Abstract
Prolonged disorders of consciousness (pDoC) in children lack objective and effective diagnostic methods to assess consciousness states, hindering targeted treatment selection and delaying recovery. It remains unclear whether EEG microstate analysis, a method capturing spatial and temporal dynamics of brain activity, can serve as a reliable tool to differentiate consciousness states in children with pDoC, warranting further investigation. Resting-state EEG data (32 channels) were collected over 10 minutes from 45 children, divided into three groups (n=15 each): Vegetative State/Unresponsive Wakefulness Syndrome (VS/UWS; 7 females, 5.9±1.2 years), Minimally Conscious State (MCS; 7 females, 5.7±1.0 years), and healthy controls (HC; 7 females, 5.8±1.3 years). Spatial and temporal properties of EEG microstates were compared across groups. Correlation analysis examined relationships between microstate parameters and Coma Recovery Scale-Revised (CRS-R) scores in children with pDoC. Support vector machine (SVM) models were trained using combined temporal and spatial microstate features, optimized via grid search and random search algorithms. Model performance was evaluated using standard metrics, and features were ranked by permutation importance. CRS-R scores differed significantly between VS/UWS and MCS (p < 0.001). The microstate (MS) C in VS/UWS showed only 1.1% topographic similarity to standard templates. HC showed stronger connectivity for MS A, C, and D, while MCS exhibited stronger functional connectivity for MS C and D compared to VS/UWS. VS/UWS had longer MS B duration, shorter MS C duration, and lower MS C coverage than MCS and HC (p < 0.05). CRS-R scores showed moderate correlations with MS B duration (r=-0.504, P=0.005), MS C coverage (r=0.679, P<0.001), and occurrence of MS C (r=0.744, P<0.001) and D (r=-0.709, P<0.001), but weak correlations with MS C duration (r=0.488, P=0.006) and MS B coverage (r=-0.376, P=0.041). Particle Swarm Optimization Support Vector Machine (PSO-SVM) classification outperformed Grid Search SVM (GS-SVM) and Quantum PSO-SVM (QPSO-SVM) (area under the curve [AUC] = 0.722), with MS C occurrence ranked as the top feature . This study demonstrates that EEG microstate analysis is an objective, user-friendly tool for differentiating consciousness states in children with pDoC. Machine learning algorithms, specifically support vector machines, revealed that MS C occurrence is a potential neurophysiological biomarker.
Similar content being viewed by others
Introduction
Diagnosing prolonged disorders of consciousness (pDoC) poses a major challenge for physicians and neuroscientists, particularly in differentiating Minimally Conscious State (MCS) from Vegetative State/Unresponsive Wakefulness Syndrome (VS/UWS). The misdiagnosis rate for VS/UWS is estimated at approximately 40%1. This high misdiagnosis rate impacts not only neurorehabilitation planning but also the roles and goals of caregivers2,3. Assessments of residual cognitive function in patients with pDoC following brain injury typically rely on standardized behavioral scales4. However, identifying behavioral responses in patients with pDoC remains a significant challenge.
Recent neuroimaging studies reveal preserved cognitive functions in individuals with pDoC, challenging assumptions based on behavior5. Diagnosing pDoC is complex due to heterogeneous neuropathology1. Accurate diagnosis is crucial for effective treatment and recovery. In children with pDoC, lower cognitive abilities and significant variations across different age groups further complicate diagnosis6. Therefore, identifying reliable methods to distinguish consciousness states in children with pDoC is particularly important.
EEG is a crucial tool for studying cerebral activity, offering higher temporal resolution than functional Magnetic Resonance Imaging (fMRI) and functional Near-Infrared Spectroscopy (fNIRS). EEG data interpretation involves analytical methods such as brain functional networks, time-frequency domain analysis, event-related potentials, entropy estimations, and microstate analysis7,8,9,10. Lehmann et al. demonstrated that resting-state EEG reveals ‘microstates’—quasi-stable states with distinct scalp potential patterns lasting a very short period11. EEG microstate analysis integrates electrode signals to map transient neural activity, revealing brain function. Each microstate reflects a unique topographic network state, with transitions signaling dynamic neural shifts12. Numerous studies demonstrate that microstate analysis offers significant advantages in adult chronic consciousness disorder research, achieving superior consciousness level classification performance when combined with machine learning algorithms13,14.The application of machine learning to microstate analysis in research on pDoC, particularly in children, remains underexplored but has the potential to improve diagnostic and prognostic models for neurological conditions15.
This study investigates brain activity differences in children with VS/UWS, MCS, and healthy children (HC) using EEG microstate analysis, leveraging spatial and temporal data for machine learning-based classification of pDoC in children. The model aims to explore potential neural biomarkers of consciousness, offering a foundation for future diagnostic and monitoring approaches in children with pDoC.
Materials and methods
The study adhered to the Declaration of Helsinki, received approval from the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (No. 2023-270-01), and was registered with the Chinese Clinical Trials Registry (ChiCTR2400080068). Researchers informed legal guardians about the study protocol and obtained their written informed consent before initiating the study.
Subjects
Participants included 45 individuals: 15 with VS/UWS (5.9±1.2 years), 15 with MCS (5.7±1.0 years), and 15 HC (5.8±1.3 years), all recruited from the Department of Rehabilitation Medicine, Third Affiliated Hospital of Zhengzhou University.
Inclusion criteria
Children with pDoC: Definitively diagnosed with pDoC over 1 month post-onset, in a stable consciousness phase, and capable of completing the Coma Recovery Scale-Revised (CRS-R) five times within ten days16. Legal guardians provided informed consent.
HC: With normal consciousness states, no neurological disorders, and legal guardians’ informed consent.
Clinical evaluation
Consciousness states in children with pDoC were assessed using the CRS-R. Three trained clinicians independently conducted each assessment, adhering to protocols for consistency and reliability. The CRS-R evaluated behavioral and physiological responses to gauge consciousness and awareness. To ensure assessment stability and reliability, patients underwent at least five CRS-R evaluations over a 10-day period before EEG recording.
EEG recording and processing
Subjects were seated in a quiet, temperature-controlled room for resting-state EEG recordings using 32 Ag/AgCl scalp electrodes arranged according to the international 10-10 system (ZhenTecBci Co. LTD, Xi’an, China). Electrode impedance was maintained below 5 kΩ for optimal signal-to-noise ratio (SNR). The signals were amplified and sampled at 500 Hz, with a recording duration of 10 minutes (see Supporting File 1).
EEG data preprocessing was conducted using EEGLAB v14.1.2b and MATLAB R2023b and included: Removal of Head Electrode for Orbital Right (HEOR) and Head Electrode for Orbital Left (HEOL) electrooculography electrodes; band-pass filtering (0.1-40 Hz) to reduce noise; manual artifact removal; segmentation into 2-second epochs; Independent Component Analysis (ICA) to isolate brain activity components, with removal of those affected by eye movements, muscle activity, and heartbeat artifacts; re-referencing to the average electrode; rejection of segments with significant muscle artifacts or extreme amplitudes; and retention of the best 90 continuous 2-second segments, totaling 180 seconds of preprocessed EEG data.
EEG microstate analysis
Before microstate analysis with Cartool, the preprocessed EEG data underwent additional bandpass filtering (2-20 Hz)17. The analysis included several key steps (see Figure 1):
-
1)
Global Field Power (GFP) Calculation: GFP, an indicator of the brain’s instantaneous electric field strength and response to events or changes in brain activity, was calculated18. The formula for GFP is:
$$\text{GFP}=\sqrt{(\sum_{\text{i}}^{\text{K}} ({\text{V}}_{\text{i}}(\text{t})-{\text{V}}_{\text{ mean }}(\text{t}){)}^{2})/\text{K}}$$(1)
Let K be the number of EEG electrodes, Vi(t) the potential of the i-th electrode at time t, and Vmean(t) the average potential across electrodes. The formula is:
-
2)
Topographic Map Representation: EEG microstates are depicted by topographic maps showing local GFP maxima. Cluster analysis categorizes these maps into types, with four to six microstate types exhibiting the highest spatial correlation across studies, thus forming the chosen clustering range19,20,21.
-
3)
Microstate Clustering: The K-means method partitioned EEG samples into clusters, with iterative optimization until reaching optimal assignments. To reduce noise, categories with two or fewer time frames (TFs) were excluded. A 20 ms smoothing algorithm was implemented during the fitting process, resulting in four distinct microstates: Microstate A (MS A), microstate B (MS B), microstate C (MS C), and microstate D (MS D)13.
-
4)
Computation of Microstate Parameters: Parameters for each segmented microstate class were derived from clustered topographies as follows: Global Explained Variance (GEV, %) Duration (ms): The average duration of a given microstate. Coverage (%): The fraction of time a given microstate is active. Occurrence (Hz): The average number of times per second a microstate is dominant. Transition probabilities: A measure of how frequently microstates of a certain class are followed by microstates of other classes.
EEG microstate source localization
The study utilized standardized Low Resolution Brain Electromagnetic Tomography (sLORETA) for microstate source localization. The sLORETA algorithm addresses the inverse problem by assuming related orientations and strengths of neighboring neuronal sources22. The four microstate templates from VS/UWS, MCS, and HC, calculated using Cartool, served as the source localization dataset. The inverse matrix was constructed from 30-channel Montreal Neurological Institute (MNI) spatial localization data, and sLORETA calculations were mapped using the Talairach human brain atlas based on digitized MRI from the MNI-152 template, detailing Brodmann area (BA) anatomical locations. The solution space was limited to cortical gray matter and hippocampus, yielding 6239 voxels at 5 mm resolution23. The MNI-152 template facilitated the detection of sLORETA calculation results, describing BA anatomical locations24.
EEG microstate brain network
The EEG data were segmented into 2-second epochs for each microstate. The HERMES toolbox was utilized to calculate brain networks within the 2-20 Hz frequency range, employing the weighted Phase Lag Index (wPLI) index to account for volume conduction effects25. Although wPLI can be applied to source-modeled EEG data, it is predominantly used at the scalp level. wPLI quantifies the alignment of phase differences between time series x(t) and y(t) align with the positive or negative imaginary axis in the complex plane, akin to the Phase Lag Index (PLI)26,27. The underlying concept is that volume conduction primarily results in 0° or 180° phase differences between signals. For estimating genuine, non-volume-conducted activity, only phase angles predominantly on the positive or negative side are considered. The PLI is calculated as the absolute value of the sum of the signs of the imaginary part of the complex cross-spectral density Sxy for signals x(t) and y(t) at a specific time or trial t.
Although PLI is insensitive to zero-lag interactions, the wPLI reduces the impact of volume conduction by scaling angle differences based on their proximity to the real axis, thereby treating near-zero-lag interactions as noise that can affect true zero-lag interactions27:
The wPLI, relying only on the cross-spectrum’s imaginary component, is more robust to noise than coherence because uncorrelated noise sources increase signal power.
A threshold was applied to the fully connected brain functional matrix, converting it into a threshold-weighted network based on connection strengths greater than one standard deviation above the median. Connections below the threshold were set to 0, while those above were retained28.
Network-based statistic (NBS) is a method specialized for statistical analysis of brain networks/functional connectivity matrices29. The NBS toolbox was utilized to identify subnetworks with statistical differences in pairwise comparisons of brain networks across distinct microstates between groups (pDoC vs HC and VS/UWS vs MCS).
Classification model establishment and feature ranking
The support vector machine (SVM) is a classification technique that maximizes the margin of the optimal hyperplane30. Unlike traditional pattern recognition algorithms, which struggle with limited training samples, SVM classifiers minimize both training and testing errors. With limited training data, classification accuracy depends on optimizing model parameters. Particle Swarm Optimization (PSO) and Quantum Particle Swarm Optimization (QPSO) are swarm intelligence algorithms that optimize solutions through iterative cooperation and competition, inspired by natural group behaviors31,32,33. This study employed grid search, PSO, and QPSO to develop three SVM models—GS-SVM, PSO-SVM, and QPSO-SVM—trained on functional connectivity and temporal microstate features derived from four microstates. Models were evaluated using 10-fold cross-validation (90% training, 10% testing per fold) with metrics including accuracy, precision, recall, F1 score, and area under the receiver operating characteristic (ROC) curve (AUC)34. Permutation feature importance, applied to the radial basis function (RBF) SVM kernel, identified the five most important features for each model35. See Figure 2.
Statistical analysis
Microstate topology similarity was analyzed using the Microstate Template Editor and Explorer Apps in Matlab R2023b36. Statistical analyses were performed using Matlab R2023b’s Statistics and Machine Learning Toolbox. The NBS method identified sub-networks with statistical differences, employing t-tests, a threshold of 10, 5000 random permutations, and an FWER-corrected P-value threshold of 0.05. Categorical demographic variables were assessed with chi-square tests, while continuous variables were evaluated using one-way ANOVA or paired t-tests. The non-parametric rank sum test compared time parameters and microstate transition probabilities. Post-hoc tests with Bonferroni correction were conducted for cross-group comparisons of significant parameters. Spearman correlation analysis was applied to explore relationships between microstate characteristics, statistical implications, and clinical scales, with significance set at P < 0.05.
Results
Demographic characteristics of the subjects
Descriptive demographic characteristics of the subjects are detailed in Table 1. Among the 30 pDoC patients, 15 were diagnosed with VS/UWS and 15 with MCS. No significant differences were observed in gender, age, right-handedness, or etiologies. However, a significant difference in CRS-R scores was noted between the VS/UWS and MCS groups (P < 0.001).
Comparison of spatial changes in four EEG microstates
Comparison of microstate topological structures
The topographies of the four microstates for subjects and standard templates are shown in Figure 3. Microstates were labeled as follows: (1) MS A, right frontal to left occipital; (2) MS B, left frontal to right occipital; (3) MS C, prefrontal to occipital; (4) MS D, frontocentral to occipital. MS C in VS/UWS showed the lowest similarity at 1.1% compared to Britz et al. (2010)15. The HC group exhibited the highest similarity across all microstates. The MCS group displayed higher similarity for MS A, B, and C, but lower for MS D, relative to VS/UWS.
Comparison of microstate source location
In the comparison between the VS and MCS groups, MS B and D shared the same source location, while the other microstates did not. Between the MCS and HC groups, MS B exhibited a different source location, whereas the remaining microstates showed consistent source localization. See Table 2 and Figure 4.
Comparison of microstate brain network
Comparison between pDoC and HC
Figure 5 shows that the HC group exhibits stronger connections than the pDoC group on the brain networks of MS A, C, and D. In contrast, there are no significant differences in connections between the HC and pDoC groups on the brain networks of MS B. The pDoC group does not show stronger connections than the HC group on any of the brain networks of MS A, B, C, and D. Detailed inter-electrode connection data are provided in Supporting File 2.
Comparison between VS/UWS and MCS
Figure 6 shows that the MCS group has stronger connections than the VS/UWS group on the brain networks of MS C and D. There are no significant differences in connections between the MCS and VS/UWS groups on the brain networks of MS A and B. The VS/UWS group does not exhibit stronger connections than the MCS group on any of the brain networks of MS A, B, C, and D. Detailed inter-electrode connection data are provided in Supporting File 2.
Comparison of microstate time parameters
Global Explained Variance (GEV) values were 72.29% for VS/UWS, 72.90% for MCS, and 72.25% for HC. In terms of microstate duration, VS/UWS was longer than MCS (P = 0.018) and HC (P < 0.001) in MS B, shorter than MCS (P = 0.009) and HC (P = 0.042) in MS C, and longer than HC (P = 0.019) in MS D. For coverage, VS/UWS was less than MCS (P < 0.001) and HC (P < 0.001) in MS C, and greater than HC (P = 0.009) in MS D. Regarding occurrence, HC was higher than VS/UWS (P < 0.001) and MCS (P = 0.002) in MS B, VS/UWS was lower than MCS (P < 0.001) and HC (P < 0.001) in MS C, and higher than MCS (P < 0.001) in MS D, while HC was higher than MCS (P = 0.024) in MS D. See Table 3 for these comparisons. Detailed time parameter data for microstates are provided in Supporting File 3.
EEG microstate transition probabilities
Transition probabilities from MS A to MS B and from MS C to MS A were lower for VS/UWS compared to MCS (P < 0.001 and P = 0.002, respectively) and HC (P < 0.001 and P < 0.001, respectively). The transition from MS A to MS C was less likely for VS/UWS than for MCS (P = 0.002) and HC (P = 0.036). Conversely, the transition from MS A to MS D was more likely for VS/UWS than for MCS (P < 0.001) and HC (P < 0.001). From MS B to MS D, VS/UWS had a higher transition probability than HC (P = 0.039). Transitions from MS C to MS B were more likely for VS/UWS than for MCS (P = 0.016), while transitions to MS D were less likely for HC than for MCS (P = 0.047). For transitions from MS D to MS A, MCS had a lower probability than VS/UWS (P < 0.001) and HC (P = 0.047). Transitions from MS D to MS B were more likely for VS/UWS than for MCS (P = 0.028) and HC (P < 0.001), and to MS C were less likely for VS/UWS than for MCS (P < 0.001) and HC (P < 0.001). Detailed results are presented in Table 4.
The correlation between microstate time parameters and clinical scales
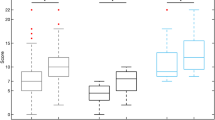
Microstate parameters showing weak correlations with the CRS-R scores include the duration of MS C and the coverage of MS B. Those exhibiting moderate correlations are the duration of MS B, the coverage of MS C, and the occurrences of MS C and D. Figure 7 displays the correlation analysis results between the CRS-R scores and EEG microstate parameters, with detailed data provided in Supporting File 4 and table 3 & 4.
The construction of consciousness classification model and features weight ranking
The ROC curve indicates that PSO-SVM has the highest AUC value of 0.72222, whereas GS-SVM and QPSO-SVM share an AUC value of 0.66667, as depicted in Figure 8A. Among the models, GS-SVM outperforms the others in terms of accuracy, precision, recall, and F1 score, as shown in Figure 8B. In the feature weight rankings across the three models, MS C occurrence is the most significant, as illustrated in Figure 9. Additional information regarding model performance can be found in Supporting File 5.
Evaluation of classification effects of different SVM models. (A) The red line overlaps with the blue line and has the same AUC value. (B) The accuracy of the GS-SVM is 0.88889, PSO-SVM is 0.86667, and QPSO-SVM is 0.86667. The precision of the GS-SVM is 0.91667, PSO-SVM is 0.89444, and QPSO-SVM is 0.90111. The recall of the GS-SVM is 0.88889, PSO-SVM is 0.86667, and QPSO-SVM is 0.86667. The F1 of the GS-SVM is 0.90220, PSO-SVM is 0.87997, and QPSO-SVM is 0.88327.
Features ranking in different SVM models. GS-SVM (A), A: PSO-SVM (B) and QPSO-SVM (C) take top five features respectively. The weight ranges of the three models were not normalized. a: MS C occurrence; b: MS D occurrence; c: The transition of MS A→D; d: The functional connectivity of MS D FC3-P4; e: The functional connectivity of MS D F8-T4.
Discussion
This study employed EEG microstate analysis to compare neural activity between children with pDoC and age- and gender-matched HC and to develop three classification models to identify neurophysiological biomarkers of consciousness. Significant differences (p < 0.01) in the four canonical microstate topographies (A–D) were observed among VS/UWS, MCS, and HC groups, with VS/UWS showing the lowest topographic similarity for MS C (1.1% to standard templates). MS B and D shared similar neural source localization in VS/UWS and MCS, whereas MS B’s source differed between MCS and HC. Weak correlations (r = 0.2–0.3, p < 0.05) were found between the CRS-R scores and MS C duration and MS B coverage. Moderate correlations (r = 0.4–0.6, p < 0.01) were observed for MS B and C duration and occurrence, and MS D occurrence. MS C occurrence may serve as a neurophysiological biomarker to differentiate consciousness states in children with pDoC.
Spatial changes in four EEG microstates
The study revealed significant spatial topology differences in MS C between VS/UWS patients and the standard template by Britz19,37,38. MS C in VS/UWS, exhibiting only 1.1% similarity, should not be classified as standard MS C, potentially due to more severe brain function impairment in these patients39. This low similarity indicates substantial abnormalities and inconsistencies in neural activity between the frontal and occipital lobes. In contrast, HC showed the highest similarity across all microstates, indicating intact and stable brain function40. Source localization of MS C in VS/UWS was found in the left temporal area, significantly differing from MCS and HC groups. The consistent maximum EEG source locations for MS A in HC and MCS groups suggest similar brain activity in the auditory network. Clinically, MCS patients can locate sound sources but usually cannot discern specific sound content6,41.
Table 2 summarizes the microstate source localization results, revealing distinct BAs associated with MS A, B, and C across groups. For MS B, HC localized to BA 30 (retrosplenial cortex, subdivision), implicated in memory integration and spatial processing, while MCS localized to BA 29 (retrosplenial cortex), linked to emotional and contextual memory processing42. These differences suggest altered retrosplenial function in MCS, potentially reflecting disrupted memory-related consciousness networks. For MS A, VS/UWS localized to BA 47 (orbital part of inferior frontal gyrus), associated with decision-making and emotional regulation, whereas MCS localized to BA 11 (orbitofrontal area), involved in reward processing and emotional integration. For MS C, VS/UWS localized to BA 11, while MCS localized to BA 7 (visuo-motor coordination), critical for integrating sensory and motor information. These findings indicate that BA 47 and BA 11 predominance in VS/UWS may reflect impaired higher-order cognitive processing, while BA 7 in MCS suggests partial preservation of sensory-motor integration, potentially aiding differentiation of pDoC states. Further studies are needed to validate these BAs as biomarkers for consciousness assessment.
In brain network analysis of microstates, HC demonstrated stronger connections than pDoC in MS A, C, and D. pDoC did not exhibit stronger connections than HC across MS A, B, C, and D. Similarly, this study found no evidence of VS/UWS having stronger connections than MCS in any of the four microstate brain networks. These variations suggest differing pathophysiological underpinnings, with distinct consciousness states (HC, MCS, VS/UWS) associated with distinct neuropathological profiles. HC’s intact brain structure and function lead to robust inter-electrode connection strength across all microstates43,44. In contrast, MCS and VS/UWS show impaired functional connectivity due to varying degrees of consciousness impairment45,46. The functional connectivity of brain networks is highly dynamic and complex, with each microstate representing unique brain network activity patterns47. These microstates capture the brain’s instantaneous functional connectivity within specific time frames, highlighting varying connection characteristics among individuals with different levels of consciousness.
Time parameters and transition probabilities in EEG microstates
In MS B, the VS/UWS duration is significantly longer than that of the MCS (P = 0.018) and the HC (P < 0.001), suggesting that vegetative state patients remain in this state for a longer period48. This prolonged duration may indicate decreased brain activity and inefficient neural connectivity. Conversely, the VS/UWS spends less time in MS C compared to the MCS (P = 0.009) and the HC (P = 0.042), implying that reduced switching between"task-positive“and”task-negative"networks is associated with less temporal conversion of internal and external perceptions49,50. Studies have also reported prolonged durations of MS B and C in pDoC patients51. In MS D, the VS/UWS’s duration and coverage are significantly higher than in the HC (P = 0.019), indicating that the vegetative state brain remains in this state longer and occupies a larger proportion of brain activity. MS D represents a specific neural activity pattern of the attention network, with its prominence in the VS/UWS suggesting abnormal brain function52.
For MS C, the VS/UWS coverage is significantly shorter than that of the MCS and HC (both P < 0.001). In contrast, MS D exhibits significantly longer VS/UWS coverage compared to the HC (P < 0.001). Prior research has shown increased MS C coverage in pDoC patients52. Additionally, increased MS D coverage has been identified as a severity indicator in pDoC53. Recent studies with extensive microstate mapping have revealed that MS C is distinct, suggesting that the networks it represents may differ54.
In MS B, HC exhibits a significantly higher occurrence than VS/UWS (P < 0.001) and MCS (P = 0.002). Conversely, in MS C, VS/UWS occurrence is significantly lower than both MCS (P < 0.001) and HC (P < 0.001). In MS D, MCS occurrence is significantly lower than HC (P = 0.024) and VS/UWS (P < 0.001). These findings contrast with Eren’s research52. Further investigation is warranted to ascertain if the occurrence of MS B, C, and D can serve as neural markers to differentiate between VS/UWS, MCS, and HC.
The study’s examination of microstate transition probabilities showed that in the VS/UWS, MS A and B predominantly transition to MS D, whereas MS C and D predominantly transition to MS B. Similar patterns were not observed in the MCS and HC. Research indicates that microstate transitions are not random19. Further research is necessary to associate these transition probabilities with functional networks.
The correlation between EEG microstate and clinical scales
EEG microstates, which represent brief periods of stable brain activity, reflect various neural processes55. Parameters of these microstates that weakly correlate with the CRS-R include the duration of MS C and the coverage of MS B. Moderately correlated parameters, indicating a more substantial relationship and a more reliable reflection of CRS-R scores changes, encompass the duration of MS B, the coverage and occurrence of MS C, and the occurrence of MS D. The correlations between CRS-R and EEG microstate parameters suggest that certain microstate metrics may be useful for assessing and monitoring consciousness states in patients with (pDoC. While weak correlations offer limited predictive value, moderate correlations could yield more significant insights. However, correlation does not imply causation, and further research is required to establish a causal link between microstate changes and CRS-R scores.
Consciousness classification model and features weight ranking
SVM, grounded in statistical learning theory, achieves structural risk minimization and is a supervised learning technique for classification and regression. It has been extensively used to construct clinical disease classification models56. Among the three models, PSO-SVM demonstrated superior classification performance. Despite the low AUC values and the atypical appearance of ROC curves for all models, this could be attributed to the small sample size57,58. Neuromarkers, biological correlates of neurological diseases, reflect disease states59. MS C occurrence was the most weighted in all models, suggesting its potential as a neural marker. However, statistical relevance does not guarantee direct biological mechanisms, necessitating further biological validation to ascertain their clinical significance.
Limation and future
This study has several limitations. First, the low incidence of pDoC restricts sample sizes in single-center studies, potentially limiting statistical power. Second, using a low-channel EEG device and a standard skull template for source localization may introduce discrepancies between estimated and actual brain areas. Nevertheless, research with low-channel devices remains valuable for accessibility and feasibility60. Third, neural markers identified by machine learning algorithms require further validation to confirm their reliability and clinical relevance.
Conclusion
This study employed EEG microstate analysis to investigate the spatiotemporal patterns of brain functional activity in children with impaired consciousness, revealing distinct temporal and spatial differences among four microstates across various levels of consciousness. Notably, certain EEG microstate parameters, especially those moderately correlated with CRS-R scores, demonstrate potential for assessing consciousness states in patients with pDoC. The PSO-SVM model outperformed others in classifying different states of consciousness. MS C occurrence emerged as a potential neural marker for distinguishing these states. These findings suggest indicate that EEG microstate analysis may serve as a convenient and objective evaluation method for monitoring the consciousness states of children with pDoC, thereby providing more sensitive guidance for clinical treatment and improving their prognosis.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Additional data can be found in the supporting files.
References
Porcaro, C. et al. Diagnostic developments in differentiating unresponsive wakefulness syndrome and the minimally conscious state. Front. Neurol. 12, 778951. https://doi.org/10.3389/fneur.2021.778951 (2022).
Estraneo, A. et al. Standard EEG in diagnostic process of prolonged disorders of consciousness. Clin. Neurophysiol. 127(6), 2379–2385. https://doi.org/10.1016/j.clinph.2016.03.021 (2016).
Bai, Y., Xia, X. & Li, X. A review of resting-state electroencephalography analysis in disorders of consciousness. Front. Neurol. 8, 471. https://doi.org/10.3389/fneur.2017.00471 (2017).
Kondziella, D. et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 27(5), 741–756. https://doi.org/10.1111/ene.14151 (2020).
Owen, A. M. & Coleman, M. R. Detecting awareness in the vegetative state. Ann. N. Y. Acad. Sci. 1129(1), 130–138. https://doi.org/10.1196/annals.1417.018 (2008).
Molteni, E. et al. Scoping review on the diagnosis, prognosis, and treatment of pediatric disorders of consciousness. Neurology 101(6), e581–e593. https://doi.org/10.1212/WNL.0000000000207473 (2023).
Müller-Putz, G. R. Electroencephalography. Handb. Clin. Neurol. 168, 249–262. https://doi.org/10.1016/B978-0-444-63934-9.00018-4 (2020).
Sanz, L. R. D. et al. Update on neuroimaging in disorders of consciousness. Curr. Opin. Neurol. 34(4), 488. https://doi.org/10.1097/WCO.0000000000000951 (2021).
Çetin, F. H. et al. A case study on EEG analysis: Embedding entropy estimations indicate the decreased neuro-cortical complexity levels mediated by methylphenidate treatment in children with ADHD. Clin. EEG Neurosci. 53(5), 406–417. https://doi.org/10.1177/15500594211064008 (2022).
Aydın, S. Alzhemimer’s disease is characterized by lower segregation in resting-state eyes-closed EEG. J. Med. Biol. Eng. 44(6), 894–902. https://doi.org/10.1007/s40846-024-00917-0 (2024).
Lehmann, D., Ozaki, H. & Pál, I. EEG alpha map series: Brain micro-states by space-oriented adaptive segmentation. Electroencephalogr. Clin. Neurophysiol. 67(3), 271–288. https://doi.org/10.1016/0013-4694(87)90025-3 (1987).
Zhang, C. et al. The temporal dynamics of Large-Scale brain network changes in disorders of consciousness: A Microstate-Based study[J]. CNS Neurosci. Ther. 29(1), 296–305. https://doi.org/10.1111/cns.14003 (2023).
Li, Y. et al. Temporal and spatial variability of dynamic microstate brain network in disorders of consciousness. CNS Neurosci. Ther. 30(2), e14641. https://doi.org/10.1111/cns.14641 (2024).
Li, H. et al. Multiple patterns of EEG parameters and their role in the prediction of patients with prolonged disorders of consciousness. Front. Neurosci. 19, 1492225. https://doi.org/10.3389/fnins.2025.1492225 (2025).
Britz, J., Van De Ville, D. & Michel, C. M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage 52(4), 1162–1170. https://doi.org/10.1016/j.neuroimage.2010.02.052 (2010).
Giacino, J. T., Kalmar, K. & Whyte, J. The JFK coma recovery scale-revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 85(12), 2020–2029. https://doi.org/10.1016/j.apmr.2004.02.033 (2004).
Brunet, D., Murray, M. M. & Michel, C. M. Spatiotemporal analysis of multichannel EEG: CARTOOL. Comput. Intell. Neurosci. 2011, 1–15. https://doi.org/10.1155/2011/813870 (2011).
Khanna, A. et al. Microstates in resting-state EEG: Current status and future directions. Neurosci. Biobehav. Rev. 49, 105–113. https://doi.org/10.1016/j.neubiorev.2014.12.010 (2015).
Michel, C. M. & Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. Neuroimage 180, 577–593. https://doi.org/10.1016/j.neuroimage.2017.11.062 (2018).
Brodbeck, V. et al. EEG microstates of wakefulness and NREM sleep. Neuroimage 62(3), 2129–2139. https://doi.org/10.1016/j.neuroimage.2012.05.060 (2012).
Bochet, A. et al. Early alterations of large-scale brain networks temporal dynamics in young children with autism. Commun. Biol. 4(1), 968. https://doi.org/10.1038/s42003-021-02494-3 (2021).
Brett, M., Johnsrude, I. S. & Owen, A. M. The problem of functional localization in the human brain. Nat. Rev. Neurosci. 3(3), 243–249. https://doi.org/10.1038/nrn756 (2002).
Fuchs, M. et al. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 113(5), 702–712. https://doi.org/10.1016/s1388-2457(02)00030-5 (2002).
Zilles, K. & Amunts, K. Centenary of Brodmann’s map—conception and fate. Nat. Rev. Neurosci. 11(2), 139–145. https://doi.org/10.1038/nrn2776 (2010).
Niso, G. et al. HERMES: Towards an integrated toolbox to characterize functional and effective brain connectivity. Neuroinformatics 11, 405–434. https://doi.org/10.1007/s12021-013-9186-1 (2013).
Vinck, M. et al. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 55(4), 1548–1565. https://doi.org/10.1016/j.neuroimage.2011.01.055 (2011).
Stam, C. J., Nolte, G. & Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 28(11), 1178–1193. https://doi.org/10.1002/hbm.20346 (2007).
Mohanty, R. et al. Rethinking measures of functional connectivity via feature extraction. Sci. Rep. 10(1), 1298. https://doi.org/10.1038/s41598-020-57915-w (2020).
Zalesky, A., Fornito, A. & Bullmore, E. T. Network-based statistic: Identifying differences in brain networks. Neuroimage 53(4), 1197–1207. https://doi.org/10.1016/j.neuroimage.2010.06.041 (2010).
Othman, MFB, Abdullah, NB, Kamal, NFB. MRI brain classification using support vector machine In fourth international conference on modeling, simulation and applied optimization. 1-4 https://doi.org/10.1109/ICMSAO.2011.5775605 (IEEE, 2011).
Kennedy, J., Eberhart, R. Particle swarm optimization In Proc. of ICNN’95-international conference on neural networks 4 1942-1948. https://doi.org/10.1109/ICNN.1995.488968.(ieee, 1995).
Kennedy, J. Swarm intelligence. In Handbook of nature-inspired and innovative computing: Integrating classical models with emerging technologies 187-219 https://doi.org/10.1007/0-387-27705-6 (Boston, MA: Springer US, 2006).
Dian, S. et al. A smooth path planning method for mobile robot using a BES-incorporated modified QPSO algorithm. Expert Syst Appl 208, 118256. https://doi.org/10.1016/j.eswa.2022.11825 (2022).
Wong, T. T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recogn. 48(9), 2839–2846. https://doi.org/10.1016/j.patcog.2015.03.009 (2015).
Altmann, A. et al. Permutation importance: A corrected feature importance measure. Bioinformatics 26(10), 1340–1347. https://doi.org/10.1093/bioinformatics/btq134 (2010).
Koenig, T. et al. EEG-meta-microstates: Towards a more objective use of resting-state EEG microstate findings across studies. Brain Topogr 37(2), 218–231. https://doi.org/10.1007/s10548-023-00993-6 (2024).
Gschwind, M. et al. Fluctuations of spontaneous EEG topographies predict disease state in relapsing-remitting multiple sclerosis. Neuroimage Clin. 12, 466–477. https://doi.org/10.1016/j.nicl.2016.08.008 (2016).
Yuan, H. et al. Spatiotemporal dynamics of the brain at rest—exploring EEG microstates as electrophysiological signatures of BOLD resting state networks. Neuroimage 60(4), 2062–2072. https://doi.org/10.1016/j.neuroimage.2012.02.031 (2012).
Demertzi, A. et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 138(9), 2619–2631. https://doi.org/10.1093/brain/awv169 (2015).
Liu, Y., Li, Z. & Bai, Y. Frontal and parietal lobes play crucial roles in understanding the disorder of consciousness: A perspective from electroencephalogram studies. Front. Neurosci. 16, 1024278. https://doi.org/10.3389/fnins.2022.1024278 (2023).
Ling, Y. et al. Cortical responses to auditory stimulation predict the prognosis of patients with disorders of consciousness. Clin. Neurophysiol. 153, 11–20. https://doi.org/10.1016/j.clinph.2023.06.002 (2023).
Aydın, S. Cross-validated adaboost classification of emotion regulation strategies identified by spectral coherence in resting-state. Neuroinformatics 20(3), 627–639. https://doi.org/10.1007/s12021-021-09542-7 (2022).
Medina Carrion, J. P. et al. Disorder of consciousness: Structural integrity of brain networks for the clinical assessment. Ann. Clin. Transl. Neurol. 10(3), 384–396. https://doi.org/10.1002/acn3.51729 (2023).
Annen, J. et al. Function–structure connectivity in patients with severe brain injury as measured by MRI-DWI and FDG-PET. Hum. Brain Mapp. 37(11), 3707–3720. https://doi.org/10.1002/hbm.23269 (2016).
Panda, R. et al. Disruption in structural–functional network repertoire and time-resolved subcortical fronto-temporoparietal connectivity in disorders of consciousness. Elife 11, e77462. https://doi.org/10.7554/eLife.77462 (2022).
Threlkeld, Z. D. et al. Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex 106, 299–308. https://doi.org/10.1016/j.cortex.2018.05.004 (2018).
Musso, F. et al. Spontaneous brain activity and EEG microstates A novel EEG/fMRI analysis approach to explore resting-state networks. Neuroimage 52(4), 1149–1161. https://doi.org/10.1016/j.neuroimage.2010.01.093 (2010).
Medina, J. P. et al. Resting-state fMRI in chronic patients with disorders of consciousness: The role of lower-order networks for clinical assessment. Brain Sci. 12(3), 355. https://doi.org/10.3390/brainsci12030355 (2022).
Ueno, D. et al. Individual differences in interoceptive accuracy are correlated with salience network connectivity in older adults. Front Aging Neurosci. 12, 592002. https://doi.org/10.3389/fnagi.2020.592002 (2020).
Goulden, N. et al. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage 99, 180–190. https://doi.org/10.1016/j.neuroimage.2014.05.052 (2014).
Toplutaş, E., Aydın, F. & Hanoğlu, L. EEG microstate analysis in patients with disorders of consciousness and its clinical significance. Brain Topogr. 37(3), 377–387. https://doi.org/10.1007/s10548-023-00939-y (2024).
Mäki-Marttunen, V. et al. Modulation of the default-mode network and the attentional network by self-referential processes in patients with disorder of consciousness. Neuropsychologia 82, 149–160. https://doi.org/10.1016/j.neuropsychologia.2016.01.022 (2016).
Stefan, S. et al. Consciousness indexing and outcome prediction with resting-state EEG in severe disorders of consciousness. Brain Topogr. 31, 848–862. https://doi.org/10.1007/s10548-018-0643-x (2018).
Custo, A. et al. Electroencephalographic resting-state networks: Source localization of microstates. Brain Connect. 7(10), 671–682. https://doi.org/10.1089/brain.2016.0476 (2017).
Jajcay, N. & Hlinka, J. Towards a dynamical understanding of microstate analysis of M/EEG data. Neuroimage 281, 120371. https://doi.org/10.1016/j.neuroimage.2023.120371 (2023).
Wang, H., Li, G. & Wang, Z. Fast SVM classifier for large-scale classification problems. IEEE Trans. Pattern. Anal. Mach. Intell. 642, 119136. https://doi.org/10.1109/TPAMI.2021.3085969 (2023).
Ling, CX., Huang, J., Zhang, H. AUC: A better measure than accuracy in comparing learning algorithms In Advances in Artificial Intelligence: 16th Conference of the Canadian Society for Computational Studies of Intelligence, AI 2003, Halifax, Canada Proceedings 16 329-341. https://doi.org/10.1007/3-540-44886-1_25 (Springer Berlin Heidelberg, 2003).
Berrar, D. & Flach, P. Caveats and pitfalls of ROC analysis in clinical microarray research (and how to avoid them). Brief. Bioinform. 13(1), 83–97. https://doi.org/10.1093/bib/bbr008 (2012).
Jollans, L. & Whelan, R. Neuromarkers for mental disorders: Harnessing population neuroscience. Front. Psych. 9, 242. https://doi.org/10.3389/fpsyt.2018.00242 (2018).
Michel, C. M. et al. EEG source imaging. Clin. Neurophysiol. 115(10), 2195–2222. https://doi.org/10.1016/j.clinph.2004.06.001 (2004).
Acknowledgments
The authors thank all participants and their parents for their assistance and cooperation with our study. The authors would like to acknowledge the use of DeepSeek (https://www.deepseek.com/) for the Chinese-English translation, English grammar checking and building classification models using code in the preparation of this manuscript.
Funding
This study was supported by key project of henan province medical science and technology research plan in 2024, project number: SBGJ202402069.
Author information
Authors and Affiliations
Contributions
YZ is the principal investigator and data custodian for this study. Data were collected by WQ, YS, ZH, GZ, YY, LY, GC, SL and MW, and the data analysis was completed by YZ, LZ. The initial draft was written by YZ, WQ, and DZ, and was revised by YZ, JZ, KS, and DZ.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval
The current study was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (No. 2023-270-01).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Hui, Z., Su, Y. et al. EEG microstate analysis in children with prolonged disorders of consciousness. Sci Rep 15, 26148 (2025). https://doi.org/10.1038/s41598-025-11038-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11038-2