Abstract
Organochlorine pesticides (OCPs) are very hazardous and persistent compounds, endangering human health and the environment. This study reports the data analysis on profile distribution, residual level, ecological risk assessment, and soil-air exchange of OCPs in environmental compartments (water, sediments, soil, and air) of Uchalli and Khabeki Lakes. The samples were analyzed through GC-MS. Ʃ13OCPs in water, sediment, soil, and air of Uchalli Lake were in the range of 0.94–10.37 µg/L, 289.26-589.03 ng/g, 247.06-480.17 ng/g, and 3.31-168.65 pg/m3, respectively. In contrast, the concentrations of Ʃ13OCPs in water, sediment, soil, and air of Khabeki Lake varied between 0.27 and 2.70 µg/L, 146.9-348.49 ng/g, 159.01-384.78 ng/g, and 187.43-1349.99 pg/m3, respectively. Among all of the analyzed OCPs, the contamination of DDTs was highest across all matrices in both studied lakes. Independent sample t test showed highly significant variation (p < 0.01) of o, pʹ-DDE, p, pʹ-DDE, o, pʹ-DDD, o, pʹ-DDT, p, pʹ-DDT in water, p, pʹ-DDD in air, while cis-chlordane was found in the soil of both lakes. In contrast, sediments represent non-significant variation (p > 0.05) in the studied lakes. The principal components of PCA of Uchalli and Khabeki Lakes revealed 73.04% and 82.57% of the total variance, indicating strong association between the sources and distribution patterns of OCPs in environmental compartments. Moreover, HCHs and DDTs represent net volatilization from soil to the atmosphere of Khabeki Lake, with the fugacity fraction greater than 0.5. In contrast, the metabolites of endosulphan and chlordane displayed ff < 0.5, showing atmospheric deposition into the soil of Uchalli and Khabeki Lakes. All of the DDT metabolites in Uchalli Lake and the sole metabolite (o, pʹ-DDE) in Khabeki Lake were shown to pose a significant threat to the ecological integrity of the studied lakes, indicating a high risk with RQ > 1. This study suggests that exposed OCP stockpiles pose a danger and should be restricted until remediation to ensure the ecological integrity of Uchalli and Khabeki Lakes. Proper disposal of banned OCPs and strict enforcement of regulations are essential to prevent illegal use.
Similar content being viewed by others
Introduction
Dichlorodiphenyltrichloroethanes (DDTs), hexachlorocyclohexanes (HCHs), chlordane, and endosulfan represent well-known organochlorine pesticides (OCPs) extensively employed for agricultural pest control and public health protection1. Despite being officially banned in Pakistan since 1994, detectable residues of DDTs and other OCPs persist in environmental compartments due to their chemical stability, lipophilicity, and resistance to degradation2. OCPs have an average half-life ranging from 60 days up to 10–15 years, depending on compound type and environmental conditions. DDT, one of the most widely used OCPs, has a half-life between 2 and 15 years, making it a dominant residue in many ecosystems3. These properties contribute to their long-term persistence in soil, sediment, and biota and their potential to bioaccumulate and biomagnify through food chains, posing risks to the ecosystem and human health4,5,6. Among these, DDTs and HCHs have been the most extensively studied, while endosulfan and chlordane are known for their endocrine-disrupting, neurotoxic, and carcinogenic effects7. Due to which, OCPs are constrained by regulatory bodies such as the Stockholm Convention, the Environmental Protection Agency (EPA), and the World Health Organization (WHO)8,9.
Lakes are considered major sinks for OCPs and, because of their high retention capacity, can also act as secondary sources of contamination within aquatic ecosystems10. Soil also plays a significant role in transmitting OCPs to other compartments. It is primarily caused by atmospheric deposition of air pollutants and volatilization from regions where pesticides are directly applied for agricultural purposes11. They are susceptible to Long Range Atmospheric Transport (LRAT), which causes them to spread swiftly through the atmosphere and tend to be redistributed globally from the source sites to remote areas because of their volatile nature12,13. Global warming and temperature fluctuations have increased pollutant volatilization from soil to the atmsophere14,15. Therefore, assessing OCP distribution through soil-air exchange is essential to understand their environmental behavior16,17.
In the context of Pakistan, reports estimate that more than 6,000 tonnes of obsolete pesticides remain stockpiled across various provinces, including Punjab, Sindh, Khyber Pakhtunkhwa, and Balochistan18. These chemicals can leach into groundwater, runoff into surface water bodies, or evaporate into the atmosphere, leading to widespread environmental dissemination19. Additionally, due to inadequate enforcement of environmental regulations, illegal use of banned pesticides persists in agricultural regions. Pesticide usage is dominated by insecticides (74%), with over 65% applied to cotton crops, followed by rice, sugarcane, maize, fruits, vegetables, and tobacco20. Such practices amplify the risk of long-term contamination and exposure for ecosystems and rural populations dependent on these environments for food and water.
The Uchalli and Khabeki Lakes in the Soan Valley, Khushab, Pakistan, are particularly vulnerable to OCP contamination due to their proximity to agricultural lands and potential exposure to pesticide runoff and atmospheric deposition. Studies have indicated that these lakes, part of the Uchalli Wetland Complex (UWC), are experiencing ecological stress, with alterations in water quality and accumulation of potentially harmful pollutants21. Land-use changes, including increased agricultural activities, have further exacerbated the situation, leading to habitat degradation and increased pollutant influx22. Given the persistence and bioaccumulative nature of OCPs, there is a pressing need for comprehensive monitoring and remediation efforts to safeguard these vital ecosystems and the communities that rely on them.
Different studies have reported high levels of OCP residues in environmental compartments of Pakistan, including the Ravi, Indus, and Baranda rivers, and regions of Azad Jammu and Kashmir, confirming their widespread distribution and persistence in the environment23,24,25,26. However, no comprehensive research has been conducted on OCP contamination in the Uchalli and Khabeki Lakes, part of UWC in Soan Valley, Khushab. This region holds ecological significance as a Ramsar site and has recently shown signs of environmental stress and biodiversity loss27,28. In light of the absence of prior baseline data and considering the ecological and public health importance of this site, this study aims to investigate the occurrence, spatial distribution, and ecological risk of organochlorine pesticides, specifically DDTs, HCHs, endosufan and chlordane in environmental compartments (water, sediment, soil, and air) of the Uchalli and Khabeki Lakes. The research is guided by the hypothesis that historical agricultural practices, legacy stockpiles, and atmospheric deposition have led to persistent OCP contamination in these lakes.
Specifically, the study will: (i) quantify OCP concentrations; (ii) evaluate their spatial distribution and fate using the soil-air exchange mechanism; and (iii) estimate ecological risk through the Risk Quotient (RQ) method. This will help the environmental health of the lakes and inform future remediation and policy strategies.
Materials and methodology
Study area
The study site includes Khabeki and Uchalli Lakes in Soan Valley, Khushab district, Pakistan. The Khabeki Lake is a shallow brackish to saline lake in the Soan Valley at 740 m above sea level (32.6219° N, 72.2141° E) with an area of 283 hectares (ha). It is 10 km from Naushera Village and 38 km northwest of Khushab District. The Uchalli Lake (32.5600° N, 72.0200° E) has a pH of more than 8, making it brackish to saline, and is approximately 943 ha in size, with a depth of 0.2 to 6 metres28. It is situated 13 km west of Naushera and 42 km north-west of Khushab District in Punjab Province, Pakistan (Fig. 1). Rainfall, seepage from adjacent irrigated land, runoff from nearby hill torrents, and springs are the primary sources of water for these naturally occurring lakes. Additionally, it receives sewage wastewater from nearby settlements due to a lack of a pipeline system28,29.
Sample collection
Water sampling
Composite water samples were collected from Uchalli Lake (n = 10) and Khabeki Lake (n = 12) to represent overall lake-wide contamination profiles. Each composite consisted of four grab samples taken from different sites across the lake, covering all four cardinal directions (north, south, east, and west) to enhance spatial representativeness. Additionally, two and four depth-stratified composite water samples were collected from Uchalli and Khabeki Lakes, respectively, at depths ranging from 1 to 5 m (Fig. 1). Both surface and deep water samples were obtained by using a calibrated water sampler and stored in pre-cleaned 1-liter bottles30. The composite sampling approach was chosen to minimize spatial heterogeneity while providing a representative evaluation of pollutant distribution with each lake31.
Sediment sampling
Composite sediment samples (n = 16) were taken separately from Uchalli and Khabeki Lakes, with each composite consisting of 4 grab samples taken from different locations within the same lake, as illustrated in Fig. 1. A stainless steel spatula was used to collect the top (0–10 cm) layer of sediments (EPA Method 5035). Sediments were collected directly into polyethylene bags using a sampler submerged in the lake30.
Soil sampling
Soil samples (n = 6) were taken from agricultural lands in Chitta and Uchalli villages near Uchalli Lake, and Naushera and Khabeki villages near Khabeki Lake (Fig. 1). A 10 cm depth of surface soil was collected using an auger (EPA Method 5035). At each site, one composite sample was prepared by thoroughly mixing four grab samples on a stainless-steel tray to ensure homogeneity. The samples were placed in bags made of polyethylene32.
Air sampling
Polyurethane Foam-Passive Air Samplers (PUF-PAS) were placed 2.5 m above the ground at specific locations surrounding the agricultural lands of Uchalli (n = 10) and Khabeki (n = 7) Lakes, respectively, as shown in Fig. 1. PUF-PAS were deployed for eight weeks from March to May 2022. Each PUF disc had a diameter of 5.5ꞌꞌ by 0.5ꞌꞌ in thickness. It was cleaned by acetone, wrapped in aluminum foil, and installed on-site into the Passive Air Sampler (PAS) to avoid contamination with a 1.5 cm intra-space diameter between two stainless steel domes of 20 cm at the deployment sites of Uchalli and Khabeki Lakes. Throughout the sample process, blanks for transportation were left open. The PUF discs were collected in June.
All samples, including water bottles, polyethylene bags of sediment and soil, and PUF discs, were securely packed, labeled on-site, and put in an ice box before being delivered to the Soil and Plant Laboratory at Government College University (GCU), Lahore, where they were kept at −4 °C until further examination33,34.
Sample preparation and extraction
A 500 ml water sample was filtered through a sieve to remove suspended solids. The samples were spiked with 10 µL of recovery indicator decachlorobiphenyl (PCB No. 209). The funnel was filled with a mixture of acetone and dichloromethane (DCM) with a ratio of 5:2 v/v to create an organic layer by shaking it upside down. 5 ml glass vials were used to remove the organic layer. About 10 g of sample was packed into a 30 × 100 mm cellulose thimble to extract sediment and soil samples. The sodium sulfate was gradually poured into the thimble using a stirrer to remove any remaining moisture from the samples. After adding 150 ml of DCM to the round-bottom flask, the thimble was kept in a Soxhlet extractor at 45 °C for 24 hours6.
For air extraction, PUFs were Soxhlet-extracted for approximately 24 h at 45 °C using 150 mL DCM. Before the extraction, all sediment, soil, and air samples were spiked with 10 µL of recovery indicator. The extract was reduced to 4–5 mL at 30 °C by rotary evaporation (DIAHAN Scientific WEV-1001 L). It was kept in 5 mL glass vials before cleanup. The extracted samples were purified using an 8 mm internal diameter silica/alumina column that was filled with 1 cm anhydrous Na2SO4, 2 cm alumina, and 2 cm silica gel (both 3% deactivated), and later eluted with 50–55 ml DCM. Then the purified samples were blown down to 1 mL by a nitrogen stream. Before the GC-MS analysis, the final extracts were spiked using 2,6,2’,6’-Tetrachlorobiphenyl (PCB No. 54) as an internal standard and later transferred into 1 ml septa glass vials. The sample name was appropriately labeled on the septa vials33.
Instrumental analysis
OCPs were analyzed using Gas Chromatography Mass Spectrometry (GC-MS) (QP2010, Shimadzu) equipped with CP-Sil 8 CB capillary column (50 m, 0.25 mm, 0.25 μm). Helium was used as the carrier gas at 1.4 mL/min flow rate under constant mode. An 1µL aliquot of the sample extract was injected using splitless mode, with an injector temperature of 250 °C and a solvent delay of 5 min. Initially, oven’s temperature was set at 60 °C for approximately one minute, and then it was raised to 295 °C at 4 °C/min rate level. The ion source, quadrupole and detector temperatures were set at 230 °C, 150 °C and, 300°C30. The mass spectrometer used electron impact ionization at 70 eV in Selected Ion Monitoring (SIM) mode, monitoring three fragment ions per compound. Calibration was performed using certified standards (≥ 99% purity). Following OCPs were examined: DDTs (o, pʹ-DDE, p, pʹ-DDE, o, pʹ-DDD, p, pʹ-DDD, o, pʹ-DDT, p, pʹ-DDT), HCHs (α-HCH, β-HCH, and γ-HCH), Chlordane (cis- and trans-chlordane), and Endosulfan (α- and β-endosulfan).
Quality assurance and quality control
The analytical processes were scrutinized by strict quality assurance in order to ensure the quality of results. The instruments were calibrated daily using six-point calibration curves (5, 10, 20, 50, 100, and 200 µg/L), with calibration standards. The standards of PCB No. 209 and PCB No. 54 were purchased from LGC. Surrogate recoveries for the analyzed OCPs ranged from 76.54 to 93.31%, with Relative Standard Deviation (RSD) maintained below 10%. To monitor contamination and method precision, transportation and laboratory blanks were analyzed through the same procedure that was used for the actual samples. The chemicals used for the analysis were acetone, dichloromethane (DCM), orthophosphoric acid (H3PO4), potassium dichromate (K2Cr2O7), diphenylamine indicator (C6H5)2NH, ferrous ammonium sulfate [(NH4)2SO4.FeSO4.6H2O] and sulfuric acid (H2SO4). DCM had purity of 99.5% and purchased from DAEJUNG while silica gel, alumina gel (high-purity grade) sodium sulfate and n-Hexane (extra pure) were purchased from SIGMA-ALDRICH. Glass wares were cleaned, rinsed with distilled deionized water, oven-dried at 105 °C for eight hours, packed in aluminum foil, and later desiccate in a furnace for four hours33. The retention times (RT) for the OCPs under analysis ranged from 4.87 to 16.25 min, depending on the compound. Three times the standard deviation of blank samples was used to compute the limits of detection (LOD), and the lowest concentration with a reasonable level of precision and recovery was used to determine the limits of quantification (LOQ). The method exhibits high sensitivity for trace-level detection of OCPs in the analyzed matrix, as evidenced by the range of LOQ values from 0.50 to 4.98 ng/g and LOD values from 0.06 to 0.432 ng/g. The values of RT, LOD, LOQ, and recoveries of analyzed of OCPs were given in S4.
Organic matter analysis
Ten grams of sediment and soil samples were taken out of each polyethylene bags and crushed to a fine consistency using a pestle and mortar, then allowed to air-dried overnight before being filtered through a stainless steel sieve mesh (2 mm). While taking 1 gram of air-dried soil, the chemicals such as 10 ml of 1 N potassium dichromate solution and 20 ml of sulphuric acid (concentrated) were added into a 500 ml beaker. Due to exothermic reaction, it was then allowed to cool for about half an hour. Later, the beaker was filled with 200 ml deionized water (DI) and 10 ml of conc. orthophosphoric acid (H3PO4) with diphenylamine indicator (10–15 drops). After inserting a Teflon-coated magnetic stirrer into the beaker, it was placed on a magnetic stirrer to be continuously mixed. The mixture was titrated with 0.5 M ferrous ammonium sulphate solution until the color transitioned from violet to green35.
The given formula (1) was used to find the molarity of the solution:
Using Eq. (2), oxidizable organic carbon was determined:
Total Organic Carbon (TOC) was calculated by Eq. (3)
Organic matter was calculated by the Eq. (4)
where “M” stands for the molarity of the solution. The volumes of the solutions used to titrate the blank and the sample are denoted by Vblank (ml) and Vsample (ml), respectively. Air-dried soil weight is represented by Wt (g). With 3 being the equivalent weight of C, 0.3 = 3 × 10−3 × 100.
Ecological risk assessment
Ecological risk assessment of individual OCPs was estimated through Risk Quotient (RQ) and calculated using the Eq. (5).
where PNEC is for expected no-effect OCP concentration to aquatic species and EC stands for individual OCP concentration. The acute toxicity data, including median effect concentration (EC50), median lethal concentration (LC50), and an assessment factor (AF), were used in Eq. (6) to compute the PNEC value.
The risks were categorized into three levels based on the RQ values: A compound had a very low potential danger if RQ ≤ 0.1, a medium potential risk if 0.1 < RQ ≤ 1, and a high ecological risk if RQ > 136.
Relative contribution (RC)
Relative contributions of each particular OCP to overall ecological risk were evaluated by equation no. (7).
RQi is the individual risk quotient of OCPs while RQm represents the sum of individual risk quotient of OCPs36.
Soil-air exchange
Fugacity fractions (ff) are a commonly used technique to assess the soil-air exchange of OCPs, hence determining their fate and distribution16. The degree to which a certain pollutant entered different environmental compartments was measured by using this technique. The fugacity fraction in soil (fs) and air (fa) for selected OCPs (DDTs, HCHs, endosulfan, and chlordane) was calculated using Eqs. (8) and (9), respectively.
Where ØOM = 1.7 times the soil’s TOC, KOA is the octanol air partition coefficient to represent the chemical partitioning between the organic phase and the atmosphere, and is improved by a factor of 0.4137. T = air temperature (K), R stands for gas constant (8.31 Pa m3/mol/K), Cs = OCPs’ concentration in soil, and Ca = OCPs’ concentration in air.
Equation (10) was used to calculate the fugacity fractions (ff) once the fugacity of the soil (fs) and air (fa) had been evaluated. Where fs = fugacity in soil, fa = fugacity in air.
Statistical analysis
Kolmogorov-Smirnov and Shapiro-Wilk test was performed using IBM Statistical Packages for the Social Sciences (SPSS) Statistics 25 to analyze the data distribution among the compartments of Uchalli and Khabeki Lakes. Values of skewness and kurtosis are based on normal distribution. Data analysis was done using parametric tests and results were expressed in descriptive statistics (median, mean, minimum range, maximum range and standard deviation). An independent sample t-test was used to determine whether the targeted analytes in Uchalli and Khabeki Lakes varied significantly or not. However, the relationship between OCPs and physicochemical parameters was assessed using the Pearson correlation. Principal Component Analysis (PCA) was carried out using XLSTAT to identify underlying patterns and potential sources of OCP contamination across four environmental compartments (water, sediment, soil, and air) for each lake. The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity were used to assess the dataset’s suitability prior to PCA. A significant Bartlett’s test and a KMO value above 0.6 validated that the data was suitable for factor analysis.
Results and discussion
Concentrations and profile distribution of OCPs in water
The range of Ʃ13OCPs concentration in the water samples of Uchalli Lake was 0.94 to 10.37 µg/L. However, the level of Ʃ13OCPs in the water samples of Khabeki Lake varied from 0.27 to 2.70 µg/L (Table 1). A global comparative analysis of OCP concentrations, including ƩDDTs, ƩHCHs, ƩEndosulfan, and ƩChlordane in water and sediment, is presented in Supplementary Table S1. The general distribution pattern of OCPs in the water of Uchalli Lake was: ƩDDTs > ƩHCHs > ƩEndosulfan > ƩChlordane, while OCPs in the water of Khabeki Lake were found in the following pattern: ƩDDT > ƩEndosulfan > ƩHCH > ƩChlordane (Table 1).
o, pʹ-DDE showed the highest concentration level among DDT metabolites both in Uchalli Lake (0.84 ± 0.47 µg/L) and Khabeki Lake (0.14 ± 0.18 µg/L). The observed dominance of o, pʹ-DDE suggest aerobic degradation of DDT isomers primarily into DDE over time. A concentration pattern of DDT metabolites in both lakes was descending, followed by o, pʹ-DDE, p, pʹ-DDE, o, pʹ-DDD, p, pʹ-DDD, o, pʹ-DDT, and p, pʹ-DDT (Table 1). The current DDE level are largely the result of past usage of DDT compound, that undergone environmental degradation in oxygen-rich environment such as surface water. DDE also highlights the environmental stability and potential for long-term ecological impact as it is more persistent and less susceptible to degrade further. ƩDDT concentrations in water are generally higher than those found in other studies conducted worldwide, including Lake Qilu38, Lake Daya39, Lake Juixi40, Lake Volta41, Lake Nansi42, Lake Honghu43 and Lake Baiyandian44 of China (See S1). While both Pakistan and China have historical usage of DDT, but the enforcement of restrictions towards DDT compounds differ. China officially banned DDT in early 1980s45, whereas, Pakistan banned DDT in 19942. The topography of the lakes surveyed may also contribute to the high level of pollution. Although the settlements and hills are steep, the Uchalli and Khabeki Lakes are low-sloping, creating a basin for the accumulation of OCPs. Moreover, the ̩ƩDDT value was found to be lower in comparison to the Pakistani Rivers’ Kabul and Sutlej46. The higher levels of DDT contamination may be due to runoff from nearby agricultural fields of Uchalli and Khabeki Lake.
Endosulfan was the second highest contributor after DDT in the water samples of Khabeki Lake, representing a greater concentration of α-endosulfan (0.10 ± 0.10 µg/L) as compared to β-endosulfan (0.08 ± 0.08 µg/L). Among chlordane isomers, the metabolite trend was: trans-chlordane > cis-chlordane. On the other hand, the concentration of α-endosulfan and β-endosulfan was in the range of 0.22 ± 0.20 µg/L and 0.15 ± 0.18 µg/L, respectively, in Uchalli Lake. Chlordane concentrations in Uchalli Lake were lower than those of other OCPs at 0.05 ± 0.02 µg/L for cis-chlordane and 0.07 ± 0.01 µg/L for trans-chlordane. Compared to other studies, such as those conducted in China’s Qilu Lake, Daya Lake, and Volta Lake, the concentrations of chlordane and endosulfan in the water of present study were found greater38,39,41. However, Imran et al.46 reported the chlordane value of River Kabul in Pakistan that was found equivalent to the current study, that might be due to the same climatic pattern.
Among HCHs, α-HCH concentration was greatest in both Uchalli and Khabeki Lakes (0.39 ± 0.31 µg/L, 0.06 ± 0.02 µg/L), respectively. The HCH metabolites pattern was the same in both lakes under study: α-HCH > β-HCH > γ-HCH. Technical HCH was widely used as a pesticide in the past, and is a mixture of several isomers: approximately 60–70% α-HCH, 5–12% β-HCH, 10–15% γ-HCH (lindane), and smaller amounts of others. Therefore, the site under study showed higher α-HCH simply due to its greater proportion in technical HCH47. Compared with other research studies globally, it was found that the lakes of China (Qilu, Daya, Juixi, Nansi, Honghu, and Baiyandian) had higher concentrations38,40,42,43,44. In contrast, Volta Lake had a concentration comparable to the current study41.
Concentrations and profile distribution of OCPs in sediment
Ʃ13OCPs concentration in sediment samples ranged from 289.26 to 589.03 ng/g in Uchalli Lake, and from 146.97 to 348.49 ng/g in Khabeki Lake, respectively. The mean concentration of OCPs in the sediments of Uchalli and Khabeki Lakes was ranked in the following order: ƩDDTs > ƩHCHs > ƩChlordane > ƩEndosulfan (Table 1).
Among DDT metabolites, o, pʹ-DDE was the most widespread compound in Uchalli and Khabeki Lakes. The trend of DDT metabolites in Uchalli Lake was: o, pʹ-DDE > p, pʹ-DDE > o, pʹ-DDD > p, pʹ-DDD > o, pʹ-DDT > p, pʹ-DDT. The consistent trend of DDT metabolites in both sediment and water is probably due to the same historical DDT inputs, such as atmospheric deposition and agricultural runoff. Since DDT degrades over time, the abundance of o, pʹ-DDE is similar across compartments, particularly in systems with low flushing rates, like lakes48. However, DDT metabolites detected in Khabeki Lake showed the following structure: o, pʹ-DDE > p, pʹ-DDE > o, pʹ-DDD > o, pʹ-DDT > p, pʹ-DDD > p, pʹ-DDT (Table 1). According to Eqani et al.49the level of DDT in the sediments of the lakes surveyed was higher than those in the Chenab River, Pakistan. Moreover, the DDT levels in this study were higher than those in Lake Daya, China39Lake Taihu, China50Lake Manzala, Egypt51Lake Nansi, China42Lake Awassa, Ethiopia51Lake Volta, Ghana41and Lake Honghu, China43. It is possible that the greater DDT concentrations in the sediments of Uchalli and Khabeki Lakes may be the due to the Pakistan’ arid and semi-arid climatic conditions, which can slow down the degradation of DDT compounds comparably to China’s diverse climatic regions that has higher humidity and microbial activity.
Among OCPs, the level of ƩHCH was found to be the second highest contributor, in which α-HCH showed greatest concentration in Khabeki (10.04 ± 3.68 ng/g) and Uchalli (14.24 ± 3.32 ng/g) Lakes. HCH isomeric trend was the same in both lakes: α-HCH > β-HCH > γ-HCH. ƩHCH concentration in the sediments of lakes under study did not seem to be consistent with those of prior studies from the River Chenab in Pakistan during the summer and winter49Hooghly river in India53Manzala Lake in Egypt51the Taihu50Honghu43Nansi42and Baiyangdian54 Lakes, as well as the Huaihe River44 in China. While it was discovered to be significantly lower than China’s Daya Lake39.
Amongst the metabolites of chlordane, trans-chlordane depicted higher levels (4.36 ± 1.88 ng/g, 2.98 ± 0.86 ng/g) in Uchalli and Khabeki Lakes, respectively. However, the highest concentration of α-endosulfan among endosulfan metabolites was found in Uchalli Lake (3.15 ± 1.45 ng/g), followed by Khabeki Lake (3.08 ± 1.28 ng/g), respectively (Table 1). The order of endosulfan was the same in both lakes as: α-endosulfan > β-endosulfan. Compared to other regions, the concentration of ƩEndosulfan and ƩChlordane in Chinese lake sediments such as Taihu Lake basin and Daya Lake was found to be relatively lesser39,55. Moreover, both studied lakes were also more concentrated than the sediment of Volta Lake in Ghana41. Increased contamination may be due to physiochemical properties of sediments, such as its organic matter and texture, which affect the mobility and retention of OCP compounds30. On the other hand, the concentration of endosulfan was found lesser than River Chenab of Pakistan49 while chlordane level was found greater than Lake Manzala of Egypt51 and Lake Honghu of China43 as shown in S1.
Concentrations and profile distribution of OCPs in soil
In Uchalli Lake, the Ʃ13OCPs concentration in soil samples ranged from 247.06 to 480.17 ng/g, whereas in Khabeki Lake, the level of ƩOCPs varied from 159.01 ng/g to 384.78 ng/g as shown in Table 2. Overall trend of OCPs in both studied lakes was: ƩDDTs > ƩHCHs > ƩChlordane > ƩEndosulphan. The DDT metabolites that showed highest mean concentration were p, pʹ-DDE (59.37 ± 8.96 ng/g) in Uchalli Lake and o, pʹ-DDT (45.75 ± 18.08 ng/g) in Khabeki Lake. The predominance of p, pʹ-DDE in the agricultural fields of nearby Uchalli Lake reflects historical DDT usage and subsequent aerobic degradation, while the higher o, pʹ-DDT levels in the vicinity of Khabeki Lake point to more recent DDT inputs, likely from dicofol applications30. The pollutant level, from greatest to lesser, followed the pattern of p, pʹ-DDE, o, pʹ-DDD, o, pʹ-DDT, p, pʹ-DDD, o, pʹ-DDE and p, pʹ-DDT in Uchalli Lake while o, pʹ-DDT, p, pʹ-DDD, o, pʹ-DDD, p, pʹ-DDE, o, pʹ-DDE, p, pʹ-DDT in Khabeki Lake. Compared with other studies, the level of DDTs in Uchalli and Khabeki Lakes was greater than that in the Ratti Galli, Subri, Shouter, Banjosa, Mangla, and Baghsar Lakes of Lesser Himalayan Region16. The LHR, being a more remote and less industrialized area, has experienced lower levels of DDT contamination. In contrast, the higher DDT contamination in UWC lakes is primarily due to extensive agricultural practices, favorable conditions for persistence with a long half-life of 10 to 15 years, and limited remedial actions56. The DDTs concentration of current study was found to be similar to that of Kalashah Kahu57 while it was found greater as compared to Indus River and Chakwal district, Pakistan32,58 and Wuhan’s agricultural soil in China55.
Among HCHs in soil, α-HCH was detected in greater amount (9.71 ± 1.13 ng/g) while the rest of the metabolites followed the descending order β-HCH > γ-HCH in Uchalli Lake. However, γ-HCH represented greatest concentration (9.48 ± 2.94 ng/g) followed by β-HCH > α-HCH in Khabeki Lake. Their higher persistence in environmental compartments resulted from pervious applications in agricultural soils. The present HCHs concentration of studied lakes was similar to that of Kalashah Kahu soil while lesser to that of Chakwal district in Pakistan58. Moreover, it was found to be significantly greater than in other lakes of Lesser Himaliyan Region and Indus Basin of Pakistan16.
Trans-chlordane and β-endosulfan represented greater concentration among their metabolites in both studies lakes (Table 2). The trend of endosulfan was same in both lakes as: β-endosulfan > α-endosulfan. Compared to other regions, chlordane and endosulfan concentrations of the current study were higher than those reported in the soils of China’s Daya Lake and Pakistan’s Indus Basin39,59. In regions like Uchalli and Khabeki Lakes with semi-arid climates, the microbial activity required for the degradation of pesticides is low due to limited moisture and high temperatures, reducing the breakdown of endosulfan in soil. Chlordane concentrations were found to be higher in the studied lakes compared to Pakistan’s cotton-growing region and Chakwal district12,58. In contrast, endosulfan levels were lower than those reported in the Chakwal district58 while higher than Kalashah Kahu and the Indus River32,57. However, the endosulfan concentrations in Khabeki Lake were comparable to those observed in the agricultural zone of Punjab, Pakistan12.
Concentrations and profile distribution of OCPs in air
Ʃ13OCPs level in air compartment of Uchalli and Khabeki Lakes ranged from 187.43 to 1349.99 pg/m3 and 3.31 to 168.65 pg/m3 as given in Table 2. OCPs trend in air samples of both studied lakes was as follows: ƩDDTs > ƩEndosulphan > ƩChlordane > ƩHCHs. Among DDT metabolites, o, pʹ-DDE was found at higher level (163.72 ± 58.50 pg/m3; 94.38 ± 49.86 pg/m3) followed by other metabolites in Uchalli and Khabeki Lakes, suggest extensive aerobic degradation of technical DDT. This pattern represents the historical usage of DDT rather than recent input. While comparing with Lesser Himalayan Regions, the concentration of DDTs was higher in current study than in the Ratti Galli Lake, the Baghsar Lake, the Shounter Lake and the Mangla Lake16. Whereas, OCP concentrations in Uchalli and Khabeki Lakes were lower than those observed in the Indus River, Pakistan32. While found comparable with the industrial zone of Lahore, Sheikhupura, Faisalabad and agricultural zone of Cheechawatni, Sahiwal, and Khanewal12. On a global scale, the reported concentration was lower than those found in Hooghly River, India, which is heavily influenced by domestic discharge and industrial runoff53 as well as in Lake Erie and Lake Ontario of North America, where historical industrial activity and long-range atmospheric transport have contributed to significant contamination61.
Among the metabolites of chlordane, trans-chlordane showed the highest level (32.82 ± 24.73 pg/m3, 66.26 ± 23.16 pg/m3) in Uchalli and Khabeki Lakes, respectively (Table 2). Both metabolites of endosulfan were found in air samples of Uchalli and Khabeki Lakes but α-endosulfan was depicted with highest level (77.62 ± 39.34 pg/m3, 56.40 ± 33.77 pg/m3) among them. However, Uchalli and Khabeki Lakes showed higher concentrations of ƩChlordane and ƩEndosulfan compared to Indus Basin60Punjab’s agricultural zone in Pakistan12Ontario and Erie Lake in North America61but lower than Hooghly River in India53. The elevated atmospheric concentration of endosulphan and chlordane in the Uchalli and Khabeki Lakes may be due to the volatilization from soil to air under warm weather conditions.
ƩHCH accounted for lowest level among all OCPs in air samples of studied lakes. Amongst HCH metabolites, α-HCH was detected in highest amount (17.43 ± 9.84 pg/m3, 19.39 ± 14.63 pg/m3) while the rest of the isomer followed the descending pattern β-HCH > γ-HCH in Uchalli and Khabeki Lakes, respectively. Both lakes under study exhibited lower concentrations than those reported for Banjosa, Subri, Mangla, and Baghsar Lakes in Pakistan16as well as Hooghly River in India, reflecting comparatively moderate atmospheric deposition53. However, the levels were higher than those found in Ratti Galli and Shounter Lakes of Pakistan’s LHR16and Erie Lake in North America61indicating regional variability influenced by climatic factors. Additionally, the concentration of 13 OCPs were compared globally to other regions and summarized in S1(supplementary material).
OCPs spatial variation and homologue composition
The concentration of OCPs were compared between the environmental compartments (water, sediment, soil, and air) of the Uchalli and Khabeki Lakes by mean of an independent sample t test as shown in Table 3. The independent sample t test is used to determine whether there is a statistically significant difference between two independent groups53. Significant differences (p < 0.05) were observed in the waters of Uchalli and Khabeki Lakes for all OCPs congeners except chlordane, while no significant differences (p > 0.05) were observed in the sediments of studied lakes. In soil, only cis-chlordane showed a very significant change, while in the air compartment of studied lakes, the DDT metabolites (o, pʹ-DDT, p, pʹ-DDD and p, pʹ-DDE) were found to be significantly different (Table 3). In other study, the spatial distribution of OCPs in the soil and air across the River Indus catchment area varied significantly among the Sukkar, Guddu, Kot Mithan and Taunsa Barrages, mainly due to differences in net volatilization and deposition of these compounds32.
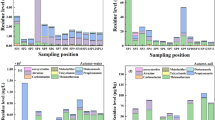
The OCP concentrations in environmental compartments were also compared among Uchalli and Khabeki Lakes (Fig. 2). Uchalli Lake had a higher concentration of DDT, HCH, endosulfan and chlordane in water, sediments, and soil compartments compared to Khabeki Lake (Fig. 2a, b and c). In the air, Uchalli Lake also had a higher level of DDT than Khabeki Lake, while the concentrations of HCH, endosulfan and chlordane were relatively higher in Khabeki Lake (Fig. 2d). Uchalli Lake may have been contaminated due to runoff from nearby agricultural areas, domestic wastewater discharge and direct waste disposal, all of which are potential sources of pollution, making it more contaminated than Khabeki Lake. Access to Uchalli Lake and the use of boats are restricted primarily due to its poor ecological condition compared to Khabeki Lake. Furthermore, no management interventions or remediation are currently in place to reverse the contamination patterns of Uchalli Lake.
Khabeki Lake
Isomers of DDT and HCH showed highest level in the studied environmental compartments of Khabeki Lake as given in Fig. 3(a). In water compartment, o, pʹ-DDE revealed highest percentage of 18.38% followed by α-endosulfan (13.07%), with the remaining compounds in descending order: p, pʹ-DDE, β-endosulfan > o, pʹ-DDD > α-HCH > p, pʹ-DDD > trans-chlordane > β-HCH > o, pʹ-DDT > cis-chlordane > p, pʹ-DDT > γ-HCH. While in sediment compartment, o, pʹ-DDE showed highest level with 21.75% followed by p, pʹ-DDE, o, pʹ-DDD, o, pʹ-DDT, p, pʹ-DDD, p, pʹ-DDT, α-HCH, β-HCH, γ-HCH, α-endosulfan, trans-chlordane, cis-chlordane and β-endosulfan. In soil, o, pʹ-DDT revealed highest percentage composition of 16.68% around the agricultural fields of Khabeki Lake followed by p, pʹ-DDD, o, pʹ-DDD, p, pʹ-DDE, o, pʹ-DDE, p, pʹ-DDT, γ-HCH, β-HCH, α-HCH, trans-chlordane, cis-chlordane, β-endosulfan and α-endosulfan. However, the percentage contribution of OCPs in the air near Khabeki Lake followed the descending trend: o, pʹ-DDE, p, pʹ-DDE, o, pʹ-DDD, α-endosulfan, trans-chlordane, β-endosulfan, p, pʹ-DDD, cis-chlordane, o, pʹ-DDT, p, pʹ-DDT, α-HCH, β-HCH and γ-HCH (Fig. 3a). Concentration of o, pʹ-DDE was highest in three compartments (water, sediment and air) while only the soil showed higher concentrations of o, pʹ-DDT, suggesting a differential degradation and partitioning behavior across environmental compartments. Bhutto et al.39 found the OCP composition in different environmental compartments of Lake Daya, which showed a higher percentage of DDT in the sediment. Moreover, the percentage of p, pʹ-DDD and α-HCH in water and sediment of Dongting Lake was also found to be highest63.
Uchalli Lake
The OCPs composition profile in studied environmental compartments of Uchalli Lake was depicted in Fig. 3(b). In water, o, pʹ-DDE showed highest percentage (19.00%) followed by p, pʹ-DDE > o, pʹ-DDD > p, pʹ-DDD > α-HCH > β-HCH > o, pʹ-DDT > α-endosulfan > p, pʹ-DDT > γ-HCH > β-endosulfan > trans-chlordane > cis-chlordane. o, pʹ-DDE was again found to be the highest contributor among other OCPs congeners in sediment with 23.46% followed by o, pʹ-DDD > p, pʹ-DDD > o, pʹ-DDT > p, pʹ-DDT > α-HCH > β-HCH > γ-HCH > trans-chlordane > α-endosulfan > cis-chlordane > β-endosulfan. In soil, p, pʹ-DDE represented highest percentage of 16.35%. o, pʹ-DDD was the second highest OCPs with 16.11% followed by o, pʹ-DDT, p, pʹ-DDD, o, pʹ-DDE, p, pʹ-DDT, α-HCH, β-HCH, γ-HCH, trans-chlordane, β-endosulfan, cis-chlordane and α-endosulfan. However, the percentage contribution of OCPs in air of Uchalli Lake revealed the following trend: o, pʹ-DDE, p, pʹ-DDE, o, pʹ-DDD, α-endosulfan, β-endosulfan, p, pʹ-DDD, trans-chlordane, o, pʹ-DDT, cis-chlordane, p, pʹ-DDT, α-HCH, β-HCH and γ-HCH (Fig. 3b). The metabolites of DDT showed highest contamination level while the metabolites of chlordane and endosulfan showed lesser contamination level in all compartments of studied lakes (Fig. 3a and b) that could be possibly because of their moderate persistence and capacity to re-volatilized back into the atmosphere. Endosulfan has a moderate persistency with a half-life of 50 days while DDT has a strong persistency with half-life of 12 to 15 years3. DDTs were the dominant congeners in all compartments of Uchalli and Khabeki Lakes, reflecting past DDTs usage in agricultural lands on a large scale.
Source identification of OCPs
DDT
The DDTs, HCHs, endosulphan and chlordane metabolites ratio were represented in Table S2. The isomeric ratios showed the recent or past applications of OCPs in water, sediments, and soil compartments. The DDE + DDD/ƩDDT ratio is used to determine whether DDT in the environmental is a degraded compound or a result of present applications. The ratio (DDE + DDD)/ƩDDT < 1 indicates historic use of DDT, while the ratio (DDE + DDD)/ƩDDT > 1 indicates the recent application of DDT55. In the current study, (DDE + DDD)/ƩDDT ratio was less than 1 in studied environmental compartments of water, sediments, and soil, representing past applications of DDT. Generally, DDT was transformed into its metabolites in environmental compartments of water, sediments, soil and later re-volatilized back into the atmosphere in case of extreme weather conditions. DDT can be degraded into DDE in aerobic condition and it can be reduced further to DDD in anaerobic condition64. So, the DDE/DDD ratio > 1 indicated aerobic degradation of DDTs in all compartments of the lakes under study, with the exception of sediment of Khabeki Lake (Table S2). Higher level of DDE was found in water and sediments of Uchalli and Khabeki Lakes due to DDE’s greater persistency than that of its parent DDT molecule65. Pakistan also has a history of using DDT to control pests60. Another study in the industrial and agricultural zone of Punjab, Pakistan also depicted the (DDE + DDD)/ƩDDT ratio lesser than 1, showing similar source identification12.
HCH
The metabolite ratio of α-HCH/γ-HCH greater than 4 indicates the usage of technical HCH while α-HCH/γ-HCH ratio < 4 specifies the utilization of lindane. The α-HCH/γ-HCH ratio is lesser than 4 in current study of environmental compartments of Uchalli and Khabeki Lakes, depicting the lindane usage in the vicinity of both studied lakes. Moreover, the higher proportion of α-HCH compared to β-HCH indicates lower isomer transformation, representing recent HCH applications (Table S2). However, the previous study reported higher concentration of β-HCH in both water and sediment samples of Daya Lake, China39.
Endosulfan and Chlordane
The ratio of α-endosulfan/β-endosulfan < 2.33 indicated a lack of endosulfan in all studied environmental compartments, except in the sediment of Khabeki Lake. Whereas, the ratio of cis-/trans-chlordane < 1 indicated the past usage of chlordane in water, sediments, and soil compartments of both studied lakes (Table S2). Another study reported the range of chlordane ratio which also indicated the presence of old sources along Pakistan’s coastal area1. The Stockholm Convention’s prohibition on the manufacturing of POPs left behind the greatest stocks of prohibited OCPs, that are caused by the destruction of landfills and manufacturing sites in different cities of Pakistan57. OCPs continue to be used illegally in many parts of Pakistan due to their effectiveness, easy accessibility, and lack of national regulatory policies, leading to widespread environmental contamination across the country19,21,49,57,60,66,67,68,69. The primary source of OCP contamination in Pakistan’s environmental compartment is the abandoned dumping of thousands of illegally used OCPs across various regions of the country18,69.
Correlation analysis between sediment OCPs and organic matter
Analysis of Pearson correlations revealed significant correlation between OCP congeners (p, pʹ-DDE and γ-HCH) and OM (Table S3). The metabolites of endosulfan (α- and β-endosulfan) and chlordane (cis- and tans-chlordane) showed a positive association, indicating that OCP adsorption increased with increasing organic matter (Table S3). The high affinity of OCPs to organic matter is due to their low water solubility and tendency to accumulate in sediments, which serve as a major sink for the compounds70. Organic matter (OM) affects the dispersal of OCPs due to their varying biodegradation rates55. The regression analysis of OCP congeners in sediment of Uchalli and Khabeki Lakes was represented in Fig. 4. The sediments of Khabeki Lake revealed that ƩOCPs was correlated positively with 59% TOC accumulation ((R2 = 0.5963). The greater adsorbance of OCPs is mostly due to total organic carbon71, as evidenced by the analysis indicating that ̩ ƩDDT, ƩHCH, ƩEndosulfans and ƩChlordane contributed 58%, 39%, 20%, and 12% towards TOC (Fig. 4a). A moderate to strong positive correlation (R2 = 0.5963) between TOC and ƩOCPs was observed in Uchalli Lake, reflecting the strong affinity of hydrophobic OCPs for OM (Fig. 4b). This suggests that sediments with higher TOC content provide more binding sites, which increases the sorption and long-term retention of OCPs. Such interactions between OM and OCPs play a critical role in controlling the mobility, bioavailability, and fate of these contaminants in aquatic ecosystems32.
Correlation analysis between soil OCPs and organic matter
Pearson correlation was used to find any potential association between the soil physiochemical parameters and OCPs concentration as given in Table S3. In current study, soil OM had a positive association with o, pʹ-DDE and p, pʹ-DDE, indicating that the adsorption of OM is higher. Whereas the significantly low and negligible positive correlation of soil OM with o, pʹ-DDD and ƩChlordane (trans-chlordane and cis-chlordane) reflects their relatively shorter half-life and greater susceptibility to degradation, which limits their long-term accumulation in organic-rich soil32,72. Comparing with previous studies, different OCPs congeners (α-HCH, γ-HCH, p, p′-DDE, o, p′-DDE and o, p′-DDD) were associated positively with soil organic carbon12. The regression analysis of OCPs in the soil of Khabeki and Uchalli Lakes was represented in Fig. 4c and d. ƩDDTs, ƩHCHs and Ʃendosulphan contributed 15%, 22% and 23% towards TOC in Uchalli Lake (Fig. 4d). In another study, the results of the site-wise regression analysis showed little or no correlation of TOC with soil of the Indus River catchment area32.
Ecological risk assessment
Table 4 displayed the Relative Contribution (RC) and Risk Quotient (RQ) of each particular OCP in Uchalli and Khabeki Lakes. o, pʹ-DDE showed a high risk with RQ > 1 in Khabeki Lake with the relative contribution of 24.54%, indicating a potential ecological risks to sensitive aquatic species. As o, pʹ-DDE exhibited the highest concentrations in almost every compartment, which suggests that it may contribute the most to the observed ecological risk. In Uchalli Lake, all 6 DDT metabolites represented high ecological risk with RQ greater than 1 by contributing 21.41% (o, pʹ-DDE), 18.29% (p, pʹ-DDE), 24.79% (o, pʹ-DDD), 18.38% (p, pʹ-DDD), 7.23% (o, pʹ-DDT) and 5.7% (p, pʹ-DDT), respectively. The dominant species in the UWC were several plants (Digitaria sanguinalis, Suaeda Fruticosa, Parthenium hysterophorus, and Heliotropium strigosum) and fish (Oreochromis niloticus and Oreochromis mossambicus)73. Several studies showed that DDT can cause bioaccumulation, reproductive toxicity, endocrine disruption, and behavioral changes in these species74,76,77. While specific sensitivity data for local species are limited, these findings underscore the need for targeted ecotoxicological studies to assess the vulnerability of regional organisms to DDT and its metabolites.
Other OCP congeners like o, pʹ-DDD, p, pʹ-DDE, p, pʹ-DDD, trans-chlordane, cis-chlordane, o, pʹ-DDT and p, pʹ-DDT with relative contribution of 20.38%, 15.51%, 14.36%, 9.49%, 6.95% 5.32% and 3.25% demonstrated a medium ecological risk in Khabeki Lake. Chlordane metabolites showed medium risk (0.1 < RQ ≤ 1) while HCH and endosulfan metabolites revealed low ecological risk (RQ ≤ 0.1) to aquatic fisheries and animals of Uchalli Lake (Table 4). Prior findings indicated that there was a comparatively significant risk of p, pʹ-DDD in Hangzhou Bay, China77. In another study, trans-chlordane, cis-chlordane, and p, pʹ-DDE also represent the most dangerous pollutant for aquatic life with high PNEC values ranging from 1.74 to 9.57 ng/L38. Whereas, in Pakistan’s Ravi River tributaries, the α-endosulfan posed a high risk during pre-monsoon and post-monsoon periods with RQ > 123.
Soil-air exchange
Soil plays a role as a primary sink in the cycle of SVOCs, absorbing and then releasing them back into the atmosphere16. The fate and environmental dispersal of OCPs were assessed by the soil-air exchange mechanism and calculated through fugacity fraction (ff). The soil-air partition coefficient (KSA) represents the soil organic matter.
(SOM) role in OCPs absorption78. According to Wang et al.79 organic matter is subsequently seen as a surrogate for OCPs absorption that regulates the SVOCs partitioning between soil and air. OCPs become volatilized from soil to air when ff > 0.5, while deposited from air to soil compartment when ff < 0.5. Furthermore, net equilibrium was reached when ff = 0.582. Figure 5 displayed the trend and net direction (deposition, volatilization and equilibrium) of OCPs in Uchalli and Khabeki Lakes. To ensure the comparability for the calculations of fugacity fraction, only six air samples taken near the corresponding soil sampling sites were used. The mean and standard deviation reported in Table S5 are based solely on these samples. The fugacity fraction calculations are elaborated in Supplementary Text (S6) and the ff value of individual OCPs were given in S7.
The wide variation of OCP congeners were witnessed regarding the fugacity fraction. The fugacity fraction of (ff > 0.5) in Khabeki Lake showed net volatilization of DDTs and HCHs from soil to air. This process is partially supported by elevated atmospheric concentrations of these compounds observed near the lake. For example, the o, pʹ-DDE concentration in air compartment (97.00 ± 54.10) was higher than in soil compartment (36.55 ± 13.5) (Table S5). However, these findings are not uniformly consistent with o, pʹ-DDT and p, pʹ-DDT, likely due to temporal variability and spatial gradients. However, all metabolites of endosulfan and chlordane in Khabeki Lake with the exception of only trans-chlordane in Uchalli Lake showed ff < 0.5, indicating the net deposition from air to soil. Although soil concentrations were not consistently higher than air concentrations, this reflects that relative fugacity of the compounds depend not only on concentration but on phase-specific fugacity capacity (Z) also80.
Numerous factors such as emission sources, atmospheric transport, ambient temperature, land use and soil properties frequently contribute to the variations in soil-air exchange processes. Soils in contaminated urban, agricultural, and industrial regions are considered secondary sources of SVOCs1,32. Remote soils, on the other hand, are believed to be an important sink for OCPs because of the organic contaminants that were deposited there16. Moreover, only p, pʹ-DDE and trans-chlordane depicted the net equilibrium in Uchalli Lake. In another study, the ff of soil-air exchange among OCP congeners (p, p’-DDD, o, p’-DDT, b-HCH, d-HCH, Heptachlor, TC and HCB) ranged from 0.3 to 0.59 in River Indus, Pakistan32. The majority of OCPs (93.3%) found to be less than 0.34 indicating soil deposition, while 2.64% of OCPs were recorded greater than 0.66, suggesting net volatilization from soil. Whereas, the ff of 4.04% OCPs were between 0.34 and 0.66, representing soil-air balance81.
Principal Component Analysis (PCA) of OCPs
Uchalli Lake
Principal Component Analysis (PCA) was conducted to identify patterns and potential sources of OCPs contamination across various environmental compartments of Uchalli Lake. The first two principal components (F1 and F2) were retained based on the Kaiser criterion (eigenvalues > 1), cumulatively accounted for 73.04% of the total variance (F1 = 50.89%, F2 = 22.15%). The bilpot revealed clustering pattern and association between OCPs and sampling sites across all four environmental compartments (water, sediment, soil, and air) as shown in Fig. 6(a). F1 was predominantly influenced by the isomers of DDT and HCH, suggesting a shared origin likely associated with the historic usage of technical DDT and lindane. Whereas, F2 differentiated other OCPs isomers such as endosulfan and chlordane, indicating the distinct usage pathways or usage patterns. The clustering of endosulfan and chlordane near sampling points (AU5-AU10) revealed elevated contamination of these compounds, favoring the atmospheric volatilization. Whereas, water samples minimal association with OCPs, reflecting lower contamination level likely due to thermodynamic dispersion and dilution. Several studies across different regions have used PCA to analyze OCP distribution in marine ecosystem, providing valuable context for the findings of our studied lakes. For instance, Zhang et al.82 identified distinct grouping of DDT and its metabolites in Taihu Lake, China, attributing them to both agricultural runoff and historic usage. This pattern is closely related to our findings of clustered DDT metabolites in soil and sediment compartment.
Khabeki Lake
PCA was performed on OCP concentrations across four environmental compartments in Khabeki Lake to explore potential sources and interrelationships among contaminants. The first two principal components (F1 and F2) explained a cumulative 82.57% of the total variance, with F1 accounting for 66.28%, and F2 for 16.30%. The PCA biplot provides clear insight into the sources and patterns of OCPs contamination (Fig. 6b). OCPs such as α-HCH, p, pʹ-DDD, o, pʹ-DDT, p, pʹ-DDT showed high positive loadings on F1, indicating strong influence on this component and likely association with two environmental compartments, particularly sediment and soil (e.g., samples SD1, SK1, SK2, SK3). Conversely, other OCPs like trans-chlordane, cis-chlordane and α-endosulfan loaded strongly on the upper right quadrant, indicating distinct distribution patters and differences in degradation behavior. The distribution of o, pʹ-DDD, p, pʹ-DDE along F2 was more associated with air samples, indicating atmospheric input and volatilization. Similarly, Alani et al.83 found a predominance of p, pʹ-DDE, p, pʹ-DDD in Nigerian Lake sediments, indicating long-term degradation of parent compounds, which aligns with our detection of these metabolites in water and air samples, suggesting atmospheric transport and environmental degradation. Ali et al.12 also reported OCP residues in Pakistan’s Indus River system, likely due to weak regulatory enforcement, a concern that supports the likelihood of ongoing or recent inputs in studied lakes despite national bans.
Conclusions and recommendations
It was concluded from this study that ƩDDT were the most dominant compound in studied environmental compartments to the total OCPs. The spatial distribution of ƩDDTs, ƩHCHs, ƩEndosulfan and ƩChlordane in environmental compartments of water, sediments, and soil is higher in Uchalli Lake as compared to Khabeki Lake. Whereas, the atmospheric level of ƩHCHs, ƩEndosulfan and ƩChlordane is higher in Khabeki Lake than Uchalli Lake. The DDE + DDD/ƩDDT ratio revealed past applications of DDT compounds in agricultural fields while the greater concentration of α-HCH and β-HCH in water and sediments of Khabeki Lake depicted lesser isomer transformation by representing the recent usage of HCH compounds. The PCA biplot provides clear insight into the sources and patterns of OCPs contamination, where F1 of both biplots was predominantly influenced by the isomers of DDT and HCH, Moreover, all studied DDT metabolites and only o, pʹ-DDE in Khabeki Lake showed high ecological risk to water bodies. Organic matter showed a strong impact on the adsorption of OCPs. Due to which it had a position association with trans-chlordane and endosulfan with the sediments of studied lake, making them more persistent into the water. Based on the current study, it was suggested that exposed stocks of OCPs sites should be considered harmful for all activities till the necessary remedial actions are implemented to safeguard the ecological integrity of Uchalli and Khabeki Lakes. The proper OCPs disposal from the banned manufacturing sites should be taken into consideration by government officials and proper rules and regulations should also be enforced in order to restrict the illegal usage of OCPs.
Limitations
The present study provides a baseline data of OCPs in different environmental compartments of two lakes of Uchalli Wetland Complex named: Uchalli and Khabeki Lakes. The comprehensive multi-media approach, coupled with the use of composite sampling techniques ensure representative measurements of localized conditions during a single season (March-May), and does not capture seasonal fluctuations in OCPs level driven by the variations in rainfall, temperature, wind pattern and land-use-cycles. Though samples were collected from overall all four cardinal directions (north, west, east, and south) at multiples points, but the sample coverage of Uchalli Lake is limited due to boating restrictions.
Data availability
Data availability statementAll data generated or analyzed during this study are included in this article (and its supplementary information file).
References
Ali, U. et al. Assessing the relationship and influence of black carbon on distribution status of organochlorines in the coastal sediments from Pakistan. Environ. Pollut. 190, 82–90. https://doi.org/10.1016/j.envpol.2014.03.024 (2014).
Eqani, A. M. A. S., Malik, S., Alamdar, R. N., Faheem, H. & A., & Status of organochlorine contaminants in the different environmental compartments of pakistan: a review on occurrence and levels. Bull. Environ Contam. Toxicol. 88, 303–310. https://doi.org/10.1007/s00128-011-0496-4 (2012).
Jayaraj, R., Megha, P. & Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary Toxicol. 9 (3–4), 90–100. https://doi.org/10.1515/intox-2016-0012 (2016).
Keswani, C. et al. Global footprints of organochlorine pesticides: A pan-global survey. Environ. Geochem. Health. 44 (1), 149–177. https://doi.org/10.1007/s10653-021-00946-7 (2022).
Guo, W., Ji, X., Yu, Z., Jiang, H. & Guan, X. Research progress and challenges on persistent organic pollutants in lakes. J. Earth Sci. 35 (2), 729–736. https://doi.org/10.1007/s12583-024-1978-8 (2024).
Hidayati, N. et al. Ecological risk assessment of persistent organic pollutants (POPs) in surface sediments from aquaculture system. Chemosphere 263, 128372. https://doi.org/10.1016/j.chemosphere.2020.128372 (2021).
Antignac, J. P., Lan, A., Bautista, J. L. & Le Bizec, B. Human exposure to persistent organic pollutants and associated health risks. Curr. Opin. Toxicol. 39, 100537. https://doi.org/10.1016/j.cotox.2023.100537 (2023).
Kamalesh, T., Kumar, P. S. & Rangasamy, G. An insights of organochlorine pesticides categories, properties, eco-toxicity and new developments in bioremediation process. Environ. Pollut. 333, 122114. https://doi.org/10.1016/j.envpol.2023.122114 (2023).
Adeyi, A. A., Babalola, B. & Akpotu, S. O. Occurrence, distribution, and risk of organochlorine pesticides in food and greenness assessment of method. Environ. Sci. Pollut. Res. 28 (25), 33433–33444. https://doi.org/10.1007/s11356-021-13047-w (2021).
Li, C., Yang, L., Shi, M. & Liu, G. Persistent organic pollutants in typical lake ecosystems. Ecotoxicol. Environ. Saf. 180, 668–678. https://doi.org/10.1016/j.ecoenv.2019.05.060 (2019).
Tzanetou, E. N. & Karasali, H. A. Comprehensive review of organochlorine pesticide monitoring in agricultural soils: the silent threat of a conventional agricultural past. Agriculture 12 (5). https://doi.org/10.3390/agriculture12050728 (2022).
Syed, J. H. et al. Organochlorine pesticides in air and soil and estimated air–soil exchange in punjab, Pakistan. Sci. Total Environ. 444, 491–497. https://doi.org/10.1016/j.scitotenv.2012.12.018 (2013).
Lee, M. et al. Assessment of organochlorine pesticides in the atmosphere of South korea: Spatial distribution, seasonal variation, and sources. Environ. Monit. Assess. 194 (10), 754. https://doi.org/10.1007/s10661-022-10335-xxie (2022).
Wu, J. & Soil-Air Partition coefficients of persistent organic pollutants decline from climate warming: A case study in Yantai county, Shandong province, China. Water Air Soil Pollut. 231. https://doi.org/10.1007/s11270-020-04718-4 (2020).
Pokhrel, B. et al. Distribution, sources, and air-soil exchange of ocps, PCBs and PAHs in urban soils of Nepal. Chemosphere 200, 532–541. https://doi.org/10.1016/j.chemosphere.2018.01.119 (2018).
Riaz, R., Malik, R. & de Wit, C. Soil-air partitioning of semivolatile organic compounds in the lesser himalaya region: influence of soil organic matter, atmospheric transport processes and secondary emissions. Environ. Pollut. 291, 118006. https://doi.org/10.1016/j.envpol.2021.118006 (2021).
Huang, H. et al. How persistent are pops in remote areas? A case study of DDT degradation in the Qinghai-Tibet plateau, China. Environ. Pollut. 263, 114574. https://doi.org/10.1016/j.envpol.2020.114574 (2020).
Tariq, M. I., Afzal, S., Hussain, I. & Sultana, N. Pesticides exposure in pakistan: a review. Environ. Int. 33 (8), 1107–1122. https://doi.org/10.1016/j.envint.2007.07.012 (2007).
Ahad, K., Mohammad, A., Khan, H., Hayat, Y. & Ahmad, I. Monitoring results for organochlorine pesticides in soil and water from selected obsolete pesticide stores in Pakistan. Environ. Monit. Assess. 166 (1–4), 191–199. https://doi.org/10.1007/s10661-009-0995-5 (2010).
Economic Survey of Pakistan. Ministry of Finance, Government of Pakistan. (2005–2006).
Ali, U., Sweetman, A. J., Jones, K. C. & Malik, R. N. Higher atmospheric levels and contribution of black carbon in soil-air partitioning of organochlorines in lesser himalaya. Chemosphere 191, 787–798. https://doi.org/10.1016/j.chemosphere.2017.10.021 (2018).
Aslam, R. W., Shu, H., Tariq, A. & Naz, I. & others. Monitoring landuse change in Uchhali and Khabeki wetland lakes, Pakistan using remote sensing data. Gondwana Research, 129 C, 252–267. (2024). https://doi.org/10.1016/j.gr.2023.12.015
Baqar, M. et al. Organochlorine pesticides across the tributaries of river ravi, pakistan: human health risk assessment through dermal exposure, ecological risks, source fingerprints and spatio-temporal distribution. Sci. Total Environ. 618, 291–305. https://doi.org/10.1016/j.scitotenv.2017.10.234 (2018).
Sohail, M. et al. Freely dissolved organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) along the indus river pakistan: Spatial pattern and risk assessment. Environ. Sci. Pollut. Res. 29 (43), 65670–65683. https://doi.org/10.1007/s11356-022-20418-4 (2022).
Taufeeq, A. et al. Assessment of organochlorine pesticides and health risk in tobacco farming associated with river Barandu of Pakistan. Environ. Sci. Pollut. Res. 28 (29), 38774–38791. https://doi.org/10.1007/s11356-021-13142-y (2021).
Ullah, R. et al. M. A. S. Assessment of organochlorine pesticides in the Himalayan riverine ecosystems from Pakistan using passive sampling techniques. Environ. Sci. Pollut. Res. 26 (6), 6023–6037. https://doi.org/10.1007/s11356-018-3987-6 (2019).
Bhatti, S. et al. Spatio-temporal variations in physico-chemical parameters and potentially harmful elements (PHEs) of uchalli wetlands complex (Ramsar site), Pakistan. Environ. Sci. Pollut. Res. 25. https://doi.org/10.1007/s11356-018-3240-3 (2018).
Dauda, T., Hafiz, M. & Anuar, M. Birds’ species diversity measurement of Uchali wetland (Ramsar site) Pakistan. J. Asia-Pacific Biodivers. 10. https://doi.org/10.1016/j.japb.2016.06.011 (2016).
Aftab, A., Aziz, R., Ghaffar, A., Rafiq, M. T., Feng, Y., Saqib, Z., … Awan, M. A.Occurrence, source identification and ecological risk assessment of heavy metals in water and sediments of Uchalli lake–Ramsar site, Pakistan. Environmental Pollution, 334, 122117. https://doi.org/10.1016/j.envpol.2023.122117 (2023).
Ali, U. et al. Significance of black carbon in the sediment–water partitioning of organochlorine pesticides (OCPs) in the indus river, Pakistan. Ecotoxicol. Environ. Saf. 126, 177–185. https://doi.org/10.1016/j.ecoenv.2015.12.024 (2016).
U.S. Environmental Protection Agency. Comparison of Multiple Point and Composite Sampling for Recreational Water Quality Monitoring. EPA/600/R-06/031. (2006).
Bajwa, A. et al. Organochlorine pesticides (OCPs) in the indus river catchment area, pakistan: status, soil-air exchange and black carbon mediated distribution. Chemosphere 152, 292–300. https://doi.org/10.1016/j.chemosphere.2016.01.024 (2016).
Aslam, R., Sharif, F., Baqar, M. & Shahzad, L. Source identification and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in air and dust samples of Lahore City. Sci. Rep. 12 (1). https://doi.org/10.1038/s41598-022-06437-8 (2022). Article 1.
Abad, E., Abalos, M. & Fiedler, H. Air monitoring with passive samplers for dioxin-like persistent organic pollutants in developing countries (2017–2019). Chemosphere 287, 131931. https://doi.org/10.1016/j.chemosphere.2021.131931 (2022).
Estefan, G., Sommer, R. & Ryan, J. Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region, 3, 65–119. (2013).
Yang, Y. et al. The challenge of micropollutants in surface water of the Yangtze river. Sci. Total Environ. 780, 146537. https://doi.org/10.1016/j.scitotenv.2021.146537 (2021).
Falconer, R. L. & Harner, T. Comparison of the octanol–air partition coefficient and liquid-phase vapor pressure as descriptors for particle/gas partitioning using laboratory and field data for PCBs and PCNs. Atmos. Environ., 34(23), 4043–4046. https://doi.org/10.1016/S1352-2310(00)00164-3 (2000).
Chen, C. et al. Spatio-temporal variations and ecological risks of organochlorine pesticides in surface waters of a plateau lake in China. Chemosphere 303, 135029. https://doi.org/10.1016/j.chemosphere.2022.135029 (2022).
Bhutto, S. et al. Occurrence and distribution of OCPs and PAHs in water, soil and sediment of Daye lake. J. Geochem. Explor. 226, 106769. https://doi.org/10.1016/j.gexplo.2021.106769 (2021).
Liu, Z., Zheng, G. & Liu, Z. Organochlorine pesticides in surface water of Jiuxi Valley, China: Distribution, source analysis, and risk evaluation. Journal of Chemistry, 1–8. (2020). https://doi.org/10.1155/2020/5101936 (2020).
Gbeddy, G., Glover, E. & Doyi, I. Assessment of organochlorine pesticides in water, sediment, African Cat fish and nile tilapia, consumer exposure and human health implications, Volta lake, Ghana. J. Environ. Anal. Toxicol. 05. https://doi.org/10.4172/2161-0525.1000297 (2014).
Zhang, G., Pan, Z., Bai, A., Li, J. & Li, X. Distribution and bioaccumulation of organochlorine pesticides (OCPs) in food web of Nansi lake, China. Environ. Monit. Assess. 186 (4), 2039–2051. https://doi.org/10.1007/s10661-013-3516-5 (2014).
Yuan, L. et al. Spatial and Temporal variations of organochlorine pesticides (OCPs) in water and sediments from Honghu lake, China. J. Geochem. Explor. 132, 181–187. https://doi.org/10.1016/j.gexplo.2013.07.002 (2013).
Dai, G. et al. Distribution of organochlorine pesticides (OCPs) and Poly chlorinated biphenyls (PCBs) in surface water and sediments from Baiyangdian lake in North China. J. Environ. Sci. 23 (10), 1640–1649. https://doi.org/10.1016/S1001-0742(10)60633-X (2011).
Zhang, Y., Qi, S., Xing, X., Yang, D., Devi, N. L., Qu, C., … Zeng, F. M. Legacies of organochlorine pesticides (OCPs) in soil of China—a review, and cases in Southwest and Southeast China. Environmental Geochemistry, 519–547. https://doi.org/10.1016/B978-0-443-13801-0.00015-3 (2024).
Imran, S., Bukhari, L. & Ashraf, M. Spatial and temporal trends in river water quality of Pakistan (Sutlej and Ravi) 2018, in: PCRWR (Ed.). Pakistan Council of Research in Water Resources (PCRWR), p. 45. (2018).
Qadeer, A., Liu, S., Liu, M., Liu, X., Ajmal, Z., Huang, Y., … Liu, Y. Historically linked residues profile of OCPs and PCBs in surface sediments of typical urban river networks, Shanghai: Ecotoxicological state and sources. Journal of cleaner production, 231, 1070–1078. https://doi.org/10.1016/j.jclepro.2019.05.203 (2019).
Qu, C., Albanese, S., Chen, W., Lima, A., Doherty, A. L., Piccolo, A., … De Vivo,B. The status of organochlorine pesticide contamination in the soils of the Campanian Plain, southern Italy, and correlations with soil properties and cancer risk. Environmental Pollution, 216, 500–511. https://doi.org/10.1016/j.envpol.2016.05.089 (2016).
Eqani, S. A. M. A. S., Malik, R. N. & Mohammad, A. The level and distribution of selected organochlorine pesticides in sediments from river chenab, Pakistan. Environ. Geochem. Health. 33 (1), 33–47. https://doi.org/10.1007/s10653-010-9312-z (2011).
Wang, D. et al. Ecological and health risk assessment of pahs, ocps, and PCBs in Taihu lake basin. Ecol. Ind. 92. https://doi.org/10.1016/j.ecolind.2017.06.038 (2017).
El-Kady, A. A., Wade, T. L., Sweet, S. T. & Sericano, J. L. Distribution and residue profile of organochlorine pesticides and polychlorinated biphenyls in sediment and fish of lake manzala, Egypt. Environ. Sci. Pollut. Res. 24 (11), 10301–10312. https://doi.org/10.1007/s11356-017-8714-1 (2017).
Yohannes, Y. B. et al. Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a rift Valley lake—Lake ziway, Ethiopia. Ecotoxicol. Environ. Saf. 106, 95–101. https://doi.org/10.1016/j.ecoenv.2014.04.014 (2014).
Khuman, S. N., Bharat, G. & Chakraborty, P. Spatial distribution and sources of pesticidal persistent organic pollutants in the Hooghly riverine sediment. Environ. Sci. Pollut. Res. 27 (4), 4137–4147. https://doi.org/10.1007/s11356-019-06973-3 (2020).
Zhonghua, Z., Zhang, L. & Wu, J. Polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in sediments from lakes along the middle-lower reaches of the Y Angtze R Iver and the H Uaihe R Iver of C Hina. Limnol. Oceanogr. 61 (1), 47–60. https://doi.org/10.1002/lno.10197 (2016).
Zhao, Z. et al. Spatial correlation analysis of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in sediments between Taihu lake and its tributary rivers. Ecotoxicol. Environ. Saf. 142, 117–128. https://doi.org/10.1016/j.ecoenv.2017.03.039 (2017).
Burgos-Aceves, M. A. et al. 1, 1, 1-trichloro-2, 2-bis (p-chlorophenyl)-ethane (DDT) and 1, 1-Dichloro-2, 2-bis (p, p’-chlorophenyl) ethylene (DDE) as endocrine disruptors in human and wildlife: A possible implication of mitochondria. Environ. Toxicol. Pharmacol. 87, 103684. https://doi.org/10.1016/j.etap.2021.103684 (2021).
Syed, J. H. & Malik, R. N. Occurrence and source identification of organochlorine pesticides in the surrounding surface soils of the Ittehad chemical industries Kalashah kaku, Pakistan. Environ. Earth Sci. 62 (6), 1311–1321. https://doi.org/10.1007/s12665-010-0618-z (2011).
Ali, S. N., Baqar, M., Mumtaz, M., Ashraf, U., Anwar, M. N., Qadir, A., … Jun, H.Organochlorine pesticides in the surrounding soils of POPs destruction facility: source fingerprinting, human health, and ecological risks assessment. Environmental Science and Pollution Research, 27, 7328–7340. https://doi.org/10.1007/s11356-019-07183-7 (2020).
Zhou, Q. et al. Distribution and sources of organochlorine pesticides in agricultural soils from central China. Ecotoxicol. Environ. Saf. 93, 163–170. https://doi.org/10.1016/j.ecoenv.2013.03.029 (2013).
Sultana, J. et al. Investigation of organochlorine pesticides from the indus basin, pakistan: sources, air–soil exchange fluxes and risk assessment. Sci. Total Environ. 497–498. https://doi.org/10.1016/j.scitotenv.2014.07.066 (2014).
Khairy, M., Muir, D., Teixeira, C. & Lohmann, R. Spatial trends, sources, and Air–Water exchange of organochlorine pesticides in the great lakes basin using low density polyethylene passive samplers. Environ. Sci. Technol. 48 (16), 9315–9324. https://doi.org/10.1021/es501686a (2014).
Kim, T. K. T test as a parametric statistic. Korean J. Anesthesiology. 68 (6), 540–546. https://doi.org/10.4097/kjae.2015.68.6.540 (2015).
Cao, F. et al. Occurrence, Spatial distribution, source, and ecological risk assessment of organochlorine pesticides in Dongting lake, China. Environ. Sci. Pollut. Res. 28 (24), 30841–30857. https://doi.org/10.1007/s11356-021-12743-x (2021).
Peng, S., Kong, D., Li, L., Zou, C., Chen, F., Li, M., … Jia, W. Distribution and sources of DDT and its metabolites in porewater and sediment from a typical tropical bay in the South China Sea. Environmental Pollution, 267, 115492. https://doi.org/10.1016/j.envpol.2020.115492 (2020).
Popa, C. L., Dontu, S. I., Carstea, E. M., Levei, E. A., Ioja, C., Popa, A. M., …Cadar, O. Organochlorine pesticides and dissolved organic matter within a system of urban exorheic lakes. Environmental Monitoring and Assessment, 192(1), 59. https://doi.org/10.1007/s10661-019-8003-1 (2020).
Zehra, A. et al. Environmental monitoring of organo-halogenated contaminants (OHCs) in surface soils from Pakistan. Sci. Total Environ. 506–507. https://doi.org/10.1016/j.scitotenv.2014.10.055 (2015).
Syed, J. H. et al. Status, distribution and ecological risk of organochlorines (OCs) in the surface sediments from the Ravi river, Pakistan. Sci. Total Environ. 472, 204–211. https://doi.org/10.1016/j.scitotenv.2013.10.109 (2014a).
Alamdar, A. et al. Organochlorine pesticides in surface soils from obsolete pesticide dumping ground in Hyderabad city, pakistan: contamination levels and their potential for air–soil exchange. Sci. Total Environ. 470–471. https://doi.org/10.1016/j.scitotenv.2013.09.053 (2014).
Eqani, S. A. M. A. S. et al. Distribution and risk assessment of organochlorine contaminants in surface water from river chenab, Pakistan. J. Environ. Monit. 14 (6), 1645–1654. https://doi.org/10.1039/C2EM11012A (2012).
Mahmood, A., Malik, R. N., Li, J. & Zhang, G. Levels, distribution pattern and ecological risk assessment of organochlorines pesticides (OCPs) in water and sediments from two tributaries of the Chenab river, Pakistan. Ecotoxicology 23 (9), 1713–1721. https://doi.org/10.1007/s10646-014-1332-5 (2014).
Gong, X. et al. The occurrence of organochlorine pesticides (OCPs) in riverine sediments of hilly region of Southern china: implications for sources and transport processes. J. Geochem. Explor. 216, 106580. https://doi.org/10.1016/j.gexplo.2020.106580 (2020).
Zhou, Y., Li, J., Wang, Y. & Chen, H. Distribution and flux of organochlorine pesticides in sediment from the East China sea: implications for sources and environmental processes. Sci. Total Environ. 778, 146208. https://doi.org/10.1016/j.scitotenv.2021.146208 (2021).
Bhatti, S. G., Tabinda, A. B., Yasin, F., Mehmood, A., Salman, M., Yasar, A., … Wajahat,R. Ecological risk assessment of metals in sediments and selective plants of Uchalli Wetland Complex (UWC)—a Ramsar site. Environmental Science and Pollution Research, 26, 19136–19152. https://doi.org/10.1007/s11356-019-04711-3 (2019).
Xing, Y. N., Guo, Y., Xie, M., Shen, R. L. & Zeng, E. Y. Bioaccumulation and maternal transfer of two understudied DDT metabolites in wild fish species. Environ. Pollut. 268, 115492. https://doi.org/10.1016/j.envpol.2020.115492 (2021).
Dasmahapatra, A. K., Powe, D. K., Dasari, T. P. S. & Tchounwou, P. B. Reproductive toxicity of DDT in the Japanese Medaka fish model: revisiting the impacts of DDT + on female reproductive health. Chemosphere 338, 139600. https://doi.org/10.1016/j.chemosphere.2024.139600 (2024).
Hawkey, A. B. et al. Neurobehavioral anomalies in zebrafish after sequential exposures to DDT and Chlorpyrifos in adulthood: do multiple exposures interact? Neurotoxicol. Teratol. 83, 106930. https://doi.org/10.1016/j.ntt.2020.106930 (2021).
Wang, X. et al. Occurrence, source, and ecological risk assessment of organochlorine pesticides and polychlorinated biphenyls in the water–sediment system of Hangzhou Bay and East China sea. Mar. Pollut. Bull. 179, 113735. https://doi.org/10.1016/j.marpolbul.2022.113735 (2022).
Degrendele, C. et al. Diurnal variations of Air-Soil exchange of semivolatile organic compounds (PAHs, pcbs, ocps, and PBDEs) in a central European receptor area. Environ. Sci. Technol. 50 (8), 4278–4288. https://doi.org/10.1021/acs.est.5b05671 (2016).
Wang, X. et al. Persistent organic pollutants in the Tibetan surface soil: Spatial distribution, air–soil exchange and implications for global cycling. Environ. Pollut. 170, 145–151. https://doi.org/10.1016/j.envpol.2012.06.012 (2012).
Mackay, D. Multimedia Environmental Models: the Fugacity Approach (2nd ed.). CRC.
Can-Güven, E., Gedik, K. & Kurt-Karakuş, P. B. Organochlorine pesticides and polychlorinated biphenyls from a greenhouse area on the Mediterranean coast of Turkey: Distribution, air-soil exchange, enantiomeric signature, and source implications. Atmospheric Pollution Research, 13(1), 101263. (2022). https://doi.org/10.1016/j.apr.2021.101263 (2001).
Zhang, H., Luo, Y., Teng, Y., Wang, J. & Christie, P. Organochlorine pesticide contamination in surface soils and sediments from a typical agricultural catchment in Taihu lake, China. Environ. Sci. Pollut. Res. 26 (25), 26064–26073. https://doi.org/10.1007/s11356-019-05662-4 (2019).
Alani, R. A., Oladapo, T. S., Levels of organochlorine & pesticide residues in biotic and abiotic components of the lagos lagoon, Nigeria. UNILAG J. Med. Sci. Technol., 3(2), 52–65. (2015).
Author information
Authors and Affiliations
Contributions
U. A. Conceptualization, methodology, GIS mapping, multivariate statistical analysis, F. S. Methods development, supervision, S. W. Investigation, Data curation, R.A. Writing, Validation, Visualization, M. S. statistical analysis, L. S. Writing, editing, R. R. Formal analysis, G. G. Writing – review, M.U. H. Review & editing, F. N. Data collection & analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aamir, U., Sharif, F., Waheed, S. et al. Profile distribution and ecological risk assessment of organochlorine pesticides (OCPs) in environmental matrices of Uchalli and Khabeki Lakes. Sci Rep 15, 33985 (2025). https://doi.org/10.1038/s41598-025-11223-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11223-3
Keywords
This article is cited by
-
Dominant riverine input of legacy organochlorine pesticides to a semi-enclosed plateau lake: distribution, source apportionment, and bioaccumulation
Environmental Monitoring and Assessment (2025)