Abstract
Rhipicephalus microplus, a tick species, causes significant economic losses in livestock and presents control challenges due to the emergence of resistance to conventional acaricides. This underscores the urgent need for effective and eco-friendly alternatives. This study evaluates the acaricidal potential of Cirsium arvense using adult immersion and larval packet tests. In silico molecular docking techniques were employed to identify biologically active compounds within C. arvense. Using Chem-Draw Ultra software (version 12.0.2, 2010), we illustrated 25 compounds derived from the plant, which were subsequently tested as ligands in docking experiments against Subolesin. Among the tested compounds, Apigenin 7-O-glucosideand Pectolinarigenin 7-glucoside exhibited significant inhibitory effects on Subolesin, with docking scores of -6.6 and − 6.3 kcal/mol, respectively. In contact bioassays using Cirsium arvense extract, various concentrations (2.5, 5, 10, 20, and 40 mg/mL) were evaluated. The results indicated an LC50 of 2.907 mg/mL and an LC90 of 47.725 mg/mL after 24 h of exposure. Notably, at the highest concentration of 40 mg/mL, the extract significantly reduced egg-laying activity in adult female ticks, yielding an oviposition index of 0.09 ± 0.02, which corresponds to a 75.68 ± 0.44% reduction in reproductive capacity. Additionally, larval mortality reached 88.33 ± 2.90%, indicating that higher concentrations not only increased larval mortality but also substantially decreased the reproductive capacity of the ticks. These findings suggest that bioactive components from Cirsium arvense show promise as candidates for the control of R. microplus. Further research is warranted to evaluate their efficacy as alternative or complementary strategies to synthetic acaricides.
Similar content being viewed by others
Introduction
Ticks are ectoparasitic arthropods that feed on the blood of birds, reptiles, and mammals, posing significant threats to livestock1,2. Among them, Rhipicephalus microplus is a major concern for the cattle industry, particularly affecting dairy cattle and resulting in global economic losses estimated at approximately $30 billion annually. These losses are primarily attributed to declines in the quality of meat, milk, and leather products3,4.R.microplus causes anemia and stunted growth and acts as a vector for parasitic protozoans such as Babesia bovis and Babesia bigemina, which cause babesiosis. It also transmits the intracellular bacterium Anaplasma marginale, responsible for bovine anaplasmosis. These infections contribute to increased morbidity and mortality in cattle5,6.
The reliance on chemical acaricides for controlling R. microplus has led to the development of resistance due to detoxification enzyme activities and mutations at target sites7,8. Repeated use of these chemicals not only fosters resistance but also results in the accumulation of chemical residues in food and adverse environmental impacts9,10Therefore, there is an urgent need for effective and eco-friendly alternatives for pest control11,12.
Plant-derived acaricides present a promising solution for managing cattle parasites, especially in rural areas. These natural compounds contain various bioactive ingredients that disrupt the physiological processes of arthropods, interfering with their life cycles (Nicoletti, 2020). Moreover, the ethnobotanical knowledge of rural communities regarding plants used to combat external parasites remains relevant today, offering potential long-term solutions to resistance issues13,14.
Canada thistle (Cirsium arvense) is a perennial plant in the Asteraceae family15. It is rich in diverse phytoconstituents, including alkaloids, glycosides, flavonoids, terpenoids, tannins, and phenolic compounds. Because Cirsium arvense contains a wealth of phytochemicals, it has a wide range of biological effects. These substances support its antibacterial, anti-inflammatory, antioxidant, and therapeutic qualities, which have been reported in both scientific and conventional medicine. C. arvense has substantial pesticidal properties in addition to its potential as a medicine. Its bioactive extracts have demonstrated notable acaricidal, insecticidal, and repellant properties against a range of pests, including insects and ticks. The plant is a viable option for use in integrated control techniques and natural pest management because of its capacity to interfere with the physiological processes of pests, which is responsible for these pesticidal effects16,17.
Subolesin is a conserved protein found in ticks and other arthropods, playing a crucial role in regulating various biological processes18. It is present in both hard and soft ticks and is considered a promising candidate for anti-tick vaccine development. Previous studies have demonstrated subolesin’s involvement in blood feeding, reproduction, development, and gene expression in hard ticks. Given its broad role in tick biology, targeting subolesin presents an attractive strategy for controlling tick populations and blocking the transmission of tick-borne pathogens19,20.In the context of molecular docking, interference with subolesin function could disrupt these vital processes, enhancing efforts to manage tick infestations21.
The study aims to evaluate the acaricidal efficacy of Cirsium arvense plant extracts against various developmental stages of Rhipicephalus microplus ticks using in vitro bioassays as well as insilico molecular docking techniques to identify specific bioactive compounds within C. arvense that exhibit potential anti-tick properties. This integrated approach seeks to develop innovative strategies for controlling the transmission of tick-borne diseases.
Materials and methods
Ticks sample collection
A total of 205 engorged adult female R. microplus ticks were collected from various cattle farms in the Charsadda district of KPK. The samples were transported to the Parasitology Laboratory at the Department of Zoology, Bacha Khan University Charsadda. Ticks were identified based on morphological characteristics following the methodology outlined by22. After identification, the ticks were divided into two groups. The first group, consisting of 150 ticks, underwent adult immersion testing (AIT) using varying concentrations of the plant extract (described below), whereas, the second group, comprising 60 ticks, was used in AIT as controls (positive and negative). The remaining ticks were placed in a BOD incubator at temperature of 28 ± 1 °C, and a relative humidity of 85 ± 5% for oviposition and eggs hatching. Following oviposition, larvae hatched from the eggs after approximately 21 days were subsequently used for the larval packet test (LPT).
Gathering plant material and extract formulation
Leaves and roots of Cirsium arvense were collected from the Charsadda district in Khyber Pakhtunkhwa (KPK), Pakistan, having coordinates 34.1682° N, 71.7504° E. The collected plant material was thoroughly washed, and sent to herbarium of the Department of Botany, BKUC for taxonomic identification. The plant material was identified by Dr. Imtiaz ahmad (Department of Botany, Bacha Khan University), under voucher No Hbku − 2930. The collected plant parts were then dried at room temperature for 14 days, and then pulverized using a plant grinder. Fifty grams of the powdered material were dissolved in 100 mL of methanol, and the solution was agitated for 10 days using shaking incubator at 250 RPM for 12 h daily. Following this, the mixture was filtered through muslin cloth followed by Whatman filter paper No. 1 twice, and the methanol was removed using a rotary evaporator (Re-LA100, Labfreez Instruments, Hunan, China). The resultant high-viscosity solution was dried in a water bath at 45 °C, and the powdered extract was measured. Different concentrations of 2.5, 5, 10, 20, and 40 mg/mL of the extract were prepared by diluting the stock solution in distilled water.
Adult immersion test (AIT)
The acaricidal efficacy of the plant extracts was assessed using a modified Adult Immersion Test (AIT) as per the standards set by23Engorged ticks were washed with purified water and dried on filter paper. A dose-response assay was conducted using methanolic extracts at concentrations ranging from 2.5 to 40 mg/mL. For each 2.5, 5, 10, 20, and 40 mg/mL concentration, 10 ticks were fully immersed in the extract for 1 to 2 min. After immersion, these ticks were placed in separate clean Petri dishes and incubated at 28 ± 1 °C with a relative humidity of 85 ± 5%. Additionally, a positive control (permethrin) and a negative control (Distilled Water) were used and each experiment were replicated three times to ensure accuracy. Mortality in adult ticks was assessed daily for 15 days post-treatment. The egg-laying index (IE) was calculated by dividing the mean weight of eggs laid by the mean weight of the female ticks. The percentage inhibition of oviposition (% IO) was determined using the formula:
(% IO) = [(IEcontrol group - IEtreated group) / IEcontrol group] × 100. This calculation quantifies the relative reduction in egg-laying activity between the control and treated groups.
Larval packet test (LPT)
The Larval Packet Test (LPT) was conducted following24. to evaluate the acaricidal effects of methanolic plant extracts on tick larvae. Filter paper was treated with varying dilutions of 2.5, 5, 10, 20, and 40 mg/mL of the plant extract, with each packet containing 100 14-day-old R. microplus larvae. The larval packets were incubated at 28 ± 1 °C and 85 ± 5% humidity for 24 h. After incubation, deceased larvae were counted to determine the mortality rate. Triplicate tests were performed for all dilutions as well as for the control group (distilled water).
Statistical analysis
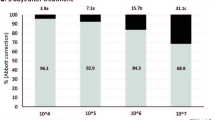
Statistical analyses were performed using R software (version 4.3.0) via R Studio25. Percent larval mortality at 24 h for each concentration was analyzed using one-way analysis of variance (ANOVA), followed by post hoc Tukey’s HSD test. The “agricolae” R package26 facilitated this analysis. Lethal concentration (LC50 and LC90) values and their corresponding chi-square values for the methanolic extract were determined through probit regression analysis27. A significance level (p-value) of 0.038 was set for heterogeneity, with a confidence limit of 95%, using the “ecotox” R package28. The results were graphically represented using the “ggplot2” R package29. The percentage inhibition of oviposition (% IO) was calculated, and the corresponding bar chart was created using Microsoft Excel (Version 2016) shown in Fig. 1A.
Rhipicephalus microplus larvae and adults respond to Cersium arvense plant extracts. A bar chart representation of exposure illustrating the percentage inhibition of oviposition (%IO) in adult female ticks subsequent to treatment with plant extracts. B: Line graph depicting the dose-fatality relationship of R. microplus larvae following 24 h exposure to plant extracts preparations. C: The evaluation of percent larval mortality at the 24-hour time point for each concentration underwent a one-way analysis of variance (one-way ANOVA), subsequently followed by a post hoc Tukey’s honest significant difference (HSD) test.
In-silico screening
Retrieval of Rhipicephalus microplusSubolesin protein sequence
The protein sequence of Subolesin from R. microplus was obtained using the Accession Number A0A9J6F3J6_RHIMP from the UniProt database (http://www.uniprot.org/). UniProt is an online resource that provides detailed information on protein sequences and their functions through the UniProt Knowledgebase tool30,31.
Validation of modelled structure by homology modelling
Homology modelling was performed using the Swiss Model program32 to construct a 3D model of the Subolesin protein. Various assessment tools, including PROCHECK33. PROSA34, and the ERRAT server35, were utilized to evaluate the quality and accuracy of the modelled structure. Among the generated models, the one with the highest validation and acceptance scores was selected for further studies.
Prediction of active site
The binding site prediction for the Subolesin protein utilized the CASTp server36. This web-based tool was instrumental in analyzing the internal cavity surfaces of the protein, identifying structural features, and pinpointing major residues located within the binding pockets.
Preparation of ligands for Docking study
In silico prediction of acetylcholinesterase (AChE) inhibitors from C. arvense extracts was conducted using the PubChem database (https://pubchem.ncbi.nlm.nih.gov/)37. A total of twelve different compounds were identified. The chemical structures of these compounds were created using Chem-Draw Ultra (version 12.0.2.1076, 2010) and saved in .mol file format. These .mol files were converted to .PDB format using Discovery Studio Biovia 2017. Subsequently, the AutoDock Tools (ADT) software was employed to prepare the ligand structures, which included adjustments for nonpolar hydrogens, Gasteiger charges, and rotatable bonds. The final Ligand.PDB files were converted to PDBQT format for use in AD4 and AutoDock Vina operations.
Docking methodology
Molecular docking was performed using the AutoDock Vina program38. Each ligand was docked independently to the receptor, utilizing appropriate grid coordinates tailored for each receptor. This process accounted for the presence of a chlorine atom in the ligand and allowed for flexibility in the ligand when it bound to the rigid macromolecule. The configuration file for AutoDock Vina was accessed using Notepad, and AutoDock Tools (ADT) were employed to process the input. PDBQT file for Subolesin, initializing the grid box’s center and volume. The Subolesin structure was prepared by including polar hydrogens and Gasteiger charges. A grid size of 23 × 23 × 23 points (x, y, and z) was used, with the grid center situated at coordinates x = 158.241, y = 192.542, and z = 204.691, and a grid spacing of 1.0 Å. After these processes, the standard .PDBQT format was maintained in the saved file. Ligands were docked using the CDOCKER method, generatingpdbqt files for each complex. The residual free energy (ΔG) scores were calculated, with changes in free energy for complexation ranging around − 99 kJ/mol. Ligands were visualized in 3D, focusing on ligand-binding sites to study the size and geometry of polar and hydrophobic regions, as well as their hydrogen-bonding interactions. Compounds were docked to the active site of Subolesin, and the binding modes of various ligands were explored. The most suitable and lowest energy binding conformations for each ligand were selected for further analysis.
ADMET analysis of the most promising phytochemicals
Understanding the metabolic disposition of potential drug candidates is essential in drug discovery and development, as it helps eliminate non-drug-like activities from bioactive agents. This analysis is typically determined by the absorption (A), distribution (D), metabolism (M), excretion (E), and toxicity (T) of the drug candidates within the body. The ADMET profiles for the selected bioactive compounds were assessed using the SwissADME online resource (swissadme.ch) and pkCSM.
Results
Potential of botanical extract against larvae
The Lethal Concentration 50 (LC50) and Lethal Concentration 90 (LC90) for the plant extracts against R. microplus larvae, hatched from isolated engorged female eggs, were calculated using regression analysis with a 95% confidence level. In addition to the established LC50 and LC90 values, a diagnostic dose of 0.6 mg/mL was determined for field applications against Rhipicephalus (Boophilus) microplus. This dose is intended to evaluate the effectiveness of the agent under real-world conditions and serves as a benchmark for monitoring tick populations. The diagnostic dose was set at approximately 20% of the LC₉₀ value to balance efficacy with safety in field applications. This dosage is intended to evaluate the effectiveness of the agent in real-world conditions and serves as a benchmark for monitoring tick populations. The diagnostic dose was set at approximately 20% of the LC50 value to balance effectiveness with safety in field applications.
Various concentrations of C. arvense extract (ranging from 2.5 to 40 mg/mL) were evaluated in the Larval Packet Test to assess their lethal effects. Probit analysis was conducted to determine the relationship between extract concentrations and the mortality rates of R. microplus larvae. The results indicated an LC50 of 2.907 mg/mL (upper limit: 3.879; lower limit: 1.922) and an LC90 of 47.725 mg/mL (upper limit: 86.559; lower limit: 32.333) for C. arvense (Table 1). As the concentrations of the botanical extract increased, the mortality rates of the larvae correspondingly increased (Fig. 1B and C).
Botanical extract role in combating adult female R. microplus ticks
The anti-tick activity of C. arvense whole-plant extract was evaluated with a focus on its effects on egg laying. Results from the Adult Immersion Test (AIT) demonstrated that the plant extract significantly suppressed egg laying compared to control groups treated with water and methanol. When fully engorged adult female ticks were exposed to the C. arvense extract in a dose-dependent manner, AIT results showed notably lower Index of Egg Laying (IE) values and higher percent inhibition of oviposition (% IO) compared to the controls (Table 2). At the highest tested dosage of 40 mg/mL, the IE for C. arvense extract was 0.09 ± 0.01 g (mean ± SE), while the water control was 0.33 ± 0.01 g and the methanol control was 0.06 ± 0.01 g. A lower IE value indicates reduced egg deposition, signifying inhibition of egg laying.The % IO at the maximum concentration (40 mg/mL) for C. arvense extract was 76.37 ± 0.44%, while the methanol control exhibited a higher % IO of 93.17 ± 1.69% (Table 2). Figure 1A graphically illustrates the increase in % IO with rising concentrations of each plant extract.
In Silico analysis
In this study, the complete sequence of the R. microplus Subolesin protein was retrieved from the UniProt protein sequence database (Accession No: A0A9J6F3J6_RHIMP). To construct the protein’s tertiary structure, templates were selected from the Protein Data Bank using the BLASTp algorithm, as no 3D data for the protein was available in the PDB database. The templates chosen were those with protein sequences closest to the query sequence. Three-dimensional modelling was performed using the Swiss Model server, a tool for homology modeling. Energy changes in the developed 3D models were optimized through stereochemical adjustments, minimizing steric clashes and strain while maintaining low energy in the model. Energy minimization was carried out using the GROMOS96 force field, and energy calculations were performed with the Swiss-PDB Viewer.
Overview and validation of computational models
The Ramachandran plot analysis was conducted using PROCHECK following the optimization of the 3D model. The secondary structure composition was estimated via structural molecular biology tools, assessing the distribution of phi (φ) and psi (ψ) angles from non-glycine and non-proline residues. The distribution of these angles indicated that the proposed models of R. microplus Subolesin are well-constructed and reliable. Results showed that 97.7% of amino acid residues were in areas of good quality, 2.3% were in acceptable quality regions, and 0.0% were in generous or disallowed quality areas. The quality factor of the model, assessed by ERRAT, was 97.143%. The degree of confidence in identifying abnormal regions was illustrated by error axes, indicating that the majority of the protein had an estimated standard error below the 95% rejection level. ERRAT’s analysis compared predicted structures to experimental data, yielding an error function value and a confidence limit range. Based on these assessments, the Swiss Model was deemed useful for further investigations and was adopted for subsequent studies (Fig. 2).
Molecular Docking analysis
Molecular docking conducted in AutoDock Tools (ADT) revealed that Apigenin 7-O-glucoside and Pectolinarigenin 7-glucoside were the most favorable candidates, with docking scores of -6.6 kcal/mol and − 6.3 kcal/mol, respectively, compared to the standard drug Deltamethrin, which had a score of -5.3 kcal/mol (Table 3) (Table 4). These results indicate that both Apigenin 7-O-glucoside and Pectolinarigenin 7-glucoside exhibit a higher affinity for the target protein.The specific interactions between Subolesin and its ligands were analyzed using AutoDock Vina (Figs. 3, 4 and 5). Apigenin 7-O-glucoside formed five hydrogen bonds with the amino acids His 157, Asp 158, Arg 162, Arg 163, and Ser 166, along with three hydrophobic interactions with Arg 162 and Arg 168 of the target receptor. In contrast, Pectolinarigenin 7-glucoside established three hydrogen bonds with Asp 158, Arg 163, and Ser 166, and interacted with aromatic residues such as Arg 162, Arg 168, and Pro 169, as well as forming a carbon-hydrogen bond with Gln 159 from a water molecule in the target receptor. Deltamethrin similarly formed two hydrogen bonds near Arg 163 and engaged in two hydrophobic interactions with Arg 162 in the target receptor (Fig. 5).
Drug-likeness and ADMET analysis
The drug-likeness of the compounds in this study was assessed according to Lipinski’s Rule of Five (Ro5), which considers molecular mass (less than 500 daltons), hydrogen bond donors (HBD, less than 5), hydrogen bond acceptors (HBA, less than 10), log P (less than 5), and refractive index (40–130)39,40. All ligands had molecular weights under 500 daltons. With the exception of Apigenin 7-O-glucoside, which has 6 hydrogen bond donors, all other ligands had fewer than 5. All ligands also possessed fewer than 10 hydrogen bond acceptors, although the values varied. The log P values ranged from 1.86 to 4.85, while the mean surface area of the ligands was between 20.23 and 170.05.Absorption predictions were made for water solubility, intestinal absorption, and skin permeability. Water solubility for the compounds ranged from − 2.559 to -7.535 log mol/L, indicating systemic solubility, as optimal values should be less than zero, ideally under − 0.5 (Falcón-Cano et al., 2020). Additionally, these compounds were classified as non-sensitizers for skin permeability, with Kp values ranging from − 2.735 to -4.823 log Kp.Drug distribution features included volume distribution (VDss) and blood-brain barrier (BBB) permeability. The data indicated favorable drug distribution in blood, with optimal VDss values ranging from 0.5 to 3 L/kg (Smith et al., 2015). The absorption classification showed moderate log BB absorption for all compounds, categorized as high absorption (> 2.0), moderate (0.1–2.0), and low (< 0.1).The analysis also considered enzyme inhibition, particularly by Cytochrome P450 (CYP). None of the compounds inhibited CYP2D6 or CYP3A4, except for Cirneyol C and Phytol, which inhibited CYP3A4. This suggests that these compounds are unlikely to adversely affect the cardiovascular system.Excretion parameters, specifically total clearance, were also evaluated. Some compounds exhibited rapid excretion with a clearance rate of 1.393 log mL/min/kg, indicating a quicker elimination process41. Finally, acute oral toxicity was assessed using the LD50 metric, which indicates the dose at which 50% of test animals die within a defined exposure period. The test compounds fell into the category of potentially harmful if swallowed, with LD50 values ranging from 1.88 to 2.478 mol/kg. Notably, all compounds showed no evidence of hepatotoxicity.
Discussion
Ticks are key vectors in spreading diseases, amplified by their adaptability to diverse climates42,43. According to the WHO’s One Health concept, interactions between domestic animals and humans facilitate the rise of zoonotic diseases, with ticks playing a pivotal role44,45. In Pakistan, where diverse tick ecology intersects with large cattle populations, monitoring and control of tick and its associated pathogens is essential. This study was thus aimed to investigate the acaricidal activity of plant extracts from C. arvense. Ticks were exposed to varying doses of methanol extract (2.5, 5, 10, 20, and 40 mg/mL). At the highest dosage of 40 mg/mL, C. arvense extracts demonstrated a mortality rate of 88.33% after 24 h of treatment. This significant level of efficacy indicates that the extract has strong potential as a natural alternative to chemical acaricides, which are often associated with resistance issues and environmental concerns.
The Index of Egg Laying (weight of saved egg mass) for the extract at this dosage was 0.09 ± 0.01 g (mean ± SE), reflecting a notable reduction in reproductive output. The percent inhibition of oviposition at this concentration was 75.68 ± 0.44%, underscoring the extract’s effectiveness in not only killing ticks but also hindering their reproductive capabilities. Such findings are crucial, as reducing tick populations and their reproductive success can significantly mitigate infestations in livestock and pets. The extract exhibited an LC50 of 2.907 mg/mL and an LC90 of 47.725 mg/mL, suggesting a relatively potent action against the target pests. The low LC50 value indicates that C. arvense can achieve significant acaricidal effects at minimal concentrations, which is advantageous for practical applications in pest management. In comparison to the present study, where Cirsium arvense methanolic extract exhibited an LC50 of 2.907 mg/mL (or 2907 ppm) and an oviposition inhibition rate of 75.68% at 40 mg/mL, the extracts of Calpurnia aurea demonstrated a significantly lower LC50 value (27–29 ppm) against adult Amblyomma variegatum and Rhipicephalus microplus (Negash et al., 2024). However, while C. aurea showed only 18.3–19.7% egg hatching inhibition at 400 ppm, C. arvense extract at a higher dose showed a much more pronounced effect on reproductive suppression, with an oviposition index of 0.09 ± 0.01 g. This indicates that although C. aurea may exhibit strong adulticidal potency at very low concentrations, C. arvense is highly effective in impairing tick reproduction, which is crucial for long-term population control.
Although the acaricidal effects of Cirsium arvense have not been thoroughly investigated, a number of papers suggest that its phytochemical constituents, including tannins, phenolic acids, and flavonoids, have broad-spectrum bioactivity against arthropods. The current investigation revealed that C. arvense methanolic extracts exhibited significant acaricidal activity against Rhipicephalus (Boophilus) microplus, indicating both fatal and reproductive inhibitory effects.
In comparison to other plant-based acaricides tested against the same tick species, including Piper rivinoides, C. arvense shows promising efficacy. P. rivinoides extract demonstrated 73.79% efficacy at a lower dose of 6.12 mg/mL in semi-natural settings and 100% mortality of both larvae and engorged adult ticks at 50 mg/mL (Lorenzetti et al., 2024). By interfering with adult viability and population regeneration, C. arvense and P. rivinoides both have an impact on tick survival and reproduction, indicating the possibility of providing long-term tick control.
Several plant extracts have been studied for their distinct acaricidal properties against mites and ticks46,47,48. The increasing interest in plant-based acaricides stems from the demand for sustainable and eco-friendly alternatives to conventional pesticides. Advances in large-scale extraction techniques for essential oils have enabled the effective use of diverse plant characteristics as repellents and anti-parasitic agents against numerous parasites49. The findings from this study add to the growing literature supporting the use of plant extracts as viable solutions for managing tick populations, potentially leading to safer and more sustainable agricultural practices.The presence of flavonoids and coumarins in C. arvense (commonly known as creeping thistle) has been well-documented, correlating these compounds with a range of medicinal properties50,51. These include diuretic effects, anti-inflammatory and hemostatic properties, astringent qualities, anti-phlogistic effects, and hepatic benefits50. Such diverse pharmacological activities underscore the potential of this plant in traditional and modern medicine.
Numerous researchers have focused on isolating specific compounds from this plant, attributing antimicrobial and medicinal properties to them52. Some noteworthy compounds include 1,2-Benzenedicarboxylic acid, mono (2-ethylhexyl) ester; 6,7-Dimethoxycoumarin; Scopoletin; 9,12-Octadecadienoic acid (Z, Z)-methyl ester; 2 H-1-Benzopyran; 6,7-dimethoxy-2,2-dimethyl-tocopherol; Ergoline-8-carboxylic acid; and 10-methoxy-6-methyl-, methyl ester (8 alpha); Nonadecane; and Camphor, among others16. These compounds not only highlight the chemical diversity of C. arvense but also its potential for further pharmacological exploration53.
Several Cirsium species have been traditionally used as herbal remedies for cardiovascular, anti-inflammatory, and diuretic purposes, with C. arvense notably applied as a hemostatic agent in clinical settings. Continued exploration of its bioactive constituents highlights its potential in developing novel therapeutics and enhancing current medical treatments54.
The use of a validated target for in vitro biochemical library screening is a critical step in discovering novel drugs. This strategy has shown promise in various fields, including cancer research, cardiovascular disease, kidney disease, and the prevention of infections caused by a range of pathogens such as bacteria, fungi, viruses, and parasites. In vitro screening has significantly advanced drug discovery and development across multiple disciplines55.
Selectivity is a crucial factor when choosing a molecular target. In this study, in silico assays were performed on the R. microplus Subolesin protein to understand the interactions between phytochemicals derived from C. arvense and the target protein. The AutoDock tool was utilized to conduct a docking study, wherein the best model obtained from homology modelling was subjected to docking simulations. In these simulations, ten phytochemical structures were used as ligands56.
The results of the docking study were employed to evaluate various combinations and identify the most favorable binding modes. A library of phytochemicals from C. arvense was screened for potential Subolesin inhibitors, interacting with different binding sites of the target protein using AutoDock Vina software. This approach facilitated the exploration of potential interactions and the assessment of binding affinities between the phytochemicals and the Subolesin protein.
Among the phytochemicals tested, Apigenin 7-O-glucoside from C. arvense emerged as the most effective inhibitor, achieving a docking score of -6.6 kcal/mol. This compound interacts uniquely with Subolesin amino acids compared to the synthetic acaricide Deltamethrin, which scored − 5.3 kcal/mol. The second most effective inhibitor identified was Pectolinarigenin 7-glucoside, also from C. arvense, with a docking score of -6.3 kcal/mol.
Natural plant-derived chemicals are gaining significant attention as promising tools in Integrated Pest Management (IPM) programs due to their acaricidal and repellent effects on ticks. This research is of paramount importance, driven by industry interest and the proven efficacy of various plant-derived chemicals, which present reduced risks to human health and the environment. Scientific advancements have led to the development of several industrial products reliant on plant bioactive compounds57.
Conclusion
This study underscores the promising acaricidal potential of Cirsium arvense, a medicinal plant native to Pakistan, against Rhipicephalus (Boophilus) microplus, a major cattle tick of significant veterinary concern. Methanolic extracts of C. arvense exhibited strong acaricidal activity, achieving up to 88.33% mortality at a concentration of 40 mg/mL and demonstrating 75.68% inhibition of oviposition, thereby effectively reducing both tick survival and reproductive capacity. These findings suggest that C. arvense could serve as an effective, eco-friendly alternative to synthetic acaricides particularly valuable in areas facing challenges of acaricide resistance and environmental toxicity. Furthermore, in silico molecular docking revealed that phytochemicals such as apigenin 7-O-glucoside and pectolinarigenin 7-glucoside possess strong binding affinities with the R. microplus Subolesin protein, a key regulator of tick development. This supports the potential of C. arvense to disrupt vital physiological processes in ticks through naturally occurring bioactive compounds. Owing to its dual mechanism of action direct mortality and reproductive inhibition—C. arvense holds considerable promise for integration into sustainable tick control strategies, particularly within Integrated Pest Management (IPM) frameworks. Based on the results, we recommend field application of C. arvense methanolic extract at concentrations between 20 and 40 mg/mL for effective management of R. microplus infestations.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Cutler, S. J. et al. Tick-borne diseases and co-infection: current considerations. Ticks tick-borne Dis. 12 (1), 101607 (2021).
Ullah, S. et al. First report of Anaplasma spp., Ehrlichia spp., and Rickettsia spp. In Amblyomma gervaisi ticks Infesting monitor lizards (Varanus begalensis) of Pakistan. Infect. Genet. Evol. 118, 105569 (2024).
Jain, P., Satapathy, T. & Pandey, R. K. Rhipicephalus microplus (acari: Ixodidae): Clinical safety and potential control by topical application of cottonseed oil (Gossypium sp.) on cattle. Exp. Parasitol. 219, 108017 (2020).
Gomes, A. F. & Neves, L. Rhipicephalus microplus (Acarina, Ixodidae) in angola: evidence of its establishment and expansion. Exp. Appl. Acarol. 74 (1), 117–122 (2018).
De Clercq, E. M. et al. Geographic distribution of the invasive cattle tick Rhipicephalus microplus, a country-wide survey in Benin. Exp. Appl. Acarol. 58, 441–452 (2012).
Pascoeti, R. et al. Parasites in dairy cattle farms in Southern Brazil. Revista MVZ Córdoba. 21 (2), 5304–5315 (2016).
Guerrero, F. D., Lovis, L. & Martins, J. R. Acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus. Revista Brasileira de Parasitologia Veterinária, 21, pp. 1–6. (2012).
Rodriguez-Vivas, R. I., Jonsson, N. N. & Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 117, 3–29 (2018).
da Silva Lunguinho, A. et al. Acaricidal and repellent activity of the essential oils of backhousia citriodora, Callistemon viminalis and cinnamodendron dinisii against Rhipicephalus spp. Vet. Parasitol. 300, 109594 (2021).
Zhou, W., Li, M. & Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contaminants, 11, 100410 (2024).
Nabil, M. et al. Acaricidal efficacy of silver nanoformulations of Commiphora molmol and Zingiber officinale against the camel tick, Hyalomma dromedarii (Ixodida: Ixodidae). Inorg. Chem. Commun. 147, 110229 (2023).
Hegazy, M. M. et al. The efficacy of Saussurea costus extracts against hematophagous arthropods of camel and cattle. Pak Vet. J. 42 (4), 547–553 (2022).
French, K. E. Plant-based solutions to global livestock anthelmintic resistance. Ethnobiol. Lett. 9 (2), 110–123 (2018).
Daniel, E. Use of medicinal plants in the control of fish parasites and problems related to their use in ethnoveterinary treatment-A review. J. Istanbul Veterinary Sci. 8 (3), 247–272 (2024).
Orhan, C. et al. The effect of Cirsium arvense extract on antioxidant status in quail. Br. Poult. Sci. 54 (5), 620–626 (2013).
Ashmita, C. et al. Cirsium arvense: A multipotent weed. Ann. Biol. 36, 442–447 (2020).
Crișan, I. et al. Current trends for lavender (Lavandula angustifolia Mill.) crops and products with emphasis on essential oil quality. Plants 12 (2), 357 (2023).
de la Fuente, J., Artigas-Jerónimo, S. & Villar, M. Akirin/Subolesin regulatory mechanisms at host/tick–pathogen interactions. Microlife 3, uqab012 (2022).
Manzano-Román, R. et al. Subolesin/akirin orthologs from Ornithodoros spp. Soft ticks: cloning, RNAi gene Silencing and protective effect of the Recombinant proteins. Vet. Parasitol. 185 (2–4), 248–259 (2012).
Nandy, K. et al. Anti-tick vaccine candidate Subolesin is important for blood feeding and innate immune gene expression in soft ticks. PLoS Negl. Trop. Dis. 17 (11), e0011719 (2023).
Grabowski, J. M. & Hill, C. A. A roadmap for tick-borne flavivirus research in the omics era. Front. Cell. Infect. Microbiol. 7, 519 (2017).
Walker, A. R. Ticks of Domestic Animals in Africa: a Guide To Identification of SpeciesVol. 74 (Bioscience Reports Edinburgh, 2003).
Sharma, A. K. et al. Comparative in vitro antimicrobial and phytochemical evaluation of methanolic extract of root, stem and leaf of Jatropha curcas Linn. Pharmacognosy J. 4 (30), 34–40 (2012).
Ticks, F. Tick-Borne disease control. A practical field manual, 1(2): p. 11. (1984).
Team, R. RStudio: integrated development environment for R. RStudio, PBC, Boston, MA, Boston, MA. (2022).
Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research (No Title), 2019).
Finney, D. Probit analysis, Cambridge University Press. Cambridge, UK, (1971).
Hlina, B. L. et al. The relationship between thermal physiology and lampricide sensitivity in larval sea lamprey (Petromyzon marinus). J. Great Lakes Res. 47, S272–S284 (2021).
Wickham, H. & Wickham, H. Programming with ggplot2. Ggplot2: elegant graphics for data analysis, : pp. 241–253. (2016).
UniProt Consortium, T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46 (5), 2699–2699 (2018).
UniProt Consortium, T. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res., 51, D523–D531 (2023).
Arnold, K. et al. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22 (2), 195–201 (2006).
Laskowski, R., MacArthur, M. & Thornton, J. PROCHECK: validation of protein-structure coordinates. (2006).
Wiederstein, M. & Sippl, M. J. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 35 (suppl_2), W407–W410 (2007).
Colovos, C. & Yeates, T. O. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 2 (9), 1511–1519 (1993).
Tian, W. et al. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res. 46 (W1), W363–W367 (2018).
Kim, S. et al. PubChem substance and compound databases. Nucleic Acids Res. 44 (D1), D1202–D1213 (2016).
Trott, O. & Olson, A. J. AutoDock vina: improving the speed and accuracy of Docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31 (2), 455–461 (2010).
Suchodolski, J. et al. A crucial role for ergosterol in plasma membrane composition, localisation, and activity of Cdr1p and H+-ATPase in Candida albicans. Microorganisms 7 (10), 378 (2019).
Dadar, M. et al. Candida albicans-Biology, molecular characterization, pathogenicity, and advances in diagnosis and control–An update. Microb. Pathog. 117, 128–138 (2018).
El-Shamy, N. T. et al. DFT, ADMET and Molecular Docking Investigations for the Antimicrobial Activity of 6, 6′-Diamino-1, 1′, 3, 3′-tetramethyl-5, 5′-(4-chlorobenzylidene) bis [pyrimidine-2, 4 (1H, 3H)-dione]. Molecules, 27(3): p. 620. (2022).
Teng, Z. et al. Molecular detection of tick-borne bacterial and protozoan pathogens in Haemaphysalis longicornis (Acari: Ixodidae) ticks from free-ranging domestic sheep in Hebei province, China. Pathogens 12 (6), 763 (2023).
Ogden, N. H. & Lindsay, L. R. Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol. 32 (8), 646–656 (2016).
Marrana, M. Epidemiology of Disease Through the Interactions between Humans, Domestic Animals, and Wildlife, in One Healthp. 73–111 (Elsevier, 2022).
Mackenzie, J. S. et al. One Health: the human-animal-environment Interfaces in Emerging Infectious DiseasesVol. 366 (Springer, 2013).
Malak, N. et al. In Silico approaches to develop herbal acaricides against R.(Boophilus) Microplus and in vitro Anti-Tick activities of selected medicinal plants. Saudi J. Biol. Sci. 29 (6), 103302 (2022).
Khan, A. et al. Structure-based in Silico design and in vitro acaricidal activity assessment of Acacia nilotica and Psidium guajava extracts against sarcoptes scabiei var. Cuniculi. Parasitol. Res. 121 (10), 2901–2915 (2022).
Ayub, S. et al. Vitro and in Silico protocols for the assessment of Anti-Tick compounds from Pinus Roxburghii against Rhipicephalus (Boophilus) Microplus ticks. Animals 13 (8), 1388 (2023).
Jaenson, T. G., Garboui, S. & Pålsson, K. Repellency of oils of lemon eucalyptus, geranium, and lavender and the mosquito repellent MyggA natural to Ixodes ricinus (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 43 (4), 731–736 (2006).
Khan, Z. U. H., Khan, F. A. S. U. & Ali, I. Phytochemical study on the constituents from Cirsium arvense. Mediterranean J. Chem. 2, 64–69 (2011).
Ullah, S., Shakir, L. & Ullah, R. Morphological and phytochemical study of Cirsium arvense from district Mardan Pakistan. J. Bioinf. Biotechnol. Res. 1 (1), 1–7 (2023).
Anand, U. et al. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites 9 (11), 258 (2019).
Aulifa, D. L. et al. A comprehensive review: mesoporous silica nanoparticles greatly improve Pharmacological effectiveness of phytoconstituent in plant extracts. Pharmaceuticals 17 (12), 1684 (2024).
Aggarwal, G. et al. Traditional uses, phytochemical composition, Pharmacological properties, and the biodiscovery potential of the genus Cirsium. Chemistry 4 (4), 1161–1192 (2022).
Deng, W., Zhu, N. & Mo, J. In vitro bioassay methods for laboratory screening of novel mosquito repellents. Entomol. Sci. 17 (4), 365–370 (2014).
Broni, E. et al. Cheminformatics-based study identifies potential Ebola VP40 inhibitors. Int. J. Mol. Sci. 24 (7), 6298 (2023).
Aware, C. B. et al. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. South. Afr. J. Bot. 151, 512–528 (2022).
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/329/46.
Funding
Researchers Supporting Project number (RGP2/376/45) King Khalid University, Abha, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, original draft writing, reviewing, and editing: Muhammad Naveed, Nosheen Malak, Zakir Ullah, Nabi Amin. Formal analysis, investigations, funding acquisition, reviewing, and editing: Shakir Ullah, Imtiaz Ahmad, Muazzam Ali Khan, Mourad Ben Said, Hanène Belkahia. Resources, data validation, data curation, and supervision: Ioannis A. Giantsis, Youssouf Ali Younous, Mohammed H AL Mughram, Adil Khan.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naveed, M., Malak, N., Ullah, Z. et al. In vitro and in silico study and pharmacokinetic analysis of the acaricidal effectiveness of Cersium arvense extract against Rhipicephalus microplus. Sci Rep 15, 25717 (2025). https://doi.org/10.1038/s41598-025-11276-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11276-4