Abstract
Homologous vaccination has proven to be an effective tool to control and eradicate lumpy skin disease. However, their use has been met with some trepidation for diverse reasons, in some regions, and has resulted in the use of heterologous vaccines (sheeppox or goatpox-based). However, conflicting data (field and experimental) raises questions about their effectiveness. As comparative data under standardized conditions are lacking, this study aimed to evaluate four sheeppox-based and one goatpox-based vaccine using a standard vaccination/challenge protocol previously used to evaluate homologous LSDV vaccines. Although some minor differences were observed between the different sheeppox-based vaccines, none of them were able to completely protect the animals against a virulent LSDV challenge, as witnessed by the development of nodules, viremia and the detection of viral genomes in the different organs and tissues. In contrast, the goatpox-based vaccine provided complete protection (no nodules nor viremia) and induced an immunological profile (seroconversion and IFNγ response) similar to the homologous vaccines. Based upon the obtained data it can be concluded that none of the tested sheeppox-based vaccines are suitable for vaccination to prevent LSDV infection, at the dose used, while the goatpox-based vaccine, Caprivac, is. It should, however, be emphasized that this cannot be extrapolated to other goatpox-based vaccines without extensive validation.
Similar content being viewed by others

Introduction
Lumpy skin disease virus (LSDV), or according to the latest ICTV taxonomy Capripoxvirus lumpyskinpox1is the causative agent of lumpy skin disease. It is a double-stranded DNA virus belonging to the genus Capripox of the Poxviridae family2. The virus has a restrictive host preference for cattle (Bos indicus and B. taurus) and water buffalo (Bubalus bubalis). However, the clinical disease has also been reported in Bos gaurus, Bos javanicus3 and Bos grunniens4. Clinical LSD has similarly been reported in several wildlife species, such as Arabian oryx (Oryx leucoryx) and springbok (Antidorcas marsupialis), and it has even been recently isolated from giraffes5. The mortality rate of LSD is generally low, between 1 and 5%, although higher mortality rates have exceptionally been reported6,7,8. Morbidity is more variable, with values between 5% and 50%8,9. Notwithstanding this variability, the disease has a major socio-economic impact due to the direct (milk loss, reduced weight gain, abortion, hide damage, etc.) and indirect income losses and/or costs (trade and market restrictions, reproductive losses, disease preventive and control measures, etc.)10,11. Therefore, it is no surprise that LSD is listed as a notifiable disease by the World Organisation for Animal Health (WOAH). With its spread to the Balkan region in 2015/201612 and its incursion into the Asian continent13,14 the last decade, LSDV has become more and more a global threat to animal health and welfare15.
Vaccination is a crucial tool in preventing, controlling, and finally eradicating many diseases. Commercial LSDV vaccines are based either on LSDV strains, which are referred to as homologous vaccines such as the Neethling vaccine strain, or on the other members of the capripox genus, sheeppox (SSPV) or goat pox virus (GTPV), which are referred to as heterologous vaccines. These can be further subdivided into live attenuated or inactivated vaccines. The efficacy of live attenuated homologous vaccines has been clearly demonstrated during LSDV outbreaks, such as in the Balkans16,17 and under standardized laboratory conditions18. However, apprehension and certain resistance have been observed to the use of these live attenuated homologous vaccines. Most of the issues are related to the safety of this type of vaccine. In general, adverse effects after vaccination have been reported to be minimal19 and mild vaccine-related disease symptoms. This includes small nodule-like structures but these are only observed with very low frequency8,18,20 and are referred to as a “Neethling response.” Also, the potential risk of reversion to virulence of the live attenuated vaccine strain by recombination with virulent strains in the field or issues during vaccine production (e.g. insufficient attenuation, contamination) raised fear as this had already occurred with vaccines for other diseases21,22,23. Despite the extensive use of Neethling-based live attenuated vaccines (LAV), no proof of such reversion to virulence or recombination with field strains under field conditions has ever been reported in the past. More recently, the emergence of recombinant LSDV strains originating from an inappropriate and insufficiently controlled vaccine production24,25 has revitalized these fears. Inactivated vaccines circumvent these issues very effectively, and promising results have been published in laboratory and field conditions26. However, as with many other inactivated vaccines, those developed against LSDV do require an initial two-dose (primer booster) application, and the immunity granted seems to be shorter than with live attenuated vaccines27. This increased vaccination effort imposes additional economic and practical burdens on farmers with small herds (buying more doses than needed) or in regions with free-roaming cattle.
Another solution to alleviate the risk of side effects and safety issues is using heterologous vaccines based on sheeppox or goatpox strains. These could constitute valid alternatives considering the antigenic relationship and the cross-reactiveness of humoral host responses to capripox viruses and the highly restrictive host preference28. This type of vaccine is currently used in several countries to control LSDV. Use of heterologous vaccines, however, leads to the loss of the SPPV/GTPV-free status of the country or zone, what has severe international trade implications. Furthermore, the protective capacity of heterologous vaccines against LSDV is not without controversy. Re-exposure of sheeppox virus RM65-vaccinated cattle to LSDV during the 2006/07 outbreak in Israel resulted in clinical manifestation of the disease29. Similar findings were obtained in Jordan30Turkey11and Egypt31. Therefore, it has been stated that the protection capacity to prevent LSD by the SPPV-based vaccine is less than their homolog counterparts32. However, an extensive comparison between both under standardized conditions is currently lacking. Therefore, this study aimed to evaluate the protective capacity of commercially available SPPV- and GTPV-based vaccines by performing vaccination/challenge animal trials identical in setup to the ones that were done for the homologous vaccines18.
Results
Unvaccinated control animals
Clinical evaluation and scoring (post-challenge)
Following the challenge with the virulent LSDV, all control animals developed a fever with the maximum body temperatures varying between 39.7 °C and 42.2 °C (Supplemental S2). The body temperatures peaked around 8 dpc and decreased afterwards. In 10 out of the 15 animals (67%), body temperatures decreased relatively fast. In these animals, the body temperatures dropped below 39.5 °C in 1 to 4 days after the peak. The remaining animals’ body temperatures remained high (above 39.5 °C) until the end of the trial (20 dpc). Overall, the fever pattern and recovery were similar across the three trials.
All 15 animals developed a local reaction at the site of the ID challenge. Three animals only showed minor local reactions that disappeared after a few days; 7 animals had moderate-sized local reactions, and 5 had large local reactions at the challenge site. For the latter, these reactions remained visible until the end of the trial.
Furthermore, 7 out of the 15 animals, equally distributed among the three trials (2, 2, and 3, respectively), developed nodules after the challenge, of which 85% (6/7) remained permanently visible. The nodules on one animal in the first trial disappeared after four days. The onset of the formation of nodules was between 6 dpc and 10 dpc, with a generalization (spreading over the entire body) seen 1 to 7 days after the first nodules appeared. Nodules were seen on all the animals with a large swelling at the intradermal challenge site. Two additional animals with a moderate-sized swelling developed nodules as well. The mean size of the swelling at the inoculation site therefore was significantly larger in animals which developed nodules than those that did not (T-test; P < 0.001). A similar association was also found between animals which developed nodules and the observed rectal temperature after the challenge. The animals with nodules had a prolonged fever and had a significantly higher fever between 6 and 9 dpc (T-test; P < 0.001). Also, the mean total clinical score during the period post-challenge was significantly higher between animals with permanent nodules and animals without nodules (T-test; P < 0.001). The total clinical score of animals with permanent nodules reached 5 (n = 1) to 7,5 (n = 5) (Fig. 1) while this did not exceed 3 for the animals which did not develop permanent nodules. The animal with the temporary nodules, in the first trial, reached a max score of 4, on 10 dpc and is situated between both groups.
Virology and serology
Viremia was seen in all animals with permanent nodules (n = 6) and in none of the animals without (n = 8) or temporary (n = 1) nodules. The onset of viremia varied between 3 dpc to 7 dpc and lasted until the end of the trial. The mean number of LSDV qPCR positive organ/tissue samples collected at necropsy (excluding skin samples as these can be externally contaminated by direct and indirect contact) was significantly higher in animals with permanent nodules (clinical, average of 64%) and those without (non-clinical, average of 11%) (T-test; P < 0.001). This is also reflected by the prevalence of the virus in the various sample types, as summarized in Table 1. In addition, the viral load in the positive samples was also higher in the clinical compared to the non-clinical animals. The Ct difference between animals with and without nodules varied between 1.9 and 12.8 with an average of 6.3 and a standard deviation of 3.8 among the different organs/tissues.
The onset of seroconversion, as detected with IPMA, varied between 3 and 12 dpc, with 100% of the control animals becoming positive. All animals remained positive until the end of the trial. The time necessary to seroconvert was similar between animals with and without nodules (Supplemental S3). Neutralizing antibodies were detected later with VNT1 and VNT2 than with the IPMA. The first detection of neutralizing antibodies with VNT1 was done at 10 and 14 dpi for the clinical and non-clinical animals. Two non-clinical animals did not seroconvert in the VNT1 before the end of the trial, while all clinical animals had neutralizing antibodies at the end of the trial. The onset of seroconversion with VNT2, was similar to VNT1, namely 11 dpi (clinical animals) and 13 dpi (non-clinical animals). While all the clinical animals became positive before the end of the trial, four of the non-clinical control animals did not. These include the two that remained negative in VNT1.
Vaccinated animals
Clinical evaluation and scoring: post-vaccination
At the moment of the vaccination, two animals in the Abic group and one in the Jovivac group had a fever and were therefore excluded when calculating the fever parameters (T, Tmax and Ntdays; Supplemental S4) in the post-vaccination period. In general, only a limited rise in body temperatures was seen in the post-vaccination period, notwithstanding the large number of days with elevated body temperatures. However, when calculating the average body temperatures on group level on those days, it never exceeded 39,4 °C. This is also reflected by the number of days the body temperature exceeded 40 °C, namely between 2 and 10 days on a group level. In other words, less than 1 day per animal (from 0.3 to 0.9 days/animal). The sole exception is in the Abic group, where this was 1.4 days per animal. Although the average number of days that animals showed fever in the 3 weeks post-vaccination varied between 3,2 days in the Jovivac group and 9,8 days in the Abic group, the important variation between individual animals made that there was no significant difference between all vaccine groups (ANOVA; p = 0.18). The average total days of elevated body temperatures on group level (Ntdays / total number of animals per vaccine group used for the calculations) varied between 3.2/4.9 (Jovivac, Romania, Caprivac), 6.6 (Penpox-M) and 9.8 (Abic).
No other clinical signs were seen in the period post-vaccination. Food uptake, general behaviour, and general health status remained normal. No enlargement of any of the lymph nodes and no local swelling at the site of vaccination was seen. This is reflected in the total clinical scoring (Supplemental S5), whereby the average daily score doesn’t surpass 0.5 on the group level and on individual level 1, with one exception of 1.5. All the scoring is attributed to elevated body temperatures. The minimal impact of the vaccination on animal health is similarly demonstrated with the minimal difference observed in total scoring compared to an untreated control group, CON3, in the period before the challenge.
Clinical evaluation of the vaccinated groups in the period post-challenge
Elevated body temperatures were seen in the first 3–4 days after the challenge which can be caused by the stress of the challenge itself and is, therefore, not necessarily due to the virus. However, a clear fever spike was seen around 7–8 dpc in almost all vaccinated animals (Supplemental S6). This fever spike is similar to the one seen upon the challenge of the unvaccinated control animals (Supplemental S7) and is caused by the challenge virus. Significant differences existed in the average number of days animals showed fever upon infection between the different groups (One-way ANOVA; P = 0,008). The mean fever period was 8, 8 and 6 days in the Jovivac, Penpox-M and Caprivac groups, respectively, and the body temperatures decreased quickly after the initial fever spike and returned to below 39.5 °C at 10 dpc in all animals. In contrast, the average fever period was 14 and 11 days in the Abic and Romania groups, respectively, with 3 and 1 animal still having a fever at 21 dpc. This is also reflected by the number of fever days (Ntdays; Supplemental S6). The average body temperatures are represented in Fig. 2.
A reduced feed uptake was only observed in one animal of the Abic group between 6 and 10 dpc, while the other vaccinated animals displayed a normal feeding pattern. With the GTPV-based vaccine Caprivac, only slight swellings were seen at the challenge site, and all disappeared by the end of the trial. This is in contrast to the SPPV-based vaccines where moderate to large (Jovivac, Penpox-M, and Abic) or even very large swellings (Romania) developed in 4, 5, 6, and 4 animals, respectively. While the slight swellings healed and disappeared, these larger swellings remained present at necropsy in all but one. This is also reflected by the fact that (i) a significant difference was found in the average swelling at the challenge site in the period post-challenge in animals from the different vaccine groups (ANOVA; p = 0.002), that (ii) T-tests showed that the mean swelling was significantly smaller in the Caprivac group compared to all other vaccines (p values < 0,05), and that (iii) also animals in the Jovivac and Romania group showed smaller swellings than those in the Abic group (p values < 0,05). The latter was not the case between the Penpox and Abic group (p = 0.10).
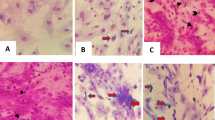
An important characteristic of LSD is the development of nodules following infection, as shown by the control animals (7 out of 15 animals showing nodules). No nodules were observed in any of the 7 animals vaccinated with the GTPV-based vaccine Caprivac. Despite the limited number of animals that could be tested in these experimental settings, this finding was borderline significant (Fisher exact test; p = 0.05). This was also in line with other clinical observations in the group of Caprivac-vaccinated animals. For the SPPV-based vaccines, however, nodules were observed in several animals in all groups. In the Abic group, six animals (86%) developed nodules corresponding to the six animals with moderate to large swellings. Generalization of the nodules occurred in 5 of the six animals (Fig. 3). The LSDV status was confirmed by RT-PCR analysis on biopsies taken from all six animals between 14 and 20 dpc with Ct-values of 13 to 19.
In the group vaccinated with Jovivac, two animals developed nodules that generalized in one animal but remained localized and limited in number in the other. In the Penpox-M group, 4 out of 7 (57%) animals developed nodules, but these remained localized. In the Romania group, three animals developed nodules, with two having generalized nodules. This means that no significant difference was found in the number of animals that developed nodules between the control and the different SPPV-based vaccine groups (Chi Square test; p = 0.268).
An overview of the individual clinical scores can be found in the figure below. For the latter three groups, the nodules were confirmed by RT-PCR analysis of biopsies taken at necropsy with Ct ranges of 14–15, 21–35, and 21–25, respectively. The Ct values in the Penpox-M group were higher than the rest and could reflect their more localized nature. Total clinical scores of all vaccinated groups are summarized in Fig. 4.
Virological analyses in vaccinated animals
Using RT-PCR, no vaccine viremia was detected in any of the vaccinated animals during the post-vaccination period.
Also, no viremia could be detected by RT-PCR in the animals vaccinated with Caprivac after the challenge. On the other hand, at least one animal tested positive on the panCapx RT-PCR in each group of animals immunized with an SPPV-based vaccine (Fig. 5). To be more precise 6, 3, 3 and 4 animals became viremic in the Abic, Jovivac, Romania, Penpox-M group, respectively, after the challenge. All the viremic animals in the Abic and Romania group developed the LSDV typical nodules. In the Jovivac group, 2 animals were clearly viremic and developed nodules. However, one additional animal had intermittent positive blood samples (on 7, 10, 14, and 15 dpi). Although the last three positives were borderline (Ct-values between 39 and 40), the first detection on 7 dpi had a Ct of 31.53 which was confirmed as wild-type LSDV by the DIVA RT-PCR. However, no nodules were observed on this animal. For the Penpox-M group, three viremic animals developed nodules, while no nodules were observed on the other animals. However, one of the animals without nodules did develop a viremia albeit with relatively high Ct-values, ranging between 36 and 40 with the exception on 11dpc with a Ct of 33. Nevertheless, these genomic detections spanned from 4 to 15 dpc and therefore this animal can be considered subclinically infected like the one identified in the Jovivac group. Furthermore, one animal had developed local nodules without developing a real viremia. At least one blood sample of each viremic animal in this group was confirmed to be wild-type LSDV by a DIVA RT-PCR. All the other animals without any nodules showed no viremia after the challenge.
A similar pattern is seen when looking at the internal organs/tissues at necropsy. A significant difference was observed in the mean number of positive organs per animal between the heterologous vaccines (One-way ANOVA; p < 0.001). For the Abic vaccine, on average 56% of the organs/tissues were positive per animal which was significantly higher than in all other groups (T-tests; p < 0.05); for Jovivac and Penpox-M this was 11 and 10%, respectively. The positivity of the organs/tissue from Romania-vaccinated animals is situated in between with 29%. All of sheeppox-based vaccines had more organs/tissue that tested positive in the Pan RT-PCR than the GTPV-based vaccine in which no internal organs were found qPCR positive. This was also significantly lower than the Romania group (T-test; p < 0.01), but not when compared to the Jovivac and Penpox groups (T-tests; p = 0.1). These results are summarized in Table 1.
There is also a significant difference in the number of positive internal organ/tissue samples between clinical and subclinical animals versus non-clinical animals when considering all vaccine groups together (T-test; p < 0.001). When looking within each vaccine group separately, this is only significant for the Romania vaccine (Mann-Whitney U test; p = 0.049), probably due to the low number of animals per group (Supplemental S8). For this calculation, skin samples, tongue and mucosa were excluded to avoid the impact of contamination from the stable environment to the animal. The sub-clinically infected animals in the Jovivac and Penpox-M group clearly had more positive organ/tissue samples than the animals without viremia and nodules. This further confirms their subclinical status. Interestingly, no internal organs/tissues were positive from the animal vaccinated with Penpox-M which had developed nodules without a viremia. Both the nodule and skin at the inoculation site collected from that animal had a Ct value of 29.
Seroconversion in vaccinated animals
The onset of seroconversion as detected by IPMA was 13, 7, 10, 17, and 9 dpv for the Abic, Jovivac, Penpox-M, Romania, and Caprivac groups, respectively. At the moment of the challenge (21 dpv), complete seroconversion was only seen in the GTPV-based vaccine (Caprivac). The groups vaccinated with SPPV-based vaccines (Abic, Jovivac, Penpox-M, and Romania) had a seroconversion rate of 29%, 57%, 71 and 29%, respectively, at the time of challenge. When considering all animals, there seemed to be a correlation between the seroconversion status (positive or negative) measured by IPMA at the moment of challenge and the presence/absence of nodules after challenge, although this was not statistically significant (Chi-square test; P = 0.08). Nevertheless, a significant difference existed in the mean titer of the seroconverted animals at challenge between vaccine groups (Kruskal-Wallis test; P = 0.0048), with the titers being rather low (varying between 1/50 and 1/120) for the SPPV-based vaccines, while being higher (between 1/120 and 1/960) for the Caprivac vaccinated group. After the challenge, all animals vaccinated with the SPPV-based vaccine seroconverted before the end of the trial and this between 6 and 10 dpc. The mean titer at the end of the trial was the lowest in the Romania group (1/2700), followed by the Jovicac group (1/3770) and the Abic group (1/4640). The highest mean titer from the SPPV-based vaccines was found in the Penpox-M group (1/5350). Like the SPPV vaccines, a booster effect was seen in the titer for the GTPV-based vaccines after the challenge. The mean titer increased from 1/720 at the time of challenge to 1/2740 at the time of euthanasia. These results are summarized in Fig. 6.
No neutralizing antibodies were seen in both VNT methods (VNT1 and VNT2) in the period post-vaccination in the animals vaccinated with the SPPV-based vaccines. The first neutralizing antibodies in those groups were detected between 6 and 10 dpc in the VNT1 and between 9 and 13 dpc in the VNT2. Even by the end of the trial, no complete seroconversion, as detected by both VNT methods, was observed in those groups. Similar to IPMA data, a different picture was seen for the GTPV-based vaccine on VNT. Neutralizing antibodies were detected prior to the challenge. One animal became positive on 15 dpv (on both VNTs) and two more on 17 dpv (in one of the two methods). By the end of the trial, 6 of 7 animals had neutralizing antibodies according to the VNT 1 and 4 out of 7 animals in the VNT2.
Cellular immune response in vaccinated animals
Clear differences can be observed in the IFN-γ responsiveness after vaccination between the different vaccines upon restimulation of the heparinized blood samples in the period post-vaccination (Fig. 7). Almost no IFN-g response could be observed after restimulation in the Abic group, with only one weak responding animal at 7/8 dpv after vaccination. More animals (5/7) became responsive in the Romania group, but the response was weak in general. In the Jovivac and Penpox-M group, all animals were responsive at least at one day, and the reaction was moderate to strong in 3 and 4 animals, respectively. A more homogenous IFN-γ response was seen in the Caprivac group. Six animals showed a moderate to strong IFN-γ secretion after stimulation which mostly lasted multiple days. Besides the difference in the number and strength of responders, Caprivac seemed to induce a longer CMI response than the SPPV-based vaccines. At the moment of challenge (21 dpv), 5 animals in the Caprivax were still responsive (2 strong, 2 moderate, 1 weak) while this was 0 animals for Abic and Romania groups, one animal (weak) for Jovivac and three animals for Penpox-M (1 strong, two weak). Despite these observed differences, the mean IFN-y response per animal in the different vaccine groups during the period of 6 to 15 dpv was just not significantly different (One-way ANOVA; p = 0.059). Only the number of days that animals where IFN-y positive in this period were significantly lower in the Abic group compared to all other vaccine groups (Fisher exact tests; p < 0.05). Furthermore, no correlation was found between the presence or absence of nodules and being at least once IFN-y positive during the period post vaccination when all groups were considered together (Chi square test; p = 0.49).
Discussion
This study evaluated five heterologous live attenuated vaccines using a standardized challenge protocol. Four of 5 heterologous vaccines were based on SPPV, more precisely RM65 (Jovivac and Abic), Romania, and Bakırköy (Penpox-M) strains. These vaccines are mainly used in the Middle East, Asia, and the Horn of Africa (RM65), India and the Maghreb region (Romania), and Turkiye (Bakırköy)33. Due to difficulties with availability, only one GTPV vaccine, based on the Gorgan strain (Caprivac), could be included. To compare these vaccines with the results from live attenuated homologous vaccines, the experimental setup for the animal trials was kept identical to that previous study18. This also includes the limitations of this type of study, namely the number of animals used per group and the fact that only young bulls were included. As explained in Haegeman et al.18there is no indication that these should impact the results. Due to the number of animals in this study, three separate animal trials were conducted, each including an unvaccinated challenged control group. The challenge can be considered successful and homogenous among the three animal trials, as two/three control animals per trial developed noduli. Furthermore, all animals developed a fever which peaked around eight dpc and a significant link was observed between the severity of the disease and local reaction at the inoculation site. This is in agreement with previous studies 51 and also similar to the data of the homologues vaccine study18further supporting the comparability of the results of both studies.
All heterologous vaccines were well supported by the vaccinated animals as no impact was seen on feeding behaviour, and no local reactions nor nodules were observed. In general, only slightly elevated body temperatures were observed during the post-vaccination period. Although the number of animals included is limited, and therefore caution is needed, it is in line with other studies using heterologous vaccines34,35,36,37,38,39. It also needs to be mentioned that certain parameters, such as milk production, could not be evaluated as all the animals in this study were young bulls. A reduction in milk production, up to 5 weeks after vaccination with an RM65-based vaccine, was reported previously40. These limited side effects of vaccination with heterologous vaccines contrast with homologues vaccines where the fever was more pronounced, with sometimes fever peaks similar as those seen in response to a challenge, and the development of nodule-like structures (aka Neethling response) in a limited number of vaccinated animals18. This suggests that heterologous vaccines provoke fewer adverse effects than LSDV-based vaccines. This could be related to a reduced capacity of the live heterologous strains to infect and replicate in cattle. This is supported by the lack of vaccine genome detection in the blood upon vaccination with the heterologous vaccines. Detection of genomic material from LSDV-based vaccine strains in the period post-vaccination was reported earlier41 and was also observed during the evaluation of the homologues vaccines18. Except for the animals with a Neethling-response, these detections were on isolated time points with high Ct -values. Therefore, it could not be excluded that the injected material was detected. However, with the heterologous vaccines in this study, no vaccine genome could be detected, although the inoculation route and the TCID50/dose were similar (for example, Lumpyvax vaccine 104 TCID50/dose versus Abic or Jovivac 103.5–4 TCID50/dose). For the Romania vaccine, the viral load was even higher, with a TCID50/dose of 105, so the lack of detection cannot be explained by a lower genomic load injected in the animals. This data is in agreement with previous published studies with heterologous vaccines37. The inability to detect injected vaccine DNA is an interesting finding as it would support diagnostics and a DIVA approach, as its detection can be misleading, cause confusion, or result in wrong interpretation42,43. In general, it can be stated that heterologous vaccines do provide a slightly better safety profile than their homologous counterparts. While the earliest onset of seroconversion with the heterologous vaccines, as determined with the IPMA, around 7 dpv is similar to that of the homologous vaccines, the proportion of animals that had seroconverted by the moment of challenge (21 dpv) was much lower for the SPPV-based vaccines. Only Penpox-M achieved a comparable rate. In contrast, the GTPV-vaccinated animals all had seroconverted by 21 dpv. The antibody titers also reflect this difference between the heterologous and the homologous vaccines at that time point. While SSPV-based vaccines had much lower titers, the GTPV-based vaccine achieved titers similar to the homologous vaccines. The lower stimulation of the humoral response by the SPPV-based vaccines was also observed in both VNTs, as none of the vaccinated animals seroconverted before the challenge in these tests. This lack of neutralizing antibodies was similarly reported for Penpox-M after 31 dpv37 and the Romania strain even after 40 dpv36. On the other hand, limited seroconversion in cattle was previously reported for RM65 and Romania vaccines around 21 dpv39,44,45,46. These data suggest that the humoral response elicited by the SPPV-based vaccines is lower than that of GTPV-based and LSDV-based vaccines. This is also supported by the data from a duration of immunity study using an SPPV-based vaccine where a clear drop in seroconversion rate was seen as early as six months post-vaccination47. This is much quicker than what was observed with the LSDV-based vaccine. For the latter, the antibodies remained present for up to 1.5 years27. However, it needs to be mentioned that protection against a capripox virus challenge can be seen in the absence of neutralizing antibodies, which is in line with the idea that immunity against capripox viruses is mainly driven by cellular immunity48. In this study, the CMI response was addressed by looking at the IFN-γ response after restimulation of heparinized blood with the virus during the post-vaccination period. The SPPV-based vaccines elicited a weak (Abic, Romania) to moderate (Jovivac, Penpox-M) IFN-γ response upon restimulation with LSDV. This is in agreement with previous published findings45. The responsiveness is generally lower than with homologous vaccines, especially at the moment of challenge (21 dpv). Similar to the humoral response, the cellular response with the GTPV-based vaccine was more pronounced and mimicked that of LSDV-based vaccines. Combining all these data, it can be stated that the induced immune response in cattle by SPPV-based vaccines is more limited than the response induced by homologous LSDV and heterologous GTPV-based vaccines. The high immunogenicity of the GTPV-based vaccine used in this study is in line with previously published findings34.
We found that the induced humoral and cell-mediated immune responses following vaccination predict the outcome of the challenge. None of the SPPV vaccines was able to protect all the animals against the virulent LSDV challenge as multiple animals, ranging from 2 to 6, developed nodules. Interestingly, the nodules that developed in the Penpox-M vaccinated group remained localized in contrast to the other vaccinated groups, where generalization of the nodules was seen in at least one animal. It is tentative to link this to the moderate IFN-γ response induced in this group compared to the low IFN-g response induced by the other SPPV-based vaccines. The failure of the SPPV-based vaccines to protect cattle also corresponded with the viremia that was detected by real-time PCR and the broad dissemination of the virus to internal tissues and organs taken at necropsy. Interestingly, besides clinical animals, also subclinical LSDV-infected animals were found in the Jovivac and Penpox-M vaccinated groups. This poses significant issues for the efficacy of these vaccines as subclinical animals can still transmit LSDV, as was recently demonstrated49,50.
The Penpox-M vaccine was administered at 3 times the dose in our study, as communicated at the time by the manufacturer, while the other SPPV-based vaccines were given at 10 times the dose. This could be an essential factor as protection was seen when administering Penpox-M at five times the dose37. However, in the study in question, the number of animals vaccinated (namely three) was very limited, especially since in most experimental conditions, only 50% of unvaccinated controls get clinical disease51,52. The failure of SPPV-based vaccines to completely protect against an LSDV challenge in our study agrees with previous data obtained under experimental conditions35,53 or in the field30,31,32,54,55. Heterologous vaccines based upon GTPV seem more promising as, in this study, they fully protected the vaccinated animals. Since only one GTPV-based was included in this study, caution is needed not to over-generalize these findings towards all GTVP-based vaccines on the market, although our data are supported by other studies34,35. The reason why GTPV-based vaccines could protect better than SPPV-based vaccines is unknown, but a closer genetic relationship to LSDV has been put forward35.
Although additional research regarding IFN-responsiveness is necessary, our results point to a tentative correlation between the number of responders and the intensity of the response on a group level, and the protective capacity of a vaccine. A strong IFN-γ response at a group level is indicative of an excellent protective capacity, as is seen with the homologous and GTPV-based vaccines, while a weak or moderate response results in partial or no protection. Such a link has also been reported for other diseases56. This link is however less straightforward at an individual animal level. For example, four animals (two in the Jovivac and Penpox-M group) reacted strongly after vaccination but nevertheless became viremic. One developed local noduli, while the other three remained sub-clinically infected. In addition, several animals remained clinically healthy in the absence of an IFN-g response. This shows again that additional and currently unknown factors play a role in LSDV immunity.
In summary, the data obtained during this study showed that heterologous SPPV-based vaccines did not offer complete protection in cattle against a virulent LSDV challenge. In contrast, the heterologous GTPV-based vaccine, Caprivac, provided complete protection similar to homologous LSDV-based vaccines.
Materials and methods
Challenge virus and cell line
The LSD/OA3-Ts.MORAN strain, kindly provided by the Kimron Veterinary Institute, Israel, and the Israeli veterinary services, was propagated on an ovine testis cell line (OA3T) according to Babiuk et al.57.
An 80–90% confluent cell culture flask (175 cm2) was inoculated with 200 µL LSDV (105/mL TCID50) in 20 mL growth medium (DMEM + 2% FCS containing fungizone 1 µg/mL and gentamycin 20 µg/mL). After four days of incubation at 37 °C and 5% CO2, a freeze/thaw cycle was done, followed by centrifugation for 10 min at 2000 g. The supernatant was collected and stored in liquid nitrogen. The titer was determined as described below.
Serological analysis
Serum samples collected during the different trials were examined for the presence of anti-LSDV antibodies utilizing the immunoperoxidase monolayer assay (IPMA) and virus neutralization tests (VNT). For the latter, two different VNT methods were applied. In the first method, the test serum was diluted in growth medium, starting from 1:2, and titrated against a constant concentration of a reference LSDV strain (100 TCID50). Each dilution is tested in duplicate. The result is expressed as the highest dilution wherefore at least 1 of the duplicates is neutralized. In the second method, the volume of test serum is kept constant but is titrated against a reference LSDV strain. The neutralization index (NI) was calculated as the log titer difference between the titer of the virus in a negative serum and in the test serum. In the text, these methods are referred to as VNT1 and VNT2 respectively. All serological test were described in detail in Haegeman et al.58. A test serum is considered to be positive on VNT 1 as the titer is 1:50 and on VNT2 as the NI is > = 1.5.
Antibody titrations were carried using a two-fold serial dilution of the test sera. The antibody titer was expressed as the highest sera dilution being positive.
Virological analysis
Viral DNA extraction
Capripoxvirus DNA from blood (200 µl), tissue and organ samples (25 mg) was extracted using the NucleoSpin Blood and NucleoSpin tissue kits (Macherey-Nagel) respectively, as described by the manufacturer except for (a) the incubation time for lysis of blood samples was prolonged to 1 h; (b) an external control (EC) was added to B3 buffer before extraction; (c) organ samples were homogenised using a TissueLyser (Qiagen) in the presence of buffer T1 and proteinase K before the 3 h incubation at 56 °C.
Real-time-PCR (RT-PCR)
The panCapx PCR panel, described in Haegeman et al.59was used to investigate the presence of the viral capripox genome in EDTA blood, or tissue. The samples were first screened with the D5R real-time PCR. Confirmation with the E3L and J6R real-time PCRs was carried out in the following cases: (1) Ct values close to the cut-off (Ct > 37) and (2) in case of sequential samples (such as EDTA blood): the first negative results preceding and succeeding a time series of positive samplings. The final status of a sample is considered positive if the Ct is < 37 or a Ct value > 37 is obtained in at least 2 out of the three real-time PCRs of the panel.
The DIVA real-time PCR (DIVA RT-PCR) described in Agianniotaki et al.60 was used to investigate whether the viral DNA was derived from a vaccine or a wild-type strain.
Virus isolation and Titration
Virus isolation was carried out on EDTA blood and organ/tissue samples. The samples were pre-treated as follows: (1) 80 mg of an organ/tissue was placed in an Eppendorf tube where 500 µl PBS and silicon carbide beads (1 mm diameter) were added. Homogenization was done in a Tissue Lyser by agitating for 6 min at 25 Hz. An additional round of homogenization was performed if necessary, after a visual inspection of the sample. Subsequently, 600 µl PBS was added to the homogenized tissue/organ. The supernatant was filtered (0,45 μm) after a short spin (max speed); (2) 1 ml sterile PBS was added to 250 µl of EDTA-blood in an Eppendorf tube and centrifuged for 2 min at 2000 rpm. The supernatant was transferred to a new Eppendorf, and 1 ml of sterile water was added.
Homogenized tissues or treated blood were further diluted 1/5 in PBS with antibiotics (Gentamycin [10 mg/ml] and Amphotericin B [250 µg/ml]).
The OA3.Ts cells were prepared in a 25 cm² flask the day before and should reach 80–90% confluency. The cell culture medium was removed, and equal volumes of pretreated sample (500 µl EDTA-blood or 1 ml organ/tissue) and new cell culture medium were added. Nine ml of culture medium (DMEM) was added after 30 min of incubation. Cells were then incubated for six days before they were tested for the presence of LSDV using the IPMA technique.
Titration of the virus was done in 96-well plates on OA3.Ts cells. Seeding was done at 104 cells/well concentration to achieve 90% confluence after 24 h at 37 °C and 5% CO2. One hundred microliters of a tenfold serial dilution of the sample, with ten replicates per dilution, were added to the plate. The visualization of potential viral plaques was done via the IPMA technique after three days of incubation. Wells were marked as positive if one or more viral plaques were seen. Virus titers were calculated as described by Spearman-Kärber61,62.
Animal trial, clinical scoring, and sampling design
Vaccines
This study used four commercially available sheeppox and one goatpox-based live attenuated vaccine: (1) Sheep pox Vaccine (SPPV-based; Abic, Phibro Vaccines; Israel; Batch 27621004), (2) Jovivac (SPPV-based; Jovac; Jordan; Batch 200115-01), (3) Penpox-M (SPPV-based; Pendik institute of veterinary control; Turkey; Batch 03/2015), (4) Romania (SPPV based; MCI Santé Animale; Morocco) and (5) Caprivac (GTPV-Based, Jovac, Jordan; batch 210115-01). An acronym was allocated to each vaccine (LAV4, 5, 8, 10 and 7) before the start, allowing anonymous testing in the animal facilities and during the statistical analysis. In this manuscript, the vaccines are referred to as Abic (1), Jovivac (2), Penpox-M (3), Romania (4), and Caprivac (5). While the Romania vaccine was produced specially to be used in cattle against LSDV, the others were so for sheep- and goatpox. By adjusting the amount of diluent used for vaccine reconstitution and/or the volume injected into cattle, these vaccines can also be applied against LSDV. For Abic, Jovivac and Caprivac, this was 10 times the dose for sheep- or goatpox while this was 3 times the dose for the Penpox-M vaccine. All the vaccines were stored, handled and applied in cattle against LSDV as described or communicated by the manufacturer.
Animal trial and design
This study regarding the efficacy of live heterologous vaccines was conducted in parallel with another, previously published, study about homologous vaccines18. As mentioned in Section Vaccines, all the vaccines received an acronym and were randomized to ensure anonymity. This resulted in some of the heterologous vaccines being tested together with homologous vaccines in the same trial. In total 3 different animal trials were conducted, for practical reasons, containing heterologous vaccines. Two out of these three also contained homologous vaccines, thereby sharing the control animals (CON1 and CON2). The results of these two control groups have been reported earlier18 but are also included in this study for clarity and comparative purposes. A third trial didn’t include homologous vaccines and the results of the controls (CON3) are reported here.
The animal trials were performed in the vector-free BSL3 stables of Sciensano. The set-up of all animal trials in this study and in the one about the homologous vaccines were identical to allow comparisons between both types of vaccines (heterologous versus homologous). Approximately 6-month-old male Holstein bulls between 250 and 300 kg were brought into the stable for an acclimatization period of 5 to 7 days. The animals were obtained from the same farm and were tested free of BVD and IBR. Seven animals were included in the study for each vaccine. Vaccination was done according to the manufacturer’s instructions at the end of the acclimatization period. Twenty-one days after vaccination, the animals were challenged with a virulent clade 1.2 Israeli LSDV field strain (LSD/OA3-Ts.MORAN; log titer 7.5–8 TCID50/mL ) by intravenous (5 mL, vena jugularis) and intradermal (ID; 1 mL) inoculation. The intradermal injection was performed at 2 locations on both sides of the neck (250 µL per site). Five additional non-vaccinated animals were included in each of the trials and were challenged at the same time via the same method. In these trials, these animals served as the challenge-control animals (Groups CON1, CON2, and CON3). All animals were monitored and sampled for 20/21 days after the challenge.
Following the fixation of the animals with a halter rope and sedation by the captive bolt, euthanasia was carried out by electrocution after moistening of the chest and head with water. Euthanasia was carried out on: 20 dpi (Abic); 29 dpi (Jovac); 27/29 dpi (Penpox-M); 23/25 dpi (Romania) and 22 dpi (Caprivac).
All animal experiments were conducted according to the European Union and Belgian regulations on animal welfare in experimentation and is in line with the ARRIVE guidelines. The Joint Ethical Committee of Sciensano approved the protocol, authorization number 20150605-01_EC_Dierproef aanvraag_LSDV_BMG_2015.
Clinical evaluation and scoring
All animals were clinically evaluated daily during all phases of the trial (acclimatization, post-vaccination [dpv], and challenge [dpc]). The clinical evaluation was based on different parameters as detailed in Haegeman et al.18 (Supplemental S1). A total clinical score was calculated based on these values.
Nodules are one of the main clinical signs of lumpy skin disease. Vaccination might sometimes cause the formation of nodules like those seen in infected cattle. However, these nodules remain smaller and disappear after 1–2 weeks. This reaction is known as the Neethling response. After the challenge, two types of nodules are seen. The first are permanent nodules. After a nodular phase, these nodules become lesions with scabs and may even become necrotic. They remain visible for a very long time. Other nodules disappear after a couple of days without the formation of lesions. These are called temporary nodules in this experiment.
Sampling
Samples for laboratory evaluation were collected: (1) once during the acclimatization period (between 3 and 5 days prior to vaccination); (2) On the day of vaccination but before the injection (0 dpv); (3) 3 times a week during the post-vaccination period; (4) on the day of challenge but before injection (0 dpc); (5) daily from 5 to 15 dpc and intermittent before and after this period. These include EDTA blood, serum, and heparin blood. Biopsies were taken when nodules first appeared to confirm the presence of LSDV. At necropsy, several tissue and organ samples were collected per animal (cf. Table 1); for the vaccinated animals, the site of vaccination was sampled as well. When the animals had developed nodules, a part of the skin with no nodules in the vicinity (more than 15 cm) was taken and is referred to as “normal skin.”
IFN-γ -release after stimulation
The cellular immune response was followed by the stimulation of heparinized blood samples collected during the animal trials. This test was described in detail by Haegeman et al.49. In brief,
whole blood (heparinzed, 1500 µl) was stimulated with either LSDV (100 µl at 106.8 TCID50/ml), PBS (100 µl) or Pokeweed Mitogen (PWM; 160 µg/ml dissolved in 1x PBS) and left to incubate overnight at 37 °C and 5% CO2. Plates were centrifuged for 10 min at 500 g, and 500 µl supernatant was collected and stored at -20 °C until testing.
Interferon-gamma (IFN-γ) was detected using the BOVIGAM® 2G kit (Thermofisher) using the manufacturer’s instructions and Parida et al.56. The obtained OD values were corrected using the negative PBS control: ODcorrected = OD virus–OD PBS. The cut-off for positivity was set to 0.3. Samples, wherefore the positive control (PWM-stimulation) was negative, were rejected. An additional classification of the observed response was carried out. The IFN-γ response was considered strong, medium and weak when the corrected OD was > 2; between 1 and 2; and < 1, respectively. The IFN-γ response was translated to points as follows: weak = 0.5, moderate = 1, and strong = 2.
Statistical analysis
One-way ANOVA tests were used to assess whether significant differences were present between different vaccine groups for parameters like total clinical score, duration of fever, swelling at the inoculation or challenge site, and number of positive organs at euthanasia. When these parameters were compared for only 2 vaccine groups or between animals with and without nodules, this was done by Student T-tests. A Kruskal Wallis test was used to compare the mean antibody response level between seroconverted animals in the different vaccine groups at the moment of challenge. For within vaccine group comparisons, non-parametric Mann-Whitney U tests were used since only 7 animals were present per vaccine. Chi-square tests and Fisher exact tests were used to evaluate potential significant differences in the number of animals that developed nodules between multiple or two vaccine groups, respectively. Chi-square tests were also used to check potential correlations between the clinical outcome and, respectively, the antibody and the IGRA response. Statistical analyses were performed in GraphPad Prism 9 and differences were considered significant if p < 0.05.
Institutional review board statement
This study was authorized and supervised by the Biosafety and Ethical Committee of Sciensano, Brussels, Belgium, approval 20150605-01.
Data availability
The main data presented in this study are available within the study itself and other data may be made available through contact with the corresponding author.
References
Current ICTV Taxonomy Release | ICTV. https://ictv.global/taxonomy.
Tulman, E. R. et al. The genomes of sheeppox and goatpox viruses. J. Virol. 76, 6054–6061 (2002).
Porco, A. et al. Case report: Lumpy skin disease in an endangered wild Banteng (Bos javanicus) and initiation of a vaccination campaign in domestic livestock in Cambodia. Front. Vet. Sci. 10, 1228505 (2023).
Sudhakar, S. B. et al. Emergence of lumpy skin disease virus (LSDV) infection in domestic Himalayan Yaks (Bos grunniens) in Himachal pradesh, India. Arch. Virol. 169, 51 (2024).
Dao, T. D. et al. Characterization of lumpy skin disease virus isolated from a giraffe in Vietnam. Transbound. Emerg. Dis. 69, e3268–e3272 (2022).
Farah Gumbe, A. A. Review on lumpy skin disease and its economic impacts in Ethiopia. J. Dairy Vet. Anim. Res. 7 (2018).
Elhaig, M. M., Selim, A. & Mahmoud, M. Lumpy skin disease in cattle: Frequency of occurrence in a dairy farm and a preliminary assessment of its possible impact on Egyptian buffaloes. Onderstepoort J. Vet. Res 84 (2017).
Tuppurainen, E. S. M. et al. Field observations and experiences gained from the implementation of control measures against lumpy skin disease in South-East Europe between 2015 and 2017. Prev. Vet. Med. 181, 104600 (2020).
Biswas, S. et al. Extended sequencing of vaccine and wild-type capripoxvirus isolates provides insights into genes modulating virulence and host range. Transbound. Emerg. Dis. 67, 80–97 (2020).
Tamire, M. Current status of lumpy skin disease and its economic impacts in Ethiopia. Res. Vaccine J. 1, 103 (2022).
Şevik, M. & Doğan, M. Epidemiological and molecular studies on lumpy skin disease outbreaks in Turkey during 2014–2015. Transbound. Emerg. Dis. 64, 1268–1279 (2017).
Mercier, A. et al. Spread rate of lumpy skin disease in the balkans, 2015–2016. Transbound. Emerg. Dis. 65, 240–243 (2018).
Azeem, S., Sharma, B., Shabir, S., Akbar, H. & Venter, E. Lumpy skin disease is expanding its geographic range: A challenge for Asian livestock management and food security. Vet. J. 279, 105785 (2022).
Putty, K. et al. First complete genome sequence of lumpy skin disease virus directly from a clinical sample in South India. Virus Genes 59, 317–322 (2023).
Bianchini, J., Simons, X., Humblet, M. F. & Saegerman, C. Lumpy skin disease: A systematic review of mode of transmission, risk of emergence and risk entry pathway. Viruses 15, 1622 (2023).
Casal, J. et al. Economic cost of lumpy skin disease outbreaks in three Balkan countries: Albania, Bulgaria and the former Yugoslav Republic of Macedonia (2016–2017). Transbound. Emerg. Dis. 65, 1680–1688 (2018).
Klement, E. et al. Neethling vaccine proved highly effective in controlling lumpy skin disease epidemics in the Balkans. Prev. Vet. Med. 181, 104595 (2020).
Haegeman, A. et al. Comparative evaluation of lumpy skin disease virus-based live attenuated vaccines. Vaccines 9, 473 (2021).
Morgenstern, M. & Klement, E. The effect of vaccination with live attenuated neethling lumpy skin disease vaccine on milk production and Mortality—An analysis of 77 dairy farms in Israel. Vaccines 8, 324 (2020).
Kumar, N. et al. Evaluation of the safety, immunogenicity and efficacy of a new live-attenuated lumpy skin disease vaccine in India. Virulence 14, 2190647 (2023).
Fox, K. A. et al. Bovine viral diarrhea in captive Rocky mountain Bighorn sheep associated with administration of a contaminated modified-live bluetongue virus vaccine. J. Vet. Diagn. Investig. 31, 107–112 (2019).
Su, Q. et al. The intracorporal interaction of fowl adenovirus type 4 and LaSota strain significantly aggravates the pathogenicity of one another after using contaminated Newcastle disease virus-attenuated vaccine. Poult. Sci. 98, 613–620 (2019).
Bumbarov, V., Golender, N., Erster, O. & Khinich, Y. Detection and isolation of bluetongue virus from commercial vaccine batches. Vaccine 34, 3317–3323 (2016).
Haegeman, A. et al. The importance of quality control of LSDV live attenuated vaccines for its safe application in the field. Vaccines 9, 1019 (2021).
Vandenbussche, F. et al. Recombinant LSDV strains in Asia: Vaccine spillover or natural emergence? Viruses 14, 1429 (2022).
Hamdi, J. et al. Development and evaluation of an inactivated lumpy skin disease vaccine for cattle. Vet. Microbiol. 245, 108689 (2020).
Haegeman, A. et al. Duration of immunity induced after vaccination of cattle with a live attenuated or inactivated lumpy skin disease virus vaccine. Microorganisms 11, 210 (2023).
Rao, T. V. S. & Bandyopadhyay, S. K. A comprehensive review of goat pox and sheep pox and their diagnosis. Anim. Health Res. Rev. 1, 127–136 (2000).
Brenner, J. et al. Appearance of skin lesions in cattle populations vaccinated against lumpy skin disease: Statutory challenge. Vaccine 27, 1500–1503 (2009).
Abutarbush, S. M. Efficacy of vaccination against lumpy skin disease in Jordanian cattle. Vet. Rec. 175, 302–302 (2014).
Abdallah, F. M., Damaty, H. M. E. & Kotb, G. F. Sporadic cases of lumpy skin disease among cattle in Sharkia province, Egypt: Genetic characterization of lumpy skin disease virus isolates and pathological findings. Vet. World 11, 1150 (2018).
Ben-Gera, J., Klement, E., Khinich, E., Stram, Y. & Shpigel, N. Y. Comparison of the efficacy of neethling lumpy skin disease virus and x10RM65 sheep-pox live attenuated vaccines for the prevention of lumpy skin disease—The results of a randomized controlled field study. Vaccine 33, 4837–4842 (2015).
EFSA Panel on Animal Health and Welfare. Scientific opinion on sheep and goat pox. EFSA J. 12, 3885 (2014).
Gari, G. et al. Evaluation of the safety, immunogenicity and efficacy of three capripoxvirus vaccine strains against lumpy skin disease virus. Vaccine 33, 3256–3261 (2015).
Zhugunissov, K. et al. Goatpox virus (G20-LKV) vaccine strain elicits a protective response in cattle against lumpy skin disease at challenge with lumpy skin disease virulent field strain in a comparative study. Vet. Microbiol. 245, 108695 (2020).
Michael, A. et al. Control of lumpy skin disease outbreak in Egypt with Romanian sheep pox vaccine. Assiut Vet. Med. J. 36, 173–180 (1996).
Uzar, S. et al. Comparison and efficacy of two different sheep pox vaccines prepared from the Bakırköy strain against lumpy skin disease in cattle. Clin. Exp. Vaccine Res. 11, 1–11 (2022).
Norian, R. et al. Evaluation of humoral and cell-mediated immunity of two capripoxvirus vaccine strains against lumpy skin disease virus. Iran. J. Virol. 10, 1–11 (2016).
Gaber, A., Rouby, S., Elsaied, A. & El-Sherif, A. Assessment of heterologous lumpy skin disease vaccine-induced immunity in pregnant cattle vaccinated at different times of gestation period and their influence on maternally derived antibodies. Vet. Immunol. Immunopathol. 244, 110380 (2022).
Abutarbush, S. M. & Tuppurainen, E. S. M. Serological and clinical evaluation of the Yugoslavian RM65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transbound. Emerg. Dis. 65, 1657–1663 (2018).
Wolff, J. et al. Development of a safe and highly efficient inactivated vaccine candidate against lumpy skin disease virus. Vaccines 9, 4 (2020).
Steinrigl, A., Revilla-Fernández, S., Eichinger, M., Koefer, J. & Winter, P. Bluetongue virus RNA detection by RT-qPCR in blood samples of sheep vaccinated with a commercially available inactivated BTV-8 vaccine. Vaccine 28, 5573–5581 (2010).
De Leeuw, I. et al. Bluetongue virus RNA detection by real-time RT-PCR in Post-Vaccination samples from cattle. Transbound. Emerg. Dis. 62, 157–162 (2015).
Norian, R., Afzal Ahangran, N., Varshovi, H. R. & Azadmehr, A. Comparative efficacy of two heterologous Capripox vaccines to control lumpy skin disease in cattle. Bulg. J. Vet. Med. 22, 171–179 (2019).
Khafagy, H., Saad, M., Abdelwahab, M. & Mustafa, A. Preparation and field evaluation of live attenuated sheep pox vaccine for protection of calves against lumpy skin disease. Benha Vet. Med. J. 31, 1–7 (2016).
Varshovi, H. R., Norian, R., Azadmehr, A. & Ahangaran, N. A. Immune response characteristics of Capri pox virus vaccines following emergency vaccination of cattle against lumpy skin disease virus. Iran. J. Vet. Sci. Technol. 9 (2017).
Hakobyan, V. et al. Duration of immunity in cattle to lumpy skin disease utilizing a sheep pox vaccine. Vet. Sci. 11, 164 (2024).
Boshra, H. et al. A lumpy skin disease virus deficient of an IL-10 gene homologue provides protective immunity against virulent capripoxvirus challenge in sheep and goats. Antiviral Res. 123, 39–49 (2015).
Haegeman, A. et al. Evidence of lumpy skin disease virus transmission from subclinically infected cattle by Stomoxys calcitrans. Viruses 15, 1285 (2023).
Shumilova, I. et al. Subclinical infection caused by a Recombinant vaccine-like strain poses high risks of lumpy skin disease virus transmission. Front. Vet. Sci. 11, 1330657 (2024).
Sanz-Bernardo, B. et al. Lumpy skin disease is characterized by severe multifocal dermatitis with necrotizing fibrinoid vasculitis following experimental infection. Vet. Pathol. 57, 388–396 (2020).
Sohier, C. et al. Experimental evidence of mechanical lumpy skin disease virus transmission by Stomoxys calcitrans biting flies and Haematopota spp. Horseflies. Sci. Rep. 9, 20076 (2019).
Hamdi, J. et al. Experimental evaluation of the cross-protection between sheeppox and bovine lumpy skin vaccines. Sci. Rep. 10, 8888 (2020).
Salib, F. & and, O. Incidence of lumpy skin disease among Egyptian cattle in Giza governorate, Egypt. Vet. World 4 (2011).
Lumpy skin disease epidemiological report IV: Data collection and analysis—2020—EFSA Journal—Wiley Online Library. https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.6010.
Parida, S. et al. Interferon-gamma production in vitro from whole blood of foot-and-mouth disease virus (FMDV) vaccinated and infected cattle after incubation with inactivated FMDV. Vaccine 24, 964–969 (2006).
Babiuk, S. et al. Evaluation of an ovine testis cell line (OA3.Ts) for propagation of capripoxvirus isolates and development of an immunostaining technique for viral plaque visualization. J. Vet. Diagn. Invest. 19, 486–491 (2007).
Haegeman, A. et al. An immunoperoxidase monolayer assay (IPMA) for the detection of lumpy skin disease antibodies. J. Virol. Methods 277, 113800 (2020).
Haegeman, A. et al. Development and validation of three capripoxvirus real-time PCRs for parallel testing. J. Virol. Methods 193, 446–451 (2013).
Agianniotaki, E. I. et al. Development and validation of a TaqMan probe-based real-time PCR method for the differentiation of wild type lumpy skin disease virus from vaccine virus strains. J. Virol. Methods 249, 48–57 (2017).
Spearman, C. The method of ‘right and wrong cases’ (‘constant stimuli’) without gauss’s formulae. Br. J. Psychol. 2, 227–242 (1908).
Kärber, G. Beitrag Zur kollektiven behandlung Pharmakologischer Reihenversuche. Naunyn Schmiedebergs Arch. Für Exp. Pathol. Pharmakol. 162, 480–483 (1931).
Acknowledgements
The author would like to thank the technical personnel of the Unit of Exotic and vector-borne diseases and the animal caretakers of the Experimental Centre of Sciensano. We are very grateful to the Kimron Veterinary Institute (Israel) and the Field Israeli Veterinary Services for providing us with the LSDV strain LSD/OA3-Ts. MORAN. M. seed pass.4. 155920/2012.20.1.13.
Funding
This research was funded by the Bill &Melinda Gates Foundation project Nr OPP1126866, the GALVmed project Nr CAO-R34A0856 on lumpy skin disease and the Belgian Federal Public Service of Health, Food Chain Safety and Environment through the contract RT 15/3 (LUMPY SKIN 1).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.H. and K.D.; methodology: A.H. and K.D.; validation: I.D.L.; formal analysis: A.H. and W.P.; investigation: A.H. and W.V.C.; resources: A.H., and W.P; data curation: A.H. and W.P.; writing—original draft preparation: A.H. and W.P.; writing—review and editing: K.D., N.D.R., I.D.L. and C.S.; visualization (figures): A.H. and W.P.; supervision: K.D., N.D.R. and L. M.; project administration, A.H. and L. M.; funding acquisition: K.D. and L. M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Haegeman, A., Philips, W., Mostin, L. et al. A goatpox but not sheeppox heterologous live attenuated vaccines provide complete protection against lumpy skin disease in cattle under experimental conditions. Sci Rep 15, 26078 (2025). https://doi.org/10.1038/s41598-025-11440-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11440-w