Abstract
There are few reported studies on post-stroke fatigue (PSF) in young and middle-aged stroke patients, however, PSF plays a key role in the patient’s disease regression. Exploring the level of PSF and the influencing factors in young and middle-aged stroke patients is crucial for determining how to reduce the level of PSF and improve the patients’ motivation for rehabilitation treatment. Therefore, this study investigated the level of PSF in young and middle-aged stroke patients and analyzed the factors influencing PSF to provide a reference or basis for healthcare professionals to develop effective and targeted PSF intervention programs. The purpose of this study was to investigate the incidence of fatigue and its related influencing factors in young and middle-aged stroke patients. A total of 300 young and middle-aged stroke patients hospitalized in the Neurology Department of a tertiary hospital in Xi’an, China, from June 13 to December 31, 2024 were consecutively recruited by convenience sampling method. According to the Fatigue Severity scale (FSS), the patients were divided into a fatigue group (FSS ≥ 36 points, 187 cases) and a non-fatigue group (FSS < 36 points, 113 cases). The general situation questionnaire, Modified Rankin Scale (mRS), Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Pittsburgh Sleep Quality Index (PSQI) and Stroke Specific Quality of Life Scale (SS-QOL), Chronic Disease Self-efficacy Scale (CDSES) were used to investigate and study them. A logistic regression model was established and stratified analysis was conducted to explore the factors influencing PSF in young and middle-aged patients. The incidence of PSF among 300 young and middle-aged stroke patients was 62.3%, Univariate analysis showed that Pre-Stroke Fatigue (PrSF), mRS score, SAS score, SDS score, PSQI score, SS-QOL score, CDSES score, marital status and occupation were related to PSF (P < 0.05). Multivariate regression analysis revealed that marital status (OR = 8.908, 95%CI 1.776–44.674), PrSF( OR = 2.909, 95%CI 1.555–5.443), SDS score (OR = 1.099, 95%CI 1.046–1.154) and SS-QOL score (OR = 0.985, 95%CI 0.972–0.998) were associated with the occurrence of PSF. Stratified analysis showed that in the group of patients with PrSF, Be married (OR = 0.438, 95%CI 0.046–4.203), SDS score (OR = 1.052, 95%CI 0.965–1.146), SS-QOL score (OR = 0.960, 95%CI 0.937–0.984), among which the SS-QOL score was associated with the risk of PSF (P < 0.001); In the group of patients without PrSF, SDS score (OR = 1.086, 95%CI 1.033–1.142) was associated with a high risk of PSF (P < 0.001), Be married (OR = 0.060, 95% CI 0.007–0.490) and SS-QOL score (OR = 0.984, 95% CI 0.974–0.995) were associated with a low risk of PSF (P < 0.05). The fatigue status of young and middle-aged stroke patients is more serious. Clinically, we should strengthen the protection of high-risk patients with the above risk factors, and corresponding intervention programs should be formulated in time to reduce the incidence of PSF, alleviate the fatigue symptoms of patients, and enhance their quality of life.
Trial registration: Registration number of China Clinical Trials Registration Center ChiCTR2500099037.
Similar content being viewed by others
Introduction
PSF is one of the unique and easily overlooked complications after stroke, which leads to physical fatigue accompanied by mental and psychological exhaustion of patients. To date, there is no broad consensus on the definition of PSF. PSF is widely regarded as a multidimensional comprehensive experience covering motion perception, emotional, and cognitive aspects. It is characterized by a sense of exhaustion early in physical or mental activity, which cannot be effectively alleviated by rest1. It can occur in the acute stage, rehabilitation stage and sequelae stage of stroke, and does not recover naturally with time2,3. PSF represents one of the most persistent physical and psychological symptoms accompanying stroke survivors, with a global prevalence of 46.79%4. Longitudinal evidence from Delva et al.5 showed that the incidence of PSF in stroke patients was 64–68% at 6 months, 70–74% at 1 year, and 58% at 3 years after the onset of the disease. Studies have shown that 16% -74% of stroke survivors suffer from the lasting effects of fatigue on their daily lives6. PSF often significantly impairs patients’ capacity to actively participate in and adhere to therapeutic interventions, which seriously hinders the recovery of patients ' physical functions and self-care ability7, limits the effectiveness of rehabilitation treatment, and affects patients ' return to normal work and life8. However, as a subjective feeling, PSF’s mild clinical manifestations is not significant, and most patients perceive their symptoms as mild, lacking awareness of the severity and importance of this complication. In addition, clinical medical staff lack awareness of screening and evaluation of PSF9, which often leads to the fact that this symptom is ignored by medical staff and their families, thus missing the best opportunity for timely intervention and causing great distress to patients. In addition, due to the difficulty in evaluating and quantifying PSF, the lack of standardized measurement criteria and the combined effects of multiple factors, it has become an important problem that is easily ignored in clinical practice but needs to be solved urgently.
Epidemiological studies10 have reported that the trend of younger stroke has covered the world. The young and middle-aged group is at a critical stage of career development, and it is also the driving force for social development and progress. At the same time, as the main economic source and the mainstay of the family, it is necessary to deal with the multi-party pressure from family, work and social interaction. The fatigue symptoms after illness are also more significant, which not only brings great pain to the patients themselves but also brings heavy family care pressure and social burden11. Therefore, the current situation of fatigue in young and middle-aged stroke patients is not optimistic and should be highly valued. It not only significantly affects the quality of life of patients, but may also exert long-term negative impact on their occupational and social activities, which urgently needs to attract extensive attention from all sectors of society. However, the current research and practice mostly focus on the fatigue problem of elderly stroke patients, and pay insufficient attention to the fatigue phenomena among younger stroke patients. There is a lack of evidence support for research and practice, and the special needs of this group are often ignored in clinical practice. Based on this, this study aims to investigate the current situation of PSF in young and middle-aged stroke patients after illness, explore and analyze its related influencing factors, provide a reference for clinical practice, help healthcare professionals understand the fatigue problems of this patient population more comprehensively, and then help to promote the development of more accurate PSF prevention and treatment strategies.
Data and methods
Research subjects
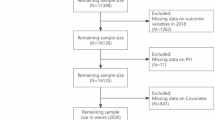
In this study, 300 young and middle-aged stroke patients admitted to the Department of Neurology of a tertiary hospital in Xi’an City, China, from June 13 to December 31, 2024 were selected by convenience sampling method. Inclusion criteria: (i) Patients who met the diagnostic criteria for stroke in the Chinese Classification of Cerebrovascular Diseases 2015 as revised by the Neurology Branch of the Chinese Medical Association and were diagnosed with stroke by brain CT or MRI; (ii) 18 years ≤ age < 65 years; (iii) No cognitive dysfunction, clear consciousness, and able to communicate through language or text; (iv) Informed consent to participate in the study. Exclusion criteria: (i) There are multiple sclerosis, systemic lupus erythematosus, Parkinson’s disease, malignant tumours and other fatigue-related diseases; (ii) Uncooperative patients with mental retardation or other malignant diseases after severe mental disorders; (iii) Patients with recurrent stroke or other serious diseases during the study; (iv) There was a history of depression before stroke.
This study received ethical approval from the Medical Ethics Committee of Shaanxi Provincial People’s Hospital (Approval Number: SPPH-LLBG-17-3.2) in accordance with the Declaration of Helsinki. All participants have obtained informed consent and signed relevant agreements. The collected data have all been anonymized to avoid errors.
Sample size calculation
This investigation utilized a two-phase analytical approach: initial descriptive analysis of the study population characteristics, followed by multivariate regression analysis to identify significant predictors. The sample size is at least 10 times that of the outcome variable.
In this study, the SAS comprised the largest number of items, Therefore, the sample size calculation was based on the SAS, which consisted of 20 items, requiring a minimum of 200 cases. To compensate for potential data loss (including 10% invalid questionnaires, disease progression, and questionnaire loss), we distributed 312 questionnaires. Following quality control procedures, 12 invalid responses were excluded, resulting in 300 valid responses and an effective response rate of 96.15%.
Description of the patient screening process
In this study, 312 patients were initially included, and 12 cases with missing basic information were excluded, leaving 300 cases for the final data analysis, as detailed in the flow chart (Fig. 1).
Research tools
General information questionnaire
It was designed by the investigators themselves and included two sections with demographic and sociological information (age, gender, marital status, occupation,
Education level, etc.) and disease-related information (stroke lesion location, course of the disease, PrSF, etc.). PrSF was measured retrospectively when the time of feeling fatigue before stroke was more than 3 months12.
Fatigue severity scale (FSS)
The scale is developed to assess the degree of fatigue of stroke patients participating in social activities and rehabilitation exercises13. The instrument comprises 9 items, each item is scored from 1 to 7 points, and the total score is the sum of the scores of each entry, i.e., 63 points. Based on established criteria, a total score of ≥ 36 points indicates clinically significant PSF, where higher scores correspond to increased fatigue severity. The scale demonstrated good reliability (Cronbach’s alpha = 0.928)14.
Modified Rankin scale (MRS)
The scale is used to evaluate the recovery of neurological function in patients15, and is divided into 6 items based on the patient’s symptoms and self-care ability, ranging from 0 ‘no symptoms at all’ to 5 ‘severe dysfunction’. A score of 0–1 indicates that patients have no symptoms at all or have symptoms but no obvious dysfunction; a score ≥ 2 indicates that the patients have different degrees of dysfunction. The higher the score, the worse the recovery of neurological function and the more severe the dysfunction. The Cronbach’s alpha is 0.95016.
Self-rating anxiety/depression scale (SAS/SDS)
The severity of anxiety/depression symptoms was evaluated according to patients’ self-reported experiences over the previous seven days. The higher the score, the more severe the symptoms. The Cronbach’s alpha were 0.800 (ICC:0.870) and 0.800 (ICC:0.880), respectively17,18.
Pittsburgh sleep quality index scale (PSQI)
The scale consists of 7 factors, with a total score ranging from 0 to 21 points, better sleep quality (≤ 7 points), and sleep disorders (> 7 points). The higher the overall evaluation score, the worse the sleep quality of the patients., The Cronbach’s alpha is 0.84219.
Stroke specific quality of life scale (SS-QOL)
This scale represents the first patient-centered, stroke-specific quality of life scale20. It contains 49 items, which belong to 12 different dimensions. Items are scored from 1 to 5, with the lowest score being 49 and the highest score being 245. The higher the score, the better the quality of life. The Kappa coefficients ranging from 0.816 to 1.000, and Cronbach’s alpha of content consistency is above 0.760, showing good reliability and validity21.
National Institute of health stroke scale (NIHSS)
The NIHSS has become the most widely adopted clinical instrument for stroke severity assessment due to its reliability, validity, and ease of administration22. The scale includes 15 items such as consciousness, eye movement, visual field range, facial movement, upper and lower limb movement, coordination, sensation and language ability. Based on established cutoff values, the severity of each item was assessed according to 0–2 points, 0–3 points or 0–4 points. The total score was 42 points. The higher the score, the more serious the degree of neurological deficit. The scale showed satisfactory internal consistency (Cronbach’s alpha = 0.797)23.
Chronic disease self-efficacy scale (CDSES)
The scale consists of 6 items, and each item is scored from 1 to 10 points, among which 1 point means ‘no confidence’ and 10 points means ‘completely confidence’, The total score is the average score of each item. The higher the total score is, the better the sense of self-efficacy and its Cronbach’s alpha is 0.880–0.95024.
Data collection methods
Within one week after admission, the researchers introduced the significance, content and confidentiality principle of this study to the patients, and conducted investigation and research after obtaining their informed consent. The general data survey of patients was obtained using case data and inquiry. The scale evaluation was completed independently by the subjects. For patients with writing or expression difficulties, the researchers dictated the questionnaire to them and filled it out according to the patient ‘s choice. The questionnaires were double-checked and confirmed by two researchers to ensure the objective accuracy of the filling in on behalf of the patient.
Following questionnaire completion, a dual-verification process was implemented: two trained researchers independently reviewed each questionnaire for completeness on the spot, with immediate follow-up to resolve any missing or ambiguous responses. A total of 312 questionnaires were distributed in this survey, with 12 invalid questionnaires were excluded during quality control for being incomplete or inconsistent, and 300 cases were finally recovered, with an effective recovery rate of 96.15%.
Statistical methods
SPSS 22.0 statistical software was used for data analysis. The general data of the patients and the scores of each scale were expressed as mean ± standard deviation for the measurements that conformed to a normal distribution, and an independent samples t-test was used for comparison between groups. Non-normal distribution measurement data were expressed as median and interquartile range, and a non-parametric test was used for comparison between groups. The count data were expressed as frequency and percentage, and the chi-square test was used for comparison between groups. A binary logistic regression model was established to perform multifactorial analysis on statistically significant variables in the univariate analysis. Stratified analysis was performed on variables with statistical significance in multivariate analysis, and the differences were statistically significant at p < 0.05.
Results
General data description of young and middle-aged stroke patients
A total of 300 young and middle-aged stroke patients were included in this study, and the baseline characteristics of the patients are shown in Table 1. According to the FSS score, the patients were categorized into the fatigue group (FSS ≥ 36 points, 187 cases) and the non-fatigue group (FSS < 36 points, 113 cases). The incidence of PSF was 62.3%. Univariate analysis showed that there were no statistically significant differences in terms of age, sex, body mass index (BMI), lesion location, NHISS score, smoking history, drinking history, stroke course, education level, medical payment method and family per capita monthly income between the two groups (P > 0.05); The independent variables PrSF, mRS score, SAS score, SDS score, PSQI score, SS-QOL score, CDSES score, marital status and occupation were associated with the occurrence of PSF, and the differences were statistically significant in between-group comparisons (P < 0.05).
Logistic regression analysis
Logistic regression analysis were performed with the occurrence of PSF as the dependent variable and marital status, occupation, PrSF, mRS score, SAS score, SDS score, PSQI score, SS-QOL score and CDSES score as the independent variables. The independent variables were assigned as shown in Table 2.
The results showed that the four variables of marital status, PrSF, SDS score and SS-QOL score were correlated with the occurrence of PSF, and the difference was statistically significant (P < 0.05). Marital status was a risk factor for predicting PSF. The risk of PSF in patients with other (unmarried/divorced/widowed) marital status was 8.908 times higher than that in married patients (OR = 8.908, 95%CI 1.776–44.674); PrSF was a risk factor for predicting PSF. The risk of PSF in patients with PrSF was 2.909 times higher than that in patients without PrSF (OR = 2.909, 95% CI 1.555–5.443). Depression was a risk factor for predicting PSF. The risk of PSF in patients with depression was 1.099 times higher than that in patients without depression (OR = 1.099, 95% CI 1.046–1.154). The SS-QOL score was negatively correlated with the risk of PSF (OR = 0.985, 95%CI 0.972–0.998), that is, the higher the degree of PSF, the lower the SS-QOL score and the worse the quality of life of patients, as shown in Table 3.
Stratified analysis
Further stratified analysis was conducted with the presence or absence of PrSF as a stratified variable, with the presence or absence of PSF used as the dependent variable, and the influencing factors (Marital status, SDS score, and SS-QOL score) screened by the aforementioned logistic regression analysis as the independent variables to explore the influencing factors and their interaction on the risk of PSF in patients with or without PrSF.
The results showed that in the group of patients with PrSF, Marital status (married) (OR = 0.438, 95%CI 0.046–4.203), SDS score OR = 1.052,95%CI 0.965–1.146), SS-QOL score (OR = 0.960, 95%CI 0.937–0.984), among which the SS-QOL score was associated with the risk of PSF (P < 0.001); in the group of patients without PrSF, SDS score (OR = 1.086, 95%CI 1.033–1.142) was associated with a high risk of PSF (P < 0.001), Be married (OR = 0.060, 95% CI 0.007–0.490) and SS-QOL score (OR = 0.984, 95% CI 0.974–0.995) were associated with a low risk of PSF (P < 0.05). All between-group interactions were significant (P < 0.05), as shown in Table 4.
Discussion
Current status of PSF in young and middle-aged stroke patients
In recent years, PSF as a common complication after stroke has been gradually understood and concerned by people25,26,27. The existing literature shows that PSF has a high incidence among stroke survivors, complex pathological mechanisms and diverse causes, It often faces many challenges in the process of treatment intervention28. The presence of PSF significantly impairs the improvement of motor function in stroke patients. The more severe the fatigue, the worse the rate of post-stroke recovery is7, which has a serious negative impact on the patient’s daily life and long-term quality of life29,30,31,32,33,34. Continuous fatigue can also cause patients to fail to return to normal work and life35. In addition, PSF is also an independent risk factor for stroke recurrence and post-stroke death36. Therefore, early identification of risk factors for PSF in young and middle-aged stroke patients is of great significance for early prevention and effective management of this symptom.
In this study, a total of 300 cases of young and middle-aged stroke patients were investigated in a tertiary hospital in Xi ‘an China, of which 187 patients had PSF. The incidence of PSF in young and middle-aged stroke patients was 62.3%. This study showed that the incidence of PSF in young and middle-aged stroke patients was relatively higher. Previous reports have shown that the incidence of PSF varies among studies33,37. The results of different scholars investigating the incidence of PSF varied, and this variability may be related to the different selection of diagnostic criteria and assessment tools in each study. In addition, the prevalence characteristics of stroke disease, dysfunction and assessment time in different regions are also important factors leading to the significant differences in the incidence of PSF. As fatigue is a subjective feeling of patients, although it is common, stroke patients are often ignored fatigue symptoms by medical staff and family members due to psychological suggestions, superimposed emotions and cognitive impairment caused by neurological deficits9, which seriously affects the later treatment of patients38. It is suggested that clinical medical workers should strengthen the routine screening and evaluation of fatigue symptoms in young and middle-aged stroke patients with the above risk factors, and formulate corresponding intervention programs to avoid and reduce the generation of PSF as much as possible.
Analysis of influencing factors of PSF in young and middle-aged stroke patients
The impact of marital status on PSF
This study showed that marital status was an influential factor of PSF, and the risk of PSF in patients with abnormal (unmarried/divorced/widowed) marital status was 8.908 times higher than that of married patients (OR = 8.908, 95% CI 1.776–44.674), which is consistent with the research results of Zhang Xuemei and other scholars39. The reason for the analysis may be that young and middle-aged stroke patients are prone to major changes in their psychological state in the face of sudden diseases and neurological deficits after the onset of the disease, and they are easy to take negative coping measures. They are in urgent need of care and support from within the family inwardly. As the most important caregivers of patients, spouses not only provide physical care to patients but also enable patients to obtain strong spiritual support and promote the correct guidance of patients to take positive coping measures. Close family relationships can give endogenous support to the family when patients face the major challenges of the disease, and the spouse’s thoughtfulness, care and understanding support of the spouse are the key to the patient’s mental victory over the disease, helping patients to reduce the stress disorder at the early stage of the disease, maintain a positive psychological state, increase the initiative of treatment, improve the expectation of the prognosis and the yearning for the future life, which in turn will help to alleviate the symptoms of their fatigue40.
As shown in Table 1 in the text, the number of married patients in the fatigue group was 169, and 18 patients with another marital status; the number of married patients in the non-fatigue group was 111, and only 2 patients with another marital status. The sample sizes of the patients with other matrimonial statuses (Unmarried/divorced/widowed) in both groups were notably small, and there was a problem of insufficient sample size. In the results of the logistic regression analysis, marital status also exhibited an unusually high OR value (OR = 8.908, 95% CI 1.776–44.674). During the collection of clinical data in this study, we observed that married patients exhibited significant advantages in terms of family psychological support and access to social resources, whereas patients with other marital statuses (unmarried/divorced/widowed) were more likely to suffer from higher levels of psychological stress or perceive stronger feelings of familial and social isolation. These individuals demonstrated varying degrees of negative emotions and psychological distress and thus were more susceptible to the symptoms of fatigue. From the perspective of clinical practice, there may be a certain degree of association between marital status and PSF. Although this study demonstrated the unusually high OR value for marital status (OR = 8.908, 95% CI 1.776–44.674) in the logistic regression analysis in this study, the directionality of its indication may have clinical significance that cannot be ignored. Furthermore, in view of the above problems, we searched for the relevant research progress41,42,43,44,45,46 on the influencing factors of PSF in recent years, and the results showed that these studies were consistent with the conclusions of this study, which revealed a significant correlation between marital status and the risk of PSF.
Stratified analysis showed that in the group of patients with PrSF, married status had no significant protective effect (OR = 0.438, 95%CI 0.046–4.203, P = 0.475). However, in the group of patients without PrSF, married status significantly reduced the risk of PSF (OR = 0.060, 95%CI 0.007–0.490, P = 0.009), and the interaction was significant (P = 0.005). The protective effect of marriage was only seen in patients without PrSF, which may be due to the fact that the fatigue symptoms in those with PrSF may be mainly driven by biological mechanisms, such as the synthesis and metabolism of neurotransmitters and receptor expression47. Under these circumstances, the superimposed influence of the post-stroke disease progression itself further obscures the positive role of psychosocial support factors such as marital status. The strong influence of biological mechanisms may weaken the role of social support, making it difficult to show its protective effect. In addition, family support (e.g., spousal care ) relieves fatigue mainly through psychological or behavioural pathways, and such patients suffer from long-term fatigue effects, making it difficult for marital support from the outside to be effective in alleviating symptoms. For patients without PrSF, the effect of family support will be more significant because they are less affected by the pathophysiological mechanisms of fatigue and have a higher degree of coping with the disease and adherence to rehabilitation. This suggests that for stroke patients who are not affected by the PrSF mechanism, family support, especially the care and companionship of spouses, should be strengthened to give full play to their positive role at the psychological and behavioural levels, so as to effectively reduce the risk of PSF and the severity of fatigue. For patients with PrSF, multi-dimensional factors such as biological characteristics and psychosocial factors must be comprehensively considered when formulating intervention strategies to improve the quality of life of patients. Therefore, clinical healthcare professionals should pay attention to the importance of the auxiliary role of the patient’s spouse, communicate with them more, and do a good job in the disease and health education of the patient’s spouse, so that they can play an effective and positive role in the process of taking care of the patients, so that patients can obtain more benefits.
In future research on topics related to PSF, we will expand the sample size, especially increase the number of cases with underrepresented sample categories of Marital status, such as other Marital status (unmarried/divorced/widowed) participants, so as to more accurately evaluate the effect of marital status on fatigue and further verify the stability and reliability of the model results.
PrSF is closely associated with the risk of PSF
The prevalence of PrSF in this study was 35.3%, and the prevalence of PSF in patients with PrSF was as high as 46.5%, which was lower than the findings of Chen et al.48. This discrepancy may stem from the current evaluation of PrSF was obtained through a historical review, which makes it difficult to achieve a unified and accurate assessment. The results of multivariate regression analysis showed that patients with PrSF were 2.909 times more likely to develop PSF than patients without PrSF, which was a risk factor for PSF, indicating that patients with fatigue before stroke are more likely to develop PSF, which was consistent with the results of previous studies by other scholars49,50,51.Further stratified analysis was conducted with the presence or absence of PrSF as a stratified variable. The results showed that PrSF status significantly revealed associations between marital status, SDS score, SS-QOL score and PSF, and all interaction tests were statistically significant, highlighting the importance of PrSF as a key effect modifier variable.
The reasons for PrSF’s influence on the occurrence of PSF were analyzed, which may include the fact that stroke patients with PrSF experience a series of imbalances in the body’s functions due to long-term fatigue, persistent endocrine disorders and decreased immunity, such as abnormal mood fluctuations, intolerance of activities, decreased willingness to engage in autonomous activities, sleep disorders and anorexia, which lead to a continuous decline in the body’s vitality, and the perception thresholds of fatigue is continuously lowered in such patients. In addition, the further damage of the disease to the body’s function after the disease may also be accompanied by complications such as limb dysfunction and cognitive impairment, which may ultimately lead to increasing fatigue in the patients52. PrSF serves not only as an independent predictor of PSF but also as a key indicator for stratified assessment. Its existence suggests that individualized intervention should be adopted according to individual differences of patients. Future research should integrate multi-omics data to deeply explore the underlying biological mechanisms behind stroke-related fatigue, thereby facilitating the development of more precise and effective therapeutic strategies.
As the current assessment of PrSF is obtained through retrospective methods, it may lead to missed diagnosis and misdiagnosis due to the influence of patients’ age, education level and other factors. Therefore, this suggests that medical workers should be aware of the significance of knowing the PrSF history of young and middle-aged stroke patients. When assessing the presence or absence of PrSF, they should use easy-to-understand language as far as possible, and make comprehensive evaluations in conjunction with the symptoms of the patients and the statements of their families. Patients with PrSF experience should be carefully asked about the cause and encouraged to actively receive treatment, to alleviate the impact of PrSF on the patients’ PSF and to reduce the level of post-stroke fatigue of the patients.
Depression is related to the risk of PSF
The relationship between PSF and depression has been repeatedly proposed in previous studies. Depression plays an important role in the development of PSF, and the severity of fatigue is closely related to depression53. The presence of depressive symptoms after stroke onset is an important risk factor for predicting the occurrence of PSF54,55,56,57. The current study also reached the same conclusion. In this study, patients with comorbid depression were 1.099 times more likely to develop PSF than those without depression. The risk of PSF in patients with depressive symptoms was significantly increased. The correlation between the two might be attributed to the fact the psychiatric symptoms of depression, would cause patients to be negatively disposed, become tired of activities and experience a decline in self-efficacy. As a result, they would prematurely withdraw from medical and social activities, and the degree of mental fatigue would increase. Depression-related physical symptoms, such as loss of appetite, can lead to nutritional deficiency, aggravating limb weakness and energy deficiency, and intensify the patient’s physical fatigue. Concurrently, the physical weakness and energy deficiency caused by PSF will in turn trigger a negative state in the patients, and the continuous lack of physical and mental energy make it difficult for the patients to cope with the blows brought by stroke during the illness period, thereby leading to the occurrence of depression and continuously increasing the negative impact of depressive symptoms58. Stratified analysis showed that in the group of patients with PrSF, the SDS score had no significant effect (OR = 1.052, 95%CI 0.965–1.146, P = 0.250). However, in the group of patients without PrSF, the SDS score was (OR = 1.086, 95%CI:1.033–1.142, P < 0.001), indicating that depressive symptoms were significantly associated with a higher risk of PSF, with the risk of PSF increasing by 8.6% for each 1-point increase in SDS score, and the interaction was significant (P < 0.001). The negative impact of depressive symptoms on PSF is particularly significant in patients without PrSF, which may be attributed to the relatively shorter period of time that such patients are subjected to the neurobiological mechanisms of fatigue. After the disease, the negative impact of the patient’s psychological condition is exacerbated by neurological function limitations, leading to impaired emotional regulation, which in turn leads to a decrease in the motivation to treat the disease and slows down the progress of rehabilitation59. Therefore, the correction of adverse psychological states demonstrates a more pronounced effect in mitigating the risk of PSF. In addition, given the regulatory effect of psychological states on fatigue perception, early implementation of psychological interventions for patients without PrSF can help to break the vicious cycle of depression and fatigue and improve the rehabilitation effect. However, patients with pre-existing fatigue mechanisms before stroke are different. Due to the early onset of inflammatory or neurometabolic abnormalities in this population, fatigue symptoms and potential negative psychological levels persist for a long time, and the physical symptoms are further aggravated after the disease. The neurological impairment caused by the disease has maximized the sense of fatigue. It is difficult to significantly improve fatigue by simply reversing the adverse psychological conditions, which depends largely on the underlying mechanism of the disease itself, and then dilutes the additional effects of depression, and to a certain extent masks the additional effects of negative psychological conditions. For such patients, the limitations of traditional psychological intervention should be recognized, and the individual differences of patients should be comprehensively considered to deal with the multiple risk factors of PSF.
Depression is a potential factor for long-term fatigue in patients. One study60 has confirmed that measures to improve the depressive state of patients after stroke can also be beneficial in eliminating their fatigue symptoms. Therefore, medical staff should pay attention to the screening and evaluation of patients ' emotional disorders while treating and caring for stroke disease itself. For patients with emotional disorders, more attention should be given, targeted interventions and effective psychological counselling and nursing care should be provided61. Early detection of stroke patients with negative emotions should be carried out, and their psychological changes should be closely monitored, emphasize the adverse effects of depressive symptoms on disease rehabilitation to patients and their families, guide patients to correctly self-venting and self-relaxing, and encourage them to take positive coping measures to confront negative emotions, thereby reducing the impact of adverse psychological states such as depression on PSF and other complications.
Quality of life level and PSF
The results of this study showed that the higher the degree of fatigue in patients, the lower their quality of life. Stratified analysis showed that in the group of patients with PrSF, lower SS-QOL scores (OR = 0.960, 95% CI 0.937–0.984, P < 0.001) were significantly associated with a higher risk of PSF. For each 1-point increase in SS-QOL score, the risk of PSF decreased by 4.0%. In the Non-PrSF group, the quality of life score (OR = 0.984, 95% CI 0.974–0.995, P = 0.003) was also negatively correlated with the risk of PSF. For each 1-point increase in SS-QOL score, the risk of PSF decreased by 1.6%, and the interaction was significant (P < 0.001). Therefore, regardless of the presence of PrSF, improvements in quality of life have a general protective effect on the risk of PSF, with a significant negative correlation. Improving the quality of life can effectively reduce the risk of PSF, which is of universal significance for preventing the occurrence of PSF, especially in the group of patients with PrSF. In line with previous research findings, many scholars have also confirmed that PSF is associated with long-term low quality of life in stroke patients62,63,64. Some scholars have found that compared with patients with simple strokes, the recovery speed of quality of life in PSF patients with the same treatment process is even slower65,66. In a review, PSF has been demonstrated to cause patients to have physical disorders, reduced participation in social activities, lead to poor rehabilitation outcomes, result in poor quality of life and increased mortality67. In the study of Ramirez-Moreno68,, fatigue was also considered to be the main predictor of health-related quality of life in patients with ischemic stroke, which implies that addressing the issue of fatigue for patients with stroke can be of great help in improving their quality of life.
The reason for analyzing the correlation between the two might be because the fatigue state has brought about non-negligible negative impacts on the patient’s activities, social roles, psychological emotions and self-care abilities. These factors are closely related to the quality of life and subsequently lead to a decrease in the patient’s quality of life. Poor quality of life inversely aggravates the patient’s fatigue and burnout, and finally forms a vicious circle of mutual influences. In clinical work, medical staff should strengthen the cognition of the adverse effects of fatigue symptoms on patients’ quality of life, correctly understand and manage PSF, and take effective intervention measures in time to improve the quality of life of stroke patients by reducing the level of fatigue, which has significant clinical significance for improving the long-term prognosis of patients as early as possible. At the same time, it provides a new basis for formulating intervention programs to improve the quality of life of patients with PSF in the future.
However, there are still some limitations to our study. First of all, only the cross-sectional survey method used to analyze the influencing factors of PSF. It is expected that a large sample size of prospective cohort studies will be carried out in the future to strengthen the strength of the argument. Secondly, this study was conducted in only one hospital, which has certain limitations in the representativeness of the sample, In future research, multi-centre large sample surveys can be conducted to provide more new perspectives and evidence-based basis for intervention. Thirdly, this study was mainly completed through scale assessment, which may have reporting bias. In the future, more objective indicators can be added to minimize potential biases. Lastly, unexamined factors such as nutritional status, and genetic predisposition may also contribute to the development of PSF. In future research, further exploration of the underlying biological mechanisms of PSF is necessary, particularly through integrating neurobiological, endocrinological, and immunological perspectives, which could identify novel biomarkers and therapeutic targets.
Conclusion
In summary, the incidence of PSF in young and middle-aged stroke patients is high and harmful. Stroke patients with PrSF and depressive symptoms are at high risk of developing PSF, and marital status and quality of life are also closely related to it, which varies with the presence or absence of PrSF. Clinical staff should pay close attention to these patients, conduct targeted assessment and judgment, and provide scientific guidance for the management of fatigue and related risk factors. At the same time, spouses and other social support systems should be encouraged to actively participate in the treatment and care of patients to reduce the incidence of PSF and Improve the degree of fatigue in patients, which is an indispensable measure to promote the recovery of patients and improve their long-term quality of life.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lanctôt, K. L. et al. Canadian stroke best practice recommendations: Mood, cognition and fatigue following stroke, 6th edition update 2019. Int. J. Stroke 15 (6), 668–688 (2020).
Miller, T. et al. Online advice for the symptomatic management of poststroke fatigue: a scoping review. J. Psychosom. Res. 162, 111039 (2022).
Wang, M. et al. Interpretation of the Canadian stroke best practice recommendations: mood, cognition and fatigue following stroke. Chin. Gen. Pract. 24 (17), 2214–2217 (2021).
Zhan, J. et al. Global prevalence estimates of poststroke fatigue: A systematic review and meta-analysis. Int. J. Stroke. 18 (9), 1040–1050 (2023).
Delva, M. Y., Delva, I. I. & Lytvynenko, N. V. Post-stroke fatigue and its dimensions over the second half year after stroke. Wiad. Lek. 71, 314–317 (2018).
Wu, Q. D., Zhou, J. & Wang, S. Mediating effect of rehabilitation self-efficacy on post-stroke depression and post-stroke fatigue of elderly stroke patients in rehabilitation period. J. Nurs. (China). 28 (17), 64–69 (2021).
Pulyk, O., Hyryavets, M. & Studeniak, T. Poststroke fatigue and motor recovery after ischemic stroke. Wiad. Lek. 75 (5 pt 2), 1328–1330 (2022).
Li, Y. et al. The incidence and related risk factors of post-stroke fatigue in patients with cerebral infarction in the early stage. Med. Philos. 39 (6), 42–45 (2018).
Teng, C. H. et al. Adaptation to poststroke fatigue in stroke survivors and their care partners: a scoping review. Disabil. Rehabil. 45 (13), 2233–2247 (2023).
O’hana, S. & Nobleza, C. Preferences of young stroke survivors to meet their unique needs: it is time to listen. Neurology 96 (13), e1809–e1811 (2021).
Aali, G. et al. Post stroke fatigue: a scoping review. F1000Research 9, 242 (2020).
Poulsen, M. B. et al. How to identify fatigue in stroke patients: an investigation of the post-stroke fatigue case definition validity. Top. Stroke Rehabil. 24 (8), 1–8 (2019).
Krupp, L. B. et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 46 (10), 1121–1123 (1989).
Chunwei, W. & Dexin, W. The application of the Chinese translation of the fatigue severity scale in the clinical practice and evaluation of patients with cerebral infarction. Chin. J. Phys. Med. Rehabilitation. 29 (9), 608–611 (2007).
Van Swieten, J. C. et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19 (5), 604–607 (1988).
Yan, L. Research on Analysis of Risk Factors and Construction of Predictive Model for Poor Prognosis after Endovascular Treatment of Different Subtypes of Acute Ischemic Stroke (Anhui Medical University, 2024).
Yuan, W. Development of the multidimensional anxiety assessment scale. Chin. J. Clin. Rehabilitation. 9 (12), 30–31 (2005).
Yanyan, W. et al. The reliability, validity and optimal cut-off value of the Chinese version of the bipolar depression assessment scale. Chin. J. Neuropsychiatric Disorders. 47 (12), 710–715 (2021).
Xianchen, L. et al. Study on the reliability and validity of the Pittsburgh sleep quality index. Chin. J. Psychiatry. 29 (2), 103–107 (1996).
Clarke, P. J. et al. Handicap in stroke survivors. Disabil. Rehabil. 21 (3), 116–123 (1999).
Chunmei, J. The Impact of Acceptance Commitment Therapy-Oriented Psychological Care on Sleep and Negative Emotions in Patients with Acute Ischemic Stroke (North China University of Science and Technology, 2024).
Roushdy, T. et al. A clinical comparative analysis between expanded NIHSS and original NIHSS in posterior circulation ischemic stroke. J. Clin. Neurosci. 114, 77–80 (2023).
Kwah, L. K. & Diong, J. National institutes of health stroke scale (NIHSS). J. Physiother. 60 (1), 61 (2014).
Meixia, Z., Hui, P. & Gaimei, Z. Research progress of self-efficacy assessment tools for chronic disease management. Chin. J. Geriatric Multiple Organ. Dis. 22 (08), 633–636 (2023).
Lai, L. et al. Visual network analysis of research hotspots and trends in post-stroke fatigue among the elderly based on bibliometrics. J. Guangxi Med. Univ. 41 (12), 1637–1643 (2024).
Li, Y. T. et al. Measuring poststroke fatigue: the psychometric properties of the Chinese version of multidimensional fatigue inventory. J. Psychosom. Res. 172, 111388 (2023).
Teng, C-H. et al. Understanding the experience of fatigue poststroke: preliminary results from a mixed-methods longitudinal study. Stroke 54 (Suppl. 1), WMP16 (2023).
Li, L., Li, X. & He, J. Analysis of the current research status of post-stroke fatigue. China Rehabilitation. 35 (06), 329–332 (2020).
Simmonds, K. P. et al. Racial and ethnic disparities in the medical management of poststroke complications among patients with acute stroke. J. Am. Heart Assoc. 13 (5), e030537 (2024).
Tremayne, J. E., Freeman, J. & Coppola, A. Stroke survivors’ experiences and perceptions of post-stroke fatigue education in the subacute phase of stroke. The FASE qualitative study. Br. J. Occup. Therapy. 84, 111–121 (2021).
Vollertsen, J. et al. The impact of post-stroke fatigue on work and other everyday life activities for the working age population—a registry-based cohort study. Ann. Med. 55 (2), 2269961 (2023).
Teng, C. H. et al. Adaptation to poststroke fatigue in stroke survivors and their care partners: a scoping review. Disabil. Rehabil. 1, 1–15 (2022).
Norlander, A. et al. Fatigue in men and women who have returned to work after stroke: assessed with the fatigue severity scale and mental fatigue scale. J. Rehabil Med. 53 (9), jrm00227 (2021).
Thomas, K. et al. How is post-stroke fatigue understood by stroke survivors and careers? A thematic analysis of an online discussion forum. BMJ Open. 9 (7), e028958 (2019).
Rutkowski, N. A., Sabri, E. & Yang, C. Post-stroke fatigue: A factor associated with inability to return to work in patients < 60 years-A 1-year follow-up. PLoS ONE. 16 (8), e0255538 (2021).
Xue, C. et al. Prevalence and trends for post-stroke fatigue in china: a meta-analysis. Chin. Gen. Pract. 27 (03), 364–374 (2024).
Lee, J. & Kim, G. Functional recovery in acute and subacute stroke patients with or without post-stroke fatigue. Brain Neurorehabil. 17 (3), e22 (2024).
Qiang, Z. et al. Visual analysis of research hotspots in the field of rehabilitation post-stroke fatigue. J. Brain Nerv. Dis. 32 (12), 767–773 (2024).
Zhang, X. et al. The status quo of self-regulated fatigue of stroke survivors in convalescence and its influencing factors. Chin. Clin. Nurs. 16 (02), 75–79 (2024).
Sun, J. Study on the Relationship Between Caregiver Preparation, Positive Feeling and Fatigue in Stroke Patients (Yanbian University, 2019).
Xianchai, H. et al. Analysis on influencing factors of post-stroke fatigue for first stroke patients. J. Nurs. Rehabilitation. 20 (06), 15–18 (2021).
Xiong, X. L. et al. Fatigue development trajectory and its influencing factors in middle-aged and elderly patients with stroke. J. Clin. Med. Pract. 28 (19), 123128133 (2024).
Jie, Z. et al. Global prevalence estimates of poststroke fatigue: a systematic review and meta-analysis. Int. J. Stroke. 18 (9), 17474930221138701 (2022).
Chao, X. et al. Meta-analysis of the prevalence and development trend of post-stroke fatigue in China. Chin. Gen. Pract. 27 (03), 364–374 (2024).
Xianchai, H. et al. Analysis of influencing factors of post-stroke fatigue in patients with first-onset stroke. Nurs. Rehabilitation. 20 (06), 15–18 (2021).
Zhu, R. et al. Post-stroke fatigue and its correlation with family functioning in patients who have experienced a first episode of stroke. Front. Aging Neurosci. 16, 1440163 (2024).
Lulu, G. et al. The mechanism of neurotransmitters and their receptors in exercise-centered fatiguej/ol. Adv. Biochem. Biophys..
Chen, Y. K. et al. Poststroke fatigue: risk factors and its effect on functional status and health-related quality of life. Int. J. Stroke. 10 (4), 506–512 (2015).
Wang, X-L. et al. Risk factors of post-stroke fatigue: a systematic review. Chin. J. Stroke. 15 (07), 759–765 (2020).
Zeng, X. et al. Risk factors for acute fatigue in patients with stroke. Practical J. Cardiac Cereb. Pneumal Vascular Disease. 28 (07), 54–58 (2020).
Zhang, Y. et al. Risk factors for post-stroke fatigue: a meta-analysis. Chin. J. Contemp. Neurol. Neurosurg. 19 (10), 726–733 (2019).
Wang, M. Prevalence of Post-stroke Fatigue after Ischemic Stroke and Research for Its Influencing Factors (Zhengzhou University, 2017).
Pedersen, A. et al. Fatigue 7 years post-stroke: predictors and correlated features. Acta Neurol. Scand. 146 (3), 295–303 (2022).
Persen, A. et al. Fatigue 7 years post-stroke: predictors and correlated features. Acta Neurol. Scand. 146 (3), 295–303 (2022).
Situ, X. & Li, Y. Analysis of risk factors for post-stroke fatigue in rehabilitation treatment of stroke patients. Mod. Practical Med. 34 (01), 82–85 (2022).
Zhang, S. et al. Related risk factors associated with post-stroke fatigue: a systematic review and meta-analysis. Neurol. Sci. 42 (4), 1463–1471 (2021).
Kwon, S. et al. Analysis of factors affecting post-stroke fatigue: an observational, cross-sectional, retrospective chart review study. Healthc. (Basel). 9 (11), 1586 (2021).
Huang, X. et al. Research progress in related factors of post-stroke fatigue. Med. Recapitulate. 25 (11), 2185–2189 (2019).
Fan, C. Y. et al. Effect of multi-disciplinary continuous nursing with the participation of nursing teachers on the rehabilitation of stroke patients with limb dysfunction. J. Nurs. Sci. 38 (6), 116–120 (2023).
Yue, M. et al. Correlation between post-stroke depression and post-stroke fatigue: a meta-analysis. Chin. Nurs. Res. 34 (02), 227–231 (2020).
Delva, I., Lytvynenko, N. & Delva, M. Factors associated with post-stroke fatigue within the first 3 month after stroke. Georgian Med. News. 267, 38–42 (2017).
Hinkle, J. L. et al. Post stroke fatigue: emerging evidence and approaches to management: a scientific statement for healthcare professionals from the American heart association. Stroke 48 (7), 159–170 (2017).
Hongmei, W. et al. Inflammatory signaling in post-stroke fatigue and depression. Eur. Neurol. 80 (3–4), 138–148 (2018).
Maaijween, A. et al. Post-stroke fatigue and its association with poor functional out-come after stroke in young adults. J. Neurol. Neurosurg. Psychiatry 86 (10), 1120–1126 (2015).
Winstein, C. J. et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for health care professionals from the American heart association/american stroke association. Stroke 47 (6), 98–169 (2016).
Qianfeng, L., Liming, H. & Mingjia, T. Research on the influence of respiratory muscle training combined with music therapy on the quality of life and motor function of patients with post-stroke fatigue. Chin. Manipul. Rehabil. Med. 12, 21–22 (2021).
Oyake, K. et al. Poststroke fatigue at admission is associated with independence levels of activities of daily living at discharge from subacute rehabilitation wards. Arch. Phys. Med. Rehabil. 102, 849–855 (2021).
Ramirez-Moreno, J. M. et al. Health-related quality of life and fatigue after transient ischemic attack and minor stroke. J. Stroke Cerebrovasc. Dis. 28 (2), 276–284 (2019).
Author information
Authors and Affiliations
Contributions
Author contributionsWriting manuscript: JK; Data extraction and statistical analysis: JK , YT , WT and ZZ; conceptualization, project administration and supervision: XM and XZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kang, J., Zhao, X., Zheng, Z. et al. An investigation of post-stroke fatigue levels and influencing factors in young and middle-aged stroke patients: a cross-sectional study. Sci Rep 15, 25046 (2025). https://doi.org/10.1038/s41598-025-10805-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10805-5