Abstract
Arabidopsis thaliana zygotes form a circumferential cortical microtubule (CMT) band that moves upward along the longitudinal axis of the cylindrical cell; however, how and why this CMT band moves remain unknown. Based on several recently proposed feedback mechanisms of CMT reorientation, we hypothesized that a directional cue (DC) changes CMT orientation. We constructed an agent-based simulation model with a DC to observe what happens during CMT formation using different parameters. We determined that the output CMT band can become larger than the input DC zone and identified two types of self-organization processes depending on the parameters used. Furthermore, using well-defined parameters that form a static CMT band, we artificially changed the position of the input DC zone, revealing that CMT band movement requires a DC to move at an appropriate speed.
Similar content being viewed by others
Introduction
Arabidopsis (Arabidopsis thaliana) zygotes are single cells formed by the fusion of egg and sperm cells during plant sexual reproduction. During zygote elongation, the cell adopts a cylindrical shape with a circumferential cortical microtubule (CMT) band near the subapical region; this CMT band maintains a uniform width and moves following the growing tip1,2. CMT band formation is critical for establishing the plant apical–basal axis because it is thought to guide the subsequent formation of the first unequal division plane. However, how and why the CMT band moves remain unknown.
As CMTs guide the deposition of cellulose microfibrils and thus, anisotropic cell growth3,4,5,6, CMT dynamics are closely linked to cell and tissue morphogenesis. The relationship between the plant cell and CMTs is often associated with mechanical feedback between the stress distribution derived from cell geometry and CMT orientation7. CMT-severing proteins cut CMTs perpendicular to stress orientation8. Several reports described the similarity between stress profiles on the cell surface and the distribution of CMT orientation9,10,11. Importantly, CMTs show an increased polymerization in the regions of increased tension12,13suggesting that CMTs may respond to tensile intensity. However, after the default CMT formation can be overwritten by the addition of tensional cues14,15CMTs return to their original orientation after 10 h, strongly suggesting that CMTs respond not only to the tension intensity itself but also to changes or fluctuations in tension15,16. Therefore, CMTs can be modified by changing the intensity or fluctuation of tension within a specific zone.
Based on experimentally obtained CMT–CMT collision probability17,18, several agent-based simulations incorporating growth and shrinkage of a single CMT and its interactions have been developed20,21,22,23,24,25including numerical studies implementing growth and/or shrinkage of a single CMT, CMT interactions (zippering, collision-induced catastrophe, and crossover), and nucleation on the existing CMT (branching)19,20,21as well as theoretical studies capturing possible CMT behaviors using mean-field theory22,23,24,25. The effect of geometry-induced catastrophe is critical for CMT band formation during embryogenesis26,27. In xylem cells, the nucleation rate at specific locations changes, resulting in a CMT pattern with gaps28,29. Thus, simulations of CMT patterns mainly modify collision-induced catastrophe, geometry-induced catastrophe, nucleation, and branching rates.
The concept of a directional cue (DC) for CMTs has also been proposed as an external or intrinsic factor that enables CMTs to reorient in response to phenomenological factors, such as mechanical stress, phytohormone signals, or polarity markers (e.g., specific proteins). Briefly, the DC modifies the properties of the CMT, including its nucleation rate, growth rate, and orientational angle within a specific zone, such as the mechanically distinguishable region discussed above. Two possible DCs have been proposed. The first is a DC that results in CMT reorientation30and the second is a dynamic instability (DI) framework at the cellular scale that results in an increased CMT growth rate without CMT reorientation31. From the physical perspective of the CMT polymerization process, a compressive force derived from tension perpendicular to the CMT promotes an increase in the CMT polymerization rate12,13.
In this study, we explored the first concept of CMT reorientation by performing an agent-based simulation of CMT movement in Arabidopsis zygotes to identify the parameters necessary for the formation of the moving CMT band. We used Arabidopsis zygote data to define a CMT dynamic space (CDS) with the width and movement speed of the moving CMT band. We defined the strength of CMTs reorientation as DC and a specific region with a DC as DC zone. We then reconstructed an agent-based model of CMTs with DC, with the aim of extracting the model parameter range that best explains the width and movement speed of the CMT band. We analyzed which parameters modify the resulting width of the CMT band. Finally, we systematically modified the movement speed of DC zone and found that it should be restricted within a certain range in order to be consistent with the observed data; otherwise, the movement of the CMT band lags behind the movement of DC. Our simulation suggests that an appropriate speed of the movement of DC zone is required to form a moving CMT band.
Methods
To understand the CMT dynamics beneath the membrane of the Arabidopsis zygote, an agent-based simulation was developed focusing on the cylindrical cell geometry during zygote elongation stage. The agent-based model incorporates the growth, shrinkage, and interaction events between agents (CMTs), including zippering, crossovers, and induced catastrophes. In addition, a DC was implemented, which modifies the angle of the newly added CMT plus end. The effect of the finite tubulin pool that restricts the total length of the CMT was taken into account. With this setting, it seems to be trivial that the CMT orientations forced to be aligned with the DC, however, we would seek to obtain non-trivial phenomena according to the strength of the microtubule-response ability and the other microtubule parameters.
Agent-based model of CMTs on the cylindrical cell surface
The agent-based model of the CMTs was based on the previous works27,30as shown in Fig. 1. In summary, reference 27 used a computational approach to study CMT dynamics on triangulated approximations of three-dimensional cell surfaces. Reference 30 used a dynamic CMT network model on 3D cell shapes, investigating to what extent CMT interactions with the membrane can influence CMT dynamics.
Schematic illustrations of cortical microtubule (CMT) dynamics, their interactions, and the effect of the directional cue (DC). The blue and green spheres in (A–F) represent \(\:{\upalpha\:}\) and \(\:{\upbeta\:}\)-tubulins, respectively. The green and magenta oval-like fragments represent the growing and shrinking ends of the microtubule, respectively. The orange region is the DC zone. (A) Microtubule (MT) growth, when the CMT plus end grows at speed \(\:{v}^{+}\). (B) MT shrinkage, when the CMT plus end shrinks at speed \(\:{v}^{-}\) while the CMT minus end undergoes shrinkage at speed \(\:{v}^{tm}\). The rescue rate \(\:{r}_{r}\), which represents the rate of change from growth to shrinkage at the plus end, and the catastrophe rate \(\:{r}_{c}\), which represents the rate of change from shrinkage to growth at the plus end, are also shown. (C) CMT zippering, when one CMT interacts with another within 40° and changes its direction to align with the other. At this angle of interaction, zippering occurs at 100% frequency. (D) Induced CMT catastrophe, when one CMT interacting with another at an angle greater than 40° is induced to depolymerize. (E) CMT crossover, when two CMTs interact with each other at an angle greater than 40° and cross one another. The induced catastrophes (D) and crossovers (E) each occur with a probability of 50% when CMTs meet at an angle greater than 40°. (F) CMTs with a DC, which causes CMTs within the DC zone to change their direction to gradually align to a specific direction (circumferential in this study).
There are six crucial parameters of CMT dynamics: (1) the growth speed of the plus end, \(\:{v}^{+}\) (Fig. 1A); (2) the shrinkage speed of the plus end, \(\:{v}^{-}\) (Fig. 1B); (3) the shrinkage speed of the minus end (treadmilling speed initiated from nucleation), \(\:{v}^{tm}\)32,33; (4) the rate of change from shrinkage to growth at the plus end (rescue rate), \(\:{r}_{r}\); (5) the rate of change from growth to shrinkage at the plus end (spontaneous catastrophe rate), \(\:{r}_{c}\); and (6) the nucleation rate of CMTs, \(\:{r}_{n}\). In more detail, the rescue rate represents the rate at which the plus end deterministically transitions from a depolymerized state to a polymerized state, while the spontaneous catastrophe rate represents the rate at which the plus end deterministically transitions from a polymerized state to a depolymerized state. The parameter values are summarized in Table 1.
In most simulations, cylindrical cell geometry was used with radius \(\:R\) and height \(\:H\). The height coordinate on the cylinder is denoted by \(\:z\). CMT dynamics were simulated on the cylindrical surface with boundaries at the top and bottom; CMTs beyond the boundary were not taken into account. The simulation was iterated \(\:N\) times with the time interval \(\:\varDelta\:t\) = 0.2 s for each step. Most of the iterations in simulations were 12,000 steps, corresponding to a total of approximately 40 min.
Interactions of growing CMTs
In addition to the dynamics of a single CMT described above, the interactions between growing CMTs were also implemented17,22,24,25,26,27. We adopted these because they were based on the experimental data in reference 17 and theoretical evaluations in the refences 24 and 25. Three interactions were considered: (1) zippering, when the growing CMT changes the direction of its growing front to be parallel to an existing CMT without changing the direction of the existing CMT, which happens when a growing CMT collides with an existing CMT at an angle smaller than 40° (Fig. 1C); (2) induced catastrophe, when a CMT begins to depolymerize from the growing front when it meets an existing CMT at an angle greater than 40° (Fig. 1D); and (3) crossover, when a growing CMT appears unaffected by the encounter of an existing CMT at an angle greater than 40° (Fig. 1E). For collisions at angles greater than 40°, this model assumes an equal probability (50%) for induced catastrophes and crossovers, based on the previous studies18,25.
Implementation of directional cue (DC)
The length of the CMT agents can be changed, owing to the growth and shrinkage of the CMT plus and minus ends, whereas the orientation of the CMTs does not change by default. With this setup, the DC zone (as shown in orange in Fig. 1F) is the region where the newly added vector (CMT plus end) is biased in a horizontal direction with weight \(\:{b}_{d}\):
where \(\:{\varvec{r}}_{\varvec{n}}\) and \(\:{\varvec{r}}_{\varvec{n}+1}\) are the current and next unit vectors of the CMT plus end, respectively, \(\:\varvec{u}\) is a circumferential unit vector, and \(\parallel \cdot \parallel\) is the standard norm. Building on the framework of the previous study30we calculated the unit vector without incorporating the stochastic fluctuation component, because our focus was on the deterministic response to the DC.
The notations for position, \(\:{\mu\:}_{T}\), and width, \(\:{\sigma\:}_{T}\), of the DC zone along the coordinate z were used (T stands for turn-on). When the input DC zone was moved, the position \(\:{\mu\:}_{T}\) was changed computationally at each step. The direction of the newly added CMT plus end depends on the position of the existing plus end, as follows:
(1) When the existing CMT plus end is located outside of the DC zone, a newly added CMT plus end will maintain the original direction.
(2) When the existing CMT plus end is located within the DC zone, a newly added CMT plus end will reorient toward the horizontal direction according to Eq. (1).
Effect of finite tubulin pool
To observe CMT dynamics effectively, a finite tubulin pool27 was used. The pool limits the CMT length by \(\:{L}_{max}\), such that the speed of a growing CMT plus end at any time \(\:t\) is dependent on the total length \(\:L\left(t\right)\) of all CMTs in the system:
where \(\:{L}_{max\:}={\rho\:}_{tub}A\) is the length of all the existing CMTs on the surface area \(\:A\) and finite tubulin density \(\:{\rho\:}_{tub}\). In reality, the growth rate of the plus end \(\:{v}^{+}\) can be variable in space; however, it was assumed to be constant in this study.
Quantification of the width of the CMT band in the data
To quantify the width of the CMT band in the zygote imaging data, cell contours were obtained from the images based on the previous study2. Using these cell contours, the cell centerline was extracted and the fluorescence intensity of the CMTs within the cell was projected to the centerline. As our focus was the gaussian type distribution of the CMT band with a higher fluorescence intensity ignoring the other lower intensity, we used the following truncated Gaussian distribution. The fluorescence intensity was fitted with the truncated Gaussian distribution as a function of position \(\:\text{z}\) on the centerline using the parameters \(\:{\upalpha\:},\:\beta\:,\mu\:,s,\text{a}\text{n}\text{d}\:b\) as follows:
where S is the curvilinear coordinate along the cell centerline, and the Gaussian probability density function is \(\:\phi\:\left(S\right)=(1/\sqrt{2\pi\:})\text{exp}\left(-{S}^{2}/2\right)\) with an average \(\:\mu\:\) and standard deviation \(\:s\). The parameter \(\:b\) is the intercept parameter, and \(\:\alpha\:\) and \(\:\beta\:\) are the lower and upper truncation limits, respectively. The cumulative distribution function is \(\:\varPhi\:\left(S\right)=(1/2)\left(1+{erf}\left(S/\sqrt{2}\:\right)\right)\). The width of the CMT band is defined as \(\:{{\upsigma\:}}_{\text{M}\text{T}}^{data}=2s\), and the CMT position is defined as \(\:{\mu\:}_{MT}^{data}=\mu\:\). When the band width could not be calculated because the probability distribution became close to a uniform distribution, the zygote was considered to lack a CMT band.
Quantification of the width of the CMT band in the model
For data analysis, the width of the CMT band can be associated with the probability density of the CMT fluorescence intensity; however, fluorescence intensity was not incorporated into the model, so another way of quantifying the CMT band needed to be defined in the model. For clarity, the term “single CMT formation” was defined as a single CMT oriented in the circumferential direction (a single ring of CMT), and the term “CMT band formation” was defined as a band formed in the circumferential direction (a bundled structure of CMTs). Using these definitions, the width of the CMT band in the simulations was calculated by quantifying the cumulative probability function \(\:P\) of the CMT segments. \(\:P\left(S\right)\) was then fitted with a sigmoid curve using five points around \(\:S=0.5\) (Fig. S1, gray dashed line). The width of the CMT band was estimated as the 68th percentile of the corresponding probability density function for the fitted sigmoidal cumulative distribution (Fig. S1).
Order parameter tensors quantifying the orientational order of the CMTs
To measure the degree of the orientational order of the CMT array in the simulations, a local order parameter tensor \(\:\varvec{q}\) and a global order parameter tensor \(\:\varvec{Q}\) were introduced27. Using the orientation of the CMT segment at position \(\:x\), \(\:\widehat{{\upomega\:}}=\text{cos}\alpha\:\:{\widehat{e}}_{x}\left(x\right)+\text{sin}\alpha\:{\widehat{e}}_{y}\left(x\right)\), where \(\:{\upalpha\:}\:\in\:\left[0,\:2{\uppi\:}\right)\:\)is the declination angle between \(\:\widehat{{\upomega\:}}\) and \(\:{\widehat{e}}_{x}\). The local order parameter tensor \(\:\varvec{q}\) at the local area \(\:dA\) is defined as
with the matrix form
Note that \(\:<\cdot\:>\) represents the sample average of \(\:\cdot\:\) within the local area \(\:dA\). The local orientational order and the local orientation of the CMT segments within \(\:dA\) were estimated by the first eigenvalue of \(\:\varvec{q}\) and by the orientation of the corresponding eigenvector of \(\:\varvec{q}\), respectively. For the characterization of global orientation, the global order parameter tensor was defined as
where \(\:\rho\:\left(x\right)\) is the local areal density of the CMT segments within \(\:dA\). The global orientational order and the global orientation of all the CMTs within the entire region were estimated by the first eigenvalue denoted by \(\:\varLambda\:\) of \(\:\varvec{Q}\) and by the orientation of the corresponding eigenvector of \(\:\varvec{Q}\), respectively. \(\:\varLambda\:\) becomes 0 for completely random CMT orientations, while for well-organized CMTs, \(\:\varLambda\:\) becomes + 1 for the horizontal array and − 1 for the vertical array. The number of local regions was \(\:{n}_{div}\) in this study, dividing the whole region into parts with height \(\:H/{n}_{div}\). A CMT band was considered to exist when Λ exceeded 0.75, and its width was determined from the slope of the probability density distribution.
Results
The CMT band moves upward with a speed of 1.5 ± 1.2 μm/hour and a width of 5.2 ± 2.4 μm
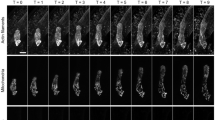
To determine the mechanism by which the CMT band moves to maintain its position as the zygote expands, we first quantified the width and movement speed of the CMT band using actual observation data (Fig. 2A and B). At the CMT-building stage, which occurs just after fertilization, the CMTs are not yet well organized; subsequently, the cell protrudes in the apical direction with the CMT band near the cell tip1. The cell maintains anisotropic elongation during the elongation stage, during which the organized CMT band is localized approximately 5–7 μm below the growing tip2. To elucidate how the CMT band is maintained, we focused on the elongation stage, assuming a cylindrical cell geometry.
Quantification of the width and movement speed of the cortical microtubule (CMT) band from live-cell imaging data of the Arabidopsis zygote. (A) Two-photon excitation microscopy (2PEM) images of the time-lapse observation of a zygote expressing a MT/nucleus marker based on the previously reported dataset2. Numbers indicate the elapsed time (h: min). Scale bar: 10 \(\:{\upmu\:}\)m. (B) Schematic illustrations of zygote growth. MT represents microtubule. The black line shows the zygote contour, and green lines show CMT organization. (C) Definition of the cell centerline S estimated from the cell contour. (D) Quantification of the cell contour and the cell centerlines. The colors of the contours represent the time from the first frame. (E) Quantification of the CMT band. The green line indicates the width of the CMT band (defined below). (F) Truncated Gaussian fitting of the fluorescence intensity projected on the centerline (black line). The red line represents the fitted truncated Gaussian curve. The position of the CMT band µ is estimated based on the median, and the width of the CMT band is estimated as \(\:{\sigma\:}_{MT}^{data}\). (G) Kymograph of the fluorescence intensity projected on the centerline of the zygote. The green and blue lines indicate the upper and lower limits of the CMT band, respectively, where \(\:s\) is the standard deviation. (H) CMT dynamic space (CDS; n = 4, indicating the speed and width of the CMT bands with their frequencies [histograms]). The vertical axis denotes \(\:{{\upsigma\:}}_{MT}^{\text{d}\text{a}\text{t}\text{a}}\), and the horizontal axis is \(\:d{\mu\:}_{MT}^{data}/dt\). The 95th percentile of the CDS is shown as a pale orange region. Typical CMT movement patterns for the cases shown in the red circles are illustrated schematically from the past band (light green) to the current band (darker green).
To standardize the dynamics of the CMT bands of different individuals to an appropriate time scale, we used the characteristic time \(\:{t}_{\text{R}\text{G}\text{S}}\) at the rapid growth stage (RGS), during which the cell length rapidly increases2. We defined the stage from \(\:{t}_{0}\) (5 h before \(\:{t}_{\text{R}\text{G}\text{S}}\)) to \(\:{t}_{\text{R}\text{G}\text{S}}\) as the elongation stage. The CMT fluorescence intensity projected on the cell centerline (Fig. 2C and D) was fitted by the truncated Gaussian distribution with mean \(\:{\mu\:}_{MT}^{data}\) and standard deviation \(\:{\sigma\:}_{MT}^{data}\:\)(Fig. 2E and F; see Methods). A kymograph of the CMT fluorescence intensity revealed that the CMT band localized to the subapical region, maintaining almost the same distance to the cell tip as the zygote elongated (Fig. 2G). The width of the CMT band also remained constant at ~ 5.2 ± 2.4 μm during elongation. The movement speed of the CMT band was 1.5 ± 1.2 μm/hour less than 2.7 μm/hour (Fig. 2H). As the typical elongation speed of zygotes was 1.8 μm/hour on average34the movement speed of the CMT band was approximately comparable to the cell elongation speed. Also, we observed four samples in Fig. 2H, showing that the width and movement of the CMT band were not constant but were fluctuated around the average \(\:(d{\mu\:}_{MT}^{data}/dt,{\sigma\:}_{MT}^{data})=\) (5.2, 1.5).
Reorientation strength parameter \(\:{\varvec{b}}_{\varvec{d}}\) affects both the width and orientational order of the CMT band
To establish correspondence between the observed data and the model parameters, we introduced a CMT dynamic space (CDS) describing the speed \(\:d{\mu\:}_{MT}^{data}/dt\) and width \(\:{\sigma\:}_{MT}^{\text{d}\text{a}\text{t}\text{a}}\) of the CMT band among the previously reported dataset2 (Fig. 2H). Eleven parameters were included in the model (Fig. 1): six CMT dynamics parameters,\(\:\:{v}^{+}\), \(\:{v}^{-}\), \(\:{v}^{tm}\), \(\:{r}_{r}\), \(\:{r}_{c}\), and \(\:{r}_{n}\); three mechanical parameters, \(\:{b}_{d}\), \(\:{\mu\:}_{T}\), and \(\:{\sigma\:}_{T}\); and two geometrical parameters, \(\:R\) and \(\:H\) (Table 1). Based on previous studies and the observed data20,25,27,33we set six parameters (\(\:{v}^{+}\), \(\:{v}^{-}\), \(\:{v}^{tm}\), \(\:{r}_{r}\), \(\:H\), and \(\:{r}_{n}\)) as fixed and observed the effects of changing the remaining five parameters (\(\:{r}_{c}\), \(\:{b}_{d}\), \(\:{\mu\:}_{T}\), \(\:{\sigma\:}_{T}\), and \(\:R\)). We analyzed the dependence of the five parameters based on the CDS.
To observe the effects of parameter perturbations, it is necessary to identify the parameters that can replicate a similar CMT band with sample-averaged data. We initially fixed the parameter \(\:{\mu\:}_{T}\) at the specific location \(\:z=10\:{\upmu\:}\text{m}\) within the range of \(\:z\:\in\:[0,\:20]\) (Fig. 2H) and used specific parameters (\(\:{r}_{c}=0.001\), \(\:{b}_{d}=0.01\), \(\:{\sigma\:}_{T}=6.67\:\left[{\upmu\:}\text{m}\right]\), and \(\:R=5\:\left[{\upmu\:}\text{m}\right]\)). Using this configuration, we examined the changes in the resulting CMT band by perturbing the parameters (\(\:{r}_{c}\), \(\:{b}_{d}\), \(\:{\sigma\:}_{T}\), and \(\:R\)).
To avoid misunderstanding, we defined the terms “single CMT formation” as a single CMT oriented in the circumferential direction (a single ring of CMT) and “CMT band formation” as a band formed in the circumferential direction (a bundled structure of CMTs). We noticed that the CMT band could be formed for the parameter \(\:{b}_{d}=0\), but it was not specifically localized (Fig. 3A). Next, we set \(\:{b}_{d}=0.001\) for the case of a weak DC, resulting in a CMT band partially localized near the DC zone (dashed line) (Fig. 3B). When \(\:{b}_{d}=0.01\), for the case of a strong DC, the CMT band covered the entire region of the DC zone (Fig. 3C, Supplementary Movie S1 and S2). We assessed the time scale of the reorientation during single CMT formation. In the case \(\:{b}_{d}<0.01\), the time required for convergence of CMT bands to the horizontal orientation becomes greater than the time scale of single CMT ring formation, roughly estimated as \(\:2\pi\:R/{v}_{+}\sim\:6.5\) min for \(\:R=5\) µm (Fig. 3D); therefore, we set the case \(\:{b}_{d}=0.01\) as a strong DC where the convergence time is fast enough to outpace single CMT ring formation.
Effect of the tension-responsive ability parameter (\(\:{b}_{d}\)) and the width of the directional cue (DC) \(\:{\sigma\:}_{T}\). The boxplots presented below are based on this definition. For all boxplots, the center line (Median): The line inside the box represents the median, which is the middle value of the dataset. Box limits (Upper and Lower Quartiles): The top and bottom of the box correspond to the 75th and 25th percentile, respectively. Whiskers: The whiskers extend to the smallest and largest values within 1.5 times the interquartile range. Points: Data points that fall outside the whiskers are considered outliers and are plotted as individual points. (A–C) Simulation results when using no DC (A), a weak DC (\(\:{b}_{d}\:=0.001\); B), and a strong DC (\(\:{b}_{d}=0.01\); C). The orientation angles of the CMT measured from the horizontal axis are represented by different colors. The DC zone is indicated by black dashed lines. (D) Change in orientation of a single CMT with vertical growth as a function of \(\:{b}_{d}\). The DC zone is highlighted in orange. (E) Box plots of CMT width \(\:{\sigma\:}_{MT}^{model}\) as a function of \(\:{b}_{d}\). Box represents the range of the central 50% of data, and small circles represent outliers. (F) The global order parameter \(\:\varLambda\:\) as a function of \(\:{b}_{d}\). (G, H) Results of simulations at \(\:t=20\) (min) for different \(\:{\sigma\:}_{T}\) values in the case of a weak DC (G) and a strong DC (H). (I) Width \(\:{\sigma\:}_{MT}^{model}\) as a function of \(\:{\sigma\:}_{T}\) in the case of a weak or strong DC. The whiskers extend to the smallest and largest values within 1.5 times the interquartile range.
To quantitatively describe the degree of CMT band formation, we evaluated the width of the CMT band \(\:{\sigma\:}_{MT}^{model}\) and the global order parameter \(\:\varLambda\:\), measuring both the orientation and density of the CMTs (see Methods). The order parameter \(\:\varLambda\:\) becomes 0 for the completely random orientation of CMTs and 1 for well-organized CMTs. We quantitatively confirmed that the width \(\:{\sigma\:}_{MT}^{model}\) tends to decrease for a certain range of \(\:{b}_{d}\), which is consistent with the above qualitative observations (Fig. 3E). Furthermore, we quantitatively confirmed that the order of CMT organization increases as \(\:{b}_{d}\) increases, which is also consistent with the observed results (Fig. 3F).
The CMT band widens as the input DC widens under both weak and strong DC
To explore whether the width of the output CMT band \(\:{\sigma\:}_{MT}^{model}\) corresponds to the width of the input DC, we focused on the perturbation of the input DC width \(\:{\sigma\:}_{T}\). In the case of a weak DC, the CMTs at\(\:\:t=20\) min were widely distributed beyond the input DC zone (Fig. 3G). In the case of a strong DC, the CMTs at \(\:t=20\) min were distributed in a region with almost the same width as that of the input DC zone (Fig. 3H). Therefore, we found that the resulting width of the CMT band can be wider than the width of the input DC zone. We quantitatively confirmed that these results were preserved independently of the parameter\(\:\:{\sigma\:}_{T}\) (Fig. 3I).
Catastrophe rate induces a phase transition for both strong and weak DC
Next, to confirm the robustness of the CMT band against CMT depolymerization, we systematically increased the catastrophe rate (\(\:{r}_{c}\)). Since depolymerization shortens CMTs, it is expected to decrease the probability of CMT collisions that may inhibit the formation of CMT bands27. Because the DC may compensate for the increased catastrophe rate by facilitating band formation, we tested the changes for both weak and strong DC conditions. In the case of a weak DC, CMT organization at \(\:t=20\) min becomes more sparse as the catastrophe rate increases because induced catastrophe occurs frequently (Fig. 4A). Moreover, quantitative evaluation of \(\:\varLambda\:\:\)revealed that the degree of orientational order was less than 0.75, indicating that the CMTs are not strongly organized (Fig. 4B). As expected, our definition of the width of the CMT band suddenly dropped to small values at \(\:{r}_{c}=0.003\), where the phase transition in terms of CMT band width occurs (Fig. 4C). This sudden decrease in the width of the CMT band occurs even in the case of a strong DC, where horizontally aligned single CMT formation is diminished by catastrophe (Fig. 4D). In this case, the index \(\:\varLambda\:\) was greater than 0.75, indicating that the CMTs are strongly organized (Fig. 4E) and a similar phase transition occurs (Fig. 4F).
Effect of the catastrophe rate \(\:{r}_{c}\) on the orientational order and width of the resulting cortical microtubule (CMT) band. (A, D) Simulation results at \(\:t\:=\:20\) [min] for different \(\:{r}_{c}\) in the case of a weak directional cue (DC; A) and a strong DC (D). (B, E) Dependence of the global order parameter \(\:\varLambda\:\) as a function of \(\:{r}_{c}\) in the case of a weak DC (B) and a strong DC (E). (C, F) Dependence of the width \(\:{\sigma\:}_{MT}^{model}\) as a function of \(\:{r}_{c}\) in the case of a weak DC (C) and a strong DC (F).
Cylinder radius does not strongly affect the CMT band but emphasizes two different types of CMT organization
Since changes in the cylinder radius alter the circumference and surface area of the cylinder, which is expected to increase the time required for CMTs to form bands, we tested the cases of R = 5.0 μm (typical zygote scale2) and R = 15 μm. In addition, we simulated weak and strong DC conditions because the DC may compensate for a delay in CMT band formation.
In the case of R = 5.0 μm, the orientational order \(\:\varLambda\:\) increased in both the strong and weak DC cases, and the resulting width of the CMT band showed an increasing trend over time (Fig. 5A-C and H-J). In the case of R = 15 \(\:{\upmu\:}\text{m}\), the trends were similar, except for the width of the CMT band in the weak DC case (Fig. 5D-F and K-M). We considered a possible mechanism for this exception as follows.
Effect of the cell radius \(\:R\) and two different types of cortical microtubule (CMT) organization. (A-C, and D-F) Simulation results for a strong directional cue (DC) with a temporal dependence of \(\:\varLambda\:\) and \(\:{\sigma\:}_{MT}^{model}\) when \(\:R\:=\:5.0\) (µm) (B, C) and \(\:R\:=\:15.0\) (µm) (E, F). The green lines represent the sigmoid function for \(\:\varLambda\:\) and the linear function for \(\:{\sigma\:}_{MT}^{model}\). (G) Schematic illustration of rapid CMT organization. Due to the rapid organization of horizontal CMTs, diagonal CMTs may be disorganized, and only the horizontal CMT band remains. The green dashed lines represent newly forming CMTs, where the red circles at the leading edge indicate those that do not collide with pre-existing CMTs, making them likely to grow. By contrast, the red crosses at the leading edge represent CMTs that collide with pre-existing CMTs, indicating a reduced likelihood of growth. The colored band represents the region under strong DC influence. (H-J, K-M) Simulation results for a weak DC with a temporal dependence of \(\:\varLambda\:\) and \(\:{\sigma\:}_{MT}^{model}\) when \(\:R\:=\:5.0\) (µm) (I, J) and \(\:R\:=\:15.0\) (µm) (L, M). For cases where the CMT band cannot be defined, the corresponding box plot is not shown. (N) Schematic illustration of gradual CMT organization. The green dashed lines represent newly forming CMTs, where the red circles at the leading edge indicate those that do not collide with pre-existing CMTs, making them likely to grow. By contrast, the red crosses at the leading edge represent CMTs that collide with pre-existing CMTs, indicating a reduced likelihood of growth. The colored band represents the region under weak DC influence. Without horizontal CMTs, diagonal CMTs can be organized so that the CMT band becomes widened.
In the case of a strong DC, the CMT band is rapidly organized, and this behavior is not strongly affected by the radius (Fig. 5A-C and H-J). Therefore, the index \(\:\varLambda\:\) and the CMT band width \(\:{\sigma\:}_{MT}\) show a similar trend (Fig. 5A, D, B, E, C and F). By contrast, in the case of a weak DC, the CMTs are not strongly aligned at first and gradually become organized (Fig. 5D-F and K-M). As a result, the index \(\:\varLambda\:\) values are lower than those in the case of a strong DC (Fig. 5E and L), and the CMT band width \(\:{\sigma\:}_{MT}\) with \(\:R=15\) µm gradually decreases, reflecting gradual organization (Fig. 5M).
The mechanism behind this might be the different frequency of CMT encounters, as summarized in Fig. 5G and N. In the case of a strong DC, the horizontal CMTs are rapidly organized, preventing the diagonal organization of the CMT band (Fig. 5G). In the case of a weak DC, the horizontal CMTs are not well organized, allowing the diagonal or spiral organization of the CMT band (Fig. 5N). Instead of rapid organization of the CMT band, the newly added CMTs do not quickly reorient but slowly reorient toward the horizontal direction. Consequently, diagonal CMT alignments are formed that do not strongly prevent the vertical organization of CMTs; thus, it takes time for the CMT band to reorganize. We call this phenomenon a gradual organization of CMTs and distinguish this from rapid organization.
A moving CMT band can form when the DC zone displays an appropriate movement speed and width
As the above results suggest that a desired CMT band can be reproduced with \(\:{r}_{c}\le\:0.002\), \(\:{b}_{d}\ge\:0.01\), \(\:{\sigma\:}_{T}\) slightly smaller than the desired band, and \(\:R=5.0\), corresponding to the observed data, we moved the designated CMT band by changing the specific parameter \(\:d{\mu\:}_{T}/dt\) (the movement speed of the DC zone). As illustrated schematically in Fig. 6A, we searched for the best fitted parameter set (\(\:d{\mu\:}_{T}/dt\), \(\:{\sigma\:}_{T}\)) from the CDS in Fig. 2H using an exhaustive search of the model parameters. As the expected DC parameters were unknown, we distributed the parameter set with \(\:d{\mu\:}_{T}/dt\in\:\left[\text{0,4.0}\right]\) µm/hour and \(\:{\sigma\:}_{T}\in\:\left[\text{0.5,3.5}\right]\) µm as a similar range to the data. As a result, \(\:{\sigma\:}_{MT}^{model}\) became wider than \(\:{\sigma\:}_{T}\), while, unexpectedly, the resulting \(\:d{\mu\:}_{MT}^{model}/dt\) became smaller or sometimes even a negative value, which is below the expected movement speed of the CMT band (Fig. 6B, D, F, H). To demonstrate what happens, we plotted the simulated CMTs from Fig. 6C: Panel (i) shows CMT behaviors with appropriate width and movement speed of the DC zone (Fig. 6D and E), (ii) shows the behavior with slower DC movement (Fig. 6F, G), and (iii) shows the behavior with faster DC movement and a wider DC (Fig. 6H). These results suggest that when the DC zone moves more quickly than the CMT growth speed, the organized CMT band lags behind the DC zone (Fig. 6H and I). (iv) shows a condition in which the movement of the CMT band exceeds that of the DC zone. This faster movement can result from stochastic CMT band formation in regions outside the DC zone.
Parameter inference by mapping input directional cue (DC) parameters to the output cortical microtubule (CMT) dynamic space (CDS). (A) Schematic illustrations of the microtubule dynamic (MTD) space in the data, with expected input DC parameters (green) and a similar range of input DC parameters to the MTD space (dashed line). (B) Simulated results in the CDS with the data range from Fig. 2H (green). (C) Discrepancy between the output and input CMT speed compared with the diagonal equivalent line (gray dashed line). Depending on the output speed of the CMT band, there are static states, states that follow the input, and states that lag behind the input. (D, F, H) CMT dynamics with the appropriate DC zone speed and length for the resulting CMT band (Case 01) (D), with a faster speed than the CMT band (Case 02) (F), and with a broader width and faster speed than the CMT band (Case 03) (H). (E, G, I) Schematic illustration of the obtained results. Depending on the input speed of the DC zone, the resulting CMT band did not move (E, i), moved appropriately (G, ii), or lagged behind the DC zone (I, iii). Region (iv) represents the case where the band speed exceeds the input speed.
In summary, we conclude that a moving CMT band can be achieved with an appropriate width and movement speed of the DC zone.
Discussion
In this study, we developed an agent-based simulation based on the CMT tension-responsive hypothesis to identify the parameters necessary for the formation of the moving CMT band, which specifically forms in the plant zygote. Through CDS analysis, we successfully compared CMT bands between the model and the observed data to estimate the necessary parameters and obtained four major new findings regarding the reproduction of the moving CMT band in Arabidopsis zygotes: (1) The width of the CMT band is ~ 5.2 ± 2.4 μm, and the movement speed is ~ 1.3 ± 1.5 μm/hour. (2) The output width of the CMT band produced can be wider than the input width of the DC zone. (3) Two types of CMT band formation can occur: rapid organization and gradual organization. (4) The formation of a moving CMT band requires an appropriate width and movement speed of the DC zone.
In addition to the CMT tension-responsive hypothesis, organization of the CMT band has been hypothesized to involve other mechanisms. The edge catastrophe hypothesis, where the cell edge induces CMT catastrophe, was proposed to explain CMT organization in early embryonic apical cells in previous studies24,25. However, this hypothesis may not apply to the zygote, especially at the elongation stage, when cell edges are lacking around the CMT band. The conceptual basis for the CMT tension-responsive hypothesis is an increase in the rate of CMT polymerization in the region of higher tension, based on previous studies12,13. However, experimental research on CMTs in protoplasts15 has shown that tension changes over time may increase the rate of CMT polymerization. Thus, the driving force might be a time derivative of the tension2. Additionally, we cannot rule out the possibility that CMTs are severed in a direction perpendicular to the tension exerted by katanin outside the DC zone8. Another possibility is an increasing growth rate without reorientation31and a possible crosstalk with actin alignments, which are formed in the longitudinal direction, which are beyond the scope of this study.
To be consistent with the parameter ranges of CMT bands in the actual observed data, the movement speed of the DC zone must be comparable to that of cell elongation (1.5 μm/hour on average). The formation of a moving, organized CMT band in the zygote may occur through a similar mechanism to the formation of CMT band structures during the tip growth of fern protonema, whose elongation rate is relatively small35. The elongation rate of root hairs is greater than 60 μm/hour36, which is more than 10 times that of the zygote, which is reported to be 2.4–3.6 μm/hour34, so it is thought that root hairs are unable to form CMT bands, as supported by our numerical results. One hypothesis is that a “lag behind” phenomenon is associated with tension where the mechanical (tensional) change during elongation becomes so fast that CMT organization does not catch up with the input mechanical perturbation. This might explain why pollen tubes do not have CMT bands, as their elongation speed is more than 10 times faster than that of the zygote37,38. Determining when and how organisms evolved a moving CMT band is of great significance, not only for cell and molecular biology, but also for understanding the evolution and diversity of plant cells.
In this study, we developed a data–model correspondence method called CDS to narrow down the range of molecular parameters such as tension-responsive ability and the catastrophe rate of CMTs, which are difficult to observe experimentally. This method can be applied not only to other cylindrical plant cells39but also to cells in general, such as the orientational order during the formation of the extracellular matrix of animal cells (for example, in fruit fly [Drosophila melanogaster] trachea40).
Data availability
The source code for the simulations presented in this study is openly available in a GitHub repository at the following URL: https://github.com/T-Nono-mathematical-biology/agent-based-model-CMT.
References
Kimata, Y. et al. Cytoskeleton dynamics control the first asymmetric cell division in Arabidopsis zygote. Proc. Natl. Acad. Sci. 113, 14157–14162(2016).
Kang, Z. C. et al. Temporal changes in surface tension guide the accurate asymmetric division of Arabidopsis zygotes. Preprint At. https://doi.org/10.1101/2024.08.07.605794 (2025).
Heath, I. B. A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J. Theor. Biol. 48, 445–449 (1974).
Paradez, A. R., Somerville, C. R. & Ehrhardt, D. W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495 (2006).
Balsuka, F. B., Samaj, J., Wojtaszek, P., Volkmann, D. & Menzel, D. Cytoskelen-plasma Membrane-cell wall continuum in plants. Emerging links revisited. Plant. Physiol. 133, 482–491 (2003).
Lloyd, C. & Chan, J. The parallel lives of microtubules and cellulose microfibrils. Curr. Opin. Plant. Biol. 11, 641–646 (2008).
Hamant, O. et al. Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 (2008).
Uyttewaal, M. et al. Mechanical stress acts via Katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 149, 439–451 (2012).
Sampathkumar, A. et al. Meyerowitz. Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 3, e01967 (2014).
Hervieux, N. et al. Mechanical shielding of rapidly growing cells buffers growth heterogeneity and contributes to organ shape reproducibility. Curr. Biol. 27, 3468–3479 (2017).
Sapala, A. et al. Why plants make puzzle cells, and how their shape emerges. eLife 7, e32794 (2018).
Inoue, D. et al. Sensing surface mechanical deformation using active probes driven by motor proteins. Nat. Commun. 7, 12557 (2016).
Hamant, O., Inoue, D., Bouchez, D., Dumais, J. & Mjolsness, E. Are microtubules tension sensors? Nat. Commun. 10, 2360 (2019).
Durand-Smet, P., Spelman, T. A., Meyerowitz, E. M. & Jönsson, H. Cytoskeletal organization in isolated plant cells under geometry control. Proc. Natl. Acad. Sci. 117, 17399–17408 (2020).
Colin, L. et al. Cortical tension overrides geometrical cues to orient microtubules in confined protoplasts. Proc. Natl. Acad. Sci. 117, 32731–32738 (2020).
Moulia, B., Douady, S. & Hamant, O. Fluctuations shape plants through proprioception. Science 372, eabc6868 (2021).
Dixit, R. & Cyr, R. Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant. Cell. 16, 3274–3284 (2004).
Dixit, R. & Cyr, R. The cortical microtubule array: from dynamics to organization. Plant. Cell. 16, 2546–2552 (2004).
Allard, J. F., Wasteneys, G. O. & Cytrynbaum, E. N. Mechanisms of self-organization of cortical microtubules in plants revealed by computational simulations. Mol. Biol. Cell. 21, 278–286 (2010).
Eren, E. C., Dixit, R., Gautam, N. A. & Three-Dimensional Computer simulation model reveals the mechanisms for Self-Organization of plant cortical microtubules into oblique arrays. Mol. Biol. Cell. 21, 2674–2684 (2010).
Deinum, E. E., Tindemans, S. H. & Mulder, B. M. Taking directions: the role of microtubule-bound nucleation in the self-organization of the plant cortical array. Phys. Biol. 8, 056002 (2011).
Shi, X-Q. & Ma, Y-Q. Understanding phase behavior of plant cell cortex microtubule organization. Proc. Natl. Acad. Sci. 107, 11709–11714 (2010).
Hawkins, R. J., Tindemans, S. H. & Mulder, B. M. Model for the orientational ordering of the plant microtubule cortical array. Phy Rev. E. 82, 011911 (2010).
Tindemans, S. H., Hawkins, R. J. & Mulder, B. M. Survival of the aligned: ordering of the plant cortical microtubule array. Phys. Rev. Lett. 104, 058103 (2010).
Tindemans, S. H., Deinum, E. E., Lindeboom, J. J. & Mulder, B. M. Efficient event-driven simulations shed new light on microtubule organization in the plant cortical array. Front. Phys. 2, 1–15 (2014).
Chakrabortty, B. et al. A plausible Microtubule-Based mechanism for cell division orientation in plant embryogenesis. Curr. Biol. 28, 3031–3043 (2018).
Chakrabortty, B., Blilou, I., Scheres, B. & Mulder, B. M. A computational framework for cortical microtubule dynamics in realistically shaped plant cells. PloS Comput. Biol. 14, e1005959 (2018).
Schneider, R. et al. Long-term single-cell imaging and simulations of microtubules reveal principles behind wall patterning during proto-xylem development. Nat. Commun. 12, 7085 (2021).
Jacobs, B., Saltini, M., Molenaar, J., Filion, L. & Deinum, E. E. Microtubule flexibility, microtubule-based nucleation and ROP pattern co-alignment enhance protoxylem microtubule patterning. Quant. Plant Biol. 6, e2, 1–13 (2024).
Mirabet, V. et al. The self-organization of plant microtubules inside the cell volume yields their cortical localization, stable alignment, and sensitivity to external cues. PloS Comput. Biol. 14, 21006011 (2018).
Li, J., Szymanski, D. B. & Kim, T. Probing stress-regulated ordering of the plant cortical microtubule array via a computational approach. BMC Plant. Biol. 23, 308 (2023).
Mitchison, T. & &’ Kirschner, M. Dynamic instability of microtubule growth. Nature 312, 237–242 (1984).
Shaw, S. L., Kamyar, R. & Ehrhardt, D. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300, 1715–1718 (2003).
Kang, Z. C. et al. Coordinate normalization of live-cell imaging data reveals growth dynamics of the Arabidopsis zygote. Plant Cell. Physiol. 64, 1279-1288 (2023).
Murata, T. & Wada, M. Organization of cortical microtubules and microfibril deposition in response to blue-light-induced apical swelling in a tip-growing Adiantum protonema cell. Planta 178, 334–341 (1989).
Grierson, C., Nielsen, E., Ketelaar, T. & Schiefelbein, J. Root hairs. Arabidopsis Book. 12, e0172 (2014).
BedingerP The remarkable biology of pollen. Plant. Cell. 4, 879–887 (1992).
Schiøtt, M., Romanowsky, S. M., Bækgaard, L. & Harper, J. F. A plant plasma membrane Ca2 + pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. 4101, 9502–9507 (2004).
Hasezawa, S., Kumagai, F. & Nagata, T. Sites of microtubule reorganization in Tabacco BY-2 cells during cell-cycle progression. Protoplasma 198, 202–209 (1997).
Öztürk-Çolak, A., Moussian, B., Araújo, S. J. & Casanova, J. A feedback mechanism converts individual cell features into a supracellular ECM structure in Drosophila trachea. eLife 5, e09373 (2016).
Acknowledgements
The authors thank Koichi Fujimoto, Katsuyoshi Matsushita, and Naoya Kamamoto (Hiroshima University), Takumi Higaki and Haruka Ono (Kumamoto University), Ishimoto Yukitaka (Saga University), and Yusuke Kimata (Tohoku University) for helpful discussions.
Author information
Authors and Affiliations
Contributions
T.N. and S.T. conceived and designed the study. T.N. and S.T. developed and implemented models and algorithms. H.M., S.N., and M.U. carried out data acquisition from live-cell imaging. T.N. and S.T. wrote the manuscript. T.N., Z.K., S.T., and M.U. edited and reviewed the manuscript. S.T., H.M., and M.U. provided research funding. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Financial support
This work was supported by JSPS KAKENHI (Grant Numbers JP20K15832, JP22K15135, JP25K18499, JP19H05670, JP19H05676, JP23H02494, JP22K21352, and JP25KJ0540), JST CREST (JPMJCR2121), the Young Researcher Challenge (YORC; to T.N. and Z.K.), the Suntory Rising Stars Encouragement Program in Life Sciences (SunRiSE; to M.U.), and the Toray Science Foundation (20-6102; to M.U.).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nonoyama, T., Kang, Z., Matsumoto, H. et al. Agent-based simulation of cortical microtubule band movement in arabidopsis zygotes. Sci Rep 15, 25787 (2025). https://doi.org/10.1038/s41598-025-11078-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11078-8