Abstract
Biodegradable chelating agents and nitrogen (N) fertilizers are widely used for the remediation of heavy metal-contaminated soils due to their ability to promote plant growth, enhance metal mobility and facilitate plant uptake. In this study, a pot experiment was conducted to investigate the effects of nitrate nitrogen (N-NO3−), ammonium nitrogen (N-NH4+) and amide nitrogen (N-amide) with different concentrations of 25, 50 and 75 mg N·kg−1 combined with (S,S)-ethylenediaminedisuccinic acid (EDDS) (1 mmol·L−1) on growth, biomass and phytoextraction efficiency of Bidens. pilosa L. and the bioavailability of cadmiun (Cd) in 15 mg·kg−1 Cd-contaminated soil. The results demonstrated that the application of 50 mg N·kg−1 N-NO3− and EDDS not only promoted plant growth and increased biomass, it also changed Cd distribution between operational fractions, increased available Cd content in soil, thereby maximizing the Cd uptake, thus enhancing the Cd enrichment capability of B. pilosa L. This combination achieved the maximum phytoextraction efficiency of 12.12% and the maximum soil Cd removal of 12.38% across all treatments. The comprehensive score obtained using the membership function method also showed that the treatment with 50 mg N·kg−1 N-NO3− and EDDS had the highest score. In conclusion, combination of 50 mg N·kg−1 N-NO3− and EDDS resulted in the greatest biomass production, and highest phytoremediation efficiency, indicating that it has great potential for application in phytoremediation with B. pilosa L. in Cd-contaminated soil.
Similar content being viewed by others
Introduction
At present, soil heavy metal pollution has become increasingly prominent and widespread due to agricultural and industrial activities, atmospheric deposition, fertilizer application, sewage irrigation and mining1,2posing substantial risks to both human health and the environment3,4. The 2021 “China Ecological Environment Report” highlighted cadmiun (Cd) as a major heavy metal pollutant affecting farmland environmental quality5. Accordingly, there is an urgent need for cost effective and sustainable methods to remediate Cd-contaminated soils. Among various methods, phytoremediation technology has garnered widespread attention due to its environmental friendliness and economic feasibility6.
B. pilosa L., as a Cd hyperaccumulator with the advantage of large biomass, short growth cycle, strong interspecies competitiveness and high remediation efficiency7has gradually attracted the attention of Chinese researchers in recent years8,9.In the mining areas, the content of Cd in soil was in the range of 50–85 mg·kg−1, B. pilosa L. showed very high Cd concentrations (112–213 mg·kg−1) in the leaves and stems, the EFs (extraction factor, ratio of Cd concentration in shoot to soil) and TFs (translocation factor, ratio of Cd concentration in shoot to root) were all higher than 110. B. pilosa L. exhibited not only better metal(loid) phytoextraction abilities but also higher shoot biomasses in a two-year field experiment on phytoremediation of soils contaminated with multiple metal(loid)s in Jiaoxi town, Liuyang city, Hunan Province, China11. B. pilosa L. also has a strong enrichment ability for Cd in Cd-contaminated farmland soil, showing stable accumulation characteristics, and the enrichment coefficient reaches 4.1612. Enhancing Cd remediation efficiency while ensuring optimal plant growth is currently a key research focus.
Nitrogen (N) is an essential macronutrient and involved in many metabolic processes of plants13,14. Although the application of N can increase the chlorophyll, leaf area index and dry biomass of plants15,16different forms and proportions of nitrogen fertilizer have different effect on plant growth, photosynthesis, nutrient absorption, and resistance to abiotic stress17,18. For Arabidopsis, NO3− and NH4+ have opposite effects on root growth, NO3− promoting growth and NH4+ inhibiting growth19,20. It has been found that N levels and forms are closely related to Cd absorption and tolerance of plants, sufficient N significantly enhanced the antioxidant capacity, inhibited the absorption and translocation of Cd in Populus euramericana21on the contrary, increasing the application of N-NO3− reduced the content of malondialdehyde (MDA) and H2O2, promoted the photosynthesis, growth and the tolerance to Cd of wheat seedlings, also promoted the absorption of Cd by wheat seedlings22. Additionally, an increased amount of N not only changed the sequestration of heavy metals by cell walls, chelation capacity and oxidative resistance to regulate Cd uptake, translocation and accumulation in plants, but also altered the Cd exchange capacity and the bioavailable Cd content in soil23,24.
Chelating agents, such as ethylenediaminetetraacetic acid (EDTA), form complexes with the heavy metal, which allows their detachment from soil particles and subsequent absorption by plant roots. However, EDTA is a persistent agent as it resists degradation by microorganisms, there is a risk of leaching and groundwater contamination, EDTA may also cause damage to soil bacteria and fungi, as well as to the plant itself. To avoid these major drawbacks, (S,S)-ethylenediaminedisuccinic acid (EDDS), as a biodegradable natural chelating agent, has been proposed to replace EDTA. Unlike EDTA, EDDS can not only activate heavy metals but also rapidly degrade (within 7–32 days) in soil, it appears to be quite less damaging to the environment since the risk of leaching heavy metals is reduced25,26. The application of EDDS increased the uranium (U) and Cd removal efficiencies of sunflower (Helianthus annuus L.)27. In addition, EDDS reduced pH and organic matter, improving microbial nutrient levels in soil28. Many studies about N fertilizers have focused on the effect of their form and concentration on plant growth and soil properties13,15. Chelating agents’ studies have concentrated on the chelating effect considering their individual use or combined with other substances7,28. While EDDS and N fertilizers have been studied separately, their combined effects on Cd fractionation and plant uptake are underexplored.
In the present study, we created an Cd-contaminated soil (15 mg·kg−1), a combined remediation technology using B. pilosa L. was utilized. Three different forms of N fertilizers (N-NO3−, N-NH4+, N-amide) with three different levels (25, 50, 75 mg N·kg−1) and EDDS (1 mmol·L−1) were provided to the soil. The objectives of this study were to determine the effects of N fertilizer combined with EDDS on (1) growth and biomass production of B. pilosa L. growing in Cd-contaminated soil; (2) the enrichment and translocation efficiency of Cd in B. pilosa L.; (3) Cd fractions and bioavailability in soil. By addressing these objectives, we aim to gain insights into the potential of combining N fertilizer and EDDS for remediating Cd-polluted soil by B. pilosa L., with a specific focus on plant uptake, bioavailability and morphological distribution of Cd in the soil. In this paper, through indoor pot experiments, form of N fertilizers combined with EDDS and the optimal application concentration which can significantly increase the content of available Cd in soil, promote the growth of B. pilosa L. and Cd absorption to enhance the remediation efficiency of Cd-contaminated soil will be explored. This is the first study to compare the effects of three nitrogen forms combined with EDDS on Cd remediation by B. pilosa L. The results will provide a valuable scientific basis and new ideas for the phytoremediation of typical high concentration Cd-polluted farmland soil in China.
Materials and methods
Experimental design
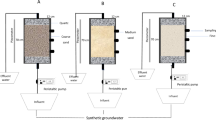
The pot experiment was performed in an artificial climate box to simulate the field environment. Temperature, humidity and illumination were 26℃, 65% and 150 µmol·m−2·s−1, respectively, for 14 h and 18℃, 70% and 0 µmol·m−2·s−1, respectively, for 10 h without light.
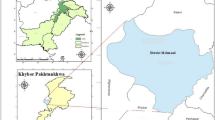
Soil used in the pot experiment was collected from the surface layer (0–20 cm) of farmland in Yuzhong District, Lanzhou, China (103°49’15"~104°34’40"E, 35°34’20"~36°26’30"N). To prepare for experimentation, the samples were air-dried, cleaned of debris, crushed and sieved using 2 mm nylon sieves for pot experiment and 100-mesh sieves for microwave clean-up to ensure consistent particle size. The total Cd content of the original soil is less than 0.001 mg·kg−1, and other basic physicochemical properties are shown in Table 1.
EDDS used in this study was obtained from Cool Chemical Science and Technology Co., Ltd Beijing, China, and applied at level: 1 mmol·L−129. CdCl2·2.5H2O and three types of N fertilizers, namely NO3−-N (Ca(NO3)2), NH4+-N ((NH4)2SO4) and amide-N (CO(NH2)2), were obtained from Damao Chemical Reagent Factory, Tianjin, China. CdCl2·2.5H2O was applied at level:15 mg·kg−130, and three types of N fertilizers were applied at levels: 25, 50 and 75 mg N·kg−1.
Cd (15 mg·kg−1) was spiked into air-dried soil by uniformly spraying an aqueous solution of CdCl2.2.5H2O onto the soil, deionized water (60% of field capacity) was added, and equilibrated for 4–5 weeks. During the equilibration period, the water content of the pot substrate was maintained at 60% of the field capacity, and the soil was mixed regularly31. 1.5 kg of treated soil was packed into each pot (18 cm diameter × 25 cm height). The B. pilosa L. seeds (provided by the Taishan Wild Vegetable Planting Base, Shandong, China) were surface-disinfected with 1% NaClO solution for 10 min (in order to improve the germination rate and disease resistance of seeds) then washed with deionized water three times, dried, and sown in pots at a depth of about 0.5–1.0 cm. The three forms of N fertilizer were separately added to the pots in the form of solution after sowing to maintain the soil N concentration at 25, 50 and 75 mg N·kg−130, respectively. The soil was regularly irrigated with deionized water, and the soil moisture of each treatment was maintained at approximately 60% of field water capacity. During the incubation period, the position of the flowerpot was changed from time to time to eliminate the marginal effect. After emergence, according to the previous research of the research group3050 seedlings of B. pilosa L. were kept in each pot. After 60 days of seedlings growth, 100 mL solution of 1 mmol·L−1 EDDS was applied to pots29. Plant and soil samples were collected for experiment on the 7th day after EDDS application. The control treatment (CK) received deionized water only, while the experiment was arranged in a completely randomized design and replicated three times for each treatment. In total, the experiment had 36 treatments shown in Table 2.
Measurement of indicators
Plant growth analysis
3 seedlings of B. pilosa L. were selected at random from each replicate and removed from the pots, then separated into shoots (aboveground parts) and roots, plant height (shoot length, Lsh) and root length (Lr) were measured with a ruler. The fresh biomass and dry biomass of plants are represented by fresh weight (FW) and dry weight (DW) respectively. Prior to the FW of shoots (FWsh) and roots (FWr) measurement, the shoots and roots were washed carefully with deionized water, followed by proper blotting between filter papers. For recording DW of shoots (DWsh) and roots (DWr), the shoots and roots were dried at 105 °C for 30 min, then transferred to 80 °C and dried until constant weight. The single plant FW and DW were calculated as the sum of FWsh and FWr, DWsh and DWr, respectively.
Determination of Cd content
A flame atomic absorption spectrometery (SpectrA 220FS, Varian, USA) was used to measure the Cd content32. At the end of the pot experiments, plant samples were collected and thoroughly washed with distilled water. Subsequently, the roots were immersed in 20 mM Na2EDTA solution for 1 h for the removal of heavy metals adsorbed on the root surface. After deactivation at 105 °C for 30 min, samples were dried to a constant weight at 80 °C. Finely ground dried shoot and root samples (0.5 g) were digested with a mixture of HNO3 and HClO4. Plant tissue samples were added with 10 ml HNO3 in each tube. Samples were kept overnight, then placed on a hot-plate set to 100 °C. When the samples changed to a yellow color, the boiling liquid samples were cooled and 5 ml HClO4 was added. Next the samples were digested at 180 °C until the digested samples turned colorless. The digested samples were diluted to a constant volume of 25 ml.
Soil samples were also collected when plants were harvested. Soil was dried and sifted (2 mm), about 0.5 g of dried soil samples were weighed and digested with an acid mixture containing 9 ml HCl, 3 ml HNO3, 3 ml HF and 1 ml HClO4, then after acid removal, constant volume (25 ml), filtration. The digested plant and soil samples were assessed with flame atomic absorption spectrophotometer (SpectrA 220FS, Varian, USA) to measure Cd content33. Standard material for analysis of effective components of loess soil (HTSB-2) was used for quality control and blank test was conducted in the whole process. The detection limit of the instrument was 0.002 mg·L−1, and the relative standard deviation (RSD) was less than 5%. During digestion, each group (20 samples) contained two standard samples (HTSB-2 and NST-2) and one blank sample, and the recovery rate of the internal standard response of the sample was above 90%.
Cd distribution between operational fractions in soil.
To evaluate the effects of N fertilizer combined with EDDS on the speciation, migration, transformation and bioavailability of Cd in soil, the Tessier’s sequential extraction method was applied to determine the speciation distribution of Cd34using 1.0 g of soil. The specific steps are shown in Table 3. Quality control measures were the same as the determination of Cd content.
Data processing and analysis
The bioconcentration factor (BCF), translocation factor (TF), phytoextraction efficiency (PE) and remove efficiency (RE) were calculated using the following Eq27..
.
RStudio 4.1.0 software was used for Pearson correlation analysis and principal component analysis (PCA), and the membership function value of the principal component score (PCS) was calculated by the membership function method using SPSS 24.0 software. Based on the ratio of principal component contribution rates, the comprehensive score of B. pilosa L. under different treatment was obtained to evaluate its Cd remediation capability35. The calculation formulas are as follows:
.
Where UPCSj represents the membership function value of the j-th PCS; xj represents the j-th PCS, xmin represents the minimum value within the j-th principal component; xmax represents the maximum value within the j-th principal component; Wj represents the contribution rate ratio of the j-th PCS; Pj represents the contribution rate of the j-th principal component; D represent the comprehensive score of Cd remediation capability for B. pilosa L., with a higher D value indicating a stronger remediation capability.
All data are shown as means ± standard deviations (SD) of at least three independent experiments with three replicates in each experiment (n = 9). Duncan’s multiple comparison test was used to evaluate significant differences between different treatments at P < 0.05. The Duncan’s test is suitable for pairwise comparisons between groups.
Results
Plant growth and biomass production
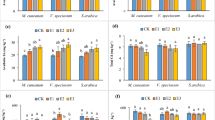
The Lsh, FWr and DWr of plants in whole pot under Cd stress increased by 10.79%, 20.93% and 34.41% (P < 0.05) over the CK, respectively, Lr and FWsh further increased by 10.52% and 11.56% with EDDS supplementation compared to Cd stress (P < 0.05), respectively (Fig. 1). The application of N fertilizers, especially N-NO3− and N-NH4+, further increased B. pilosa L. growth and biomass. The largest Lsh and Lr among all treatments were observed in plants treated with 25 mg N·kg−1 N-NH4+ combined with EDDS, which increased by 11.24% and 23.13% over the Cd + EDDS treatment, 12.19% and 36.09% over the Cd treatment, respectively (P < 0.05) (Fig. 1a, b). The highest biomass was observed in plants treated with 75 mg N·kg−1 N-NO3− combined with EDDS, FWsh and DWsh, FWr and DWr of plants in the whole pot (FWsh: 327.86 mg·pot−1, DWsh: 41.53 mg·pot−1, FWr: 25.02 mg·pot−1, DWr: 6.65 mg·pot−1) increased by 27.04%, 45.59%, 25.45% and 38.11% over the Cd + EDDS treatment, 41.72%, 24.01%, 20.48% and 19.46% over the Cd treatment, respectively (P < 0.05) (Fig. 1c, d, e, f). The use of N-amide significantly inhibited plant growth and biomass production (Fig. 1).
Plant Cd accumulation and translocation
Under Cd stress, the addition of EDDS increased the Cd content in the shoot and root by 54.76% and 76.26% (P < 0.05), respectively (Fig. 2a, b), also increasing the total Cd content absorbed by the plants by 34.78% (P < 0.05) (Fig. 2c). Compared to the Cd treatment, combination of N fertilizers and EDDS increased the plant’s extraction of Cd. The maximum of Cd content in the shoot, root and total Cd in plants of the whole pot were observed in plants treated with 50 mg N·kg−1 N-NO3− combined with EDDS, there was an increase of 125.72%, 181.10% and 97.83% over the Cd treatment, an increase of 45.85%, 59.48% and 46.77% over the Cd + EDDS treatment, respectively (P < 0.05) (Fig. 2a, b, c). Meanwhile, treatment of 50 mg N·kg−1 N-NO3− combined with EDDS decreased the total Cd in soil by 43.81% and 88.51% over the Cd + EDDS and Cd treatments, respectively (P < 0.05), achieving the lowest value (19.71 mg·kg−1) among all treatments (Fig. 2d).
B. pilosa L. under Cd stress demonstrated a strong Cd accumulation capability, both BCFsh and BCFr are greater than 2. The plant TF was relatively low, with the maximum (1.15) observed in the treatment of 25 mg N·kg−1 N-NO3− combined with EDDS. Compared to the Cd treatment, the application of EDDS significantly increased the BCFsh, BCFr, PE and RE by 54.39%, 76.26%, 34.53% and 30.80% (P < 0.05), respectively. The addition of N fertilizers increased the plant’s Cd remediation efficiency. The enhancement effect of N-NO3− is greater than that of N-NH4+ and N-amide. The highest Cd accumulation was observed in plants treated with 50 mg N·kg−1 N-NO3− and EDDS, BCFsh, BCFr, PE and RE increased by 46.07%, 59.59%, 46.73% and 43.62% over the Cd + EDDS treatment, and increased by 125.52%, 181.28%, 97.39% and 87.86% over the Cd treatment (P < 0.05), respectively (Table 4).
Cd distribution between operational fractions in the soil
The Tessier five-step extraction method divided Cd in soil into five fractions: exchangeable fraction (F1), carbonate-bound fraction (F2), Fe-Mn oxides bound fraction (F3), organic-bound fraction (F4) and residual fraction (F5). The bioavailability of F1-Cd, F2-Cd and F3-Cd is higher than that of F4-Cd and F5-Cd. Addition of EDDS increased the content of F3-Cd by 27.31%, decreased the content of F5-Cd by 13.99% (P < 0.05), compared to the Cd treatment. The combination of N fertilizer with EDDS reduced the F5-Cd content. Compared to EDDS treatment, the addition of 50 mg N·kg−1 N-NO3− resulted in a reduction of 5.78% in the total Cd content across all fractions (Fig. 3a, b, c, d, e).
According to the sequential extraction, Cd in the soil was predominantly distributed into F2 and F3 (Fig. 3f). The application of EDDS enhanced Cd solubility and availability in soil, the proportion of the F1-Cd + F2-Cd + F3-Cd increased by 3.65%, to the contrary, the proportion of the F4-Cd + F5-Cd decreased by 11.73%, compared to the Cd treatment (P < 0.05). Among all the treatments of N fertilizer combined with EDDS, treatments of 50 mg N·kg−1 N-NO3− combined with EDDS had the most significant effect on distribution of Cd in the soil, it decreased the proportion of the F1-Cd + F2-Cd + F3-Cd by 10.58%, to the contrary, increased the proportion of the F4-Cd + F5-Cd by 15.47%, compared to the Cd + EDDS treatment (P < 0.05) (Fig. 3f).
Correlation analysis
To further investigate the mechanism of the combined effect of N-NO3− and EDDS on Cd accumulation, Pearson correlation analysis was conducted on variables. The correlation between different varieties of B. pilosa L. under different treatments of N-NO3− combined with EDDS in the Cd-contaminated soil was shown in Fig. 4. As shown in Fig. 4, the N-NO3− concentration, which was significantly positively correlated with Lr, FWsh, Cdr and BCFr (P < 0.05), was positively correlated with the FWr, DWsh and DWr, Cd-plant, PE, RE, F1-Cd and F2-Cd content (P > 0.05), while being significantly negatively correlated with TF, F3-Cd and F4-Cd content (P < 0.05), and also negatively correlated with Cdsh, Cd-soil, Lsh, BCFsh and F5-Cd content (P > 0.05).
Relationship between different varieties of B. pilosa L. under different treatments of N-NO3- combined with EDDS in the Cd-contaminated soil. Different abbreviations used in the figure are as follow: N-Nitrate: concentration of N-NO3-; FWsh: fresh weight of shoot; FWr: fresh weight of root; DWsh: dry weight of shoot; DWr: dry weight of root; Cdsh: Cd content in shoot; Cdr: Cd content in root; Cd-plant: total amount of Cd accumulated by the plant; Cd-soil: total amount of residual Cd in soil; Lsh: length of shoot; Lr: length of root. The remaining indicators have been mentioned in the earlier table and figure.
Evaluation of the plant remediation capability for Cd-contaminated soil
Since F1-Cd and F2-Cd in soil had the highest availability, seventeen indicators were selected for evaluating the Cd remediation capability of B. pilosa L. by using the combination of PCA and membership function method: Lsh, Lr, FWsh, FWr, DWsh, DWr, Cdsh, Cdr, Cd-plant, Cd-soil, BCFsh, BCFr, TF, PE, RE, F1-Cd and F2-Cd. The PCA results indicated that there were 4 principal components with eigenvalues greater than 1(see Supplementary Table S1), with a cumulative contribution rate of 92.39%, reflecting most of the information. Among these, plant Cd content and enrichment capability contributed the most to the remediation ability of B. pilosa L., followed by soil Cd fractions and plant biomass (Fig. 5).
Loading plots of PCA on various attributes of B. pilosa L. under different treatments of N-NO3− combined with EDDS. Different abbreviations used in the figure are as mentioned in Fig. 4.
Using x1 to x17 to correspond to the seventeen indicators mentioned above, and Y1, Y2, Y3 and Y4 to represent the first, second, third and fourth PCS, respectively, formulas (8), (9), (10) and (11) were derived based on the loading matrix and principal component eigenvalues. The membership function values Uj were then calculated based on the PCS, with the principal component contribution ratios Wj as weights, to obtain the comprehensive scores for different treatments (see Supplementary Table S2). The evaluation results showed that the treatment of N-NO3− combined with EDDS had the highest remediation capability for B. pilosa L., followed by N-NH4+ combined with EDDS, then EDDS alone, and the least effective treatment was N-amide combined with EDDS. Among all treatments, the B. pilosa L. treated with 50 mg N·kg−1 N-NO3− combined with EDDS exhibited the best capability for remediating Cd-contaminated soil.
Discussion
Biomass of hyperaccumulator plants and heavy metal concentration in plants are the key factors for successful phytoextraction36. EDDS as biodegradable natural chelating agent, can not only activate heavy metals but also degrade rapidly in soil ensuring safety for human health and environment26. In addition, EDDS can reduce pH and organic matter, improving microbial nutrient levels in soil281 mmol·L−1 EDDS is equivalent to 28 mg·kg−1 of N37. Application of EDDS increased the uranium (U) and Cd removal efficiencies of sunflower27. In this study, Lsh and DWr increased under Cd stress, Lr and FWsh further increased with EDDS supplementation. Similar studies have shown that Cd-contaminated soil induces a hormesis effect in Coronopus didymus (Brassicaceae), resulting in increased growth and biomass, EDDS addition leads to a further increase in the root length over the control38. It indicated that Cd stress induced biological resistance in B. pilosa L., with EDDS providing certain N and C source for the plant by many microorganisms and enhance microbial activity, directly or indirectly, improving plant growth39. As a macronutrient, N is the main organic component of most plant biochemical compounds and plays an important role in plant photosynthesis40. N mitigated the effect of metal stress and increased the accumulation of Cd in the aboveground parts of (A) lentiformis plants, the fertilization with N could be an effective tool to enhance Cd-phytoextraction from polluted sites15,23. In present study, application of N fertilizers, especially N-NO3− and N-NH4+, further increased (B) pilosa L. growth and biomass. The plants treated with 75 mg N·kg−1 N-NO3− + EDDS showed the highest biomass among all treatments. The use of N-amide significantly inhibited plant growth and biomass production. Different forms and proportions of N fertilizer have different effect on plant growth, photosynthesis, nutrient absorption, and resistance to abiotic stress17,18. Our result further proved that different forms of N fertilizer have different effect on plant growth17,18. It also indicated that B. pilosa L. was a nitrate-loving plant. High concentrations of N-NO3− were stored in vacuoles by the plant, which did not harm the plant41. Nitrate served as an important signaling molecule involved in maintaining the key physiological processes necessary for optimal plant growth and development. Improving nitrate uptake and translocation through the activation of nitrate sensing, signaling, and regulatory processes promoted plant growth42. The combination of 75 mg N·kg−1 N-NO3− and EDDS provided a more suitable growth environment and N source for the plant.
Recent studies indicated that the Cd accumulation in C. didymus shoots, leaves and roots were increased by application of EDDS in Cd-contaminated soil38. The similar findings were found in this study. Under Cd stress, the addition of EDDS increased the Cd content in the shoot and root of B. pilosa L., the total Cd accumulation in the plants of the whole pot also increased. EDDS had chelating properties and formed a stable EDDS-Cd chelate with Cd2+, which helped to release Cd2+ from the soil. The Cd2+ became more available in the soil solution, making it easier for plants to absorb43,44. Additionally, N levels and forms are closely related to Cd absorption and tolerance of plants, sufficient N significantly inhibited the absorption and translocation of Cd in Populus euramericana21on the contrary, increasing the application of N-NO3− promoted the absorption of Cd by wheat seedlings22. Compared to other form of N, nitrate is more effective in promoting the absorption of Cd22,23. Similarly, in the present study, different forms of N fertilizers combined with EDDS increased the Cd content in the shoot and root, but the best effect observed in the N-NO3− treatment. The Cd content in the shoots and roots, as well as the total Cd accumulation in plants of the whole pot, reached their maximum (0.83 mg·kg−1, 92.37 mg·kg−1, and 2.73 mg·pot−1) under the treatment of 50 mg N·kg−1 N-NO3− combined with EDDS. Meanwhile, this treatment reduced the total residual Cd in soil, achieving the lowest value (19.71 mg·kg−1) among all treatments. Many studies have found that the supply of N-NO3− enables plants to absorb more Cd than N-NH4+, N-amide, and N-organic forms, the reasons might be as follows: (1) N-NO3− stimulates the secretion of organic acid into the rhizosphere, which increases the soil H+ concentration and acidify the soil under low pH-buffer soil conditions, which increases the rate of bioavailability of total Cd in polluted soil23,24; (2) The Ca2+ released from nitrate nitrogen (Ca(NO3)2) has a similar ion radius to Cd2+, leading to competition for adsorption sites in the soil, as a result, the concentration of available Cd2+ in the soil increases45; (3) NO3− transport across the plasma membrane temporarily polarises and hyperpolarises the membrane potential, which results in enhanced Cd transport23; (4) N might adjusts non-Cd special gene expressions and divalent cation transporters (i.e. Fe, Mn, and Zn transporters) to regulate Cd uptake and transport in plants, such as application of excessive NO3 − increased Fe and Cd content in rice by upregulating OsIRT1 (Iron-regulated transporter 1) and OsNramp1 (Natural Resistance- Associated Macrophage Protein 1) gene expression46. Furthermore, Cd2+ or EDDS-Cd chelates are accumulated in the roots, translocated through the xylem to the plant shoots and concentrated in the cell walls or stored in the vacuoles47. This alleviated the impact of Cd stress and enabled B. pilosa L. to accumulated more Cd. However, some studies have found that N-NH4+ is more likely to promote Cd accumulation than N-NO3− in the case of equal amounts of N48. These different conclusions can be caused by different plant species and different cultivated land types. Generally speaking, in paddy fields, N-NO3− is more likely to promote more Cd accumulation than N-NH4+, while in dry land the opposite is true. Collectively, N-NO3− represents a more efficient fertilizer for hyperaccumulator species used in Cd bioremediation, N-NH4+ can be used to reduce Cd accumulation in crop production49. However, more experimental evidence is needed to clarify the effects of N-NO3− and N-NH4+ on Cd uptake and accumulation in different plants25.
BCF, TF, PE and RE values are often used to assess the accumulation, translocation, phytoextraction and remediation efficiency of plants50. In this study, B. pilosa L. demonstrated a strong Cd accumulation capability (BCF > 2 in shoot and root). Under Cd stress, the addition of EDDS significantly increased the BCFsh, BCFr, PE and RE of B. pilosa L. The similar findings were reported by Wang et al.39indicated that 1 mmol·L−1 EDDS significantly promotes the translocation and absorption of Cd. Similarly, in this study, N fertilizer application increased the plant’s Cd accumulation. The N-NO3− combined with EDDS treatment significantly enhanced the plant’s Cd remediation efficiency more than N-NH4+ or N-amide. The plants treated with the combination of 50 mg N·kg−1 N-NO3− and EDDS showed the highest Cd accumulation characteristics (BCFsh: 5.39, BCFr: 6.16, PE: 12.12%, RE: 12.38%). This was consistent with findings of Yotsova et al.22 and Yang et al.23. The reason might have been that 50 mg N·kg−1 N-NO3− in combination with EDDS, provided an appropriate N source to upregulate the expression of divalent cation transporter protein genes Nramp1, HMA2 (heavy metal transporting ATPase 2) and IRT1 so as to promote Cd absorption from the rhizosphere to the roots and Cd translocation from roots to shoots46. In addition, 50 mg N·kg−1 N-NO3− in combination with EDDS might alter the cell wall components and structure to accommodate Cd in plants by increasing the pectin and hemicellulose content in the root cell wall51. It also might enhance the chelation capacity by suppling the nutrient pool for the consumption of glutathione (GSH) and phytochelatins (PCs), both of which have been extensively reported in Cd stabilisation and translocation in plants, alleviate oxidative stress by regulating antioxidative systems to enhance resistance52ultimately regulating the plant’s Cd remediation efficiency23. However, these physiological, biochemical and molecular mechanisms need to be further studied. Furthermore, the plant TF was relatively low, with the maximum (1.15) observed in the treatment of 25 mg N·kg−1 N-NO3− combined with EDDS. This indicated that the Cd absorbed by B. pilosa L. was mainly concentrated in the roots. This was consistent with the results of correlation analysist. It demonstrated that Cd translocation capacity of B. pilosa L. was weak, it may be because there is no specific Cd2+ channel and transporter in B. pilosa L., and the root system acts as a barrier for the transfer of Cd to the above-ground part of the plant, and Cd accumulation in the root system protects the plant from oxidative stress53.
The Tessier five-step extraction method divided Cd in soil into five fractions34. Among these fractions, the F1-Cd had high bioactivity. It had a weak binding capacity with solid adsorbents and could easily be released into a highly mobile free fraction, which was readily absorbed by plant organisms. The F2-Cd was susceptible to weathering and is highly sensitive to pH. Both F1-Cd and F2-Cd were the most easily absorbed by plants54. In our study, Cd existed predominantly in the F2 fraction in the soil, next was the F3 fraction. Chelating agents can directly change the solubility of heavy metals, transform the residual fraction from the most stable to the active state, and enhance their bioavailability, thereby facilitating the restoration of heavy metal-contaminated soil by chemical reactions such as acidification, complexation, precipitation and oxidation reduction55.Chelating agents can also affect the heavy metals in soil indirectly by influencing soil microbial community diversity and enzyme activity. This change is usually in a dynamic equilibrium, with the increase of the soluble state, other fractions of heavy metals are increasingly reduced44,56. In addition, Cd in soil can be re-mobilized by rhizosphere acidification caused by organic acid efflux48N-NO3− has been also reported to stimulate the secretion of organic acid into the rhizosphere, which increases the soil cation exchange capacity (CEC) and H+ concentration, the water soluble Cd content, and Cd accumulation in rice57. The promotion effect of N fertilisation is more notable under acidic (low pH) soil conditions58. Based on our results, addition of EDDS increased the content of F3-Cd, decreased the content of F5-Cd, the combination of N fertilizer with EDDS reduced the F5-Cd content, the addition of 50 mg N·kg−1 N-NO3− and EDDS decreased the proportion of the F1-Cd + F2-Cd + F3-Cd, to the contrary, increased the proportion of the F4-Cd + F5-Cd, reduced the total Cd content across all fractions, this was consistent with the results of correlation analysist. These results indicated that treatment with 50 mg N·kg−1 N-NO3− and EDDS was most efficient and decreased the amount of residual Cd in the soil, thereby activating Cd2+ in the soil, promoting the accumulation and translocation of Cd by plants. Similar results have been found in previous studies2.
Currently, the combination of PCA and membership function method for comprehensive evaluation of plant capabilities is a popular approach35,59. The PCA results indicated that plant Cd content and enrichment capability contributed the most to the remediation ability of B. pilosa L., followed by soil Cd fractions and plant biomass, indicating that the addition of N fertilizer and EDDS effectively altered soil Cd fractions and increased plant biomass to accumulate more Cd, consistent with previous results. The comprehensive evaluation results showed that the B. pilosa L. treated with 50 mg N·kg−1 N-NO3− combined with EDDS exhibited the best capability for remediating Cd-contaminated soil. This result was consistent with the results described above, and further proved the strengthening effect of N fertilizers combined with EDDS on remediation efficiency of B. pilosa L. in Cd-contaminated soils. However, as biodegradable chelator, although EDDS is less persistent in the environment than non-biodegradable chelators, it can still be toxic to some plants and microorganisms and can potentially cause long-term harm to the ecosystem, such as, the activated heavy metals may migrate downward into groundwater through the leaching effect, thereby polluting groundwater and damaging the aquatic ecosystem6. Slow-release chelating agents have been developed to control the release speed of chelating agents to avoid a sudden increase of soil bioavailable heavy metals60.
Conclusions
The combination of 50 mg N·kg−1 N-NO3− and EDDS not only promoted growth and increased biomass of B. pilosa L., it also changed Cd fraction and increased available Cd content in soil, thereby achieved the maximum soil Cd removal of 12.38% across all treatments. The comprehensive score obtained using the membership function method also showed that the treatment with 50 mg N·kg−1 N-NO3− and EDDS had the highest score. Comprehensively considering factors such as environmental risk, plant growth, and phytoremediation efficiency, 50 mg N·kg−1 N-NO3− combined with EDDS is the most promising strategy for phytoremediation to remediate 15 mg·kg−1 Cd-contaminated farmland soil through continuous planting and harvesting B. pilosa L.
In this study, pot experiments were adopted only, as a next step, it is necessary to confirm pot experiment results and demonstrate the feasibility of its practical application through field cultivation methods. It is also recommended to further explore the physiological, biochemical and molecular mechanisms of the combined application of N fertilizer and EDDS on the growth and Cd absorption and accumulation of B. pilosa L. by methods such as molecular biology and metabolomics.
Experimental research and field studies on plants statement
In the study only cultivated plants were used which are neither endangered nor at risk of extinction. We confirm that their handling was performed in compliance with relevant institution, national and international guidelines and legislation.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Xiang, M. T. et al. Assessment of heavy metal pollution in soil and classification of pollution risk management and control zones in the industrial developed City. Environ. Manage. 66, 1105–1119. https://doi.org/10.1007/s00267-020-01370-w (2020).
Yang, Q., Yang, C., Yu, H., Zhao, Z. Q. & Bai, Z. K. The addition of degradable chelating agents enhances maize phytoremediation efficiency in Cd-contaminated soils. Chemosphere 269, 129373. https://doi.org/10.1016/j.chemosphere.2020.129373 (2021).
Chen , H. X. et al. Effect of silicon spraying on rice photosynthesis and antioxidant defense system on cadmium accumulation. Sci. Rep. 14, 15265. https://doi.org/10.1038/s41598-024-66204-9 (2024).
Sikdar, A. et al. Cadmium contamination in the soil environment: impact on plant growth and human health. In Agrochemicals in Soil and Environment: Impacts and Remediation (eds. Naeem, M., Bremont, J. F. J., Ansari, A. A. & Gill, S. S.) 367–408. (Singapore: Springer Nature Singapore, 2022). https://doi.org/10.1007/978-981-16-9310-6_16
Ministry of Ecology and Environment. China’s ecological environment status report. Ministry of Ecology and Environment of the People’s Republic of China (2021). https://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/202205/P020220608338202870777.pdf (2022).
Yin, F. W., Li, J. B., Wang, Y. L. & Yang, Z. Y. Biodegradable chelating agents for enhancing phytoremediation: mechanisms, market feasibility, and future studies. Ecotoxicol. Environ. Saf. 272, 116113. https://doi.org/10.1016/j.ecoenv.2024.116113 (2024).
Yang, Q., Xie, J. T., Liu, H. J. & Fang, Z. G. The addition of exogenous low-molecular-weight organic acids improved phytoremediation by Bidens pilosa L. in Cd-contaminated soil. Environ. Sci. Pollut Res. 51, 76766–76781. https://doi.org/10.1007/s11356-022-20686-0 (2022).
Zhang, X. Y. et al. Effect of crop straw biochars on the remediation of Cd-contaminated farmland soil by hyperaccumulator Bidens pilosa L. Ecotoxicol. Environ. Saf. 219, 112332. https://doi.org/10.1016/j.ecoenv.2021.112332 (2021).
Dou, X. K., Dai, H. P., Skuza, L. D. & Wei, S. H. Bidens pilosa L. hyperaccumulating cd with different species in soil and the role of EDTA on the hyperaccumulation. Environ. Sci. Pollut Res. 26, 25668–25675. https://doi.org/10.1007/s11356-019-05831-6 (2019).
Dai, H. P., Wei, S. H. & Skuza, L. D. Effects of different soil pH and nitrogen fertilizers on Bidens pilosa L. Cd accumulation. Environ. Sci. Pollut Res. 9, 9403–9409. https://doi.org/10.1007/s11356-019-07579-5 (2020).
Yu, F. M. et al. Phytoextraction of metal(loid)s from contaminated soils by six plant species: A field study. Sci. Total Environ. 804, 150282. https://doi.org/10.1016/j.scitotenv.2021.150282 (2022).
Zhang, Y. X., Zhou, L., Xiao, N. C., Pang, R. & Song, B. Remediation potential of B. pilosa L. in cadmium-contaminated farmland. Acta Ecol. Sin. 40 (16), 5805–5813. https://doi.org/10.5846/stxb201812242790 (2020).
Ye, X. X., Hu, H. X., Li, H. Y., Xiong, Q. Z. & Gao, H. J. Combined nitrogen fertilizer and wheat straw increases the cadmium phytoextraction efficiency of Tagetes patula. Ecotoxicol. Environ. Saf. 170, 210–217. https://doi.org/10.1016/j.ecoenv.2018.11.135 (2019).
Ye, T. Y. et al. Interaction effects of irrigation and nitrogen on the coordination between crop water productivity and nitrogen use efficiency in wheat production on the North China plain. Agric. Water Manag. 271, 107787. https://doi.org/10.1016/j.agwat.2022.107787 (2022).
Eissa, M. A. & Roshdy, N. M. K. Nitrogen fertilization: Effect on Cd-phytoextraction by the halophytic plant quail bush [Atriplex lentiformis (Torr.) S. Wats]. S. Afr. J. Bot. 115, 126–131. https://doi.org/10.1016/j.sajb.2018.01.015 (2018).
Koppensteiner, L. J. et al. Yield and yield components of facultative wheat are affected by sowing time, nitrogen fertilization and environment. Eur. J. Agron. 140, 126591. https://doi.org/10.1016/j.eja.2022.126591 (2022).
Yu, X., Keitel, C., Zhang, Y., Wangeci, A. N. & Dijkstra, F. A. Global meta-analysis of nitrogen fertilizer use efficiency in rice, wheat and maize. Agric. Ecosyst. Environ. 338, 108089. https://doi.org/10.1016/j.agee.2022.108089 (2022).
Shilpha, J., Song, J. N. & Jeong, B. R. Ammonium phytotoxicity and tolerance: an insight into ammonium nutrition to improve crop productivity. Agronomy 13, 1487. https://doi.org/10.3390/agronomy13061487 (2023).
Giehl, R. F. H. Von wirén, N. Root nutrient foraging. Plant. Physiol. 166, 509–517. https://doi.org/10.1104/pp.114.245225 (2014).
Li, B. H., Li, G. J., Kronzucker, H. J., Baluˇska, F. & Shi, W. M. Ammonium stress in Arabidopsis: signaling, genetic loci, and physiological targets. Trends Plant. Sci. 19, 107–114. https://doi.org/10.1016/j.tplants.2013.09.004 (2014).
Song, J. Y. et al. Mechanisms underlying enhanced cd translocation and tolerance in roots of Populus euramericana in response to nitrogen fertilization. Plant. Sci. 287, 110206. https://doi.org/10.1016/j.plantsci.2019.110206 (2019).
Yotsova, E. et al. Effects of cadmium on two wheat cultivars depending on different nitrogen supply. Plant. Physiol. Biochem. 155, 789–799. https://doi.org/10.1016/j.plaphy.2020.06.042 (2020).
Yang, Y. J. et al. Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: a review. Sci. Total Environ. 708, 135186. https://doi.org/10.1016/j.scitotenv.2019.135186 (2020).
Chen, K. X. et al. Effects of nitrogen forms on cd uptake and tolerance in wheat seedlings. Sci. Total Environ. 936, 173451. https://doi.org/10.1016/j.scitotenv.2024.173451 (2024).
Yoo, J. C. et al. A combination of ferric nitrate/edds enhanced washing and sludge-derived Biochar stabilization of metal-contaminated soils. Sci. Total Environ. 616, 572–582. https://doi.org/10.1016/j.scitotenv.2017.10.310 (2018).
Wang, Y. L., Lu, Y. H., Lu, J. Y., Zhang, Z. N. & Yang, Z. Y. Research progress on the biosynthesis and bioproduction of the biodegradable chelating agent (S,S)-EDDS. Process. Biochem. 116, 38–48. https://doi.org/10.1016/j.procbio.2022.02.025 (2022).
Chen, L., Yang, J. Y. & Wang, D. Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus annuus L.) enhanced with biodegradable chelating agents. J. Clean. Prod. 263, 121491. https://doi.org/10.1016/j.jclepro.2020.121491 (2020).
Wang, X. L. et al. Biodegradation and effects of EDDS and NTA on Zn in soil solutions during phytoextraction by alfalfa in soils with three Zn levels. Chemosphere 292, 133519. https://doi.org/10.1016/j.chemosphere.2022.133519 (2022).
Yang, B. et al. Effects of EDDS on growth, antioxidant enzyme, and cd accumulation of Bidens pilosa L. seedlings under cd stress. J. Agro-Environ. Sci. 5, 875–882. https://doi.org/10.11654/jaes.2017-1116 (2018).
Zheng, Y. et al. Study on the synergism of nitrogenfertilizer and EDDS to enhance the remediation of cadmium contamiated soil by Bidens Pilosa L. J. Lanzhou Jiaotong Univ. 4, 111–118. https://doi.org/10.3969/j.issn.1001-4373.2022.04.015 (2022).
Chen, Y. P. et al. Effects of combined treatments of exogenous NO and EDDS on growth, physiology and Cd accumulation of Medicago sativa under Cd stress condition. J. Plant Resour. Environ. 6, 1–14. https://doi.org/10.3969/j.issn.1674-7895.2022.06.01 (2022).
MEEPRC (Ministry of Ecology and Environment of the People’s Republic of China). Soil Quality-Determination of Lead, Cadmium-Graphite Furnace Atomic Absorption Spectrophotometry. GB/T 17141-1997. https://www.mee.gov.cn/image20010518/1949.pdf (1997).
Xu, Y. S., Xia, X., Li, X., Wang, J. & Yu, W. Effect of digestion systems on determination of heavy metals in soil. Environ. Eng. 5, 66–69. https://doi.org/10.13205/j.hjgc.201905013 (2019).
Gao, H. F. et al. Speciation analysis of lead-contaminated soil treated with sodium dihydrogen phosphate using Tessier and BCR. Chin. J. Environ. Eng. 11 (10), 5751–5756. https://doi.org/10.12030/j.cjee.201611189 (2017).
Feng, L. J., Chen, Z. Q., Luo, L. J. & Wang, J. Effects of bacterial agent application on physiological characteristics of photosynthesis and stress resistance in Dicranopteris pedata under high temperature stress. Guihaia 44 (11), 2089–2100. https://doi.org/10.11931/guihaia.gxzw202310003 (2024).
Huang, R. et al. Evaluation of phytoremediation potential of five cd (hyper) accumulators in two cd contaminated soils. Sci. Total Environ. 721, 137581. https://doi.org/10.1016/j.scitotenv.2020.137581 (2020).
Wang, Y. L. et al. Effects of S, Sethylenediamine disuccinic acid on the phytoextraction efficiency of Solanum nigrum L. and soil quality in Cd-contaminated alkaline wheat soil. Environ. Sci. Pollut Res. 28, 42959–42974. https://doi.org/10.1007/s11356-021-13764-2 (2021).
Raina, R., Sharma, P., Batish, D. R., Kohli, R. K. & Singh, H. P. Comparative assessment of two biodegradable chelants, S, S-ethylenediamine disuccinic acid and nitrilotriacetic acid, in facilitating cd remediation by lesser swine Cress (Coronopus didymus, Brassicaceae). Environ. Monit. Assess. 195 (12), 1526. https://doi.org/10.1007/s10661-023-12073-0 (2023).
Wang, Y. L. et al. Effects of EDDS on the cd uptake and growth of Tagetes patula L. and Phytolacca americana L. in cd-contaminated alkaline soil in Northern China. Environ. Sci. Pollut Res. 27, 25248–25260. https://doi.org/10.1007/s11356-020-08877-z (2020).
Lin, Y. J., Feng, Y. X. & Yu, X. Z. The importance of utilizing nitrate (NO3–) over ammonium (NH4+) as nitrogen source during detoxification of exogenous thiocyanate (SCN–) in Oryza sativa. Environ. Sci. Pollut Res. 4, 5622–5633. https://doi.org/10.1007/s11356-021-15959-z (2022).
Coskun, D., Britto, D. T., Shi, W. M. & Kronzucker, H. J. How plant root exudates shape the nitrogen cycle. Trends Plant. Sci. 22 (8), 661–673. https://doi.org/10.1016/j.tplants.2017.05.004 (2017).
Kant, S. Academic Press,. Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. In Seminars in Cell & Developmental Biology (ed. Mao, Y.) 74, 89–96. (2018). https://doi.org/10.1016/j.semcdb.2017.08.034
Zhao, Y. P. et al. Role of chelate on Cu distribution and speciation in Lolium multiflorum by synchrotron techniques. Sci. Total Environ. 621, 772–781. https://doi.org/10.1016/j.scitotenv.2017.11.189 (2018).
Wang, M. M., Song, G. F., Zheng, Z. H., Mi, X. & Song, Z. X. Exploring the impact of fulvic acid and humic acid on heavy metal availability to alfalfa in molybdenum contaminated soil. Sci. Rep. 14, 32037. https://doi.org/10.1038/s41598-024-83813-6 (2024).
Ren, C. et al. Study on the passivation effect of different passivators on alkaline cadmium contaminated soil. Environ. Sci. Technol. 44 (3), 71–78. https://doi.org/10.19741/j.issn.1673-4831.2021.0098 (2021).
Yang, Y. J. et al. Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa). Environ Exp. Bot 122, 141–149. https://doi.org/10.1016/j.envexpbot.2015.10.001(2016).
Wångstrand, H., Eriksson, J. & Öborn, I. Cadmium concentration in winter wheat as affected by nitrogen fertilization. Eur. J. Agron. 26, 209–214. https://doi.org/10.1016/j.eja.2006.09.010 (2007).
Xu, Z. Nitrogen fertilizer affects rhizosphere cd re-mobilization by mediating gene AmALM2 and AmALMT7 expression in edibleamaranth roots. J. Hazard. Mater. 418, 126310. https://doi.org/10.1016/j.jhazmat.2021.126310 (2021).
Wu, Z. C. et al. Increasing ammonium nutrition as a strategy for Inhibition of cadmium uptake and xylem transport in rice (Oryza sativa L.) exposed to cadmium stress. Environ. Exp. Bot. 155, 734–741. https://doi.org/10.1016/j.envexpbot.2018.08.024 (2019).
Moslehi, A., Feizian, M., Higueras, P. & Eisvand, H. R. Assessment of EDDS and vermicompost for the phytoextraction of cd and Pb by sunflower (Helianthus annuus L). Int. J. Phytorem. 21, 191–199. https://doi.org/10.1080/15226514.2018.1501336 (2019).
Chai, M. W. et al. Does ammonium nitrogen affect accumulation, subcellular distribution and chemical forms of cadmium in Kandelia obovata? Ecotox Environ. Safe. 162, 430–437. https://doi.org/10.1016/j.ecoenv.2018.07.031 (2018).
Zhang, F., Wan, X. Q. & Zhong, Y. Nitrogen as an important detoxification factor to cadmium stress in Poplar plants. J. Plant. Interact. 9, 249–258. https://doi.org/10.1080/17429145.2013.819944 (2014).
Liu, B. Q., Wang, J., Xu, M., Zhao, L. & Wang, Z. F. Spatial distribution, source apportionment and ecological risk assessment of heavy metals in the sediments of Haizhou Bay National ocean park, China. Mar. Pollut Bull. 149, 110651. https://doi.org/10.1016/j.marpolbul.2019.110651 (2019).
Zhao, M. et al. Microplastics promoted cadmium accumulation in maize plants by improving active cadmium and amino acid synthesis. J. Hazard. Mater. 447, 130788. https://doi.org/10.1016/j.jhazmat.2023.130788 (2023).
Bian, X. G., Cui, J., Tang, B. P. & Yang, L. Chelant-induced phytoextraction of heavy metals from contaminated soils: a review. Pol. J. Environ. Stud. 27, 2417–2424. https://doi.org/10.15244/pjoes/81207 (2018).
Segun, O. O., Blessing, B. O., Rasheedat, A. & Bukola, F. D. Emerging contaminants: evaluation of degradable chelators towards enhancing cadmium phytoextraction efficiency of bioenergy crop grown on polluted soil. Emerg. Contam. 7, 139–148. https://doi.org/10.1016/j.emcon.2021.05.001 (2021).
Hassan, M. J., Wang, F., Ali, S. & Zhang, G. Toxic effects of cadmium on rice is affected by nitrogen fertilizer form. Plant. Soil. 277, 359–365. https://doi.org/10.1007/s11104-005-8160-6 (2005).
Zaccheo, P., Crippa, L. & Pasta, V. D. Ammonium nutrition as a strategy for cadmium mobilisation in the rhizosphere of sunflower. Plant Soil. 283, 43–56. https://doi.org/10.1007/s11104-005-4791-x (2006).
Zhu, Y. D. et al. Evaluation and identification index of heat tolerance in different summer maize varieties at V12 stage. Acta Agron. Sin. 48 (12), 3130–3143. https://doi.org/10.3724/SP.J.1006.2022.13079 (2022).
You, Y., Dou, J. F., Xue, Y., Jin, N. F. & Yang, K. Chelating agents in assisting phytoremediation of uranium-contaminated soils: a review. Sustainability 14 (10), 6379. https://doi.org/10.3390/su14106379 (2022).
Acknowledgements
We thank Lanzhou Jiaotong University, China for providing the facilities to carry out the experimental work presented in this study.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 32260316), 2023 Open Fund Project of Key Laboratory of Strategic Mineral Resources in the Upper Yellow River of the Ministry of Natural Resources of the People’s Republic of China (Grant No. YSMRKF202306) and Gansu Provincial Science and Technology Plan Funding (Grant No. 24JRRA1171).
Author information
Authors and Affiliations
Contributions
Y.C. (Yinping Chen) wrote the main manuscript text, B.C. (Bo Cao) prepared Figs. 1, 2, 3, 4 and 5 and Y.L. (Yuzhi Lu) conducted a format analysis, Y.Z. (Yan Zheng) and Y.S. (Yong Sun) prepared Tables 1, 2, 3 and 4 and Tables. S1-2, Q.L. (Qian Li), Q.Y. (Qiaoling Yuan) and X.Z. (Xiaolan Zhang) conducted supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Cao, B., Lu, Y. et al. The combination of nitrogen fertilizers and EDDS enhances remediation efficiency of Bidens pilosa L. in Cd-contaminated soils. Sci Rep 15, 25310 (2025). https://doi.org/10.1038/s41598-025-11538-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11538-1