Abstract
Enamel prisms possess a unique microstructure, and their damage due to erosion is irreversible, making enamel restoration through non-invasive regeneration a significant challenge. This in-vitro study aimed to reconstruct the prism-like structure of enamel damaged by citric acid erosion through non-invasive biomimetic remineralization. Grape seed extract (GSE), combined with ethylenediaminetetraacetic acid (EDTA) and agarose hydrogel, was prepared via a hydrothermal technique. Additionally, a separate solution containing monoethanolamine (MEA) and potassium phosphate dibasic (K2HPO4) was prepared. Both solutions were applied as a treatment protocol for 30 h on citric acid-eroded enamel surfaces. Three groups were compared: the control non-eroded enamel group (G0), the eroded non-treated enamel group (G1), and the treated enamel group (G2). The enamel surfaces were analyzed using atomic force microscopy (AFM), scanning electron microscopy coupled with energy dispersive X-ray analysis (SEM-EDX), and transmission electron microscopy (TEM). Significant topographic changes were observed in the G2 group compared to the G0 and G1 groups. AFM analysis revealed the formation of a new layer on the eroded surface as revealed by the increased arithmetical mean deviation of the roughness (Sa = 255.7 ± 40.61 nm) and the smoother surface profile (Sku = 2.98 ± 0.53) of G2 compared to both G0 and G1. SEM examination showed the presence of uniform, prism-like, regenerative tissues, and EDX analysis confirmed the formation of hydroxyapatite (HAp) with a predominant calcium oxide (1.93 Ca/P molar ratio) phase on the treated enamel surface. TEM analysis indicated a crystal size of 12–15 nm. In conclusion, the application of GSE/EDTA agarose hydrogel and MEA/ K2HPO4 solution successfully repaired the eroded enamel surface, generating a uniform, prism-like enamel structure by providing the necessary inorganic mineral ions and the organic protein matrix template required for biomimetic remineralization.
Similar content being viewed by others
Introduction
Enamel, the hardest tissue in the human body, is composed of hierarchically structured, tightly packed prisms, with hydroxyapatite (HAp) crystals arranged parallel within each prism. These HAp crystals have diameters ranging from 25 to 100 nm and lengths between 0.1 and 100 μm. Enamel damage, whether caused by caries, erosion, or other factors, is considered irreversible. Depending on the lesion’s size, restoration is typically achieved through artificial materials or non-invasive remineralization techniques1,2. Non-carious enamel lesions, which arise from the loss of hard tissue not caused by caries, can result from prolonged exposure to acids in food and beverages, such as citric acid, phosphoric acid, and carbonic acid. These exposures can lead to enamel erosion and demineralization. Previous treatment protocols for curing enamel erosion, including fluoride varnishes3, dentifrices4, casein phosphopeptide–amorphous calcium phosphate pastes5,6, and bioactive glass-based7 and calcium silicate-based restorations8 have been explored. While many of these products can remineralize enamel, the resulting crystals are often morphologically irregular with disrupted alignment compared to the structured, prism-like hydroxyapatite of natural enamel9.
The pH of therapeutic agents plays a critical role in their interaction with enamel surfaces. An acidic environment promotes demineralization, while a neutral to slightly basic pH favors remineralization by stabilizing calcium and phosphate ion activity. Fluoride, a widely studied cariostatic agent, exerts its protective effect not only by enhancing remineralization but also by reducing enamel solubility in acidic conditions through the formation of fluorapatite. Studies have shown that both ion availability and enamel surface reactivity are pH-dependent10,11.
In an attempt to replicate the unique structure of enamel prisms, scientists have explored synthetic approaches involving high temperature, pressure, and acidic pH; however, these methods are impractical for routine dental practice. Consequently, new strategies focusing on biomimetic remineralization of non-carious lesions have been developed12.
The development of biomimetic remineralization techniques for treating non-carious lesions requires a comprehensive understanding of dental tissue biomineralization. Biomineralization is the process by which inorganic ions accumulate in a synchronized manner, guided by protein molecules, to form hierarchical structures. Both proteins and inorganic ions play critical roles in driving the systematic biomimetic mineralization process13. Three primary theories explain the mechanisms behind biomineralization. The first, the “classical nucleation theory,” suggests that individual ions combine based on kinetic and thermodynamic principles to form ordered clusters, which then evolve into crystal lattices. Protein molecules in this theory help lower the energy barrier, facilitating the formation of properly organized nucleating clusters14. The second, the “non-classical nucleation theory,” posits that pre-nucleation clusters aggregate to form well-hydrated, amorphous mineral phases that subsequently crystallize into higher-order structures. These clusters are particularly sensitive to pH and the presence of protein molecules15,16. The third, “crystallization by particle attachment,” combines elements of the previous two theories, where mineral phases are created through interactions between ion clusters, gels, and oligomers, leading to the formation of larger crystal assemblies. This process is regulated by energy kinetics, phase stability, and the presence of gels and polymers17. Based on these theories, various studies have sought to replicate the biomineralization process using different methodologies18,19,20,21.
One such approach is the use of metastable amorphous calcium phosphate phases, which support natural enamel remineralization by directly transforming into HAp crystals22. Another strategy involves the application of monetite, a calcium phosphate phase known for its ability to resorb quickly in vivo, making it effective in bone regeneration23. Monetite, the anhydrous form of brushite, shares similar calcium and phosphorus content with brushite but is more acidic. Both phases have been used as precursors for HAp production and as dental mineralizing agents in products such as chewing gums and toothpastes24,25,26. Given the proven biological adaptability of calcium phosphate phases, numerous studies have investigated novel and cost-effective strategies for synthesizing these phases. In vitro procedures for enamel-like tissue regeneration using various calcium phosphate phases have been well-documented, often involving harsh chemical conditions, such as high-temperature hydrothermal methods27,28,29.
The hydrothermal process is a highly effective and efficient chemical approach for producing various inorganic crystal structures, including HAp in forms such as nanoparticles, nanofibers, nanorods, and plates, as well as monetite crystals30,31,32,33. In this method, calcium and phosphate salt solutions are typically combined with ammonia or urea to adjust the pH. Organic additives, such as EDTA, are introduced to control the rate of calcium ion release and regulate the solution’s degree of supersaturation. Surfactants are also employed to direct the alignment of nano-apatite rods into a prism-like structure, serving as reverse micro-emulsions. Recently, electrospun nanofibers of amorphous calcium phosphate/poly(vinylpyrrolidone) have been introduced as hydrogel templates to facilitate enamel remineralization in vitro34,35,36. In a related study, agarose hydrogel has been proposed as a biomimetic template for mineralization, mimicking the organic matrix required for mineral deposition in enamel. Agarose is a naturally occurring polysaccharide composed of repeating units of d-galactose and 3,6-anhydro l-galactose37. As per the biomineralization theories, the interaction of mineral ions with a gel-like protein matrix is essential for enamel apatite deposition in vivo.
Xie et al.38 hypothesized that grape seed extract (GSE) could stabilize the exposed collagen matrix, promoting mineralization by interacting with the organic dentin structure through Proanthocyanidin-collagen binding. Proanthocyanidins (PAs), a class of plant metabolites, have a molecular structure consisting of flavonoids, oligomers, and catechins, and have been shown to support collagen synthesis and prevent ischemia. However, their role in human tooth remineralization remains under investigation38,39. Additionally, GSE contains significant amounts of essential minerals such as calcium, potassium, magnesium, sodium, phosphorus, and sulphur, which may aid in mineral precipitation on tooth lesions38,40. Monoethanolamine (MEA), an ethanolamine compound with one primary amine group, is an odorless, colorless liquid known for stabilizing solutions and maintaining homogeneity across a wide pH range. A study by Suchanek41 demonstrated that MEA could be used to synthesize highly crystalline, pure calcium phosphate structures, including monetite plate-like microcrystals. By adjusting the concentration of MEA, various crystal shapes, including hydroxyapatite plates and nanofibers, could be selectively synthesized.
Despite the promising potential of these materials, many challenges remain in achieving effective enamel regeneration. This study aims to explore an improved biomimetic mineralization strategy for the uniform remineralization of acid-eroded enamel surfaces. We hypothesize that the application of a biomimetic hydrogel containing grape seed extract (GSE) and EDTA, in combination with a monoethanolamine and phosphate solution, can provide protein-guided mineralization models that induce the formation of enamel-like hydroxyapatite crystals on eroded enamel surfaces, thereby mimicking the hierarchical structure of native enamel.
Materials and methods
Materials
Red grape seeds (V. vinifera Syrah) were obtained from a grape juice factory (the Gianaclis Grape Juice Factory, Alexandria, Egypt). Citric acid solution (Rasayan Laboratories, was obtained from Anand, Gujarat, India, Batch No. 0396-196-160316). Ethylene diamine tetraacetic acid (EDTA) was obtained from sigma Aldrich, Egypt. Agarose powder (BioWest Regular Agarose G-10, was gotten from Gene Company, Spain). Monoethanolamine (MEA) (Oswal Chemicals, Gujarat, India, CAS No. 75-04-7) and potassium hydrogen phosphate (K2HPO4) (Thomas-Baker Lab Chemicals, India).
Preparation of enamel samples and erosive challenge
All procedures were conducted in accordance with the ethical guidelines set forth by the Helsinki Declaration. The experimental protocols were approved by the Medical Research Ethics Committee, National Research Centre of Egypt (Reference number: 1436072023). The isolated teeth were obtained from patients undergoing orthodontic extractions at the dental clinic of the Medical and Scientific Centre of Excellence, National Research Centre, Egypt. Informed consent was obtained from all subjects and/or their legal guardians.
Based on power analysis using G*Power software (Faul et al.42), a minimum of 25 samples was required to detect a medium effect size (f = 0.25) with 80% power and α = 0.05 in a repeated measures design (within-subject comparison across three conditions). Therefore, 25 sound human premolars were collected and used sequentially in three experimental conditions: G0 (baseline, sound enamel), G1 (after citric acid erosion), and G2 (after biomimetic remineralization). This self-controlled design allowed each tooth to serve as its own control, thereby reducing variability and increasing the study’s sensitivity. Teeth with caries, developmental defects, or surface abnormalities were excluded. Defect-free teeth were ultrasonically cleaned with deionized water and stored in artificial saliva7.
For the erosive challenge, the samples were exposed to 50 mL of 0.03 g/10mL citric acid solution (Rasayan Laboratories, Anand, Gujarat, India, Batch No. 0396-196-160316) adjusted to pH 2.77. The samples were incubated for 2 h at 35 °C in a shaking incubator (30 rpm - shaking incubator SI-100R HYSC, Korea)43,44.
Red grape seed extract (GSE) and GSE/EDTA agarose hydrogel preparation
Grape seed extract (GSE) was prepared following the method outlined by Li45. The pH-value of the red grape seed extracts was 4.8. Briefly, 200 g of powdered grape seeds were extracted with 800 mL of a 70:30 (v/v) ethanol: water mixture in a shaking incubator at 45 °C for 2 h. The mixture was then centrifuged at 5000 g for 10 min, and the supernatant was decanted. The residue was re-extracted for another 2 h, and the supernatants were collected, evaporated using a rotary evaporator, and stored at −20 °C until further use. A 1.9 mL portion of the prepared GSE was diluted into 100 mL of deionized water. Ethylene diamine tetraacetic acid (EDTA) was added at a 1:0.5 molar ratio, followed by the addition of 0.5 g of agarose powder (BioWest Regular Agarose G-10, Gene Company, Spain). The pH was adjusted to 6.5 using 0.1 M NaOH and 0.1 M HCl. The mixture was allowed to swell at 25 °C for 30 min. For hydrothermal treatment, the mixture was transferred to a 40 mL Teflon-lined stainless-steel reactor, which was placed in a furnace at 100 °C for 12 h. Afterward, the reactor was immersed in an ethylene glycol bath at 0–4 °C for rapid cooling. The hydrogel was then kept at 60 °C until use36.
MEA/K2HPO4 solution preparation
A solution of monoethanolamine (MEA) (Oswal Chemicals, Gujarat, India, CAS No. 75-04-7) and potassium hydrogen phosphate (K2HPO4) (Thomas-Baker Lab Chemicals, India) was prepared by adding 2 × 10⁻³ mol/L MEA to a 0.05 mol/L K2HPO4 solution. The pH of the solution was adjusted to 6.5 using 0.1 M NaOH46.
Biomimetic mineralization model for eroded enamel surfaces using GSE/EDTA agarose hydrogel and MEA/K2HPO4 solution
For the G2 group (treated enamel), 10 mL of the GSE/EDTA agarose hydrogel was applied to the enamel surface. After allowing the hydrogel to gel for 10 min, then 5 mL of the MEA/K2HPO4 solution was added, forming a 2-mm-thick layer. Both solutions were maintained on the enamel surface for 30 h at 37 °C in a shaking incubator (30 rpm - shaking incubator SI-100R HYSC, Korea). Following the incubation, the samples were removed, rinsed with deionized water, and air-dried47.
Structural characterization and compositional analysis of the formed crystals forremineralized enamel
Surface characterization using AFM
Surface roughness and morphology of the enamel samples before and after acid erosion, as well as after remineralization treatment, were examined using an Atomic Force Microscope (AFM) (Anton Paar - Tosca™ 200, USA). Three-dimensional images of the enamel surfaces were obtained at random sites with a scanning size of 10 μm × 10 μm using an arrow NCR tapping cantilever at 400 × 400 resolution. AFM data were analyzed using Tosca Analysis software according to ISO 25,178 for surface roughness. The roughness parameters evaluated were Sa, Sq, and Sku. Sa refers to the arithmetical mean deviation of the roughness, Sq is the root mean square deviation of the roughness, and Sku refers to the coefficient of kurtosis, which measures the spikiness of the 3D surface texture. A histogram of all measured height points is obtained, with Sku representing the deviation from a normal distribution. If Sku = 3, the surface exhibits a Gaussian distribution, while Sku > 3 indicates a spiky surface and Sku < 3 indicates a bumpy surface48,49.
SEM-EDX analysis of the remineralized enamel surface
The enamel samples from all three groups were examined using a JEOL JSM-5800 scanning electron microscope under high vacuum conditions, with a chamber pressure maintained at approximately 10⁻⁶ Torr to ensure optimal imaging performance. Sputter coating was not applied in this study because the enamel samples, both before and after treatment, were analyzed under identical conditions, serving as their own control in this self-controlled experimental design. While uncoated biological samples can sometimes lead to charging effects, the enamel surfaces provided sufficient conductivity for imaging due to their inherent mineral content and smoothness. An Energy Dispersive X-ray Spectrometer (EDX) with a SiLi X-ray detector (Oxford Instruments, UK) was employed for elemental analysis of the enamel surfaces33.
TEM analysis for identification of the obtained crystals on the remineralized enamel surface
The tooth samples were fixed using a mixture of 2.5% glutaraldehyde and 1.5% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 30 min. After washing three times with 0.1 M cacodylate buffer, post-fixation was carried out using osmium tetroxide for 1 h. The samples were dehydrated in an ascending ethanol series and post-stained with uranyl acetate. Enamel samples were sectioned into 3 × 3 mm slices with a thickness of 250–500 μm using a diamond disc. These were cleaned in a solution of isopropanol and acetone (60%:40%, v/v) for 15 min50. The transmission electron microscope (TEM) (JEOL JEM-210) system, equipped with a field emission.
Statistical analysis
Data were statistically analyzed using Microsoft Excel® 2016, Statistical Package for Social Sciences (SPSS®) Version 24, and Minitab® Version 16. One-way Analysis of Variance (ANOVA) was performed to compare the outcomes (remineralization effects on enamel) across the different groups. Post-hoc tests were conducted when appropriate to identify specific group differences. Data from three replicates are presented as mean ± standard deviation (SD).
Results and discussion
AFM analysis
AFM analysis revealed significant alterations in the topography of the enamel surface after citric acid erosion and subsequent remineralization treatment (Fig. 1 and Table 1). The enamel surface, following citric acid erosion, appeared highly rugged, with increased surface roughness. However, after remineralization treatment, this ruggedness was mitigated, and the enamel surface became even smoother than its baseline condition before acid erosion. Specifically, the mean roughness parameters (Sq and Sa) increased from 96.31 ± 54.79 nm and 77.26 ± 44.78 nm in the G0 (control) group to 170.48 ± 128.1 nm and 111.03 ± 79.28 nm in the G1 (acid-eroded) group, respectively. Upon remineralization (G2 group), a new layer was deposited, significantly elevating the enamel surface, with higher Sq and Sa values of 318.05 ± 52.45 nm and 255.7 ± 40.61 nm, respectively.
Regarding roughness profile sharpness, as measured by kurtosis (Sku), the value for the G0 group was 3.01 ± 0.4, indicating a normal distribution of surface heights. However, this value increased to 5.99 ± 1.5 for the G1 group, suggesting that the height distribution became more spiked due to acid erosion. After remineralization treatment in the G2 group, Sku values decreased to 2.98 ± 0.53, indicating a smoother surface profile. This shift from sharp spikes to round bumps reflects the deposition of a new layer, which helped restore the enamel surface topography. The citric acid erosion significantly altered the surface of the enamel, as evidenced by the increased roughness values in G1 group, caused by the dissolution of HAp crystals within the enamel prisms. After remineralization treatment in the G2 group, a new mineralized layer was deposited, which elevated the surface height, as seen in the increased Sq and Sa values.
These results reveal the impact of biomimetic remineralization on enamel surface and suggest that the remineralization treatment effectively restored the enamel surface to a condition similar to its baseline before erosion. The kurtosis (Sku) values provided additional insight into the surface profile. While the G1 group exhibited a highly spiked surface profile after acid erosion, the G2 group showed a smoother, more rounded surface profile following remineralization. This indicates that the remineralization process not only restored mineral content but also improved the overall surface morphology of the enamel.
SEM-EDX analysis
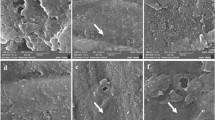
SEM images magnified at 1000× and 15,000× of the non-eroded (G0), eroded (G1), and remineralized (G2) enamel surfaces are shown in (Fig. 2). The G0 group exhibited a relatively smooth surface with intact enamel prism cores. After citric acid erosion (G1 group), then the enamel surface showed dissolution of the prism cores, leaving the prism periphery largely intact. In the G2 group, remineralization treatment led to the closure of open prism cores by uniformly distributed insoluble crystalline precipitates, which were formed within the prism cores and across the enamel surface. These SEM images explain the previously obtained AFM results where G1 revealed increased roughness parameters (Sa and Sq) as a result of the resolved prism core that increased the depth of the enamel surfaces, however, G2 showed increase in the surface height due to the new deposited minerals inside the prism core.
Energy-dispersive X-ray (EDX) analysis showed no significant differences in the calcium and phosphorus content (weight%) between the groups (Fig. 3). The calculated Ca/P molar ratios were 1.85, 1.97, and 1.93 for the G0, G1, and G2 groups, respectively, suggesting a similar mineral composition across all groups. These findings are consistent with previous studies that reported comparable Ca/P ratios in enamel-like materials51,52. The lack of significant differences in the Ca and P weight percentages across the groups, as observed in EDX analysis, may be due to the presence of residual Ca2+ ions on the surface of the eroded enamel (G1 group) and the similarity in Ca/P content between the remineralized (G2) and control (G0) groups. The slight differences in the Ca/P molar ratios (1.85 for G0, 1.97 for G1, and 1.93 for G2) are in line with the typical Ca/P ratio of enamel, which is approximately 2.13 ± 0.1752. A previous study53 examined samples prepared with intentionally adjusted Ca/P ratios ranging from 0.5 to 2.5. The study found that samples with a 2.0 Ca/P ratio predominantly presented the HAp phase, with the detection of CaO formation.
TEM analysis
TEM images (Fig. 4) of the remineralized enamel surfaces revealed the formation of hydroxyapatite (HAp) crystals, which were approximately 12–15 nm in size and aggregated into clusters. Selected-area electron diffraction (SAED) patterns indicated the crystalline nature of the deposited minerals, which corresponds with the SEM images (Fig. 2). The TEM analysis provides essential insight into the morphology and crystallinity of the mineral phase generated by the hydrogel, thereby supporting the proposed biomimetic mechanism of enamel remineralization. Including this figure establishes the hydrogel’s intrinsic capacity to nucleate and grow enamel-like apatite, which is critical for interpreting its remineralizing effect observed in the treated enamel samples.
The presence of a dark region in the central area of the crystals could suggest a transition from octacalcium phosphate (OCP) to hydroxyapatite (HAp), as both phases exhibit mismatched layers54. However, the more plausible explanation is that this dark area represents a Ca-rich region within the crystals, which aligns with known observations that enamel crystals begin to dissolve at their central regions during acid exposure55. Therefore, in the present study, the erosive citric acid attack began centrally, after which the remineralization process, facilitated by the GSE/EDTA agarose hydrogel and MEA/K2HPO4 solution, promoted the deposition of minerals over these lesions, thereby restoring the enamel’s integrity. In addition, previous studies56,57 have stated that calcium atoms in the HAp unit cell reveal a higher defect density and atomic overlap, as human enamel crystals are susceptible to electron beam radiation damage during TEM observation. As a result, the TEM electron beam amplified the overlap of HAp atoms during crystal examination58. Since the HAp crystals had nanometric dimensions, as revealed in the electron diffraction pattern, the incident electron beam’s minimum cross-section was beyond 10 nm, meaning the crystals’ nanometric region, from which the electron diffraction patterns originated, essentially encompassed the entire human tooth enamel crystal.
Implications of the remineralization approach
While the current study provides strong morphological evidence for enamel remineralization using AFM, SEM, and TEM imaging, it is important to acknowledge that mineral content was not quantified using transverse microradiography (TMR), additionally, substance loss during the erosion-remineralization cycle was not directly measured. These aspects represent important limitations, as TMR could have offered more accurate insight into lesion depth changes and true mineral gain. Future studies incorporating TMR and profilometry or optical coherence tomography (OCT) are needed to quantitatively assess mineral uptake and enamel loss.
However, the surface and subsurface changes observed via AFM, SEM-EDX, and TEM in this study align with previous reports indicating that surface morphology improvements often correlate with remineralization efficacy, even in the absence of TMR. For instance, a previous study59 demonstrated that gum containing calcium fluoride significantly reinforced enamel subsurface lesions in-situ by promoting mineral diffusion, despite using microradiography as a semi-quantitative measure. Their findings underscore the relevance of morphological improvements in supporting remineralization potential, particularly when accompanied by high-affinity mineral-binding agents like proanthocyanidins in grape seed extract (GSE).
Furthermore, the remineralization of enamel via the biomimetic approach using GSE/EDTA agarose hydrogel and MEA/K2HPO4 solution has shown promising results in restoring enamel integrity. This process mimics the natural enamel biomineralization process and is particularly effective in sealing the eroded enamel prisms, as observed through both AFM and SEM analyses. The incorporation of GSE, rich in proanthocyanidins, further enhances the remineralization process by facilitating calcium ion deposition and strengthening the organic matrix of the enamel38,60,61.
The pH of the remineralizing environment is a critical determinant of the efficacy of enamel repair. In this study, the use of monoethanolamine (MEA) served not only as a pH buffer but also contributed to stabilizing the ionic environment favorable for hydroxyapatite (HAp) formation. A previous study41 found that, MEA allows the stabilization of the initial solution across a wide pH range and therefore expands the ability of the synthesis method to a broader selection of calcium phosphate phases.
A slightly basic pH, as maintained by MEA, promotes the supersaturation of calcium and phosphate ions, thereby favoring mineral precipitation over demineralization. This aligns with fluoride-based research, where pH modulation was shown to significantly influence mineral uptake. Kitasako et al.59, demonstrated that chewing gum containing calcium fluoride enhanced enamel subsurface remineralization and emphasized the importance of maintaining an optimal pH to support ion diffusion and crystal formation. Similarly, in the present study, the controlled alkaline conditions supported by MEA facilitated the nucleation of enamel-like HAp nanocrystals, as observed under TEM. This highlights the synergistic role of pH not just as a passive medium but as an active modulator of mineralization kinetics and phase stability.
However, the roles of acidic and alkaline pH in fluoride’s dental interactions are distinct and complementary, representing two sides of the demineralization-remineralization cycle. Under highly acidic conditions, as demonstrated by Scholz et al.62, a primary interaction is the formation of a thick calcium fluoride (CaF2) precipitate on the enamel. This process is critically dependent on the acid itself, which first dissolves the enamel surface to release the necessary calcium ions that then react with fluoride to form this protective layer. In contrast, an alkaline or neutral pH is the essential driving force for remineralization, as highlighted by Kitasako et al.59, where stimulated saliva raises the oral pH to this alkaline state, creating an environment supersaturated with calcium and phosphate ions that are driven back into the tooth to repair subsurface lesions. These two pH-dependent mechanisms are linked: the CaF2 layer formed during an acid attack can later act as a pH-controlled reservoir, releasing fluoride ions to strengthen and enhance the natural remineralization process that occurs under favorable alkaline conditions.
As a result, the remineralization strategy presented in this study effectively regenerated enamel surfaces that had been eroded by citric acid. The deposition of a new mineralized layer, the restoration of surface roughness, and the sealing of enamel prism cores all point to the potential of this biomimetic approach in preventive dental care and enamel regeneration. Our hypothesis suggests that the GSE/EDTA agarose hydrogel and MEA/K2HPO4 solution act synergistically to promote mineral deposition on the acid-eroded enamel, facilitating the formation of a new, structured mineralized layer that mimics the natural enamel surface. The results support the notion that this remineralization strategy not only restores the mechanical and topographical properties of the enamel but also provides a promising method for enhancing the resilience of enamel against further erosive attacks.
Conclusion
The biomimetic synthesis process presented in this study, utilizing GSE/EDTA agarose hydrogel and MEA/K2HPO4 solution, offers a novel approach for generating prism-like enamel structures through the complex nucleation and uniform growth of inorganic crystal formations, guided by organic protein matrix templates. While further in vivo studies are needed to evaluate the efficacy of this remineralization treatment in situ, this research represents a significant step toward the development of innovative biomaterials for reparative and restorative dentistry, with the potential to regenerate enamel-like structures.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GSE:
-
Grape seed extract
- EDTA:
-
ethylene diamine tetra acetic acid
- MEA:
-
monoethanolamine
- K2HPO4 :
-
potassium phosphate dibasic
- AFM:
-
atomic force microscope
- SEM-EDX:
-
scanning electron microscopy coupled with energy dispersive X-ray analysis
- TEM:
-
transmission electron microscopy
- Hap:
-
hydroxyapatite
- PA:
-
Proanthocyanidin
References
Moradian-Oldak, J. Protein-mediated enamel mineralization. Front. Biosci. 17, 1996 (2012).
Lacruz, R. S., Habelitz, S., Wright, J. T. & Paine, M. L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 97, 939–993 (2017).
Marinho, V. C., Worthington, H. V., Walsh, T. & Clarkson, J. E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 7, 1465–1858 (2013).
Tschoppe, P. & Meyer-Lueckel, H. Effects of regular and highly fluoridated toothpastes in combination with saliva substitutes on artificial enamel caries lesions differing in mineral content. Arch. Oral Biol. 57, 931–939 (2012).
Cao, Y. et al. Biomimetic mineralisation of phosphorylated dentine by CPP-ACP. J. Dent. 41, 818–825 (2013).
Elgamily, H. et al. Antibacterial and remineralization efficacy of casein phosphopeptide, glycomacropeptide nanocomplex, and probiotics in experimental toothpastes: an in vitro comparative study. Eur. J. Dent. 13, 391–398 (2019).
Safwat, E. M. et al. Preparation and characterization of dental pit and fissure sealant based on calcium sodium silicate bioactive glasses. Silicon 15, 6785–6800 (2023).
Malkondu, Ö., Karapinar Kazandağ, M. & Kazazoğlu, E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed. Res. Int. 160951 (2014). (2014).
Fan, Y. et al. Amelogenin-assisted ex vivo remineralization of human enamel: effects of supersaturation degree and fluoride concentration. Acta Biomater. 7, 2293–2302 (2011).
ten Cate, J. M. & Duijsters, P. P. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 16 (3), 201–210 (1982).
Buzalaf, M. A. R. et al. Mechanisms of action of fluoride for caries control. Monogr. Oral Sci. 22, 97–114 (2011).
Zhou, Y. Z., Cao, Y., Liu, W., Chu, C. H. & Li, Q. L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces. 4, 6901–6910 (2012).
Sharma, V., Srinivasan, A., Nikolajeff, F. & Kumar, S. Biomineralization process in hard tissues: the interaction complexity within protein and inorganic counterparts. Acta Biomater. 120, 20–23 (2021).
De Yoreo, J. J. et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760 (2015).
Gebauer, D., Kellermeier, M., Gale, J. D., Bergström, L. & Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 43, 2348–2371 (2014).
Rao, A., Berg, J. K., Kellermeier, M. & Gebauer, D. Sweet on biomineralization: effects of carbohydrates on the early stages of calcium carbonate crystallization. Eur. J. Mineral. 26, 537–552 (2014).
Wallace, A. F. et al. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions. Science 341, 885–889 (2013).
Veis, A. A window on biomineralization. Science 307, 1419–1420 (2005).
George, A., Sabsay, B., Simonian, P. A. & Veis, A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J. Biol. Chem. 268, 12624–12630 (1993).
He, G. et al. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry 44, 16140–16148 (2005).
George, A. & Arthur, V. Phosphorylated proteins and control over apatite nucleation, crystal growth and Inhibition. Chem. Rev. 108, 4670–4693 (2008).
Gebauer, D. & Co¨lfen, H. Prenucleation clusters and non-classical nucleation. Nano Today. 6, 564–584 (2011).
Tamimi, F. et al. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: brushite vs. monetite. Acta Biomater. 8, 3161–3169 (2012).
Cama, G. et al. Structural changes and biological responsiveness of an injectable and mouldable monetite bone graft generated by a facile synthetic method. J. R Soc. Interface. 11, 20140727 (2014).
Medvecky, L., Giretova, M. & Sopcak, T. Preparation and properties of tetracalcium phosphate–monetite biocement. Mater. Lett. 100, 137–140 (2013).
Zou, Z., Liu, X., Chen, L., Lin, K. & Chang, J. Dental enamel-like hydroxyapatite transformed directly from monetite. J. Mater. Chem. 22, 22637–22641 (2012).
Chen, J. D., Wang, Y. J., Wei, K., Zhang, S. H. & Shi, X. T. Self-organization of hydroxyapatite nanorods through oriented attachment. Biomaterials 28, 2275–2280 (2007).
Yamagishi, K. et al. A synthetic enamel for rapid tooth repair. Nature 433, 819 (2005).
Wang, X. et al. Direct growth of human enamel-like calcium phosphate microstructures on human tooth. J. Nanosci. Nanotechnol. 9, 1361–1364 (2009).
Zhang, X. & Vecchio, K. S. Hydrothermal synthesis of hydroxyapatite rods. J. Cryst. Growth. 308, 133–140 (2007).
Costa, D. O., Dixon, S. J. & Rizkalla, A. S. One-and three-dimensional growth of hydroxyapatite nanowires during sol–gel–hydrothermal synthesis. ACS Appl. Mater. Interfaces. 4, 1490–1499 (2012).
Liu, X., Lin, K. & Chang, J. Modulation of hydroxyapatite crystals formed from α-tricalcium phosphate by surfactant-free hydrothermal exchange. CrystEngComm 13, 1959–1965 (2011).
Jokić, B. et al. Synthesis and characterization of monetite and hydroxyapatite whiskers obtained by a hydrothermal method. Ceram. Int. 37, 167–173 (2011).
Chen, H. F., Clarkson, B. H., Sun, K. & Mansfield, J. F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid Interface Sci. 288, 97–103 (2005).
Wu, D. et al. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 34, 5036–5047 (2013).
Fletcher, J., Walsh, D., Fowler, C. E. & Mann, S. Electrospun Mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. Crystengcomm 13, 3692–3697 (2011).
Cao, Y., Mei, M. L., Li, Q. L., Lo, E. C. M. & Chu, C. H. Agarose hydrogel biomimetic mineralization model for the regeneration of enamel Prism like tissue. ACS Appl. Mater. Interfaces. 6, 410–420 (2014).
Xie, Q., Bedran-Russo, A. K. & Wu, C. D. In vitro remineralization effects of grape seed extract on artificial root caries. J. Dent. 36, 900–906 (2008).
Sarni-Manchado, P., Cheynier, V. & Moutounet, M. Interactions of grape seed tannins with salivary proteins. J. Agric. Food Chem. 47, 42–47 (1999).
Ozcan, M. M. Mineral contents of several grape seeds. Asian J. Chem. 22, 6480–6488 (2010).
Suchanek, K., Bartkowiak, A., Perzanowski, M. & Marszałek, M. From monetite plate to hydroxyapatite nanofibers by monoethanolamine assisted hydrothermal approach. Sci. Rep. 8, 15408 (2018).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A. G. Statistical power analysis using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 41 (4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149 (2009).
Eisenburger, M., Addy, M., Hughes, J. A. & Shellis, R. P. Effect of time on the remineralisation of enamel by synthetic saliva after citric acid erosion. Caries Res. 35, 211–215 (2001).
Wang, Y. L. et al. Erosive potential of soft drinks on human enamel: an in vitro study. JFMA 113, 850–856 (2014).
Li, H., Wang, X., Li, P., Li, Y. & Wang, H. Comparative study of antioxidant activity of grape (Vitis vinifera) seed powder assessed by different methods. J. Food Drug Anal. 16, 1–8 (2008).
Jawale, K. D., Kamat, S. B., Patil, J. A., Nanjannawar, G. S. & Chopade, R. V. Grape seed extract: an innovation in remineralization. J. Conserv. Dent. 20, 415–418 (2017).
Fan, Y., Sun, Z. & Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 30, 478–483 (2009).
Yong, Q. et al. Matt polyurethane coating: correlation of surface roughness on measurement length and gloss. Polymers 12, 326 (2020).
Hammam, R. E., Safwat, E. M., Abdel-gawad, S. A., Shoeib, M. & Shimaa, E. H. Influence of anodization conditions on deposition of hydroxyapatite on α/β Ti alloys for osseointegration: atomic force microscopy analysis. Trans. Nonferrous Met. Soc. China. 34, 3629–3649 (2024).
Elgamily, H. M., El-Sayed, S. M., El-Sayed, H. S. & Youssef, A. M. Laboratory evaluation of anti-plaque and remineralization efficacy of sugarless probiotic jelly candy supplemented with natural nano prebiotic additive. Sci. Rep. 13, 1–14 (2023).
Poorni, S., Kumar, R. A., Shankar, P., Indira, R. & Ramachandran, S. Effect of 10% sodium ascorbate on the calcium: phosphorus ratio of enamel bleached with 35% hydrogen peroxide: an in vitro quantitative energy-dispersive X-ray analysis. Contemp. Clin. Dent. 1, 223 (2010).
Abbassy, M. A., Masoud, A. I., Alsulaimani, F. F. & Bakry, A. S. Effect of citric acid erosion on enamel and dentin and possible protection by a novel bioactive Borate adhesive system. J. Dent. 124, 104208 (2022).
Liu, H., Yazici, H., Ergun, C., Webster, T. J. & Bermek, H. An in vitro evaluation of the ca/p ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta Biomater. 4, 1472–1479 (2008).
Reyes-Gasga, J., Arellano-Jiménez, M. J. & Garcia-Garcia, R. On the observation of the HAP-OCP interface by electron microscopy. Acta Microsc. 19, 279–284 (2010).
Brès, E. F., Barry, J. C. & Hutchison, J. L. A structural basis for the carious dissolution of the apatite crystals of human tooth enamel. Ultramicroscopy 12, 367–372 (1983).
Arends, J. Dislocations and dissolution of enamel. Caries Res. 7, 261–268 (1973).
Reyes-Gasga, J. & Garcıa-Garcıa, R. Analysis of the electron-beam radiation damage of TEM samples in the acceleration energy range from 0.1 to 2 mev using the standard theory for fast electrons. Radiat. Phys. Chem. 64, 359–367 (2002).
Reyes-Gasga, J. & Brès, E. F. High resolution STEM images of the human tooth enamel crystals. Appl. Sci. 11, 7477 (2021).
Kitasako, Y., Sadr, A., Hamba, H., Ikeda, M. & Tagami, J. Gum containing calcium fluoride reinforces enamel subsurface lesions in situ. J. Dent. Res. 91, 370–375 (2012).
Kosasi, S., Hart, L. A., van Dijk, H. & Labadie, R. P. Inhibitory activity of Jatropha multifida latex on classical complement pathway activity in human serum mediated by a calcium-binding Proanthocyanidin. J. Ethnopharmacol. 27, 81–89 (1989).
Kim, S., Don, S. & Mainwaring, D. E. Effect of ion-binding on the formation of temporary viscoelastic networks of Proanthocyanidin biopolymers. J. Appl. Polym. Sci. 65, 1795–1805 (1997).
Scholz, K. J. et al. EDX-analysis of fluoride precipitation on human enamel. Sci. Rep. 9, 13442. https://doi.org/10.1038/s41598-019-49742-5 (2019).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
H. M.E., E.M.S., and A.M.Y: Conceptualization, methodology, investigation, writing—original draft preparation, review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Tests were performed according to the ethical guidelines of the medical research ethics committee of the National Research Centre, Egypt, with approval number 1436072023. An informed consent was obtained from all subjects and/or their legal guardian(s).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elgamily, H.M., Safwat, E.M. & Youssef, A.M. Development of a novel Agarose/Nano-Hydroxyapatite/Grape seed extract hydrogel for biomimetic remineralization of demineralized human enamel (An In-Vitro Study). Sci Rep 15, 26086 (2025). https://doi.org/10.1038/s41598-025-11490-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11490-0