Abstract
Guava (Psidium guajava L.) leaves are deemed promising reservoir of phytoconstituents, with their characteristics potentially influenced by the timing of harvest and the dynamics of soil-plant interactions. The study revealed varying concentrations of minerals and vitamins in guava leaves, predominantly featuring vitamins B and C. Assessment of pigments using HPLC revealed that guava leaves collected in March had higher pigment concentration (461.233 mg/100 g) than that collected in August (447.084 mg/100 g). Quantification of total phenolics in guava leaves collected in March and August resulted in measurements of 435.21 ± 0.17 mgGAE/g and 294.31 ± 0.14 mgGAE/g, respectively. HPLC analysis demonstrated a diverse array of phenolic and flavonoid compounds present in Psidium guajava, with greater abundance and concentration of phenolic and flavonoid compounds in the samples harvested in March compared to those collected in August. For biological evaluation, guava leaves harvested in March demonstrated strong scavenging effect on DPPH and ABTS radicals, and considerable inhibition of carbohydrate-metabolizing enzymes (α-amylase, α-glucosidase, and β-galactosidase) in a dose-dependent manner. Furthermore, the March-collected guava leaves exhibited notable inhibition of COX-2 and 5-LOX enzyme activities, surpassing the effects of leaves collected in August. The study’s outcomes demonstrate richness of phytoconstituents in guava leaves, which underpin various biological functions, particularly during spring relative to the summer. This highlights the importance of the timing of collection in assessing phytochemical properties and their biological implications, highlighting the necessity of considering this aspect when sampling guava leaves.
Similar content being viewed by others
Introduction
Guava (Psidium guajava L.), a member of the Myrtaceae family, is extensively cultivated and valued as a significant fruit in tropical regions such as India, Indonesia, and South America. This traditional plant is esteemed for its wide-ranging medicinal and nutritional benefits. The Egyptian Bassateen El Sabahia guava holds significant value in Egypt for its health-promoting and nutritional properties. The leaves of this guava are utilized in the treatment of gastrointestinal disorders, such as diarrhea, as well as respiratory conditions, owing to their antimicrobial characteristics. The fruit itself, abundant in Vitamin C and antioxidants, enhances immune function and is commonly consumed fresh or incorporated into various products, including juices and jams1,2. Various parts of the guava tree, including its leaves, stems, and fruits, have been utilized in numerous countries for the treatment of ailments such as stomach pain, diabetes, diarrhea, and other health issues3. Psidium guajava leaves are characterized by their dark green hue and elliptical to oval shape, featuring an obtuse apex. These leaves have been employed in the management of numerous respiratory and gastrointestinal conditions4. Additionally, they are widely acknowledged for their properties as antispasmodics, cough suppressants, anti-inflammatories, and agents for managing diabetes5. Recent studies conducted on animal models have demonstrated the potential of P. guajava leaves as effective antitumor, anticancer, and cytotoxic substances6,7,8. The bioactive and therapeutic characteristics of P. guajava leaves are largely attributed to a distinctive array of bioactive polyphenolic compounds, including quercetin, flavonoids, and various phenolic acids such as ferulic, caffeic, and gallic acids9. A multitude of studies highlight the advantageous effects of integrating P. guajava leaf extract into food products as a functional ingredient, due to the presence of numerous compounds like rutin, naringenin, gallic acid, catechin, epicatechin, kaempferol, isoflavonoids, and vitamins, all of which are recognized for their positive impacts on human health10.
The fluctuations in primary metabolic processes in plants across different seasons are well documented11. The production and accumulation of secondary metabolites in plants influenced by the stages of cultivation. Throughout the various growth phases, numerous enzymes are involved in the biosynthetic pathways of phenolic compounds. Additionally, environmental factors significantly impact these processes. Factors such as soil characteristics and cultivation practices can modify the expression of various enzymes that participate in phenolic biosynthesis, thereby affecting the interactions with plant secondary metabolites. These enzymes are subject to complex regulatory controls and demonstrate specific responses to environmental changes, particularly those related to seasonal variations12.
The characteristics of soil, including its nutrient composition, play a crucial role in shaping the phytochemical profile of plants. Soils that are rich in organic matters and essential nutrients are associated with an increased production of beneficial phytoconstituents13. Thus, both the optimal timing for harvest and the quality of soil are vital for optimizing the beneficial effects of the plant. Ignoring these factors can lead to erroneous or inconsistent evaluations of the potency and composition of phytochemical extracts. Therefore, to ensure a commercially reliable output of P. guajava leaves with consistent high quality and functionality, the evaluation of functional constituents, along with antioxidant, antidiabetic, and anti-inflammatory activities, was performed, in relation to harvest timing and the soil-plant interaction.
Methodology
Chemicals and reagents
All chemicals utilized in this study were of analytical grade. Standards and solvents used in the study were sourced from Merck and Co., Inc., USA and Sigma-Aldrich. TLC plates (analytical and preparative), were purchased from Sigma Aldrich, Darmstadt, Germany. Additionally, 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) sourced from Aldrich Chemie in Germany, as well as 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) from Fluka Biochemika, Sigma-Aldrich, were employed. Additionally, the carbohydrate-metabolizing enzymes, which include purified α-amylase, α-glucosidase, and β-galactosidase, were obtained from Sigma Chemical Company in New York, USA.
Plant collection, authentication and extraction
Intensely green leaves of Psidium guajava “Egyptian Bassateen El Sabahia guava”, devoid of any diseases, insect infestations, or mechanical injuries, were harvested from a five-year-old tree located on a private farm in El-Salheya Elgdeda, Sharkia Governorate, Egypt. Permission and informed consent were obtained from the private farm owner before collecting the leaves of Psidium guajava for this study. The collection occurred just before the flowering phase in March and during the fruiting period in August 2024. During this timeframe, the environmental conditions were characterized by an average maximum temperature of 25 °C (77 °F) during the day and a minimum of 15 °C (59 °F) in the early mornings or evenings in spring. In August, daytime temperatures reached highs of 35 °C to 40 °C (95 °F to 104 °F), while nighttime lows ranged from 23 °C to 28 °C (73 °F to 82 °F). Precipitation levels varied from 0 to 0.6 mm, and the daily light saturation duration ranged between 8.45 and 10.30 h.
The taxonomical identification of the leaves was performed by Prof. Dr. Gamal Farag at the Horticulture Research Centre of the Ministry of Agriculture. A specimen was archived in the Herbarium of the National Research Centre in Egypt, where it has been assigned the registration number M259.
Leaves of P. guajava that were gathered underwent a gentle rinsing with distilled water to eliminate any dust particles. Following this, they were shade-dried and ground into a fine powder using a blender. To achieve a consistent particle size, the powder was subsequently passed through an aluminum sieve with a mesh size of 1 mm. The extraction of phenolic compounds was conducted in accordance with the methodology established by14. In summary, two separate batches of dried powdered P. guajava leaves, each weighing 350 g and collected in March and August, were extracted using a solvent mixture of ethanol and water in a 75:25 ratio (v/v) at room temperature, with a total volume of 3 L for six extractions. The extracts were then concentrated using a rotary evaporator (Heidolph, Germany) at a temperature of 50 °C, resulting in dark green residues weighing 46 g dry weight (13.4% W/V) and 43.5 g dry weight (12.43%W/V), respectively. The concentrated extracts were further defatted through partitioning with petroleum ether (300 ml, four times), yielding defatted residues of 7.3 g and 6.9 g dry weight, respectively. The remaining extracts were subjected to extraction with ethyl acetate, leading to the concentration of the phenolic-rich fractions, which provided dried residues of 19.5 g and 17.6 g dry weight, respectively, for subsequent phytochemical and biological evaluations.
Soil sampling
Soil type criteria were defined to evaluate the influence of soil composition on the phytoconstituents of dried P. guajava leaves15. The collection of soil samples was facilitated through the use of a soil auger and compositing soil samples followed. Soil samples were obtained from 0 to 150 cm depth range. The soil samples were collected, air dried, sealed, and stored in a polypropylene container in which was analysed for soil properties and other physical and chemical analyses after which the characteristic tests were conducted. Saturation soil paste extract analyses which covered most of the methods specified in the soil survey laboratory methods handbook (USDA, 2004) customs were made of the samples. These included soil texture, CaCO3 content, salinity EC, sodicity pH, soluble cations and anions, organic matter, and gypsum content analyses. Samples were first crushed and then examined through a set of sieves 2 mm mesh size first, then 0.5 mesh thereafter the powders were left to dry at room temperature while analysing the mid-infrared diffused reflected spectrum.
Soil samples were tested for TN, P and S, exchangeable cations (Ca, Mg, Na and K), soil micronutrients (Fe, Cu, Mn, Zn, and B), and cation exchange capacity (CEC). The Mehlich-3 multi-nutrient extraction method (1984) was also used to determine some of these parameters in soil samples. An Inductively coupled plasma (ICP) spectrometer was utilized to quantify the concentration of the extracted components. Using MIR-diffused reflectance spectral analysis, TAN and CEC were also measured.
Assessment of disease incidence
The determination of disease incidence in P. guajava leaves, collected in the months of March and August, was performed by employing the formula specified by Iqra et al.16: Disease Incidence (D.I.) = Number of diseased leaves / Total number of examined leaves × 100.
Phytochemical constituency
Macro and micro‑elements in P. guajava L. leaves
The nutritional composition of dried P. guajava leaves, collected in March and August, was assessed separately for both macro and microelements. The analysis encompassed macroelements such as Phosphorus, Potassium, Calcium, Sodium, and Magnesium, alongside microelements including Zinc, Copper, Iron, and Manganese. The concentrations of Potassium, Calcium, Sodium, and Magnesium, as well as the microelements, were measured using an atomic absorption spectrophotometer (Perkin Elmer, 1100B), adhering to the methodology proposed by Aboulthana et al.17. Furthermore, the Phosphorus content was evaluated using a spectrophotometer (Perkin-Elmer Lambda 2) in accordance with the vanado-molybdate method established by Jackson18.
Vitamins contents in P. guajava leaves
The quantification of vitamins in dried P. guajava leaves, collected during the months of March and August, was conducted utilizing high-performance liquid chromatography (HPLC) with a Shimadzu-UFLC Prominence system. This system featured an ultraviolet-visible (UV-Vis) detector (Model-SPD 20 A), in accordance with the methodology established by Hasan et al.19. The analysis of the results was performed following the protocol detailed by Aboulthana et al.20.
HPLC analysis of natural pigments
The HPLC analysis procedures used to determine the levels of chlorophyll a, b, and β-carotene in the ethyl acetate fraction of dried P. guajava leaves, which were collected in March and August, were carried out according to the guidelines set by Siong and Lam21.
Quantifiable valuation of total phenolics
The total polyphenol concentration in the ethyl acetate fraction of dried P. guajava leaves, collected in March and August, was quantified as mg of gallic acid per 100 g. This was achieved using the Folin-Ciocalteu reagent, based on the method described by El-Feky et al.,22. The results were presented as equivalents of gallic acid.
Quantifiable valuation of total flavones and flavonols
The total flavones and flavonols in the ethyl acetate extract of dried P. guajava leaves, collected in March and August, were quantified as described by Bahloul et al.23 using 2% AlCl3-ethanol solution. Absorbance readings were taken at 420 nm, and the results were presented in terms of mg rutin equivalent.
Quantifiable valuation of total flavanones and dihydroflavonols
The total content of flavanones and dihydroflavonols in the ethyl acetate extract of dried P. guajava leaves, collected in March and August, was evaluated following the method outlined by Bahloul et al.23. The assessment utilized a 2,4-dinitrophenylhydrazine solution, and absorbance was measured at 486 nm, with results expressed as mg of naringenin equivalent.
HPLC identification of phenolics and flavonoids
High-performance liquid chromatography (HPLC) analysis was performed on the ethyl acetate extract of dried P. guajava leaves collected in March and August at the Central Labs in NRC, Cairo, Egypt. An Agilent 1260 series instrument equipped with a C18 column (4.6 mm x 250 mm i.d.) was used for the analysis. The mobile phase was a mixture of water (A) and 0.05% trifluoroacetic acid in acetonitrile (B), flowing at a rate of 0.9 ml/min. A linear gradient program was set with the following parameters: 0 min (82% A); 0–5 min (80% A); 5–8 min (60% A); 8–12 min (60% A); 12–15 min (82% A); 15–16 min (82% A); and 16–20 min (82% A). The analysis utilized a multi-wavelength detector set to 254, 280, 320, and 360 nm. The identification of phenolic compounds was accomplished by comparing their retention times with those of standard phenolics and flavonoids24.
Isolation of major flavonoids
The extracts from dried P. guajava leaves, collected in the months of March and August, were each processed through Preparative TLC chromatography on an Aluminum TLC plate coated with silica gel 60G and a fluorescent indicator F254 (Merck, dimensions 20 × 20 cm, thickness 250 μm). The chromatographic development employed a solvent system comprising ethyl acetate, dichloromethane, formic acid, acetic acid, and water in a ratio of 100:10:10:10:11 (v/v/v/v/v)25. The chromatograms, once developed and dried, were subjected to scanning in fluorescence mode at a wavelength of 365 nm, both before and after treatment with a 1% ethanolic solution of AlCl3. Bands that indicated a positive reaction to the spraying reagent were collected individually. The purification process continued on thin-layer chromatography (TLC) plates, employing a solvent system of methanol, chloroform, and hexane in a 7:2:1 (v/v/v) ratio, and the retention factor (Rf) values for the distinct bands were subsequently determined26. Flavonoid-containing bands were carefully excised with surgical blades, dissolved in ethanol, and subsequently filtered using Whatman No. 42 filter paper. The structural elucidation of the isolated compounds was conducted through Nuclear Magnetic Resonance (NMR) spectroscopy using JEOL instruments at frequencies of 270 MHz, 400 MHz, and 125 MHz for the respective analyses of 1H-NMR and 13C-NMR.
Biological investigation
Free radical scavenging assay
Two distinct antioxidant assays were performed to evaluate the radical scavenging potential of the ethyl acetate fraction obtained from dried P. guajava leaves collected in March and August. The assays involved the scavenging of DPPH and ABTS radicals at various concentrations (10, 50, 100 µg), following the methods suggested by Rahman et al.27. Ascorbic acid served as the reference standard.
In vitro anti-diabetic activity
The anti-diabetic effects of the ethyl acetate fraction from dried P. guajava leaves, collected in March and August, were compared in vitro. This comparison involved measuring the inhibition of carbohydrate hydrolyzing enzymes, including α-amylase, α-glucosidase, and β-galactosidase, as detailed in Naguib et al.28.
In vitro antiinflammatory evaluation
The anti-inflammatory effects of the ethyl acetate fraction of dried P. guajava leaves, collected in March and August, were evaluated in vitro using different concentrations (1,10,100 µg). This evaluation involved measuring the inhibition of COX-2 using Inhibitor Screening Kits (Milpitas, CA, USA), with absorbance readings taken at UV-410 nm alongside a blank. Indomethacin was used as a reference drug for this assay. Furthermore, an inhibition assay was performed with the 5-LOX enzyme (human recombinant) using a 5-LOX kit (Sigma-Aldrich), and results were compared to those obtained with Zileuton as a reference drug. Absorbance was measured at UV-490 nm against a blank. The assay methodology was based on the work of El-Feky and El-Rashedy29. IC50 values were derived from the inhibition curves using the sigmoid dose-response model and linear regression analysis in Microsoft Office Excel 2010.
Statistical analyses
All experiments were conducted three times, and the data is presented as mean ± standard deviation. Statistical analysis was carried out using one-way analysis of variance (one-way ANOVA) with the Statistical Package for Social Sciences (SPSS for Windows, version 11.0).
Results and discussion
Soil characterization
In the area studied, texture and CEC are vital components wherein crop could acquire information on nutrients and water in the root zone. Results represented further Depict these features of the soil sample that had been examined. Moreover, the presence of soil texture is important in maintaining soil moisture, which is helpful during the growing period of crop and also in connection with the existing irrigation practices. However, the soil is homogenous in area and from the surface and subsurface layers and/or horizons30. The physical and chemical properties of the soil were conducted, and the findings are detailed in Tables 1 and 2. Soil pH, value of 7.9. Therefore, it is considered neutral to moderately alkaline. There were no appreciable variations between horizons. The pH of the soil is 2.1 dS/m. Percent organic matter is 0.91%. Calcium carbonate is 1.5%. Alluvial deposit-growing soils contain very little. Mainly, the dominant soil textural types were clay loam in a relatively uniform pattern. Pedological analyses showed that agricultural and sustainable crop production in this region is ideal. For that reason, the water and minerals supplying to crops was considered acceptable; deep soils and good drainage offer the ability to have crops with good root development, thus a positive growth condition. Total N in soil comprises inorganic and organic nitrogen, the latter dominating. Plants, however, only utilize the inorganic fraction of N. This then implies that optimum TN could not be regarded as a guarantee with respect to plant productivity. Low levels of TN might be due to reduced soil OC content, insufficient and unbalanced fertilizer use, intensive cropping systems, and N losses through leaching15,30. Moreover, nutrient mining and low OM applications which were frequent in the studied area, could lead to a decline in TN level. High variability in P concentration might result in variation of soil management practices31,32,33,34,35,36. The obtained value showed a K deficiency. Hence, K fertilizer application should be implemented. The basic cations on the exchange site. The result showed in the order of Ca > Mg > K.
Disease incidence
The incidence of disease in P. guajava leaves harvested in March was 16.43 ± 1.25, compared to 21.37 ± 1.16 in August. This shows that P. guajava leaves are less affected by disease before flowering than they are during the fruiting stage.
Macro and micro‑elements of P. guajava leaves
The macro and micro-element contents of dried P. guajava leaves collected at two different harvest times are shown in Table 3. There were variable concentrations of minerals in P. guajava L. leaves collected in March and August. The nitrogen content in dried P. guajava leaves from March and August was found to be notable, at 2.19% and 1.97%, respectively. Similar findings regarding nitrogen content in dried P. guajava leaves were reported by Tomar et al.37.
The levels of potassium and calcium in dried P. guajava leaves were found to be adequate. Specifically, the potassium concentration in leaves harvested in March and August was recorded at 1.96% and 1.38%, respectively. The potassium levels in the majority of dried P. guajava leaves fell within the optimal range. These findings are consistent with those reported by Tomar et al.37. Furthermore, the calcium content in P. guajava leaves collected during the same months was measured at 1.87% and 1.65%, respectively, with calcium being the most abundant mineral present. This suggests that guava leaves may serve as a beneficial dietary supplement for addressing calcium deficiencies, as noted by Gurusamy et al.38.
The magnesium levels in P. guajava leaves gathered in March and August were found to be moderate, measuring 0.53% and 0.47%, respectively. Additionally, phosphorus and sodium were present in reasonable quantities. Specifically, the phosphorus concentrations in the P. guajava leaves collected during these months were 0.31% for March and 0.27% for August, aligning with the findings of Singh and Singh39. In terms of sodium, the levels recorded were 0.24% in March and 0.19% in August.
In terms of micro-elements analysis, the iron levels in P. guajava leaves collected in March and August were determined to be 72.10 ppm and 56.13 ppm, respectively. The majority of these leaves contained iron concentrations that fell within the optimal range of 50–120 ppm, corroborating the findings of Parihar et al.40. Furthermore, Manganese content in dried P. guajava leaves for the same months was recorded at 43.62 ppm and 41.05 ppm, which aligns with the optimal range reported by Kotur et al.41. The copper levels in P. guajava leaves collected in March and August were found to be 12.50 ppm and 10.63 ppm, consistent with the observations of Singh and Singh39, who noted copper levels between 4.0 and 14.0 ppm dried P. guajava leaves. Lastly, the zinc concentrations in P. guajava leaves for March and August were the lowest at 9.76 ppm and 8.54 ppm, respectively, indicating a deficiency in zinc as highlighted by Tomar et al.37.
The study’s findings affirm that guava leaves are an abundant source of macro and micro-elements, positioning them as a highly beneficial option for human nutrition and as an effective animal feed to address micronutrient deficiencies42. The calcium-rich nature of dried P. guajava leaves may assist in reducing the likelihood of deficiency-related ailments such as hypocalcemia and osteoporosis43. A daily consumption of 100 g of dried guava leaves, whether prepared as tea or taken in capsule form, can yield 34.85%, 79.34%, and 46.11% of the recommended daily intake for potassium, iron, and zinc, respectively, which is greater than the amounts provided by the fruit44.
Another notice that the levels of macro and microelements in dried P. guajava leaves exhibit considerable variation, which can be attributed to the physicochemical characteristics of the soil, the fertility status, and various agricultural practices, resulting in differing nutrient profiles across plantations45. Nitrogen and potassium, due to their mobile nature, are translocated from older leaves to the meristematic tissues, with peak levels observed in March, followed by a decline from April until the fruit harvesting period.
The observed spike in foliar nitrogen and potassium levels in March may be attributed to the emergence of new leaves and the mobilization of nutrients from storage tissues to active growth sites, with 63–77% of the nitrogen required for spring growth and flowering sourced from the tree’s reserves. Developing fruits act as sinks for nitrogen, phosphorus, and potassium, leading to the mobilization of these nutrients from the leaves to the fruits, which explains the decreasing trend of these elements during August and September46. Consequently, it is recommended that P. guajava leaves be collected prior to flowering in March to ensure a high mineral concentration.
Vitamins contents in dried P. guajava leaves
The vitamin content in dried P. guajava leaves, collected during two different times of the year, is presented in Table 4. The results demonstrated varying concentrations of vitamins B and C in P. guajava L. leaves harvested in March compared to those collected in August, with the March samples exhibiting superior concentrations than those from August. This evidence underscores the notion that the seasonal timing of plant harvesting can significantly influence the nutritional quality of the leaves.
In the current study, the concentrations of vitamins B1, B2, B3, and B6 in dried P. guajava leaves collected in March were found to be 18.24, 29.43, 13.65, and 9.76 mg/100 g, respectively, surpassing the levels recorded in August, which were 15.07, 24.11, 9.63, and 7.85 mg/100 g. Furthermore, the Vitamin C concentration in the March samples was notably high at 96.42 mg/100 g, compared to 87.15 mg/100 g in August. These results are consistent with the findings of Thomas et al.47. The data obtained from this investigation indicate that guava leaves represent a significant source of vitamins B and C, thereby validating their diverse therapeutic uses in folk medicine, as highlighted by Pandian and Jayalakshmi48. It has been established that vitamin B is crucial for enhancing blood circulation, facilitating nerve relaxation, and stimulating cognitive functions, while the higher vitamin C content in dried P. guajava leaves may contribute to immune system enhancement and the maintenance of healthy blood vessels43.
HPLC analysis of natural pigments
The pigment profile of the ethyl acetate fraction from dried P. guajava leaves collected in March and August was evaluated through HPLC. The values for Chlorophyll a, b, β-Carotene, and the total identified pigments are summarized in Table 5. The analysis demonstrated that the dried P. guajava leaves which collected in March had a higher pigment content (461.233 mg/100 g) compared to those collected in August (447.084 mg/100 g). In March, the concentrations of chlorophyll a, b, and β-Carotene were found to be 167.039, 293.251, and 0.943 mg/100 g, respectively, whereas the August samples showed chlorophyll a, b, and β-Carotene levels of 159.264, 287.034, and 0.786 mg/100 g.
The fluctuations in the abundance and composition of pigments were influenced by the timing of the harvest. Gaining insight into the temporal variations in phytochemical composition is essential for elucidating the regulatory mechanisms involved and ultimately enhancing the quality of phytochemical products.
Quantifiable valuation of total phenolics and flavonoids
The assessment of total phenolics and flavonoids in the ethyl acetate fraction of dried P. guajava leaves, gathered in March and August, was performed, with the comprehensive results illustrated in Fig. 1. The total phenolic content in the ethyl acetate fraction of dried P. guajava leaves collected in March and August was found to be 435.21 ± 0.17 mg GAE/g and 294.31 ± 0.14 mg GAE/g, respectively. The analysis also indicated that the flavones and flavonols were present at concentrations of 2986.13 ± 0.17 mg rutin equivalent/100 g fraction and 2651.40 ± 0.14 mg rutin equivalent/100 g fraction, for the months of March and August, respectively. However, the concentrations of flavanones and dihydroflavonols were significantly lower, recorded at 2135.17 ± 0.12 mg naringenin equivalent/100 g fraction and 2018.07 ± 0.10 mg naringenin equivalent/100 g fraction, respectively, for the months of March and August.
The results of this study substantiate the notable concentration of total phenolics and flavonoids in dried P. guajava leaves, which underpins various biological functions, especially during the spring season prior to flowering in March, as compared to their levels during the fruiting phase in August. These findings align with the research conducted by Qian and Nihorimbere49.
The observed variation in phenolic content in the ethyl acetate fraction from dried P. guajava leaves, which collected in March and August may be linked to climatic conditions. The high temperatures prevalent in August could potentially lead to the degradation and decomposition of certain thermolabile phenolic and flavonoid compounds. Phenolic compounds, which exhibit a range of chemical structures, typically contain at least one aromatic ring, with one or more hydrogen atoms replaced by hydroxyl groups. These compounds are recognized for their diverse biological effects, which include anti-inflammatory, antimicrobial, hypolipidemic, anticancer activities, as well as antioxidant properties50,51.
Phenolic and flavonoids identification by HPLC
Phenolic and flavonoid compounds are pivotal bioactive constituents that contribute significantly to the management of various metabolic and physiological functions in the human body52. The increasing interest in bioactive compound-rich diets in recent years can be attributed to their potential to decrease the risk of numerous chronic diseases53,54. The impact of agronomic and environmental conditions on the levels of phenolic compounds in plants and their bioactivities has been extensively discussed in the literature55,56. As a result, this evaluation seeks to explore the variability of phenolic and flavonoid compounds in P. guajava leaves across different harvesting times.
The HPLC analysis conducted on the ethyl acetate fractions of dried P. guajava leaves collected in March and August demonstrated a diverse array of phenolic and flavonoid compounds present during both harvesting periods. Specifically, 15 distinct compounds were identified in the March samples, whereas 3 compounds were detected in the August samples. Comprehensive results are available in the accompanying Table 6.
Among the compounds detected in the ethyl acetate fraction of P. guajava leaves gathered in March, syringic acid (0.43 mg/g) and methyl gallate (0.32 mg/g) were identified as the main phenolic acids. The analysis also indicated that the predominant flavonoids in this fraction included rutin (0.94 mg/g), quercetin (0.65 mg/g), and apigenin (0.78 mg/g). Conversely, the samples collected in August showed the presence of methyl gallate (0.29 mg/g), rutin (0.73 mg/g), and apigenin (1.76 mg/g).
The findings indicate a greater abundance and concentration of phenolic and flavonoid compounds in dried P. guajava leaves which harvested in March compared to those collected in August. This variation highlights the significant impact of harvest timing on the levels of phytoconstituents, as demonstrated by the fluctuations in phenolic and flavonoid concentrations over time57,58.
The findings are consistent with those of Díaz-de-Cerio et al.59, who reported that quercetin constitutes a key bioactive phenolic compound in P. guajava leaves. Additionally, Wang et al.60 highlighted the presence of various other compounds, including gallic acid, rutin, chlorogenic acid, avicularin, isoquercitrin, and kaempferol. In contrast, another study revealed that catechin (2.25%) and epicatechin (1.45%) were found in greater concentrations, whereas lower amounts of gallic acid, chlorogenic acid, quercetin, caffeic acid, and epigallocatechin gallate were detected in P. guajava leaf extract61. The HPLC chromatograms for the ethyl acetate fraction of P. guajava leaves, obtained in both March and August, are illustrated in Fig. 2.
Characterization of the isolated compounds
The thin-layer chromatographic investigation of extracts from dried P. guajava leaves, collected in March and August, led to the identification of six compounds (1–6). The compounds quercetin (1), gallocatechin (2), and kaempferol 7-O-glucoside (3) were identified by comparing their Co-TLC profiles with those of known authentic standards. The structural elucidation of morin (4), quercetin 3-O-arabinoside (5), and morin-3-O-araboinoside (6) was achieved using 1H-NMR and 13C-NMR techniques. The characterization of compounds 4, 5, and 6 is described as follow:
Compound (4); Morin was obtained as a yellow powder of melting point 300 °C, 31 mg, Rf=0.79, 1H-NMR (400 MHz, CD3OD-d4): 6.41 (1H, d, J=2.7 Hz, H-6), 6.23 (1H, d, J=2.7 Hz, H-8), 7.12 (1H, s, H-3’), 6.85 (1H, d, J=7.6 Hz, H-5’), 7.60 (1H, d, J=7.6 Hz, H-6’). 13C-NMR (125 MHz, CD3OD-d4): 155.32 (C-2), 133.47 (C-3), 177.81 (C-4), 161.96 (C-5), 97.95 (C-6), 164.98 (C-7), 92.87 (C-8), 157.47 (C-9), 104.86 (C-10), 122.11 (C-1’), 145.61 (C-2’), 115.37 (C-3’), 149.10 (C-4’), 114.96 (C-5’), 121.65 (C-6’). The isolated compound, which had been identified earlier, demonstrated results that were consistent with the documentation provided by Tachakittirungrod et al.62.
Compound (5); Quercetin 3-O-arabinoside was isolated as yellow needles, 24.5 mg, m.p. 255ͦ C; Rf=0.72, 1H-NMR (400 MHz, CD3OD-d4): 6.42 (1H, d, J=2.4 Hz, H-6), 6.20 (1H, d, J=2.4 Hz, H-8), 7.69 (1H, s, H-2`), 6.73 (1H, d, J=7.9 Hz, H-5`), 7.61 (1H, d, J=7.9 Hz, H-6`), 5.23 (1H, d, J=7.6 Hz, H-1``), 3.76 (1H, d, J=8.7 Hz, H-2``), 3.59 (1H, d, J=2.9 Hz, H-3``), 3.77 (1H, m, H-4``), 3.90 (2 H, m, H-5``); 13C-NMR (125 MHz, CD3OD-d4): 162.35 (C-2), 135.47 (C-3), 181.36 (C-4), 159.03 (C-5), 100.21 (C-6),162.48 (C-7), 94.14 (C-8), 105.35 (C-9), 158.46 (C-10), 121.85 (C-1`), 114.71 (C-2`), 143.93 (C-3`), 151.24 (C-4`), 117.26 (C-5`), 122.43 (C-6`), 104.58 (C-1``), 72.84 (C-2``), 72.05 (C-3``), 69.02 (C-4``), 66.11 (C-5``). The findings associated with the previously identified isolated compound were in agreement with those outlined in the literature by Metwally et al., 63.
Compound (6); Morin-3-O-arabinoside was isolated as yellow amorphous powder, 27 mg, m.p. 260 ͦ C; Rf= 68, 1H-NMR (400 MHz, CD3OD-d4): 6.39 (1H, d, J=2.9 Hz, H-6), 6.18 (1H, d, J=2.9 Hz, H-8), 7.09 (1H, s,H-3`), 6.81 (1H, d, J=8.4 Hz, H-5`), 7.57 (1H, d, J=8.4 Hz, H-6`), 5.19 (1H, d, J=7.8 Hz, H-1``), 3.90 (1H, d, J=9.2 Hz, H-2``), 3.64 (1H, d, J=3.4 Hz, H-3``), 3.79 (1H, m, H-4``), 3.84 (2 H, m, H-5``). 13C-NMR (125 MHz, CD3OD-d4): 158.41 (C-2), 133.59 (C-3), 178.74 (C-4), 162.43 (C-5), 98.67 (C-6), 165.48 (C-7), 93.06 (C-8), 158.36 (C-9), 105.14 (C-10), 122.15(C-1’), 146.32 (C-2’), 115.36 (C-3’), 149.52 (C-4’), 115.01 (C-5’), 122.03 (C-6’), 102.97 (C-1``), 72.85 (C-2``), 71.64 (C-3``), 68.43 (C-4``), 65.81 (C-5``). The isolated compound, identified in prior research, showed results that were consistent with the findings reported by Arima and Danno 64.
The compounds quercetin (3,3′,4′,5,7-Pentahydroxy flavone) and gallocatechin (Flavan-3,3′,4′,5,5′,7-hexol) were previously detected in the leaves of Psidium guajava, as reported by Sampath et al.65. Moreover, Kaempherol 7-O-glucoside was isolated by Batubara et al.66. Quercetin has been documented to exhibit antioxidant, anti-inflammatory, and anti-allergic effects67. Gallocatechin is recognized for its antioxidant and anti-cancer properties68. Furthermore, kaempherol 7-O-glucoside has also been associated with antioxidant and anti-inflammatory activities69.
Upon examining the two extracts from dried P. guajava leaves, it was determined that gallocatechin (2) and kaempferol 7-O-glucoside (3) were present in the ethyl acetate extract from samples collected in both March and August. However, the compounds quercetin (1), morin (4), quercetin 3-O-arabinoside (5), and morin-3-O-arabinoside (6) were identified solely in the extract from the leaves collected in March.
Biological investigation
The dried leaves of P. guajava contain phytochemical constituents that exhibit diverse biological activities, including antioxidant, hypoglycemic, and anticancer effects, among others43. Notably, phenolic and flavonoid compounds are pivotal in regulating various physiological functions, such as cell proliferation, enzymatic processes, cellular redox balance, and signal transduction pathways, thereby playing a significant role in the prevention of chronic diseases52,70.
-
1.
Free radical scavenging activity
The production of free radicals during metabolic processes is a critical factor in the emergence of various health problems within the human body. These problems range from inflammatory diseases and ischemic disorders to neurological conditions, hemochromatosis, emphysema, acquired immunodeficiency syndrome, and several other medical issues71.
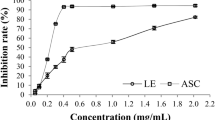
A comparative analysis of the free radical scavenging activity of the ethyl acetate fraction from dried P. guajava leaves collected in March and August was performed against DPPH and ABTS radicals at serial concentrations of 10, 50, and 100 µg, as shown in Table 7. The findings revealed that the ethyl acetate fraction from the March collection demonstrated enhanced scavenging capabilities, with recorded values of 69.84 ± 0.20 and 72.16 ± 0.06 for DPPH and ABTS radicals, respectively, at 10 µg/ml. while, at concentration of 50 µg/ml, the values rose to 78.53 ± 0.12 and 78.96 ± 0.13, and at 100 µg/ml, they reached 87.30 ± 0.21 and 83.12 ± 0.09. These results were markedly higher than those obtained from the August collection. The observed concentration-dependent effects are attributed to the higher concentrations of phenolic and flavonoid compounds found in the leaves harvested during the spring.
The antioxidant properties of dried P. guajava leaves can be attributed to the presence of various phenolic compounds, including chlorogenic acid, caffeic acid, gallic acid, protocatechuic acid, and ferulic acid, among others9. In addition to flavonoids such as rutin, quercetin, apigenin, and kaempferol72. Numerous studies have highlighted the antioxidant, anticancer, antidiabetic, and anti-inflammatory properties of flavonoids29,73. The effectiveness of flavonoids is closely linked to their molecular structure, particularly the number and positioning of hydroxyl groups, as well as the conjugation and resonance characteristics74,75.
The role of antioxidant compounds found in P. guajava leaves in mitigating the adverse effects of free radicals has been substantiated by a variety of research studies. Chen and Yen5 identified a linear relationship between the potency of antioxidants, their capacity to neutralize free radicals, and the phenolic content in P. guajava leaves. In addition, a subsequent study demonstrated the synthesis of silver nanoparticles from crude polysaccharides of P. guajava leaves, which exhibited remarkable scavenging activities against DPPH radicals and ABTS radical cations60. These results indicate that P. guajava leaf extracts could be valuable as antioxidant resources in both food preservation and cosmetic applications.
Values are represented by mean ± SD of three replicate. The different superscript letters between groups are significant differences at P values < 0.05.
In vitro antidiabetic activity
Diabetes represents a significant chronic health issue, affecting approximately 10% of the global population with a disorder of blood glucose metabolism, primarily marked by a state of hyperglycemia. This condition can arise from either a deficiency in insulin production by the β-cells of the pancreatic islets, as seen in type 1 diabetes, or from the cells’ failure to respond adequately to the insulin that is secreted, characteristic of type 2 diabetes76. The sustained hyperglycemic state results in overproduction of reactive oxygen species (ROS) and dyslipidemia, leading to significant cellular damage and a range of complications77.
The in vitro assessment of the antidiabetic activity of the ethyl acetate fraction from dried P. guajava leaves was conducted using three varying concentrations (10, 50, and 100 µg/mL). The results, detailed in Table 8, reveal that the ethyl acetate extract demonstrated significant inhibitory activity against carbohydrate-metabolizing enzymes, including α-amylase, α-glucosidase, and β-galactosidase, in a dose-dependent manner. Notably, the antidiabetic effects were found to be more substantial in dried P. guajava leaves which harvested in March than in those collected in August. This observation highlights the critical role of harvesting time in influencing the phytochemical profiles and biological properties of P. guajava leaves.
The bioactive constituents found in dried P. guajava leaves have demonstrated efficacy in mitigating the risk of diabetes. Several studies have documented the antidiabetic effects of flavonoids derived from P. guajava leaves, reporting substantial improvements in the functionality of β-cells in pancreatic islets and the morphology of hepatocytes in diabetic mice78,79. Furthermore, research focusing on the hypoglycemic properties of dried P. guajava leaf extract has indicated that its phenolic compounds can enhance vascular function in mice with obesity induced by diet80.
A different study assessing the food-drug interactions of P. guajava leaf tea found no potential interactions with medications, thereby supporting the conclusion that guava leaf tea is safe regarding food-drug interactions. Individuals with borderline diabetes often consume guava leaf tea to control the swift rise in blood sugar levels post-meal, as the tea is rich in carbohydrates and dietary polyphenols that bind to digestive enzymes, which are believed to promote health by limiting the absorption of dietary sugars and lipids81. Furthermore, a recent analysis of herbal teas also confirmed that guava tea does not exhibit any interactions with pharmaceuticals82.
In vitro antiinflammatory evaluation
Cyclooxygenase-2 (COX-2) and lipoxygenase (5-LOX) are recognized as significant pro-inflammatory enzymes that play a crucial role in the metabolism of arachidonic acid83. In recent years, there has been considerable interest among researchers in developing selective inhibitors for COX-2 and 5-LOX, aiming to mitigate the adverse effects associated with non-steroidal anti-inflammatory drugs (NSAIDs) and to explore novel therapeutic strategies for various conditions, including cancer and chronic inflammatory diseases84. This study aims to identify a dual inhibitor of COX-2 and 5-LOX by examining the anti-cyclooxygenase and anti-lipoxygenase activities of dried P. guajava leaves through an enzymatic assay. The results, including percentage inhibition and IC50 values for two ethyl acetate fractions of dried P. guajava leaves which collected in March and August, alongside reference drugs, are detailed in Table 9.
It was found that the ethyl acetate fraction from dried P. guajava leaves which collected in March showed a superior inhibition of COX-2 enzyme activity (Inhibition 78.34 ± 0.17%; IC50 3.95 ± 0.14 µg/mL) and 5-LOX enzyme activity (Inhibition 68.11 ± 0.12%; IC50 6.23 ± 0.10 µg/mL) relative to P. guajava extract gathered in August. This was evaluated against the standard medications Indomethacin and Zileuton.
The anti-inflammatory properties of dried P. guajava leaves can be attributed to their significant flavonoid content. Flavonoids are characterized by a benzene ring (A) that is fused with a six-membered ring (C) and features a phenyl ring (B) at the 2-position85. Research indicates that these compounds exhibit anti-inflammatory effects during both the proliferative and exudative stages of inflammation by inhibiting various enzymes, including nitric oxide synthase, xanthine oxidase, lipoxygenase (LOX), and cyclooxygenase (COX)86. The proposed mechanism of action involves the formation of hydrogen bonds with the active site of COX enzymes, as well as π–π interactions between the phenyl ring of the flavonoid and the Tyr355 residue at the entrance of the COX binding site. This binding is further enhanced by hydrophobic interactions within the hydrophobic and lobby regions of the COX enzyme’s binding site. In the case of 5-LOX inhibition, the inhibitory effect is attributed to hydrogen bonding with the Ala424 residue87.
Data are mean ± SD of 3 replicates, different superscript letters between groups are significant differences at P values < 0.05.
Conclusion
Psidium guajava leaves are deemed a promising reservoir of natural compounds that are easily accessible. Their significant levels of minerals and vitamins encourage their use as a direct source of nutrition. Furthermore, P. guajava leaves contain a variety of secondary metabolites, including flavonoids and other phenolic compounds. These phytochemicals have the potential to enhance and stabilize various physiological and metabolic processes within the human body, serving as important immune stimulators and modulators for chronic conditions such as diabetes, cancer, and diseases affecting the gastrointestinal, neurodegenerative, and cardiovascular systems.
The investigation of phyto-constituents in P. guajava leaves revealed significant differences in their composition and concentration, which were influenced by the timing of harvest and the relationship between soil and plant. Specifically, P. guajava leaves harvested in March exhibited higher phytoconstituents concentrations than those collected in August. These results highlight the importance of harvest timing in assessing phytochemical content and biological effects, indicating that this variable must be taken into account during the sampling of P. guajava leaves. Additionally, careful monitoring of soil nutrient deficiencies is vital for optimizing P. guajava yield.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Gutiérrez, R. M. P., Mitchell, S. & Solis, R. V. Psidium guajava: A review of its traditional uses, phytochemistry and Pharmacology. J. Ethnopharmacol. 117, 1–27 (2008).
Hendrarti, W., Robiansyah, M., Nur, S., Amin, A. & Abidin, H. L. Potential of ethanol extract of guava (psidium Guajava L.) leaves as adjuvant-antibiotic on salmonella typhi characterized. Bull. Pharm. Sci. Assiut Univ. 46 (2), 1259–1268 (2023).
Oh, W. K. et al. Antidiabetic effects of extracts from Psidium guajava. J. Ethnopharmacol. 96, 411–415 (2005).
Laily, N., Kusumaningtyas, R. W., Sukarti, I. & Rini, M. R. D.K. The potency of guava Psidium guajava (L.) leaves as a functional immunostimulatory ingredient. Procedia Chem. 14, 301–307 (2015).
Chen, H. Y. & Yen, G. C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium Guajava L.) leaves. Food Chem. 101, 686–694 (2007).
Ashraf, A. et al. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm. Biol. 54, 1971–1981 (2016).
Jiang, L., Lu, J., Qin, Y., Jiang, W. & Wang, Y. Antitumor effect of guava leaves on lung cancer: A network Pharmacology study.arab. J. Chem. 13, 7773–7797 (2020).
Lok, B. et al. The anticancer potential of Psidium guajava (Guava) extracts. Life 13 (2), 346 (2023).
Farag, R. S., Abdel-Latif, M. S., Abd El Baky, H. H. & Tawfeek, L. S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 28, e00536 (2020).
Shaheena, S., Chintagunta, A. D., Dirisala, V. R. & Kumar N.S.S. Extraction of bioactive compounds from Psidium guajava and their application in dentistry. AMB Express. 9, 1–9 (2019).
Farre´, E. M. & Weise, S. E. The interactions between the cir Cadian clock and primary metabolism. Curr. Opin. Plant. Biol. 15, 293–300 (2012).
Rutkowska, M., Balcerczak, E., ´ Swiechowski, R., Dubicka, M. & Olszewska, M. A. Seasonal variation in phenylpropanoid biosynthe Sis and in vitro antioxidant activity of Sorbus domestica leaves: harvesting time optimisation for medicinal application. Ind. Crops Prod. 156, 112858 (2020).
Ryu, D. H., Cho, J. Y., Yang, S. H. & Kim, H. Y. Effects of harvest timing on phytochemical composition in Lamiaceae plants under an environment-controlled system. Antioxidants 12 (11), 1909 (2023).
Amal, M., El–Feky, Nadia, A. & Mohammed Potential antioxidant and cytotoxic impacts of defatted extract rich in flavonoids from Styphnolobium japonicum leaves growing in Egypt. Sci. Rep. 14, 18690 (2024).
AbdelRahman, M. A. E. & Metwaly, M. M. Digital soil characteristics mapping for aiding site-specific management practices in the West nile delta, Egypt. Discov Sustain. 4, 47. https://doi.org/10.1007/s43621-023-00162-6 (2023).
Iqra, H. I., Khan Qadri, W., Amrao, R. & Ijaz, L. S. Effect of environmental conditions (temperature and precipitation) on severity of guava die-back caused by Colletotrichum spp. Under Climatic conditions of Pakistan. J. Plant. Pathol., 1–2 (2022).
Aboulthana, W. M. et al. Phyto-and biochemical study on cape gooseberry (physalis Peruviana L.) extract incorporated with metal nanoparticles against hepatic injury induced in rats. Nat. Prod. Res. 1, 1–4 (2024).
Jackson, M. L. Soil Chemical Analysis (Prentice Hall of India Pvt. Ltd., 1973).
Hasan, M., Akhtaruzzaman, M. & Sultan, M. Estimation of vitamins B-Complex (B2, B3, B5 and B6) of some leafy vegetables Indigenous to Bangladesh by HPLC method. J. Anal. Sci. Methods Instrum. 3 (3A), 24–29 (2013).
Aboulthana, W. M. et al. Evaluation of antioxidant efficiency of Croton tiglium L. Seeds extracts after incorporating silver nanoparticles. Egypt. J. Chem. 62 (2), 181–200 (2019).
Siong, T. & Lam, L. C. Analysis of carotenoids in vegetables by HPLC. Asean Food J. 7 (2), 91–99 (1992).
El-Feky, A. M., Aboulthana, W. M., El-Sayed, A. B. & Ibrahim, N. E. Chemical and therapeutic study of Nannochloropsis oculata on spleen of streptozotocin induced diabetes in rats. Der Pharma Chem. 9, 36–43 (2017).
Bahloul, N. et al. Aqueous extracts from Tunisian diplotaxis: phenol content, antioxidant and anti-acetylcholinesterase activities, and impact of exposure to simulated Gastrointestinal fluids. Antioxidants 5 (2), 12 (2016).
El Sawi, S. A., Elbatanony, M. M., El-Feky, A. M., Ibrahimb, M. E. & Aly, H. F. Restorative antiaging influence and chemical profile of Prunus domestica L. (European plum) seed extract in a D-galactoseinduced rat model. Egypt. Pharm. J. 23, 328–338 (2024).
Kowalska, T. & Sajewicz, M. Thin-layer chromatography (TLC) in the screening of botanicals–its versatile potential and selected applications. Molecules 27 (19), 6607 (2022).
Gwatidzo, L., Dzomba, P. & Mangena, M. TLC separation and antioxidant activity of flavonoids from Carissa bispinosa, Ficus sycomorus, and Grewia bicolar fruits. Nutrire 43, 1–7 (2018).
Rahman, M. M., Islam, M. B. & Biswas, M. Khurshid alam, A. H. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 8 (1), 1–9 (2015).
Naguib, A. M. et al. M.Phytochemical screening of Nepetacataria extracts and their in vitro inhibitory. Effects on free radicals and carbohydratemetabolising enzymes. Nat. Prod. Res. 26 (23), 2196–2198 (2013).
ElFeky, A. M. & El-Rashedy, A. A. Sterols and flavonoids in strawberry calyx with free radical scavenging, anti-inflammatory, and molecular dynamic study. Beni-Suef Univ. J. Basic. Appl. Sci. 12 (1), 108 (2023).
AbdelRahman, M. A. E., Engel, B., Eid, M. S. M. & Aboelsoud, H. M. A new index to assess soil sustainability based on Temporal changes of soil measurements using geomatics–an example from El-Sharkia, Egypt. All Earth. 34 (1), 147–166 (2022).
Ali, M. Pruning management in Psidium guajava L. Orchards—an overview. Gesunde Pflanzen. 75, 1441–1447 (2023).
Bandyopadhyay, S. & Maiti, S. K. Development of nutrient stocks and soil properties of restored solid waste dump after 5 years of afforestation with guava fruit orchard. In: (eds Kumar, S., Ghangrekar, M. M. & Kundu, A.) Sustainable Environmental Engineering and Sciences. SEES 2021. Lecture Notes in Civil Engineering, vol 323. Springer, Singapore. https://doi.org/10.1007/978-981-99-0823-3_10 (2023).
Dwivedi, V. & Agnihotri, S. Effect of integrated nutrient management on growth, yield and economics of guava (Psidium Guajava L.) cv. Allahabad Safeda. Int. J. Curr. Microbiol. App Sci. 7 (6), 3449–3453 (2018).
Kate, P. A., Kadam, A. S., Shinde, S. B. & Shriram, J. M. Effect of soil and foliar application of micronutrients on quality and economics of guava (Psidium Guajava L). J. Pharmacognosy Phytochemistry. 9 (6), 1647–1650 (2020).
Mitra, S. K., Gurung, M. R. & Pathak, P. K. Integrated nutrient management in high density guava orchards. InII International Symposium on Guava and other Myrtaceae 849, 349–356 (2008).
Preet, M. S. et al. Minkina, T. Soil nutrient status and morphometric responses of guava under drip irrigation and high-tech horticultural techniques for sustainable farming. Hydrology 9 (9), 151 (2022).
Tomar, G., Chauhan, J. M., Kumar, A. & Singh, B. Nutritional status of leaves of guava orchards of Western Uttar Pradesh. Annals Plant. Soil. Res. 22 (1), 66–69 (2020).
Gurusamy, D. et al. Multi-phenotype CRISPR-Cas9 screen identifies p38 kinase as a target for adoptive immunotherapies. Cancer Cell. 37 (6), 818–833 (2020).
Singh, S. & Singh, V. Nutritional status of soils and leaves of guava (Psidium guajava) orchards of Agra district, Uttar Pradesh. Annals Plant. Soil. Res. 24 (3), 355–359 (2022).
Parihar, P., Singh, V. & Bhadauriya, U. P. S. Status of micro nutrients in guava orchards soils and plants of Kymore plateau and satpura hills of Madhya Pradesh. J. Indian Soc. Soil Sci. 61 (1), 44–46 (2013).
Kotur, S. C., Kumar, R. & Singh, H. P. Influence of nitrogen, phosphorus and potassium on composition of leaf and its relationship with fruits yields in Allahabad Safeda Guajava (Psidium guava L.) on an Alfisol. Indian J. Agric. Sci. 67, 568–570 (1997).
Adrian, J. A. L., Arancon, N. Q., Mathews, B. W. & Carpenter, J. R. Mineral composition and soil-plant relationships for common guava (Psidium Guajava L.) and yellow strawberry guava (Psidium cattleianum var. Lucidum) tree parts and fruits. Commun. Soil. Sci. Plant. Anal. 46, 1960–1979 (2015).
Kumar, M. et al. Guava (Psidium Guajava L.) leaves: nutritional composition, phytochemical profile, and health-promoting bioactivities. Foods 10 (4), 752 (2021).
Freire, J. M., Abreu, C. D., Santos, C. D., Santos, C. D. & Simão, A. A. Assessment of mineral substances level and antioxidant potential in leaves of three guava tree varieties. J. Med. Plants Res. 7 (32), 2365–2369 (2013).
Bhargava, B. S. Leaf analysis for nutrient diagnosis, recommendation and management in fruit crops. J. Indian Soc. Soil Sci. 50, 362373 (2002).
Chetri, K., Sanyal, D. & Kar, P. L. Changes in Nutrient Element Composition of Guava Leaves in Relation To Season, Cultivar, Direction of Shoot, and Zone of Leaf Sampling30121–128 (COMMUN. SOIL SCI. PLANT ANAL, 1999). l&2
Thomas, L. A. et al. Biochemical and mineral analysis of the undervalued leaves–Psidium Guajava L. Int. J. Adv. Sci. Res. 2, 16–21 (2017).
Pandian, R. S. & Jayalakshmi, M. HPLC analysis of water soluble vitamin B in leaves Psidium guava. Asian J. Pharm. Pharmacol. 5 (1), 69–72 (2019).
Qian, H. & Nihorimere, V. Antioxidant power of phytochemicals from Psidium guajava leaf. J. Zhejiang Univ. Sci. 5 (6), 676–683 (2004).
Wojdylo, A., Oszmianski, J. & Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105 (3), 940–949 (2007).
Batanony, E. L. & El-Feky, M. Manipulation of different phytochemical classes against inflammation induced by radiations. Egypt. J. Chem. 65 (3), 81–96 (2022).
Hamed, M. A. et al. Phytoconstituents of red grape seeds extract as inflammatory modulator in adjuvant arthritic rats: role of IL-1 and its receptor blocking. J. Biologically Act. Prod. Nat. 12 (3), 254–275 (2022).
El-Feky, A. M. & Aboulthana, W. M. Chemical composition of lipoidal and flavonoidal extracts from Egyptian Olive leaves with in vitro biological activities. Egypt. J. Chem. 66 (13), 1903–1913 (2023).
El Sawi, S. A., Elbatanony, M. M., El-Feky, A. M. & Farghaly, A. A. Antimutagenic and cytotoxic potential of Punica granatum L. and Opuntia ficusindica L. Peels. Egypt. J. Chem. 67 (1), 267–283 (2024).
Moussa, M. I. et al. Fertilizer micro-dosing and harvesting time of Indigenous leafy vegetables affect in vitro antioxidant activities. J. Food Bioactives, 6 (2019).
Misra, D., Dutta, W., Jha, G. & Ray, P. Interactions and regulatory functions of phenolics in soil-plant-climate nexus. Agronomy13(2):280 (2023).
Pandey, A. & Savita, R. Harvesting and post-harvest processing of medicinal plants: problems and prospects. Pharma Innov. J. 6 (12), 229–235 (2017).
Liebelt, D. J., Jordan, J. T. & Doherty, C. J. Only a matter of time: the impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 18, 1409–1433 (2019).
Díaz-de-Cerio, E., Gómez-Caravaca, A. M., Verardo, V., Fernández-Gutiérrez, A. & Segura-Carretero, A. Determination of guava (Psidium Guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J. Funct. Foods. 22, 376–388 (2016).
Wang, L., Bei, Q., Wu, Y., Liao, W. & Wu, Z. Characterization of soluble and insoluble-bound polyphenols from Psidium guajava l.leaves co-fermented with Monascus Anka and Bacillus sp. and their bio-activities. J. Funct. Foods. 32, 149–159 (2017).
Liu, C. W., Wang, Y. C., Lu, H. C. & Chiang, W. D. Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process. Biochem. 49, 1601–1605 (2014).
Tachakittirungrod, S., Ikegami, F. & Okonogi, S. Antioxidant active principles isolated from Psidium guajava grown in Thailand. Sci. Pharm. 75 (4), 179–193 (2007).
Metwally, A. M., Omar, A. A., Harraz, F. M. & El Sohafy, S. M. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacognosy Magazine. 6 (23), 212 (2010).
Arima, H. & Danno, G. I. Isolation of antimicrobial compounds from guava (Psidium Guajava L.) and their structural Elucidation. Biosci. Biotechnol. Biochem. 66 (8), 1727–1730 (2002).
Sampath Kumar, N. S. et al. Extraction of bioactive compounds from Psidium guajava leaves and its utilization in Preparation of jellies. AMB Express. 11, 1–9 (2021).
Batubara, I., Suparto, I. H. & Wulandari, N. S. The best extraction technique for kaempferol and quercetin isolation from guava leaves (Psidium guajava). InIOP Conference Series: Earth and Environmental Science 2017 Mar 1 (Vol. 58, No. 1, p. 012060). IOP Publishing.
Sharma, A. & Gupta, H. Quercetin—a flavanoid. Chron. Young Sci. 1, 10–15 (2010).
Legeay, S., Rodier, M., Fillon, L., Faure, S. & Clere N Epigallocatechin gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients 7, 5443–5468 (2015).
Alam, W., Khan, H., Shah, M. A., Cauli, O. & Saso, L. Kaempferol as a dietary Anti-Inflammatory agent: current therapeutic standing. Molecules 25 (18), 4073 (2020).
Luca, S. V. et al. Bioactivity of dietary polyphenols:the role of metabolites. Crit. Rev. Food Sci. Nutr. 60, 626–659 (2020).
Stefanis, L., Burke, R. E. & Greene, L. A. Apoptosis in neurodegenerative disorders. Curr. Opin. Neurol. 10, 299–305 (1997).
Taha, T. F., Elakkad, H. A., Gendy, A. S. H., Abdelkader, M. A. I. & Hussein, S. S. E. Vitro bio-medical studies on Psidium guajava leaves. Plant. Arch. 19, 199–207 (2019).
El-Feky, A. M., Elbatanony, M. M. & Mounier, M. M. Anti-cancer potential of the lipoidal and flavonoidal compounds from Pisum sativum and Vicia faba peels. Egypt. J. Basic. Appl. Sci. 5 (4), 258–264 (2018).
Ullah, A. et al. Important flavonoids and their role as a therapeutic agent. Molecules 25 (22), 5243 (2020).
El-Feky, A. M. & Batanony, E. L. Potential phytoconstituents of some fruit and vegetable peels against oxidative damage, inflammatory and cytotoxic diseases. Egypt. J. Chem. 65 (3), 261–272 (2022).
Mazumdar, S., Akter, R. & Talukder, D. Antidiabetic and antidiarrhoeal effects on ethanolic extract of Psidium guajava (L.) bat.leaves in Wister rats. Asian Pac. J. Trop. Biomed. 5, 10–14 (2015).
Hu, X. F. et al. Evaluation of in vitro/in vivo anti-diabetic effects and identification of compounds from Physalis alkekengi. Fitoterapia 127, 129–137 (2018).
Luo, Y., Peng, B., Wei, W., Tian, X. & Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules 24, 1343 (2019).
Zhu, X. et al. Anti-hyperglycemic and liver protective effects of flavonoids from Psidium guajava L. (guava) leaf in diabetic mice. Food Biosci. 35, 100574 (2020).
Díaz-de-Cerio, E. et al. The hypoglycemic effects of guava leaf (Psidium Guajava L.) extract are associated with improving endothelial dysfunction in mice with diet-induced obesity. Food Res. Int. 96, 64–71 (2017).
Kaneko, K. et al. Evaluation of food-drug interaction of guava leaf tea. Phyther Res. 27, 299–305 (2013).
Matsuda, K., Nishimura, Y., Kurata, N., Iwase, M. & Yasuhara, H. Effects of continuous ingestion of herbal teas on intestinal CYP3A in the rat. J. Pharmacol. Sci. 103, 214–221 (2007).
Kutil, Z. et al. Impact of wines and wine constituents on cyclooxygenase-1, cyclooxygenase-2, and 5lipoxygenase catalytic activ Ity. Mediat Inflamm. 2014, 1–8 (2014).
Osmaniye, D., Sağlık, B. N., Levent, S., Özkay, Y. & Kaplancıklı, Z. A. Design, synthesis and biological evaluation of new N-acyl hydrazones with a Methyl sulfonyl moiety as selective COX‐2 inhibitors. Chem. Biodivers. 18 (11), e2100521 (2021).
Feng, W., Hao, Z. & Li, M. Isolation and structure identification of flavo noids. In: (ed Justino, G. C.) Flavonoids. IntechOpen, Rijeka, 2 (2017).
Jiang, X. et al. Molecular design opportunities presented by solvent-exposed regions of target proteins. Med. Res. Rev. 39, 2194–2238 (2019).
Md Idris, M. H. et al. Flavonoids as dual inhibitors of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX): molecular Docking and in vitro studies. Beni-Suef Univ. J. Basic. Appl. Sci. 11, 117 (2022).
Acknowledgements
The scientific investigation was achieved in the laboratories of the National Research Centre, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No financial assistance was received either from government or any private agency for the present work.
Author information
Authors and Affiliations
Contributions
Amal M. El-Feky demonstrated meticulous planning in choosing the research topic, systematically gathered relevant literature, conducted a thorough analysis of the data, and ultimately produced the final version of the manuscript. Mohamed A. E. AbdelRahman played a significant role in data collection, supported the study design, assessed the data, composed sections of the manuscript, and provided valuable interpretations of the findings.
Corresponding author
Ethics declarations
Consent for publication
The authors hereby declare that there are no conflicting interests that could have influenced the research results presented in this paper.
Competing interests
The authors declare no competing interests.
Ethics
The research study was conducted in accordance with the ethical guidelines established by the Medical Research Ethical Committee at the National Research Centre in Egypt.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Feky, A.M., AbdelRahman, M.A.E. Harvest time and soil-plant relationship effects on phytochemical constituency and biological activities of psidium guajava L. leaves. Sci Rep 15, 25907 (2025). https://doi.org/10.1038/s41598-025-11323-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11323-0