enteromyxum spp. (Myxozoa) in experimentally infected tiger puffer, Takifugu rubripes. J Fish Dis. 29, 561–567 (2006). Temminck & Schlegel
Marcogliese, D. J. & Cone, D. K. Myxozoan communities parasitizing Notropis hudsonius (Cyprinidae) at selected localities on the St. Lawrence River, Quebec: possible effects of urban effluents. J Parasitol. 87, 951–956 (2001).
Sitjà-Bobadilla, A., Schmidt-Posthaus, H., Wahli, T., Holland, J. W. & Secombes, C. J. Fish immune responses to Myxozoa. In: (eds Okamura, B., Gruhl, A. & Bartholomew, J. L.) Myxozoan Evolution, Ecology and Development, 253–280 (Springer, Cham, (2015).
Schmidt-Posthaus, H. & Wahli, T. Host and environmental influences on development of disease. In: (eds Okamura, B., Gruhl, A. & Bartholomew, J. L.) Myxozoan Evolution, Ecology and Development, 281–293 (Springer, Cham, (2015).
González, F. U. T., Herrera-Silveira, J. A. & Aguirre-Macedo, M. L. Water quality variability and eutrophic trends in karstic tropical coastal lagoons of the Yucatán Peninsula. Estuar Coast Shelf Sci. 76, 418–430 (2008).
Acknowledgements
The authors thank Francisco de Atocha Puc Itza and Arturo Centeno Chalé for fish collection and maintenance. We are grateful to J. Mirella Hernández de S. for identifying the fish species.
Funding
Open access funding provided by HUN-REN Veterinary Medical Research Institute. This study was supported by the Stipendium Hungaricum Program. Graciela Colunga-Ramírez is supported by SECIHTI, Mexico (grant number: CVU 769732).
Author information
Authors and Affiliations
Contributions
Colunga-Ramírez Graciela: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Aguirre-Macedo M. Leopoldina: Conceptualization, Writing – review, Resources, field and laboratory support in Mexico. Kálmán Molnár: Writing – review & Editing. Csaba Székely: Conceptualization, Supervision, Resources, Project administration, Funding. Boglárka Sellyei: Writing – review & editing, Conceptualization, Methodology, Supervision, Resources. Gábor Cech: Writing – review & editing, Conceptualization, Methodology, Supervision, Resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colunga-Ramírez, G., Cech, G., Aguirre-Macedo, M.L. et al. Description of Ellipsomyxa prima n. sp. in the gallbladder of Gambusia yucatana (Cyprinodontiformes: Poeciliidae) from freshwater springs in the Yucatán Peninsula, Mexico. Sci Rep 15, 25213 (2025). https://doi.org/10.1038/s41598-025-10781-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10781-w