Abstract
In the process of tea garden management, the pruned tea plant branches usually return directly to the field, which may interfere adjacent plant growth and resource waste. Therefore, it is essential to properly manage and economically utilize pruned tea plant branches. This study was combined physiology, transcriptomics, and metabolomics to analyze the effects of uncomposted and composted tea plant branches (UT and CT, respectively) on seed germination and seedling growth of Brassica napus L. The results demonstrated that the phenolic chemicals (tea polyphenols and catechins) and alkaloid (caffeine) in CT were reduced by over 84.56–99.57% compared to UT. The CT extracts solution promoted seed germination and seedling growth, while the UT extracts solution had the opposite effect. Comprehensive transcriptomic and metabolomic analyses revealed that the primary pathways affected were phenylpropanoid biosynthesis, plant hormone signal transduction, and MAPK signal pathway - plant. These results showed that UT extracts solution may affect the B. napus L. growth by disrupting the plant hormonal balance and antioxidant system. These results of this study indicate that in situ aerobic composting is a plant-friendly method for converting pruned tea plant branches into organic fertilizer and provides a theoretical basis for the resource utilization of pruned tea plant branches.
Similar content being viewed by others
Introduction
The tea plant (Camellia sinensis (L.) Kuntze) serves as the primary cash crop in the southwestern region of China. According to statistical data from the Food and Agriculture Organization of the United Nations (FAO), the global area dedicated to tea cultivation was estimated to be approximately 8.67 million hm2 by 2022. In the cultivation process includes tea garden management, processing, and the renewal and replacement of tea plant varieties, China annually generates over 5.8 million tons of tea plant branch residues1. Notably, the tea planting area in Guizhou exceeds 46.66 million hm22. Typically, the waste branches from tea plants are either returned to the tea garden or burned onsite, primarily due to the mountainous terrain in the local area. However, this practice may promote the spread of diseases in tea plants, lead to inefficient utilization of organic resources, and contribute to serious air pollution3,4. As such, it is imperative to investigate effective methods for the harmless treatment and resource utilization of pruned tea plant branches.
Tea plants are generally integrated with annual crops in an intercropping system during the initial planting phase5. This intercropping approach can improve soil quality, increase crop yields, optimize land use, and enhance economic returns by promoting complementarity, mutual benefits, and ecological balance between the crops3,5,6. However, the decomposition of pruned tea plant branches may release chemicals (as phenolic and alkaloid) into the environment, thereby influencing the growth of neighboring plants and potentially leading to reduced yields7,8. Tea plant is an important economic crop in Guizhou Province, the pruned tea plant branches have a high biological yield, excellent nutrient absorption capacity, and are rich in organic matters (7.93 g kg− 1), nitrogen (15.8–19.0 g kg− 1), phosphorus (1.24–2.11 g kg− 1), potassium (8.34–9.42 g kg− 1), and trace elements (as aluminum 8.01–8.22 g kg− 1)9,10, making them a valuable source of organic fertilizer.
In agricultural practices, the use of organic fertilizers is a crucial strategy for replenishing soil nutrients, enhancing soil fertility, and improving productivity. Meanwhile, returning straw to the field is an important method for increasing soil organic matter, further contributing to soil health and sustainability11,12,13. Research indicates that applying organic fertilizers and returning straw to the soil can help balance nitrogen, phosphorus, potassium, and trace elements. This practice maintains or enhances organic matter content in moist and black soils, improves soil structure, promotes microbial activity, and increases the biological availability of soil nutrients11,12,13,14. However, the pruned tea plant branches are often left to decompose naturally on the surface. Due to their large size, high lignification, extended decomposition period, and potential to harbor pathogens, these pruned branches have not been effectively utilized15. Most tea gardens in Guizhou province are in hilly and mountainous regions, where the site conditions and soil quality are often poor, with an average organic matter content below 3.45%16. The primary method for replenishing nutrients involves the application of organic fertilizers, which help improve soil quality and increase productivity in tea gardens17.
In the present study, the effects of uncomposted and composted extract of tea plant branches (UT and CT, respectively) on seed germination and seedling growth of B. napus L. were evaluated. The objectives of this research are: (1) to elucidate the CT production by in-site aerobic composting; (2) to characterize the seed germination and seedling physiological change in response to UT and CT extracts; (3) to investigate the transcriptional changes of genes under UT and CT extracts treatment; and (4) to elucidate the alterations in significant metabolic pathways and key regulatory genes.
Materials and methods
Production of CT by in-site aerobic composting
The in-site aerobic composting experiment was conducted in Guiyang, Guizhou Province (26°24’ N, 106°40’ E, at an altitude of 1100 meters). This area is characterized by a subtropical monsoon humid climate, with an average annual temperature of 16.7°C and total annual precipitation of 923.40 mm (http://gz.cma.gov.cn). The tea plant (var. Fuding Dabai) branches used in our study were collected from a local tea plantation in Guiyang city (26°24’ N, 106°40’ E). A portion of tea plant branches was air-dried and prepared for analyze, while the remaining branches were treated with a decomposing agent derived from Clostridium cellulosum under elevated temperatures. For every 1000 kg of fresh tea plant pruning branches, 2.0 kg of maturing bacteria and composting aids were added. Approximately 1500 kg of this mixture was then formed into a cone shape (approximately 5 m in diameter and 1.5 m in height) and covered with plastic film to minimize water and heat loss during the composting process. Additionally, ten air holes, each with a diameter of 10 cm, were created in the plastic film. The experiment was conducted in triplicate, with a total duration of 60 days. The resulting fermented products were subsequently used in further experiments18.
Seed germination and seedling growth test
A 10.00 g aliquot of both uncomposted and composted samples was mixed with 100 mL of distilled water (1:10 ratio) and incubated at 37 °C for 48 h. The mixture was then agitated at 6000 rpm and 25 °C for 10 min, followed by filtration through a 0.45 μm filter membrane to collect the supernatant. The water extracts from the uncomposted and composted tea plant pruning branches were designated as UTE (UT extracts) and CTE (CT extracts), respectively. A concentration of 0.1 g mL− 1 was prepared and diluted with deionized water to create solutions of 0.00 (blank control), 0.50, 2.00, 8.00, and 32.00 g L− 1 for both UTE and CTE, which were subsequently stored at 4 °C for future use. Keep nitrogen, phosphorus, and potassium equal. The suspensions were filtered to remove solids. At this point, there were 16.34 g TP, 1.98 g N, 0.20 g P, and 0.85 g K on average in each liter of filtrate from UT, while the allelochemicals and nutrients in the CT filtrate averaged 1.23 g TP, 2.52 g N L− 1, 0.33 g P L− 1, and 1.27 g K L− 1. Given the substantial nutrient content present within the CT filtrate, which has the potential to exert an additional stimulatory influence on plant growth processes, (NH4)2SO4, NH4H2PO4, and K2SO4 were incorporated into the UT filtrate. This addition was carried out with the aim of rendering the nutrient concentration in the UT filtrate equivalent to that of the CT filtrate, as per the findings reported by19.
The 50 seeds of rape (Brassica napus L. from Guizhou Jinong Technology Co., Ltd) were disinfected with a 3.0% hydrogen peroxide solution for 1 min, rinsed with sterile water, and then placed on 10 cm diameter Petri dishes lined with three layers of Whatman® qualitative Grade 1 No. 1 filter paper (Merck KGaA), immersed in 5 mL UTE and CTE solution of different concentrations. A control treatment using distilled water was also included for comparative purposes. Each treatment was repeated six times. The seeds were germinated in a climate chamber set at 25 °C, with a 12-hour light/12-hour dark photoperiod and a light intensity of 5000 lx. After a culture period of 12 days, the lengths of the taproot and plant height were measured using a vernier caliper.
Determination of nutrient and chemical composition in UT and CT
For the determination of nitrogen, the Kjeldahl method was implemented. Concentrated sulfuric acid was added to the sample, and the mixture was refluxed until the solution turned clear. After cooling, the nitrogen content was directly determined using a Kjeldahl nitrogen analyzer, following established procedures18. Phosphorus analysis was performed via the molybdenum-antimony resistance colorimetric method. The sample underwent high-temperature digestion with sodium hydroxide. Subsequently, after cooling, the digested sample was dissolved in distilled water and filtered. The resulting filtrate was mixed with a molybdenum-antimony color-developing solution, and the absorbance was measured at 700 nm to calculate the phosphorus content20. Potassium quantification was achieved using the flame photometry method21. The assessment of humic acid was conducted in accordance with the methodology outlined by Su et al., (2024)22. For the quantification of tea polyphenols, the Folin-phenol method was employed. Specifically, 7 mL of water, 0.5 mL of Folin-Ciocalteu reagent, and 0.5 mL of the sample extract were sequentially added and thoroughly mixed. After a 3-minute incubation period, 2 mL of 20% sodium carbonate solution was introduced. The mixture was then subjected to a brief heating process at 100 °C for 1 min, followed by cooling. The absorbance was measured at 685 nm, and the results were reported as milligrams of gallic acid equivalent per 100 g of dry weight23. The free amino acids were analyzed via the (GB/T8314-2013) Hydration Yin triketone color method. A mixture consisting of 0.1 g of the sample and 0.9 g of magnesium oxide was refluxed in 60 mL of boiling water for 20 min. Subsequently, the solution was diluted to 100 mL. Then, 1 mL of the diluted mixture was mixed with phosphate buffer and 0.5 mL of phycocyanin. After heating for 15 min, the solution was cooled, adjusted to a final volume of 25 mL, allowed to stand for 10 min, and the absorbance was measured at 570 nm23. The determination of caffeine, catechins, and catechin monomers was performed using the analytical approach described by Jiao et al., (2024)23.
Chemical and biochemical analysis
Seeds were classified as germinated when the length of the radicle exceeded 1 mm. The number of germinated seeds was counted every 24 h until no new germination was observed for three consecutive days. The seed germination rate, seed germination index, and seed vitality index were calculated following the methodology outlined by Wang et al. (2022)24. The starch content in both leaves and roots was assessed through acidolysis followed by anthrone colourimetry. Specifically, 0.1 g of leaves were placed into a test tube filled with distilled water, boiled at 100 °C for 20 min, and then cooled before being transferred to a 100 mL volumetric flask for constant volume adjustment. Subsequently, 1 mL of the solution was pipetted into a new tube, followed by the addition of 5 mL of anthrone reagent. After boiling the mixture, the absorbance was measured at 620 nm, and the starch content was calculated based on the standard curve. Nitrate reductase activity was evaluated in vitro. The determination of free amino acids was performed using the ninhydrin colorimetric method. Soluble protein content was quantified using Coomassie brilliant blue colorimetry. The soluble sugar content was measured via the anthrone-sulfuric acid colorimetric method, which involved boiling the sample and subsequently cooling it with a mixture of ultrapure water and anthrone-sulfuric acid, with absorbance readings taken at 620 nm. The root activity of B. napus L. was assessed using the TTC method. 0.2 g root tips were incubated with TTC solution (0.4%, 10 mL), vacuum-treated (10 min), and kept at 37 °C (2 h, dark). After acid quenching (H₂SO₄), methanol extraction (7 h, 30 °C) was performed, and absorbance was read at 484 nm25. the chlorophyll content in the leaves of B. napus L. was determined through a 95% ethanol grinding and filtration technique. The concentrations of jasmonic acid (JA), salicylic acid (SA), abscisic acid (ABA), and indole-3-acetic acid (IAA) were measured using an enzyme-linked assay kit (Shanghai Keqiao Biological Technology Co., LTD.).
The roots and leaves of B. napus L. seeds were finely ground using liquid nitrogen. A precise amount of 0.2 g of the fresh samples was placed in a 10 mL centrifuge tube, followed by the addition of 8 mL of 0.05 mol L− 1 sodium phosphate buffer (PBS, pH 7.8). The mixture was shaken vigorously for 15 min and then centrifuged at 4 °C at 8000 rpm for 20 min. The resulting supernatant was utilized to assess the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), employing methods previously outlined by Duan et al., (2022)26Additionally, the malondialdehyde (MDA) content was determined using the TBARS-TCA method as described26.
Transcriptome sequencing of B. napus L. in root
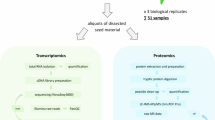
Transcriptome sequencing was conducted using the Illumina NovaSeq Reagent Kit to explore gene expression changes in the root tissues of B. napus. treated with UTE and CTE. The observed alterations in physiological and biochemical parameters suggested that each treatment significantly impacted on the roots. Therefore, sterile water (as a blank control), 32 g L− 1 UTE, and 32 g L− 1 CTE treatment were selected for transcriptome analysis. A sample of 0.5 g of B. napus. root tissue was collected and promptly frozen in liquid nitrogen, with three replicates for each treatment. RNA extraction was performed using TRIzol® Reagent (Qiagen, Germany) and subsequently purified with Plant RNA Purification Reagent (Shanghai Meiji Biomedical Technology Co., LTD). Sequencing was carried out on the Illumina NovaSeq 6000 platform by Shanghai Meiji Biomedical Technology Co., LTD, China27.
Metabolome analysis of B. napus L. in root
Approximately 1 g of B. napus L. seedling root tissue was collected and promptly frozen using liquid nitrogen. Each treatment was conducted in triplicate. A 1 g sample of the root tissue was placed into 2 mL centrifuge tube, along with a 6 mm diameter of abrasive bead. To facilitate metabolite extraction, 400 µL of an extraction extract solution (methanol: water in a 4:1 ratio, v:v) containing 0.02 mg mL−1 of the internal standard (L-2-chlorophenylalanine) was added. The sample was then ground for 4 min (-10℃, 60 Hz) using a frozen tissue grinder, followed by low-temperature ultrasound extraction for 30 min (5℃, 40 kHz). Afterwards, the sample was stored at -20 °C for 40 min, centrifuged for 10 min (4℃, 12000 rpm), and the supernatant was carefully collected and filtered through a 0.2 μm filter. Finally, the filtered solution was transferred to a 2 mL injection vial for LC-MS analysis. Chromatographic separation was achieved using a BEHC18 column (100 × 2.1 mm, 1.7 μm) prior to mass spectrometric analysis. The mobile phase system comprised (A) 2% acetonitrile in water containing 0.1% formic acid and (B) acetonitrile with 0.1% formic acid. A gradient elution program was employed as follows: initial 2% B (0–0.5 min), linear increase to 35% B (0.5–7.5 min), further increase to 95% B (7.5–13 min), isocratic hold at 95% B (13–14.4 min), rapid return to 2% B (14.4–14.5 min), followed by column re-equilibration at 2% B (14.5–16 min). The flow rate was maintained at 0.40 mL/min with the column temperature controlled at 40 °C. The ion spray voltage fluctuation (ISVF) was set at − 2800 V in negative mode and 3500 V in positive mode. The normalized collision energy was applied in MS/MS mode with rolling values of 20 V, 40 V, and 60 V. The LC-MS raw data were processed using Progenesis QI software (Waters Corporation, Milford, USA) for comprehensive data analysis, including peak detection, chromatographic alignment, and intensity normalization. Mass spectrometric data were acquired in data-dependent acquisition (DDA) mode with a scan range of m/z 70-105027,28.
Statistic analysis
All data were analyzed using Excel 2021 software (Microsoft Corporation, WA, USA). One-way analysis of variance (ANOVA), and least significant difference (LSD) tests were conducted using SPSS 26.0 software (IBM Corporation, NY, USA). Visualizations were generated using GraphPad Prism 8.0.2 software (GraphPad Software, San Diego, CA, USA), and images were refined and combined using Adobe Photoshop 2023 (Adobe Systems Software Ireland Ltd, USA). All transcriptomic and metabolomic visualizations were created using the online platform Majorbio (www.majorbio.com). After evaluating the gene expression levels using RSEM, each gene’s fragments per kilobase of transcript per million mapped reads (FPKM) were obtained depending on the length of the gene. Heat maps and figures were generated utilizing TBtools (South China Agricultural University, Guangzhou, China). The VIP values were calculated using the PLS-DA model, and the screening criterion for important variables was set at a threshold of ≥ 1.0. In the volcano plot, the criteria were p < 0.05 and ∣log2 FC∣ ≥ 1, which were used as the criteria for screening differential metabolites. O2PLS analysis was performed based on the differential metabolite screening conditions (p-value < 0.05, VIP > 1, fold change > 1) and the differential gene screening conditions (p-adjust < 0.001, fold change > 2).
Results
Nutrient and chemical composition in UT and CT
At the end of the composting process, the CT exhibited higher nutrient levels compared to the UT, with values of 25.90 g kg− 1 N, 3.50 g kg− 1 P, and 13.70 g kg− 1 K, along with a pH of 7.28. In contrast, the UT had nutrient levels of 20.20 g kg− 1 N, 2.20 g kg− 1 P, and 8.70 g kg− 1 K, with a pH of 5.24. Additionally, the concentrations of tea polyphenols (TP), caffeine, total catechins, Epigallocatechin (EGC), Epigallocatechin gallate (EGCG), Catechin gallate (CG), and Gallocatechin (GC) in the CT treatment showed significant reductions of 92.42%, 99.10%, 99.57%, 99.73%, 99.66%, 84.56% and 98.69%, respectively. Notably, the UT did not contain any humic acid (HA), whereas the CT had a substantial amount of 212.1 g kg− 1 of HA (Table 1).
Seed germination and seedling growth performance
The Fig. 1a illustrates the varying responses of B. napus L. seedling growth to UTE and CTE treatments. As the concentration of UTE increased, a significant inhibition in the growth of B. napus L. seedlings was observed compared to the control group. Specifically, the root systems became progressively shorter and thinner, and the leaves exhibited yellowing. In contrast, CTE showed beneficial effects to varying degrees. The key growth parameters (including seed germination rate, seed germination index, seed vigour index, plant biomass, plant height, and root length) declined with increasing concentrations of UTE. Conversely, when exposed to a 32 g·L− 1 CTE solution, these same metrics improved compared to the control, with increases of 3.31% in seed germination rate, 16.75% in germination index, 46.53% in seed vitality index, 48.57% in plant biomass, 53.77% in plant height, and 23.94% in root length (Fig. 1b-g).
Effects of the uncomposted and composted tea plant branch (UTE and CTE, respectivatly) on Brassica napus L. growth. (a) growth change, (b) biomass, (c) seed germination rate (GR), (d) seed germination index (GI), (e) plant height, (f) root length, (g) seed vigor index. CK, deionized water, UTE, uncomposted tea plant branch extracts, CTE, composted tea plant branch extracts. The vertical bar represents the standard deviation of three replicates, and for the same parameter, the different letters above the bar indicate significant differences (p < 0.05).
Physiological index
The application of UTE led to a reduction in several physiological indicators in B. napus L. seed leaves and roots, including nitrate reductase activity, free amino acids, soluble protein, soluble sugar, starch content, root activity index, and chlorophyll levels, with the most pronounced decreases occurring at higher UTE concentrations. In contrast, CTE exhibited a positive effect on growth across these parameters. In the 32 g L− 1 CTE solution nitrate reductase activity and root activity increased by 41.82% and 61.39% compared to the control group respectively; root free amino acids, soluble protein, soluble sugar and starch contents increased by 55.13%, 11.03%, 64.24%, and 70% respectively (Fig. 2). Similarly, in the leaves, while nitrate reductase activity and chlorophyll content improved by 39.03% and 35.34%, respectively, compared to the control; free amino acid, soluble protein, soluble sugar and starch contents increased by 21.79%, 17.26%, 31.88%, and 46.67% respectively (Fig.S1a-f).
Antioxidant physiological index
To alleviate the impact of adverse conditions, plant antioxidant systems are capable of triggering a series of defense mechanisms. Plants have an antioxidant system that plays a role in neutralizing reactive oxygen species (ROS), thus maintaining a dynamic balance between the generation and elimination of ROS29. In this study, the levels of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) in the leaves and roots of B. napus L. seedlings subjected to CTE treatment exhibited a decreasing trend with increasing concentration. In comparison to the control group, the SOD, POD, and CAT levels in the seedling roots at varying CTE concentrations were significantly diminished by 44.55–75.33%, 21.56–82.93%, and 4.31–74.98%, respectively (except for the CAT level under the 0.5 g L− 1 treatment, which had no significant change) (Fig. 3a-c). The levels of SOD, POD, and CAT in the leaves also significantly decreased by 5.71–32.05%, 2.24–42.07%, and 15.88–60.83%, respectively (except for the POD activity under the 0.5–2 g L− 1 treatment, which remained unchanged) (Fig. S1g-i). As the concentration of UTE increased, the malondialdehyde (MDA) content in the leaves and roots of B. napus L. exhibited a progressive increase, significantly surpassing the levels observed in the CK treatment (p < 0.05). Specifically, compared with the control, the MDA content of B. napus L. seedlings at a concentration of 32 g L− 1 increased by 0.24% and 5.41%, respectively. Conversely, a distinctly different trend was observed with progressively increasing concentrations of CTE. Compared to the CK group, treatment with CTE at a concentration of 32 g·L− 1 resulted in reductions in MDA levels by 64.01% and 37.95%, as shown in Fig. 3d and Fig. S1j. These findings suggest that UTE stress can disrupt normal physiological processes in plants, with elevated concentrations significantly inhibiting plant growth and compromising the plants’ stress response mechanisms30.
Endogenous hormone content
As the increase of UTE concentration, the contents of Abscisic acid (ABA), Salicylic acid (SA), and Jasmonic acid (JA) in the leaves and roots in UTE treatment exhibited a general upward trend. Compared with the CK treatment, the ABA, SA, and JA contents in the leaves treated with 32 g L− 1 UTE treatment increased by 296.43%, 157.95%, and 342.62%, respectively, while the root system showed increases of 258.74%, 11.56%, and 115.21%. Notably, Indole acetic acid (IAA) levels first increased before decreasing as the concentration of UTE increased, reaching their highest values at the 0.5 g L− 1 UTE treatment, with measurements of 70.06 µg g− 1 (leaves) and 106.28 µg g− 1 (roots). Conversely, as CTE concentration increased, both ABA and JA levels, along with SA in the seedling leaves and roots, exhibited a downward trend, while IAA levels continued to increase refer to Fig. 4a to d (Fig. S1k-n). In addition, by measuring the ethylene content in the roots, it was found that the ETH content significantly increased with the increase in UET concentration (Fig. 4e).
Based on the physiological indicators measured in the previous stage, B. napus L. seedlings exhibited a more pronounced impact on the root system when exposed to uncomposted tea plant branches and compost leachate. Consequently, transcriptome and metabolome sequencing were conducted on the root system of the B. napus L. seedlings to further investigate these effects.
Transcriptome analysis
Using the Illumina platform, B. napus L. root samples were used for RNA extraction, cDNA library construction and sequencing. Approximately 58.39 Gb clean data were obtained from nine samples. The Q20 and Q30 values of all clear reads were greater than 98.19% and 94.67%, respectively, illustrating the high reliability of the sequencing results (Table S1). In addition, the PCA analysis indicated that the expression of gene clusters in CK, UTE and CTE were distinguishable (Fig. S2). The heat maps showed that the gene expression of different treatments was significantly different (Fig. S3). As shown in Fig. 5b and Table S2, transcriptome sequencing analysis identified 6,453 (4,179 upregulated and 2,274 downregulated), 3,542 (1,494 upregulated and 2,048 downregulated), and 3,939 (1,196 upregulated and 2,743 downregulated) differentially expressed genes (DEGs) in the comparisons UTE vs. CK, CTE vs. CK, and CTE vs. UTE, respectively. Venn analysis indicated that 46,097 genes were shared by CK, UTE and CTE, 185 DEGs were shared by UTE vs. CK, CTE vs. CK and CTE and UTE (Fig. 5a and Table S3). The largest number of differentially expressed genes was observed in UTE vs. CK, indicating that the uncomposted tea plant branches had the most significant impact on the growth of B. napus L.
Enrichment analyses were performed using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases to further evaluate the biological function of DEGs in B. napus L. roots treated with UTE and CTE. GO annotation analysis showed that the DEGs of the 3 comparison groups could be divided into three ontologies: biological processes (BPs), molecular functions (MFs), and cellular components (CCs), as illustrated in Fig. S4. For KEGG enrichment analysis, the UTE vs. CK comparison revealed significant enrichment in the pathways of plant hormone signal transduction (114 genes), plant-pathogen interaction (100 genes), and starch and sucrose metabolism (85 genes). In the CTE vs. CK comparison, significant enrichment was observed in plant-pathogen interaction (77 genes), plant hormone signal transduction (75 genes), and starch and sucrose metabolism (50 genes) pathways. Lastly, in the CTE vs. UTE comparison, pathways with significant enrichment included plant hormone signal transduction (88 genes), phenylpropanoid biosynthesis (57 genes), and starch and sucrose metabolism (40 genes), as shown in Fig. 5c.
Transcriptomic analysis. (a) Venn analysis. (b) Volcano plots of upregulated and downregulate differential expression genes (DEGs), Green represents differentially down-regulated genes, red represents differentially up-regulated genes, blue represents nonsignificant differentially differentiated genes. (c) Top 20 KEGG pathway enrichment analysis.
Metabolome analysis
To elucidate the response mechanisms of plant roots to 32 g L− 1 UTE and CTE, non-targeted metabolomics (LC-MS) was employed to study the metabolism of B. napus L. roots and identify distinct plant metabolites. Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA) demonstrated clear distinctions among the samples, as shown in Fig. S5a and b. Fig. S5c further validates the reliability of the PLS-DA data. A total of 1252 metabolites were detected in the three B. napus L roots, which could be classified into 17 different categories. Among these, lipids and lipid-like molecules (283 metabolites, 22.60%), organoheterocyclic compounds (225 metabolites, 17.97%), and organic acids and derivatives (214 metabolites, 17.09%) comprised the largest proportions (Fig. S6).
Volcano plot analysis identified 232 upregulated and 184 downregulated Differentially accumulated metabolites (DAMs) in the UTE vs. CK group, 171 upregulated and 121 downregulated DAMs in the CTE vs. CK group, and 205 upregulated and 218 downregulated DAMs in the CTE vs. UTE group (Fig. 6a). Cluster heatmap and VIP (VIP > 1 and p < 0.05) analyses revealed significant differences in metabolites between treatments (Fig. S7 and S8). Based on fold changes in metabolite accumulation, the top 20 DAMs with the greatest fold differences were identified in the UTE vs. CK, CTE vs. CK, and CTE vs. UTE treatment groups (Fig. 6b). A Venn diagram showed 51 common DAMs across control groups, primarily including organic acids and derivatives, phenylpropanoids and polyketides, lipids and lipid-like molecules, and ten additional compounds (Fig. S9 and Table S4). The top 20 most enriched metabolic pathways were selected from the KEGG database, identifying key metabolic pathways by which tea plant branch extracts impact root growth in B. napus L. seedlings. These pathways primarily included tryptophan metabolism, phenylpropanoid biosynthesis, and plant hormone signal transduction (Fig. 6c).
Metabolomic analysis. (a) Volcanic maps of up and down-regulated differential metabolites in each comparison group. (b) The top 10 up-regulated and down-regulated differential metabolites in each comparison group. (c) The top 20 pathways of significant of the differentially accumulated metabolites (DAMs) on KEGG, The X-axis is enrichment factor and the Y-axis is pathway name. The size of the bubbles indicates the number of DAMs involved. The color of the bubble represents the enrichment of the pathway.
Combined transcriptomic and metabolomic analysis
Transcriptomic and metabolic data were integrated for O2PLS analysis in Fig. S10a. The nine-quadrant diagram shows the correlation between UTE vs. CK, CTE vs. CK and CTE vs. UTE genes and compounds (Fig. S10b). The common pathways of transcription and metabolism were analyzed by Venn. There are 49, 41 and 55 paths in UTE vs. CK, CTE vs. CK and CTE vs. UTE, respectively (Fig. 7a). Further, through the KEGG pathway annexe gene set and metabolic set KEGG pathway enrichment, it was found that mainly phenylpropanoid biosynthesis, plant hormone signal transduction, MAPK signal pathway - plant, and biosynthesis of various plant secondary metabolites (Fig. 7b). Based on the results of previous studies and in combination with the present study, it was determined that phenylpropane biosynthesis, plant hormone signal transduction, MAPK signal pathway - plant were the main pathways affecting the growth of B. napus L. seed27.
Phenylpropane biosynthetic pathway
In the phenylpropanoid biosynthesis pathway, 44 DEGs were identified, including four PAL (phenylalanine ammonia-lyase), five 4CL (4-coumarate–CoA ligase), six CCR (cinnamoyl-CoA reductase), three COMT (caffeic acid 3-O-methyltransferase), three CYP84A (ferulate-5-hydroxylase), three CAD (cinnamyl-alcohol dehydrogenase), four HCT (shikimate O-hydroxycinnamoyltransferase), two C3’H (5-O-(4-coumaroyl)-D-quinate 3’-monooxygenase), eleven CSE (caffeoylshikimate esterase), and two BRT1 (sinapate 1-glucosyltransferase). Expression of these genes showed differential changes depending on treatment. Compared to CK, UTE treatment led to a down-regulation of several genes, including PAL, 4CL, CCR, CYP84A, C3’H, and CSE, while CTE treatment resulted in the up-regulation of genes such as PAL, 4CL, CCR, COMT, CAD, and C3’H. The up- or down-regulation of these genes influenced the levels of 13 metabolites within the pathway, including phenylalanine, cinnamaldehyde, coniferyl aldehyde, sinapoyl aldehyde, coniferyl alcohol, 5-hydroxyconiferyl alcohol, sinapyl alcohol, 4-coumaroylshikimate, dattelic acid, ferulic acid, sinapic acid, 1-O-sinapoyl-beta-D-glucose, and sinapoyl malate (Fig. 8a and Table S5). Moreover, PAL is a rate-limiting enzyme that catalyzes the first step of the phenylpropanoid metabolic pathway and participates in various physiological activities during plant growth and development. Therefore, this study analyzed the correlations between the expression of PAL differentially expressed genes in the phenylpropanoid synthesis pathway and root length, antioxidant enzyme activity, and hormone levels under different extraction solutions. The results showed that BnaA02G0015500ZS and BanA04G0070500ZS were significantly positively correlated with root length. BnaC05G0498400ZS was significantly positively correlated with JA content, while BnaC08G0333400ZS was positively correlated with root length, SOD activity, and SA content (Fig. S11).
Plant hormone signal transduction pathway
Key genes in the hormone signal transduction system were identified and analyzed, revealing varied expression patterns across treatments. In the ABA signaling pathway, most genes were downregulated under UTE treatment but upregulated under CTE treatment. This trend extended to other hormone signaling pathways, where gene expression patterns in gibberellin, ethylene, and salicylic acid pathways resembled those observed in ABA signaling, while expression patterns in auxin, brassinosteroid, and jasmonic acid pathways were similar to those in cytokinin signaling (Fig. 8b and Table S5).
MAPK signal pathway - plant
In this study, the MAPK signal pathway was widely confirmed to play a crucial role in plant growth, development, and responses to diverse biotic and abiotic stresses. Reactive oxygen species (ROS) served as a key factor for activating the MAPK signal pathway, where oxidative stress triggered the expression of related genes by elevating ROS levels. A total of 44 differentially expressed genes (DEGs) were identified in the MAPK signal pathway. Compared with the CTE treatment, the UTE treatment activated most genes in the MAPK signal pathway (except ATP7 and MPK8), suggesting that UTE treatment influenced the antioxidant system by activating genes within the MAPK signal pathway (Fig. 8c).
qRT-PCR validation
To verify the reliability of the transcriptome, a total of 10 differentially expressed genes (DEGs) were selected. The expression data of these DEGs were then presented through bar charts and line graphs (Fig. S12). The results showed that the qRT-PCR and RNA-seq data of these 10 DEGs were highly consistent, thus indicating that the transcriptome data of this study was accurate and reliable.
Discussion
The decomposition of accumulated residues and litter from tea plants results in the production of chemicals, primarily phenolic compounds and alkaloids, which inhibit microbial activity in the rhizosphere soil7,34. Following the pruning of tea plant branches, tea polyphenol, catechin compounds and caffeine exert indirect inhibition effects on the long-term cultivation systems of tea plants7,8,34,35. After composting, the tea polyphenol content, total catechins, and caffeine levels were significantly reduced, while nitrogen, phosphorus, and potassium levels increased, likely due to the addition of Clostridium cellulosum19,36. Microorganisms decompose organic matter, reducing material volume and creating a “concentration effect” for nutrients within the material19,37,38. Additionally, the incorporation of exogenous nitrogen sources adjusts the carbon-to-nitrogen (C/N) ratio, enhancing nutrient conversion and release20,39. The humic acid concentration also increased to 212.10 g kg⁻¹ post-decomposition, indicating improved maturity and reduced plant toxicity (Table 1)40,41. In conclusion, through reasonable composting treatment, the content of allelochemicals (tea polyphenols, catechins and caffeine) in tea plants branches can be reduced and the utilization rate of nutrients can be improved.
Tea plants contain over 1,370 secondary metabolites, making it impractical to assess the phytotoxicity of each metabolite individually. The seed germination test is a detection method used to assess the overall toxicity of foreign substances to plants24. Studies indicate that phenolic compounds and alkaloid can interfere throughout the seed germination process, as well as disrupt organ nutrient absorption and development7,8,42,43. In B. napus L. seeds, biomass decreased significantly with increasing UTE concentration, likely due to the elevated levels of phenolic compounds in UTE, which impact nutrient accumulation, transport processes, and disrupt normal physiological metabolism in plants44. In contrast, microbial composting of tea plant branches reduces phenolic compound content, increases nutrient levels, and raises humic acid concentration, all of which support improved plant growth.
UTE treatment with different concentrations inhibited the contents of soluble sugar, soluble protein and soluble starch in roots and leaves of B. napus L. to varying degrees. Soluble protein, as an important substance in osmoregulation, could regulate soluble sugar to resist stress to a certain extent. In this study, soluble protein and soluble sugar in roots and leaves of B. napus L. treated with 32 g L− 1 UTE showed significant differences compared with CK (p < 0.05), which may be due to the decreased osmotic regulation ability and enhanced hydrolysis of plants under stress beyond the normal range, resulting in the inhibition of protein synthesis and ultimately a significant decrease in soluble protein content in B. napus L. (Fig. 2d-f and Fig. S1d-f).
Enzymatic and non-enzymatic antioxidant systems are the mechanisms by which most plants respond to stress. In the present study, the activity of three antioxidant enzymes (SOD, POD, CAT and MDA) was measured with the increase in UTE concentration. Elevated UTE concentrations led to an imbalance in reactive oxygen metabolism in B. napus L., compromising cell membrane integrity and ultimately affecting plant growth (Fig. 3 and Fig. S1g-j)43,45 This effect may be attributed to polyphenols and alkaloid present in the uncomposted extract46. These findings highlight the physiological response of B. napus L. to UTE, particularly within its antioxidant enzyme system.
In this study, UT treatment increased the contents of ABA, SA, and JA in plants, inhibited plant growth and development, and further suppressed the growth of roots and stems. This phenomenon might be attributed to the biotic stress (allelochemicals) induced by UT47,48. The elevation in hormone levels represents a protective mechanism initiated by plants in response to this biotic stress. Plant allelochemicals, primarily comprising tea polyphenols, caffeine, and catechins from pruning residues, are released into the soil environment through multiple pathways including leaf leaching, root exudation, volatilization, and residue decomposition. These compounds exhibit significant allelopathic effects by inhibiting seed germination and growth of neighboring plants. In continuous cropping tea plantations, the accumulation of these allelochemicals induces characteristic replant problems through two distinct mechanisms: (1) triggering reactive oxygen species (ROS) bursts that lead to increase in malondialdehyde (MDA) content in tea leaves, indicating severe membrane lipid peroxidation damage; and (2) disrupting ROS metabolic homeostasis by suppressing the activity of crucial antioxidant enzymes (SOD, POD). To counteract this oxidative stress, tea plants employ a dual defense strategy, activating both enzymatic antioxidant systems (SOD, POD, CAT) to restore redox balance and preserve physiological functionality8.
In the present study, transcriptome analysis revealed that the roots of B. napus L. primarily influenced plant growth by enhancing responses to abiotic stress through pathways such as starch and sucrose metabolism, cell wall metabolism via phenylpropanoid biosynthesis, and plant hormone signaling pathways49.
It has been reported that ABA can regulate plant growth and development, especially playing a significant role in resisting abiotic stress. In this study, UT treatment increased the ABA content, and protein phosphatase 2 C (PP2C) plays a crucial role in plant signal transduction, development process, and responses to biotic and abiotic stresses. In this study, transcriptome sequencing revealed that UT treatment activated the expression of PP2C-related genes, suggesting that PP2C genes may regulate the development process and stress response of rapeseed50. Additionally, related studies have reported that ABRE-binding factors (ABF) also play an important role in plant responses to abiotic stress51. In this study, the expression levels of ABF-related genes were upregulated under UT treatment, indicating that UT treatment activated the expression of ABF genes, further affecting the balance of ABA. When plants are subjected to abiotic stress, the content of jasmonic acid (JA) in the plant body will significantly increase. This change activates the expression of Jasmonate ZIM-domain (JAZ) related genes in the jasmonic acid signaling pathway. With the activation of JAZ genes, the transcription factor Myelocytomatosis proteins (MYC), which were originally inhibited by JAZ proteins, are released from their constraints. This further activates downstream responses and participates in the corresponding physiological processes to enhance the plant’s stress resistance, enabling the plant to survive and grow better under adverse environmental conditions52.
Ethylene has played an important role in plant growth and development (such as organ senescence) and stress response. However, the regulation of ethylene by MAPK has interacted with other hormone pathways (such as ABA and JA) to form a complex regulatory network. For example, MAPK has activated both ethylene and ABA signals under drought stress53,54. In this study, under UTE treatment, the differentially expressed genes ETR, MKK, EIN, EBF, and ERF downstream of ethylene in rapeseed were significantly down-regulated, while the ethylene content in roots increased, indicating that these differential genes may negatively regulated ethylene synthesis.
Through metabolomics analysis, the main enriched differential metabolites in B. napus L. were mainly enriched in tryptophan metabolism, phenylpropanoid biosynthesis and plant hormone signal transduction pathways. Research by55 has shown that these pathways are essential for regulating plant growth and adversity stress27,56. For example, tryptophan is an essential amino acid for the synthesis of a large number of bioactive molecules such as auxin, plant antitoxins, indole glucosinolates and terpenoid indole alkaloids. It plays a crucial role in the regulation of plant growth and development and stress responses55,57,58. Phenylpropanoid biosynthesis plays a vital role in plant growth, development, and resilience, significantly contributing to the plant’s ability to respond to environmental factors, including tolerance and resistance to both abiotic and biotic stresses53. In summary, UTT and CTE treatments may further influence the growth and development of B. napus L. through abiotic stress and altering phenolic compound levels.
Phenylpropanoid biosynthesis is closely related to seed germination59. Under UTE treatment, the roots of B. napus L. exhibited downregulation of genes associated with PAL (phenylalanine ammonia-lyase), resulting in reduced conversion of phenylalanine to cinnamic acid60. At the same time, the expression of 4CL and CCR decreased, affecting lignin synthesis61,62which may be a significant factor limiting the growth and development of B. napus L. The decrease in C3’H expression would affect the growth and development of plants63. The decrease in CSE expression not only directly reduced the content of ferulic acid, but also indirectly affected the composition and structure of plant cell wall, which further affected the mechanical strength and stress resistance of plants64. Studies have shown that CSE is also involved in plant growth and development. A Medicago truncatula mutant with a transposon insertion in the CSE gene displayed significant dwarfism and developmental changes65. These results suggest that CSE may affects plant growth and development. In contrast, CTE treatment produced the opposite effect on B. napus L. roots. Upregulated expression of genes related to PAL, 4CL and CCR supported lignin synthesis, promoting plant growth, development, and increased stress resistance52,66. The up-regulation of COMT and CYP84A genes ensured the synthesis of Coniferylaldehyde and Sinapaldehyde, and enhanced the growth potential and stress resistance of plants67,68.
Related studies have shown that genes such as transport inhibitor response 1, gibberellin receptor GID1, transcription factor TGA, abscisic acid receptor PYL family and xyloglucan: xyloglucosyl transferase TCH4 can enhance plant stress resistance69,70. In addition, the auxin response factor is involved in auxin signaling pathways to regulate tissue differentiation71. The regulation of hormone signaling pathways is crucial for the development of plant root72. When treated with UTE, the expression of genes related to PYL decreased, the expression of PP2C (protein phosphatase 2 C) was suppressed, and the genes associated with SnRK2 (threonine-protein kinase SRK2) were further activated. This disruption directly affected the normal functioning of the ABA (abscisic acid) signaling pathway, leading to impeded root growth73.
Thus, owing to the geographical and climatic characteristics of tea plant distribution in southwest China, the effects of uncomposted and composted tea plant branches on seed germination and seedling growth were evaluated using aqueous extracts. The relatively high experimental concentrations used in this study were selected to meet specific scientific objectives, allowing for the exploration of chemical interactions and enhanced understanding of bioactive compounds under controlled conditions. However, it is acknowledged that these concentrations may not fully represent natural exposure levels due to factors such as soil dilution, microbial interactions, and degradation processes. Therefore, future research should emphasize comprehensive field studies to investigate the presence and roles of these compounds in natural environments. This approach would yield a more accurate and realistic understanding, addressing current limitations and deepening insights into the mechanisms of action of these compounds within ecosystems.
Conclusions
In this study, the effects of uncomposted and composted tea plant branches on seed germination and seedling growth of B. napus L. were analyzed by physiology, transcriptomics and metabolomics. The results demonstrated that the phenols chemicals (tea polyphenols and catechins) and alkaloid (caffeine) in CT were reduced by over 84.56–9.57% compared to UT. The CT aqueous solution promoted seed germination and seedling growth, while the UT solution had the opposite effect, likely due to disruption of the plant hormonal balance and antioxidant system. Comprehensive transcriptomic and metabolomic analyses revealed that the primary pathways affected were phenylpropanoid biosynthesis, plant hormone signal transduction, and MAPK signal pathway. Therefore, in situ aerobic composting is a plant- friendly method for converting tea plant pruning branches into organic fertilizer.
Abbreviations:
UT, Uncomposted tea plants; CT, Composted tea plants; UTE, Uncomposted extracts of tea plants; CTE; Composted extracts of tea plants; N, Nitrogen; P, Phosphorus; K, Potassium; HA, Humic Acid; EGC, Epigallocatechin; EGCG, Epigallocatechin gallate; CG, Catechin gallate; GC, Gallocatechin; TP, Tea Polyphenol; GR, Germination rate; GI, Germination index; NR, Nitrate reductase; SOD, Superoxide dismutase; POD, Peroxidase; CAT, Catalase; MDA, Malondialdehyde; ABA, Abscisic acid; SA, Salicylic acid; JA, Jasmonic acid; IAA, Indole acetic acid; DEGs, Differentially expressed genes; DAMs, Differentially accumulated metabolites.
Data availability
Data will be made available on request. If someone wants to request the data from this study, they can directly contact the primary corresponding author (Jiao Yujie, jiaoyujie9@163.com).
References
Guo, S. S., Awasthi, M. K., Wang, Y. F. & Xu, P. Current Understanding in conversion and application of tea waste biomass: A review. Bioresour Technol. 338, 125530. https://doi.org/10.1016/j.biortech.2021.125530 (2021).
Ge, Y. H., Wang, L., Huang, Y. J., Jia, L. Y. & Wang, J. H. Characteristic flavor compounds in Guizhou green tea and the environmental factors influencing their formation: investigation using stable isotopes, electronic nose, and headspace-gas chromatography ion migration spectrometry. LWT-Food Sci. Technol. 196, 115887. https://doi.org/10.1016/j.lwt.2024.115887 (2024).
Wang, T. et al. Tea plantation intercropping green manure enhances soil functional microbial abundance and multifunctionality resistance to drying-rewetting cycles. Sci. Total Environ. 810, 151282. https://doi.org/10.1016/j.scitotenv.2021.151282 (2022).
Phiri, R., Rangappa, S. M. & Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 434, 139989. https://doi.org/10.1016/j.jclepro.2023.139989 (2024).
Wang, S. S. et al. Different changes of bacterial diversity and soil metabolites in tea plants-legume intercropping systems. Front. Plant. Sci. 14, 174225. https://doi.org/10.3389/fpls.2023.1110623 (2023).
Huang, Z. et al. Tea plant-legume intercropping simultaneously improves soil fertility and tea quality by changing bacillus species composition. Hortic. Res. 9, 46. https://doi.org/10.1093/hr/uhac046 (2022).
Ye, J. H. et al. Autotoxicity of the soil of consecutively cultured tea plantations on tea (Camellia sinensis) seedlings. Acta Physiol. Plant. 38, 195. https://doi.org/10.1007/s11738-016-2216-5 (2016).
Arafat, Y. et al. Soil sickness in aged tea plantation is associated with a shift in microbial communities as a result of plant polyphenol accumulation in the tea gardens. Front. Plant. Sci. 11, 601. https://doi.org/10.3389/fpls.2020.00601 (2020).
Waqas, M. et al. Unlocking integrated waste biorefinery approach by predicting calorific value of waste biomass. Environ. Res. 237, 116943. https://doi.org/10.1016/j.envres.2023.116943 (2023).
Borgohain, A. et al. Temperature effect on biochar produced from tea (Camellia sinensis L.) pruning litters: A comprehensive treatise on physico-chemical and statistical approaches. Bioresour Technol. 318, 124023. https://doi.org/10.1016/j.biortech.2020.124023 (2020).
Tian, S. Y. et al. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil. Biol. Biochem. 165, 108533. https://doi.org/10.1016/j.soilbio.2021.108533 (2022).
Anning, D. K. et al. Stimulation of soil organic matter fractions by maize straw return and nitrogen fertilization in the loess plateau of Northwest China. Appl. Soil. Ecol. 191, 105061. https://doi.org/10.1016/j.apsoil.2023.105061 (2023).
Chen, L. M. et al. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant. Sci. 13, 986763. https://doi.org/10.3389/fpls.2022.986763 (2022).
Yu, Y. T. et al. Effects of organic fertilizers on plant growth and the rhizosphere microbiome. Appl. Environ. Microbiol. 90(2), 1719–1723. https://doi.org/10.1128/aem.01719-23 (2024).
Wang, Z. et al. Recent advances review in tea waste: High-value applications processing technology and value-added products. Sci. Total Environ. 946, 174225. https://doi.org/10.1016/j.scitotenv.2024.174225 (2024).
Yang, X.D., Yi, X.Y., Ni, K., Zhang, Q.F., Shi, Y.Z., Chen, L.B., Zhao, Y.Y., Zhang, Y.L., Ma, Q.X., Cai, Y.J., Ma, L.F., Ruan, J.Y. Patterns and abiotic drivers of soil organic carbon in perennial tea (Camellia sinensis L.) plantation system of China. Environ Res 237, 116925.https://doi.org/10.1016/j.envres.2023.116925. (2023)
Yin, R. X. et al. Organic fertilizer, and bio-organic fertilizer improve soil fertility and tea quality. Agronomy. 14(10), 2339. https://doi.org/10.3390/agronomy14102339 (2024).
Jiao, Y. J. et al. In situ aerobic composting eliminates the toxicity of Ageratina adenophora to maize and converts it into a plant- and soil-friendly organic fertilizer. J. Hazard. Mater. 410(8), 124554. https://doi.org/10.1016/j.jhazmat.2020.124554 (2021).
Jiao, Y. J., Li, Y., Yuan, L. & Huang, J. G. Allelopathy of uncomposted and composted invasive aster (Ageratina adenophora) on ryegrass. J. Hazard. Mater. 402, 123727. https://doi.org/10.1016/j.jhazmat.2020.123727 (2021).
Zhang, Q. et al. Effects of pruning on growth, rhizosphere soil physicochemical indexes and bacterial community structure of tea tree and their interaction. Agriculture 13, 1972. https://doi.org/10.3390/agriculture13101972 (2023).
Cao, Z. L. et al. Bioaugmentation on humification during co-composting of corn straw and biogas slurry. Bioresour Technol. 374, 128756. https://doi.org/10.1016/j.biortech.2023.128756 (2023).
Su, J. et al. Phosphate additives promote humic acid carbon and nitrogen skeleton formation by regulating precursors and composting bacterial communities. Bioresour Technol. 399, 130617. https://doi.org/10.1016/j.biortech.2024.130617 (2024).
Jiao, Y. J. et al. Impact of spreading time on flavor quality in Duyun Maojian summer green tea. LWT https://doi.org/10.1016/j.lwt.2024.117103 (2024). 214.117013.
Wang, G. Y. et al. Key factors affecting seed germination in phytotoxicity tests during sheep manure composting with carbon additives. J. Hazard. Mater. 421, 126809. https://doi.org/10.1016/j.jhazmat.2021.126809 (2022).
Zhu, Q. et al. Effects of biochar on seedling root growth of soybeans. Chil. J. Agric. Res. 78(4), 0718–5839. https://doi.org/10.4067/S0718-58392018000400549 (2018).
Duan, Y. P., Zhang, Y. & Zhao, B. Lead, zinc tolerance mechanism and phytoremediation potential of Alcea rosea (Linn.) Cavan, and Hydrangea macrophylla (Thunb.) Ser. and ethylenediaminetetraacetic acid effect. Environ. Sci. Pollut. Res. 27(29), 41329–41343. https://doi.org/10.1007/s11356-021-18243-2 (2022).
Zhao, W. et al. Integrated transcriptomics and metabolomics reveal key metabolic pathway responses in Pistia stratiotes under cd stress. J. Hazard. Mater. 452, 131214. https://doi.org/10.1016/j.jhazmat.2023.131214 (2023).
Hu, S. S. et al. Pu-erh tea increases the metabolite cinnabarinic acid to improve circadian rhythm disorder-induced obesity. Food Chem. 394, 133500. https://doi.org/10.1016/j.foodchem.2022.133500 (2022).
Li, C. T., Cao, W., Wu, W. Q., Xin, X. W. & Jia, H. L. Transcription-metabolism analysis of various signal transduction pathways in Brassica chinensis L. exposed to PLA-MPs. J. Hazard. Mater. 486, 136968. https://doi.org/10.1016/j.jhazmat.2024.136968 (2025).
Đorđević, T. et al. Phytotoxicity and allelopathic potential of Juglans regia L. leaf extract. Front. Plant. Sci. 13, 986740. https://doi.org/10.3389/fpls.2022.986740 (2022).
Kanehisa, M. & Goto, S. K. E. G. G. Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Cao, P. R., Liu, C. Y. & Li, D. Autointoxication of tea (Camellia sinensis) and identification of its autotoxins. Allelopathy J. 28(2), 155–166 (2011).
Cao, P. R. & Luo, S. M. Studies on the allelopathy of tea plant Camellia sinensis (L.) Kuntze. J. South. China Agricultural Univ. 2, 129–133 (1994). (in Chinese).
Harindintwali, J. D., Zhou, J. L. & Yu, X. B. Lignocellulosic crop residue composting by cellulolytic nitrogen-fixing bacteria: A novel tool for environmental sustainability. Sci. Total Environ. 715, 136912. https://doi.org/10.1016/j.scitotenv.2020.136912 (2020).
Nigussie, A. et al. Effect of microbial inoculation on nutrient turnover and lignocellulose degradation during composting: A meta-analysis. Waste Manage. 125, 220–234. https://doi.org/10.1016/j.wasman.2021.02.043 (2021).
Zhang, X. et al. Enhancing aerobic composting performance of high-salt oily food waste with Bacillus safensis YM1. Bioresour Technol. 397, 130475. https://doi.org/10.1016/j.biortech.2024.130475 (2024).
Qu, J., Zhang, L. J., Zhang, X., Gao, L. H. & Tian, Y. Q. Biochar combined with gypsum reduces both nitrogen and carbon losses during agricultural waste composting and enhances overall compost quality by regulating microbial activities and functions. Bioresour Technol. 314, 123781. https://doi.org/10.1016/j.biortech.2020.123781 (2020).
Chen, Y. F. et al. Microbial inoculants reshape structural distribution of complex components of humic acid based on spectroscopy during straw waste composting. Bioresour Technol. 359, 127472. https://doi.org/10.1016/j.biortech.2022.127472 (2022).
Sajid, S. et al. Pretreatment of rice straw by newly isolated fungal consortium enhanced lignocellulose degradation and humification during composting. Bioresour Technol. 354, 127150. https://doi.org/10.1016/j.biortech.2022.127150 (2022).
Pereira, J. C. et al. Allelopathic potential and identification of secondary metabolites in extracts of Canavalia ensiformis L. Rev. Ceres. 65, 243–252. https://doi.org/10.1590/0034-737X201865030004 (2018).
Bieser, J. M. H., Maria, A. Z., Murtada, J. & Thomas, S. C. Biochar mitigation of allelopathic effects in three invasive plants: evidence from seed germination trials. Can. J. Soil. Sci. 102(1), 213–224. https://doi.org/10.1139/cjss-2020-0160 (2022). (2022).
Wang, C. et al. Effects of autotoxicity and allelopathy on seed germination and seedling growth in Medicago truncatula. Front. Plant. Sci. 13, 908426. https://doi.org/10.3389/fpls.2022.908426 (2022).
Wang, S., Cheng, H. Y., Wei, M., Wu, B. D. & Wang, C. Y. Litter decomposition process dramatically declines the allelopathy of Solidago canadensis L, on the seed germination and seedling growth of Lactuca sativa L. Int. J. Phytorem. 22(12), 1295–1303. https://doi.org/10.1080/15226514.2020.1765140 (2020).
Šamec, D., Karalija, E., Šola, I., Vujčić Bok, V. & Salopek-Sondi, B. The role of polyphenols in abiotic stress response: the influence of molecular structure. Plants 10(1), 118. https://doi.org/10.3390/plants10010118 (2021).
Cao, P. R., Liu, C. Y. & Li, D. Effects of different autotoxins on antioxidant enzymes and chemical compounds in tea (Camellia sinensis L.) Kuntze. Afr. J. Biotechnol. 10(38), 7480–7486 (2011).
Khamare, Y., Chen, J. J. & Marble, S. C. Allelopathy and its application as a weed management tool: A review. Front. Plant. Sci. 28(13). https://doi.org/10.3389/fpls.2022.1034649 (2022).
Dong, Q., Wallrad, L., Almutairi, B. O. & Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant. Biol. 64(2), 287–300. https://doi.org/10.1111/jipb.13228 (2022).
Qiu, J. F. et al. Genome-wide analysis of the protein phosphatase 2 C genes in tomato. Genes 13(4), 604. https://doi.org/10.3390/genes13040604 (2022).
Yoshida, T. et al. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant. Cell. Environ. 38(1), 35–49. https://doi.org/10.1111/pce.12351 (2015).
Zhao, X. Q., He, Y. Q., Liu, Y. X., Wang, Z. F. & Zhao, J. JAZ proteins: key regulators of plant growth and stress response. Crop J. 12, 1505–1516. https://doi.org/10.1016/j.cj.2024.11.001 (2024).
Zhou, J. G. et al. Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis. Plant. Cell. 34(8), 3066–3087. https://doi.org/10.1093/plcell/koac139 (2022).
Tu, C. Y., Zheng, L., Yan, J., Shen, R. F. & Zhu, X. F. ACS2 and ACS6, especially ACS2 is involved in MPK6 evoked production of ethylene under cd stress, which exacerbated cd toxicity in Arabidopsis thaliana. Plant. Sci. 352, 112354. https://doi.org/10.1016/j.plantsci.2024.112354 (2025).
Liu, W. C. et al. Coordination of plant growth and abiotic stress responses by tryptophan synthase β subunit 1 through modulation of Tryptophan and ABA homeostasis in Arabidopsis. Mol. Plant. 15(6), 973–990. https://doi.org/10.1016/j.molp.2022.04.009 (2022).
Rahim, M. A., Zhang, X. B. & Busatto, N. Editorial: Phenylpropanoid biosynthesis in plants. Front. Plant. Sci. 14, 1230664. https://doi.org/10.3389/fpls.2023.1230664 (2023).
Hildebrandt, T. M., Nesi, A. N., Araújo, W. L. & Braun, H. P. Amino acid catabolism in plants. Mol. Plant. 8(11), 1563–1579. https://doi.org/10.1016/j.molp.2015.09.005 (2015).
Tzin, V. & Galili, G. New insights into the Shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant. 3(6), 956–972. https://doi.org/10.1093/mp/ssq048 (2010).
Yang, J. et al. Dynamic transcriptome and metabolome analyses of two types of rice during the seed germination and young seedling growth stages. BMC Genom. 21, 07024–07029. https://doi.org/10.1186/s12864-020-07024-9 (2020).
Yang, H. Y. et al. Transcriptomic and metabolomic investigation of the adaptation mechanisms of blueberries to nitrogen deficiency stress. Sci. Hortic. 321, 112376. https://doi.org/10.1016/j.scienta.2023.112376 (2023).
Sewalt, V. et al. Reduced lignin content and altered lignin composition in Transgenic tobacco down-regulated in expression of L-Phenylalanine Ammonia-Lyase or cinnamate 4-Hydroxylase. Plant. Physiol. 115(1), 41–50. https://doi.org/10.1104/pp.115.1.41 (1997).
Muro-Villanueva, F., Mao, X. Y. & Chapple, C. Linking phenylpropanoid metabolism lignin deposition and plant growth Inhibition. Curr. Opin. Biotechnol. 56, 202–208. https://doi.org/10.1016/j.copbio.2018.12.008 (2019).
Ma, Q. X. et al. Knockdown of p-Coumaroyl shikimate/quinate 3′-Hydroxylase delays the occurrence of post-harvest physiological deterioration in cassava storage roots. Int. J. Mol. Sci. 23(16), 9231. https://doi.org/10.3390/ijms23169231 (2022).
Yu, Y. B. et al. Characterization of caffeoyl shikimate esterase gene family identifies CsCSE5 as a positive regulator of Podosphaera xanthii and Corynespora Cassiicola pathogen resistance in cucumber. Plant. Cell. Rep. 42(12), 1937–1950. https://doi.org/10.1007/s00299-023-03074-x (2023).
Ha, C. M. et al. An essential role of caffeoyl shikimate esterase in monolignol biosynthesis in Medicago truncatula. Plant. J. 86(5), 363–375. https://doi.org/10.1111/tpj.13177 (2016).
Fu, Z. P. et al. Proanthocyanidin-aluminum complexes improve aluminum resistance and detoxification of Camellia sinensis. J. Agric. Food Chem. 68(29), 7861–7869. https://doi.org/10.1021/acs.jafc.0c01689 (2020).
Xing, Q. F. et al. Melatonin-induced plant adaptation to cadmium stress involves enhanced phytochelatin synthesis and nutrient homeostasis in Solanum lycopersicum L. J. Hazard. Mater. 456, 131670. https://doi.org/10.1016/j.jhazmat.2023.131670 (2023).
Wang, H. et al. Glucose enhanced lignin accumulation in grapevine stems via promoting phenylpropanoid biosynthesis. Chem. Biol. Technol. Agric. 11(1), 152. https://doi.org/10.1186/s40538-024-00676-9 (2024).
An, Y. L. et al. Genome-wide identification of the PYL gene family of tea plants (Camellia sinensis) revealed its expression profiles under different stress and tissues. BMC Genom. 24(1), 362. https://doi.org/10.1186/s12864-023-09464-5 (2023).
Gong, W. L. et al. Physiological and transcription analyses reveal the regulatory mechanism in oat (Avena sativa) seedlings with different drought resistance under PEG-Induced drought stress. Agronomy 12(5), 1005. https://doi.org/10.3390/agronomy12051005 (2022).
Xu, C. Z. et al. Auxin-mediated Aux/IAA-ARF-HB signaling cascade regulates secondary xylem development in Populus. New. Phytol. 222(2), 752–767. https://doi.org/10.1111/nph.15658 (2019).
Jia, Z. T., Giehl, R. F. H. & Wirén, N. Nutrient–hormone relations: driving root plasticity in plants. Mol. Plant. 15(1), 86–103. https://doi.org/10.1016/j.molp.2021.12.004 (2022).
Waadt, R. et al. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell. Biol. 23(10), 680–694. https://doi.org/10.1038/s41580-022-00479-6 (2022).
Acknowledgements
This work was supported by the Guizhou Provincial Science and Technology Projects (QKHJC[2024]youth091), Guizhou Provincial Basic Research Program (Natural Science) No. MS(2025)644, Guizhou University Natural Science Special Scientific Research Fund Project (2022-26).
Author information
Authors and Affiliations
Contributions
M. C (first author). wrote the main manuscript text, X. Z. and Z. F. prepared figures and tables. X.Z. Y. and L.T. L. prepared materials. Y.J. J (primary corresponding author) obtained financing for the research. Y.J. J (primary corresponding author) and W.D. W (the joint corresponding author) revised the main manuscript text. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, M., Zhang, X., Feng, Z. et al. Integrated physiological, transcriptomic and metabolomic analysis of the response of Brassica napus L. to pruning residues from tea plant. Sci Rep 15, 27246 (2025). https://doi.org/10.1038/s41598-025-11425-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11425-9