Abstract
Higd-1a is under expressed in colorectal cancer cells and its overexpression impaires the proliferation, migration, and invasiveness of colorectal cancer cells. However, the mechanism and clinical potential of Higd-1a in colorectal cancer remains to be further studied. This study investigated the potential of antitumor mechanism and clinical potential of Higd-1a in progression in colorectal cancer. We found that overexpression of Higd-1a in HCT-8 cancer cells suppressed tumor growth in nude mice. Higd-1a overexpression induced cle-caspase-3 and cle-caspase-9 and reduced Bcl-2 expressions, while p-AKT, p-JAK2 and p-STAT3 levels were significantly increased after downregulating Higd-1a in HCT-8 cancer cells. Ruxolitinib co-treatment completely enhanced the anticancer effect induced by inhibiting JAK2/AKT/STAT3 pathway in HCT-8 cells that downregulated Higd-1a. The expression rate in high Higd-1a expression group in stage I–II was considerably greater than that in stage III–IV, and Higd-1a expression and T stage were independent factors affecting the survival time of colorectal cancer. Higd-1a overexpression suppressed tumor growth in vivo and the molecular mechanisms may relate activation of apoptosis-related proteins and suppression JAK2/AKT/STAT3 pathway, and Higd-1a expression is helpful to evaluate the prognosis of colorectal cancer.
Similar content being viewed by others
Introduction
HIG1 domain family member 1 A (Higd-1a) is an important mitochondrial functional protein. As a subunit of cytochrome C oxidase, it can catalyze the reduction of oxygen to water and plays an important role in the assembly of respiratory complex1,2. Higd-1a gene was initially described in cultured human cervical epithelial cells and subsequently induced in hypoxic primary cultures of neurons, as well as in mouse embryonic fibroblasts3. Human Higd-1a gene, located on chromosome 3p22.1, is a 10.4 kDa mitochondrial intima protein with two transmembrane domains at the N-terminal and C-terminal4. The arrangement of these two domains caused the N-terminal and C-terminal to face outward into the membrane gap, while the remaining proteins form rings within the matrix. Although the N-terminal domain is not required to guide Higd-1a localization, it is required for protein survival5,6.
The function of Higd-1a is mainly focused on mitochondrial metabolic pathways and oxidative phosphorylation, so the research of Higd-1a is mainly focused on metabolic diseases1,7,8,9. Recent studies have shown that Higd-1a plays an important role in the development and proliferation of tumor cells and is considered to be a key protein in tumor recurrence10. Higd-1a interacted with the mitochondrial electron transport chain to reduce oxygen consumption and AMP-activated protein kinase (AMPK) activity, promoting tumor cell survival7. Higd-1a reduced γ secretase activity by binding to the mitochondrial γ secretase complex, thereby reducing reactive oxygen species (ROS) production and mitochondrial dysfunction and ultimately activating tumor chemotherapy resistance8. Higd-1a also bound to the cytochrome C oxidase complex and increased its activity, thereby protecting tumor cells from a capacity crisis2. In addition, Higd-1a can induce the proliferation of pancreatic cancer cells by activating the extracellular signal-regulated kinase (ERK) signaling pathway9. Other studies have shown that Higd-1a is the target gene of miR-375 and miR-489-3p, the expression of Higd-1a was increased by regulating the expression of miR-375 and miR-489-3p, and then the occurrence of glioma can be accelerated11,12. These studies indicate that Higd-1a, as a mitochondrial functional protein, is widely involved in the occurrence and development of tumors. JAK2/STAT3 signaling pathway plays a critical role in cell proliferation and differentiation by affecting the activation state of downstream effector molecules. The activation of JAK2/STAT3 signaling pathway is involved in tumorigenesis and development. Suppressing the JAK2/STAT3 signaling pathway can restrict the proliferation and metastasis of colorectal cancer. We predict that Higd-1a may be related to JAK2/STAT3 pathway.
Our previous studies had shown that Higd-1a was underexpressed in colorectal cancer cells and its overexpression weakened the proliferation, migration, and aggressiveness and increased apoptosis of colorectal cancer cells in vitro13. However, the in vivo anti-colorectal cancer effect, underlying mechanisms and clinical potential of Higd-1a remain unclear. In the present study, we examined the effect of Higd-1a on colorectal cancer in vivo with underlying mechanisms and clinical potential.
Results
Upregulation of Higd-1a suppresses tumor growth in vivo
Firstly, the regulatory effect of Higd-1a overexpression on HCT-8 cells in nude mice with subcutaneously implanted tumors was verified. We injected HCT-8 cells transfected in control group (Ctrl) and Higd-1a overexpression group (Higd-1a OE) subcutaneously into nude mice (Fig. 1A). Tumor weight and volume were measured every 4 days form daily from day 14 to day 34, and then the tumor tissue was surgically removed and photographed. Compared to the ctrl group, the expression of Higd-1a was significantly higher in Higd-1a OE group (Fig. 1C, D). Moreover, the results showed that the weight and volume of tumor-bearing tissue were much decreased after Higd-1a overexpression (Fig. 1B, E and F). After tumor tissues were stained with immunohistochemistry, the amount of cancer cells was decreased when Higd-1a overexpression treatment (Fig. 1G). Furthermore, as shown in Fig. 1H, K, the analysis of tumor specimens demonstrated that Higd-1a overexpression-treatment was associated with induction of apoptosis and reduced proliferation (ki67 protein). Our findings collectively demonstrated that the overexpression of Higd-1a in nude mice appeared to slow the growth of tumors.
Higd-1a overexpression suppresses tumor growth in vivo. (A). Higd-1a expression levels in cells overexpressing Higd-1a (Higd-1a OE) were detected by western blotting. (B) After transfection, cells were subcutaneously injected into mice, and formation of subcutaneous tumors was compared on the 35th day. (C). Higd-1a expression levels in tumor tissue were detected by western blotting. (D) Quantification of Higd-1a protein expression. (E) Tumor mass. (F) The subcutaneous tumor volume was calculated every 4 days starting on the 14th day of inoculation, and a formed volume curve was drawn. (G) Immunohistochemical staining of tumor specimens. (H) Apoptosis levels in in tissues. (I) Quantification of apoptosis rate. (J) Ki-67 protein expression in tissues. (K) Quantification of ki-67 cells. **, P < 0.01. ***, P < 0.001. ****, P < 0.0001.
Upregulation of Higd-1a induces activation of expression of apoptosis-related proteins
See Fig. 2
Downregulation of Higd-1a mediated JAK2/AKT/STAT3 pathway
In order to further explored Higd-1a mediated potential anti-tumor mechanism, we screened multiple potential signaling pathways that had been reported to regulate the progression of colorectal cancer. Interestingly, the Western blotting results indicated that protein levels of p-AKT, p-JAK2 and p-STAT3 in the downregulating Higd-1a group in HCT-8 cells were significantly increased in comparison with the control group (Fig. 3A, D). These findings suggested that Higd-1a mediated colorectal cancer development may be related to JAK2/AKT/STAT3 pathway.
JAK2/AKT/STAT3 pathway is involved in Higd-1a mediated colorectal cancer progression
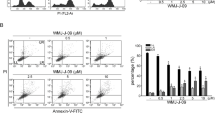
To further validate the involvement of JAK2/AKT/STAT3 signaling in Higd-1a mediated colorectal cancer progression, janus kinase inhibitor ruxolitinib was applied. Interestingly, the results showed that treatment with ruxolitinib effectively reversed the decreasing Higd-1a-induced increase in cell proliferation in HCT-8 cells (Fig. 4A). Additionally, we found that ruxolitinib treatment significantly promoted Higd-1a-induced apoptosis (Fig. 4B, C). Furthermore, compared to the siHigd-1a group, the protein levels of p-AKT, p-JAK2 and p-STAT3 were significantly decreased in the siHigd-1a + ruxolitinib group (Fig. 4D, E). Then, the increase of ruxolitinib induced the transition of cell cycle from S phase to G0/G1 phase (Fig. 4F, G). These findings indicated that Higd-1a mediated colorectal cancer proliferation, apoptosis and cell cycle is related to JAK2/AKT/STAT3 pathway.
JAK2/AKT/STAT3 pathway is involved in Higd-1a mediated colorectal cancer progression. After Higd-1a was knocked down in HCT-8 cells, then the cells were treatment with ruxolitinib. (A) The effect of cell proliferation was analyzed by CCK-8 assay. (B) Representative images showing the effect of cell apoptosis. (C) Quantification of the percentage of cell apoptosis. (D) The expression of p-AKT, p-JAK2 and p-STAT3 in cells were detected by western blotting. (E) Quantification of p-AKT, p-JAK2 and p-STAT3. (F) The effect on cell cycle was detected using PI staining. (G) Quantification of the proportion in different stages of the cell cycle. **, P < 0.01.
The associations between clinicopathological features and Higd-1a expression
Then, we compared Higd-1a expression with the clinicopathological features of patients with colorectal cancer. The results showed that in high Higd-1a expression group, the expression rate in stage I–II was 62.26%, which was considerably greater than that in stage III–IV(34.7%). However, the high Higd-1a expression were not significantly associated with gender, age, depth of tumor invasion, lymph node metastasis, nerve invasion, vascular cancer embolus (Table 1).
The potential value of Higd-1a expression in prognostic assessment of patients with colorectal cancer
Furthermore, the COX regression model was used to investigate the factors affecting overall survival of colorectal cancer patients. Cox regression model of single factor analysis and multiple-factor analysis both showed that there was significant association between the T stage/ Higd-1a level and prognosis of colorectal cancer, suggesting that Higd-1a expression(p = 0.001, HR = 5.951) and T stage (p = 0.016, HR = 0.546) are helpful to evaluate the prognosis of colorectal cancer (Table 2).
Discussion
This study firstly investigated the potential antitumor mechanism and clinical potential of Higd-1a in colorectal cancer. Our findings revealed that upregulation of Higd-1a significantly increased colorectal cancer tumor growth in vivo. Subsequent investigations in vitro revealed that this anticancer effect was primarily achieved by activation of apoptosis-related proteins and suppression JAK2/AKT/STAT3 pathway. Furthermore, our study demonstrated that Higd-1a level could be used as an independent risk factor to evaluate the prognosis in colorectal cancer patients.
Nowadays colorectal cancer is considered as the third most common cancer in the world. Hepatic metastases often already happen earlier before colon cancer is diagnosed because of its dormant clinical symptoms14. Traditionally, chemotherapy is regarded as the most important treatment of colon cancer. However, the limitations of chemotherapy should not be underestimated, such as low selectivity, deficiency in tumor tissues, drug resistance, and systemic toxicity, etc. Thus, the survival rate for those diagnosed with colorectal cancer is dismal14,15. Studies have shown that tumor development is an evolutionary process involving several genes and stages16,17. Moreover, rapid advances in molecular biology have allowed researchers to uncover gene molecules and signaling pathways connected to colorectal cancer, providing crucial insight into the disease’s etiology and pathogenesis18. In our previous study found that Higd-1a was underexpressed in colorectal cancer, and overexpression of Higd-1a inhibits the proliferation, migration ability, and invasiveness of colorectal cancer and promotes apoptosis13. However, the anticancer effects in vivo, mechanism and clinical potential of Higd-1a in colorectal cancer remains to be further studied.
Apoptosis is a type of programmed cell death accompanied by the activation of endogenous apoptosis-related proteins19. In our study, it was found that protein levels of cle-caspaes-3 and cle-caspase-9 in the Higd-1a overexpression group were markedly increased, whereas those of Bcl-2 was decreased in comparison with the control group. These results imply that Higd-1a regulates cellular apoptosis together with apoptosis-related proteins activation. Meanwhile, consistent with our in vitro findings, Higd-1a overexpression prevents tumor development through induction of apoptosis and reduced ki67 protein level.
Janus kinase (JAK)/signal transducer and activator of transcription (STAT) is an intracellular signal transduction pathway that is widely expressed in cells. It is involved in cellular immune regulation, proliferation, differentiation and apoptosis20. JAK2 is an important regulator of inflammatory response and cell proliferation and differentiation, and it can regulate downstream transcription factors such as STAT1, STAT3 and STAT5. In addition, JAK2 can induce the phosphorylation of STAT3, which, as a cytoplasmic signaling transcription factor, plays an important role in mediating cancer21,22. STAT3 can also participate in the transcriptional upregulation of many genes, acting on the one hand through direct DNA binding, and on the other hand acting as a co-activator of transcription factors and participating in related inflammatory responses. AKT, a serine/threonine kinase, is a key player in regulating cell signaling that is important for cell death and survival23. In addition, AKT is a major regulator involved in transcriptional regulation of the anti-apoptotic protein Bcl⁃2, which plays a key role in the prevention of cell death24. There is a mutual network between JAK and AKT, and JAK regulates the activation of AKT25. Our study found that downregulating Higd-1a can increase the levels of p-AKT, p-JAK2 and p-STAT3, while ruxolitinib co-treatment completely reversed the anticancer effect induced by JAK2/AKT/STAT3 activation, colorectal cancer death, apoptosis and cell cycle. These findings suggested that JAK2/AKT/STAT3 pathway plays a crucial role in the anticancer effect of Higd-1a.
Moreover, the expression rate in high Higd-1a expression group in stage I–II was 62.26% was considerably greater than that in stage III–IV. The detection of Higd-1a combined with postoperative pathological staging can help improve the clinical evaluation of tumors. Since TNM stage is directly related to the overall survival of tumor patients, the expression level of Higd-1a has statistical significance in different postoperative clinical stages, suggesting that the expression level of this gene is somewhat correlated with the overall survival. However, there was no significant correlation between other common clinicopathological factors (age, gender, lymph node metastasis, nerve invasion, vascular cancer embolus and depth of tumor invasion, etc.). In univariate and multivariate analysis, Higd-1a expression and T stage were an independent factor affecting the survival time of colorectal cancer, and patients with high Higd-1a expression had better prognosis. The results of our study indicate that the expression of Higd-1a in colorectal cancer tissues is significantly different in patients with different clinical stages, which has certain reference value for predicting the prognosis of colorectal cancer.
In conclusion, upregulation of Higd-1a suppresses tumor growth in vivo, further investigation of the molecular mechanisms revealed that activation of apoptosis-related proteins and suppression JAK2/AKT/STAT3 pathway-related anticancer potential. Our results suggest that Higd-1a level could be used as a potential predictive biomarker to help identify novel prognosis targets for colorectal cancer.
Methods
Clinical sample collection
A total of 125 tissue specimens stored in liquid nitrogen tanks at the Affiliated Hospital of Jiangnan University were obtained from fresh tumors. All patients underwent radical resection of colorectal cancer and had complete pathological data and survival data. The clinical case data and follow-up data of these 125 patients were collected, including gender, age, depth of tumor invasion, lymph node metastasis, nerve invasion, vascular cancer embolus and TNM staging.
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University (LS2019050) and informed consent was taken from all the patients.
Cell lines and culture
The colorectal cancer cell lines used in this study, HCT-8 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, 20110 USA). HCT-8 was cultured in McCoy’s 5 A Medium with 10% fetal bovine serum (FBS), RPMI-1640 Medium with 10% horse serum and Leibovitz’s L-15 Medium with 10% FBS, respectively. The culture media were supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 µg/mL streptomycin. as previously described13.
Higd-1a silencing and overexpression in HCT-8 cells
The vector pCDH-EF1α-MCS-T2A-puro was purchased from Antihela BioTech (Xiamen, Fujian, China) for the preparation of the plasmid encoding the Higd-1a protein (Gene ID: 25,994) and named Higd-1a OE. 1.2 × 106cells/well HCT-8 cells in 6-well plate were transfected with Higd-1a OE mid (5 µg/well) using Lipofectamine 2000, according to the manufacturer’s protocol. After 48 h, cells were analyzed. A small interfering RNAs (siRNA) of Higd-1a, si Higd-1a was obtained from GenePharma (Shanghai, China). 1.2 × 106 cells/ well HCT-8 cells in 6-well plate were transfected with siRNA (400 pmol/well) using Lipofectamine 2000, according to the manufacturer’s protocol. After 48 h, cells were analyzed. Nonspecific siRNA was used as a negative control (siNC), as previously described13.
Nude mice subcutaneous tumor formation experiment
12 male BALB/c nude mice aged 4–6 weeks and weighing 18–20 g each from the Laboratory Animal Center, Jiangnan University, and the animal experiments were done in the animal Laboratory of Jiangnan University. In order to study the effects of Higd-1a on the development of nude mice, 12 mice were bred and raised in two groups. Each set of cells was trypsin-digested, centrifuged, counted, resuspended at a density of 4 × 106/mL, and then subcutaneously injected into the nude mice in a volume of 100 µL. Tumor growth was observed in nude mice every 4 days for volume using Vernier calipers. 34 days later, the mice were uniformly euthanized through intraperitoneal injection of 0.3% pentobarbital sodium, and the tumor that had been implanted subcutaneously was removed, sized, and photographed. Our experiments protocol on live vertebrates were approved by Ethics Committee on Laboratory Animal Management and Animal Welfare of Jiangnan University(IACUC Issue No: JN.No20220930m1200424[373]). All methods were carried out in accordance with relevant guidelines and regulations. And all methods are reported in accordance with ARRIVE guidelines.
Tissue staining
Tumor specimens were fixed in 4% paraformaldehyde and subsequently embedded in paraffin for immunohistochemical analysis. The sections, with a thickness of 5 μm, were stained using antibodies targeting Ki-67. Proteins were detected using HRP-conjugated secondary antibodies and diaminobenzidine (DAB). Apoptosis levels in the frozen tumor tissue sections were detected using immunofluorescence.
Cell proliferation assay
HCT-8 cells with knockdown of Higd-1a (3 × 103 cells/well) were seeded in 96-well plates to assess colorectal cancer cell viability. The cells were exposed to the ruxolitinib for 48 h. Then, 10 µL of CCK-8 reagent was added to the cells and cultured for 2 h. Formazan crystals were dissolved in DMSO. Absorbance of the solution was measured at 450 nm on a microplate reader (Tecon, Switzerland). IC50 values were determined using the logit method.
Cell apoptosis analysis
HCT-8 cells with knockdown of Higd-1a (2 × 105 cells/well) were seeded in 6-well plates to assess colorectal cancer cell apoptosis. The cells were incubated for 24 h. Subsequently, the cells were exposed to the ruxolitinib for 48 h. Cells were then harvested and stained with FITC-conjugated Annexin V and PI. The Accuri C6 Plus flow cytometer (BD Biosciences, CA, USA) was used for cell analysis, as previously described13.
Cell cycle assay
HCT-8 cells with knockdown of Higd-1a (2 × 105 cells/well) were seeded in 6-well plates to assess colorectal cancer cell apoptosis. The cells were incubated for 24 h. Subsequently, the cells were exposed to the ruxolitinib for 48 h. the cells were harvested, fixed in 70% ethanol, and stored at 4 °C overnight. After washing twice with PBS, the fixed cells were permeabilized by adding 0.2% Triton X-100 containing 10 µg/mL RNase at 37 °C. Cells were stained with propidium iodide (PI, 20 µg/mL) and analyzed using a flow cytometer, as previously described13.
Western blot analysis
Tumor tissues and cells were lysed to obtain lysates. Protein levels in the lysates were quantified using Bradford assay (Bio-Rad, Hercules, CA, USA). Proteins were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The blots were blocked for 1.5 h using a 5% nonfat milk/TBST solution. The PVDF membranes were treated with primary antibodies and incubated overnight at 4 °C. The proteins were detected using HRP-conjugated secondary antibodies and an ECL substrate (Bio-Rad). Protein levels were quantified using Images J software (version 1.38e). Values were normalized to those of the respective controls, as previously described13.
Statistical analysis
SPSS 23.0 statistical software was used to analyze the differences between two groups throguh Mann-Whitney test and Student’s t-test (unpaired) for nonparametric and parametric data, respectively. Survival analysis was carried out by KaplanMeier method and survival curve was drawn. Univariate and multivariate analyses were performed using COX quadrat models. hazardratio (HR) and 95% confidence interval (95% CI) were used to assess the association between Higd-1a expression level and the risk of death in patients with colorectal cancer. P < 0.05 was considered to be statistically significant difference.
Data availability
Data used to support the findings of this study are available from the corresponding author upon request.
References
Hayashi, T. et al. Higd1a is a positive regulator of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 112, 1553–1558. https://doi.org/10.1073/pnas.1419767112 (2015).
Klammt, C. et al. Facile backbone structure determination of human membrane proteins by NMR spectroscopy. Nat. Methods. 9, 834–839. https://doi.org/10.1038/nmeth.2033 (2012).
Salnikow, K. et al. The involvement of hypoxia-inducible transcription factor-1-dependent pathway in nickel carcinogenesis. Cancer Res. 63, 3524–3530 (2003).
Ameri, K. et al. Nuclear localization of the mitochondrial factor HIGD1A during metabolic stress. PLoS One. 8, e62758. https://doi.org/10.1371/journal.pone.0062758 (2013).
Wang, J. et al. Pancreatic beta cells lack a low glucose and O2-inducible mitochondrial protein that augments cell survival. Proc. Natl. Acad. Sci. USA. 103, 10636–10641. https://doi.org/10.1073/pnas.0604194103 (2006).
Timon-Gomez, A., Bartley-Dier, E. L., Fontanesi, F. & Barrientos, A. HIGD-Driven regulation of cytochrome c oxidase biogenesis and function. Cells 9, 2620 https://doi.org/10.3390/cells9122620 (2020).
Ameri, K. et al. HIGD1A regulates oxygen consumption, ROS production, and AMPK activity during glucose deprivation to modulate cell survival and tumor growth. Cell. Rep. 10, 891–899. https://doi.org/10.1016/j.celrep.2015.01.020 (2015).
Nagao, T. et al. Higd1a improves respiratory function in the models of mitochondrial disorder. FASEB J. 34, 1859–1871. https://doi.org/10.1096/fj.201800389R (2020).
Li, T. et al. Higd1a protects cells from lipotoxicity under High-Fat exposure. Oxid. Med. Cell. Longev. 2019, 6051262. https://doi.org/10.1155/2019/6051262 (2019).
An, H. J. et al. Higd-1a regulates the proliferation of pancreatic cancer cells through a pERK/p27(KIP1)/pRB pathway. Cancer Lett. 461, 78–89. https://doi.org/10.1016/j.canlet.2019.07.007 (2019).
Guo, J. et al. MiR-375 induces ROS and apoptosis in ST cells by targeting the HIGD1A gene. Gene 685, 136–142. https://doi.org/10.1016/j.gene.2018.10.086 (2019).
Cheng, Z., Wang, G., Zhu, W., Luo, C. & Guo, Z. LEF1-AS1 accelerates tumorigenesis in glioma by sponging miR-489-3p to enhance HIGD1A. Cell. Death Dis. 11, 690. https://doi.org/10.1038/s41419-020-02823-0 (2020).
Xu, Z. et al. HIG1 domain family member 1A disrupts proliferation, migration, and invasion of colon adenocarcinoma cells. Bioengineered 12, 10501–10511. https://doi.org/10.1080/21655979.2021.1999368 (2021).
Fabregas, J. C., Ramnaraign, B. & George, T. J. Clinical updates for Colon cancer care in 2022. Clin. Colorectal Cancer. 21, 198–203. https://doi.org/10.1016/j.clcc.2022.05.006 (2022).
Cappell, M. S. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol. Clin. North. Am. 37, 1–24. https://doi.org/10.1016/j.gtc.2007.12.002 (2008).
Chen, S. & Shen, X. Long noncoding rnas: Functions and mechanisms in colon cancer. Mol. Cancer. 19, 167. https://doi.org/10.1186/s12943-020-01287-2 (2020).
Durai, R., Yang, S. Y., Seifalian, A. M. & Winslet, M. C. Principles and applications of gene therapy in colon cancer. J. Gastrointestin Liver Dis. 17, 59–67 (2008).
Ahmed, F. E. Colon cancer: Prevalence, screening, gene expression and mutation, and risk factors and assessment. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 21, 65–131. https://doi.org/10.1081/GNC-120026233 (2003).
Huerta, S., Goulet, E. J. & Livingston, E. H. Colon cancer and apoptosis. Am. J. Surg. 191, 517–526. https://doi.org/10.1016/j.amjsurg.2005.11.009 (2006).
Bousoik, E. & Montazeri Aliabadi, H. Do we know Jack about JAK? A closer look at JAK/STAT signaling pathway. Front. Oncol. 8, 287. https://doi.org/10.3389/fonc.2018.00287 (2018).
Huynh, J., Etemadi, N., Hollande, F., Ernst, M. & Buchert, M. The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Semin Cancer Biol. 45, 13–22. https://doi.org/10.1016/j.semcancer.2017.06.001 (2017).
Mengie Ayele, T., Tilahun Muche, Z., Behaile Teklemariam, A. & Bogale Kassie, A. Chekol abebe, E. Role of JAK2/STAT3 signaling pathway in the tumorigenesis, chemotherapy resistance, and treatment of solid tumors: A systemic review. J. Inflamm. Res. 15, 1349–1364. https://doi.org/10.2147/JIR.S353489 (2022).
Revathidevi, S. & Munirajan, A. K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 59, 80–91. https://doi.org/10.1016/j.semcancer.2019.06.002 (2019).
Ricciardi, M. R., Mirabilii, S., Licchetta, R., Piedimonte, M. & Tafuri, A. Targeting the akt, GSK-3, Bcl-2 axis in acute myeloid leukemia. Adv. Biol. Regul. 65, 36–58. https://doi.org/10.1016/j.jbior.2017.05.002 (2017).
Choi, Y. et al. HER2-induced metastasis is mediated by AKT/JNK/EMT signaling pathway in gastric cancer. World J. Gastroenterol. 22, 9141–9153. https://doi.org/10.3748/wjg.v22.i41.9141 (2016).
Funding
This article is supported by Wuxi Taihu Lake Talent Plan, Supports for Leading Talents in Medical and Health Profession.
Author information
Authors and Affiliations
Contributions
Yong Mao was responsible for the concept and design of the work. Zhenyu Xu was responsible for the data analysis, interpretation of results, and the first draft of the manuscript. Hanyue Zhang assisted with collecting data and Yang Chen was responsible for data analysis.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Z., Zhang, H., Chen, Y. et al. The potential antitumor mechanism and clinical potential of Higd-1a in colorectal cancer. Sci Rep 15, 33987 (2025). https://doi.org/10.1038/s41598-025-11670-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11670-y