Abstract
Carbon dioxide (CO2) is the main greenhouse gas contributing to the rise in global temperature. Polymeric membranes often face a permeability-selectivity trade-off, limiting their effectiveness in meeting the demands of modern membrane technology. Mixed matrix membranes (MMMs) suggest advantages in energy consumption, cost, and operation for gas separation by overcoming the limitations of pure polymeric membranes through the incorporation of inorganic–organic hybrid material fillers. Our goal in this research was to improve the separation performance of polyvinyl chloride (PVC) membrane by creating an IL@AC/MOF filler by impregnating ionic liquid (IL) with an activated carbon/NH2-MIL-53(Al) (AC/MOF) composite. This filler was then added to the PVC membrane, offering a possible way to address the trade-off problems in membranes of polymeric by fusing the high gas separation efficiency of inorganic fillers with the processability of polymers. TGA, FESEM, EDX, FTIR spectroscopy, X-ray Diffraction (XRD), and Brunauer–Emmett–Teller (BET) surface area analysis were used to characterize the produced MMMs. At pressures up to 4 bar for CO2 and N2, the permeability, diffusion coefficients, and solubility of MMMs were determined at 288.15, 298.15, 308.15, and 318.15 K. The results indicate that IL@AC/MOF/PVC MMMs with 15 wt.% IL@AC/MOF loading had CO2 permeabilities that were 12.4 times greater than those of the PVC membrane. Furthermore, the CO2/N2 selectivity of IL@AC/MOF/PVC MMMs with 15 wt.% IL@AC/MOF loadings was 1.3 times greater than the selectivity of the pure PVC membrane at 298.1 5 K and 3 bar. The AC/MOF composite successfully improved gas diffusion in the PVC membrane owing to the porous nature of the MMM nanocomposites. The obtained data demonstrate that the separation performance of IL@AC/MOF/PVC MMMs is approached the Robeson upper-bound limit.
Similar content being viewed by others
Introduction
Gas separation with polymeric membranes has gained popularity because of its energy-saving with reduced manufacturing and expenses for equipment. The trade-off between gas permeability and selectivity makes it challenging to achieve outstanding separation performance for polymeric membranes1. Mixed matrix membranes (MMMs) are complex membranes make up of a polymer matrix and an inorganic–organic hybrid substance in the form of micro- or nanoparticles (the discrete or dispersed phase; filler or additive). They have emerged as a material that could solve the issue of polymeric membranes2,3. Traditionally, zeolites have been used as fillers for the fabrication of zeolite/polymer MMMs, however, difficulty of preparing defect-free zeolite crystals and poor adhesion between the polymer and zeolite particles limit the applicability of these MMMs4.
To improve the gas separation capabilities of polymer membranes, metal–organic frameworks (MOFs), a relatively new family of unique organic–inorganic materials, have recently been employed as fillers in MMMs5. MOFs have large porosities, high surface areas, controllable pore sizes, good mechanical and thermal stabilities. The most important advantage of MOFs over other traditional fillers is that their physical and chemical properties can be easily tuned by choosing the proper metal–ligand pair for a target application6,7,8,9. Numerous experimental investigations on MOF/polymer MMMs have been documented for a range of gas separation applications, such as CO2/CH4, CO2/N2, and H2/CO210,11,12,13,14. Incorporation of MOFs into polymers generally improve either the gas permeability or the selectivity of the polymer membrane15.
Ionic liquids (ILs) can enhance the separation performance of MOFs owing to their strong affinity towards various gasses, like CO2, in addition to modifying the pore structure of MOF. Incorporation of ILs into MOFs to prepare IL/MOF composites can combine the advantages of both constituents, such as high affinity of ILs towards desired gas molecules and tunable structure of both materials16,17,18. IL/MOF composites have proven to be highly CO2 selective over N2 and CH4 gasses through adsorption studies which leads to improved selectivities compared to pristine MOFs19,20,21,22,23. As a result, utilization of IL/MOF composites in MMM applications has attracted considerable interest and recent studies showed that IL/MOF composites can significantly improve the selectivity of the membranes depending on the well-controlled location of IL molecules24,25. For instance, Li et al.26 examined the CO2/O2 permeability of [Bmim][Tf2N]/ZIF8 composite in polysulfone (PSF). Their results show that MMM permeability increased by 48% for CO2 and 39% for O2. Ferreira et al.27 incorporated, 1-propyl-3-methylimidazolium bromide, [PMIM][Br]/MIL101(Cr) composite into Matrimid and the IL/MOF/polymer MMMs exhibited CO2 permeability of 44 Barrer, which was 4-times higher than that of pure Matrimid (9.8 Barrer). Habib et al.28 investigated CuBTC/Pebax MMMs and [EMIM][OAc]/CuBTC/Pebax MMMs with 10, 15, and 20 wt.% loadings of CuBTC and [EMIM] [OAc]/CuBTC fillers. They found that at 15 wt.% [EMIM][OAc]/CuBTC loading, the MMM showed significantly high CO2 permeability (335 Barrer) and CO2/N2 selectivity of 176 compared to pure Pebax having a CO2 permeability (135 Barrer) and CO2/N2 selectivity of 32. The CO2 permeability and CO2/N2 selectivity of [EMIM][OAc]/CuBTC/Pebax MMM was 2.5 and 5.6 times higher than the pure Pebax membrane. However, CO2/N2 and CO2/CH4 selectivities of the MMM were only slightly improved compared to that of the pure polymer. These results highlight that IL/MOF composites can be highly promising filler particles for MMM applications owing to their high CO2 affinities. Therefore, the synthesis of novel IL/MOF composites and their use in polymers to generate MMMs can lead to new membranes with exceptional CO2 separation performances. The development of advanced mixed matrix membranes (MMMs) incorporating activated carbon (AC), metal–organic frameworks (MOFs), ionic liquids (ILs), and polymers has gained significant attention as a strategy to enhance CO₂ separation performance. AC, characterized by its inert carbon skeleton and large specific surface area, provides an economical and eco-friendly component for gas adsorption applications29. Its tunable pore architecture and exceptional thermal and chemical stability make it an ideal filler in membrane systems. The integration of AC with MOFs into polymeric membranes aims to overcome the limitations of pure MOF or polymer systems, such as poor mechanical strength or limited selectivity. Kayal et al.30 studied the incorporation of activated carbon to MIL-101(Cr) MOF composites to store CH4 and CO2. According to the experimental data,the CH4 adsorption on MAX-MIL composite MOF is 12% (in gravimetric basis) and 9% (in volumetric basis) higher than that of parent MIL-101(Cr) MOF at 300 K. Nevertheless, it has been demonstrated that the substantial empty space within the MOF matrix does not fully aided to the adsorption and separation processes. Adding more porous structures, especially carbon-based ones, to the MOF matrix is a useful strategy to improve the weak interactions between the interior pores and the guest molecules. Accordingly, expanding the study of filler materials composed of multiple functional components such as ionic liquids (ILs) and AC/MOF composites is highly desirable to better understand how structural modifications influence the gas separation performance of mixed matrix membranes (MMMs). The present study addresses existing gaps in the literature by exploring a novel IL@AC/MOF system that has not yet been extensively studied. The primary objective is to design and fabricate a high-performance, cost-effective MMM capable of significantly enhancing CO₂ separation from gas mixtures, with potential applicability in industrial gas processing.
To clarify how the objective of developing a high-performance, cost-effective mixed matrix membrane (MMM) for industrial CO₂ separation has been achieved, the present study provides direct performance benchmarks and comparative data. In our previous investigations, we explored CO2/N2 separation performance using PVC-based mixed matrix membranes (MMMs) incorporating IL-coated coke/NH₂-UiO-66 and IL@AC/NH₂-MIL-101 nanocomposites. These studies demonstrated that incorporating IL@AC/MOF fillers significantly enhanced the permeability of the about 19.2 times and the CO2 /N2 selectivity increases 2.94 times at 298.15 K and 4 bar compared to the pure PVC membrane compared to pure PVC membranes31. In other study we was investigated CO2/N2 separation into PVC-based mixed-matrix membranes based on IL@AC/NH2-MIL-101 nanocomposites; the outcome indicate that the addition of the IL([Cho][Pro]) filler, the permeability of the AC/MOF/PVC MMMs is increased about 17.6 times and the CO2 /N2 selectivity increases 2.94 times at 298.15 K and 4 bar compared to the pure PVC membrane32.
In this study, first cholinium amino acid ionic liquids ([Cho][AA]s) were impregnated into an AC/NH2-MIL-53(Al) composite using the vacuum method. Subsequently, MMMs were prepared by incorporating the [Cho][AA]/AC/NH2-MIL-53(Al) composite into a PVC matrix. XRD, TGA, BET analysis, FESEM with EDX, and FTIR spectroscopy were used to analyze the MMMs. Furthermore, the impact of critical operational parameters like pressure and temperature on the permeability of MMMs for CO2 and N2 was examined. The novelty of this study lies in the innovative use of a newly synthesized IL@AC/MOF composite, which has been specifically tailored for enhanced CO2 selectivity and stability in MMMs, representing a significant advancement membrane technologies. Moreover, the tunability of NH2-MIL-53(Al) and compatibility with [Cho][AA]s offers new opportunities for tailoring membrane properties toward specific industrial gas separation applications. In the current study, we extended this concept by synthesizing a novel [Cho][AA]/AC/NH₂-MIL-53(Al) composite and incorporating it into PVC-based MMMs. The use of low-cost and scalable materials such as activated carbon, cholinium-based ionic liquids, and PVC further contributes to the economic feasibility of these membranes. Together, these findings substantiate the claim of achieving a high-performance, cost-effective MMM suitable for industrial CO₂ separation, effectively addressing a critical gap in the current literature.
Experimental
Materials
Polyvinyl chloride (PVC) with K-value 69-71,Choline chloride (purity > 99 wt %), Aluminium nitrate nonahydrate (Al(NO3)3·9H2O) (purity > 99 wt %), 2-amino terephthalic acid (NH2-H2BDC)(purity > 99 wt %), L-glutamic acid (Glu) (purity > 99 wt %), hydrochloric acid (HCl) (37 wt %), Tetrahydrofuran (THF) (purity > 99.5 wt %), and potassium hydroxide (KOH) (> 95% purity) were obtained from Sigma–Aldrich products. Ethanol (purity > 99 wt %) and N,N-Dimethylformamide (DMF) (purity > 99 wt %) were purchased from Merck. Activated carbon obtained from Almond shell. In gas absorption testing, CO2 gas (purity > 99.9 wt %) was utilized.
AC activation
Almond shell was utilized to prepare the activated carbon. Chemical activation of ground almond shell with 1 M KOH resulted in particle sizes of 2 to 3 mm. The sample was combined with a 1 M KOH solution to obtain a slurry with a KOH/sample weight ratio of 5. After 12 h of heating at 333 K, the slurry was dried at 383 K. Then, the product was pyrolyzed under N2 flow (200 cm3/min) for 2 h at 573 K. Afterwards, the activated carbon (AC) was kept at 1073 K for 3 h.
Synthesis of AC/NH2-MIL-53 (Al) composite
The AC/NH2-MIL-53 (Al) composite was synthesized using the hydrothermal technique33. To synthesize AC/NH2-MIL-53(Al), 6.71 g of Al(NO3)3·9H2O was mixed with 3.74 g of NH2-H2BDC and 0.1 g of AC and 50 mL of deionized water in an autoclave lined with Teflon for 5 h at 423 K. The final product was repeatedly washed with acetone. To remove unreacted NH₂-H₂BDC from the pores, the synthesized AC/NH₂-MIL-53(Al) was activated by treatment in DMF at 423 K for approximately 48 h. Then, synthesized AC/NH2-MIL-53 (Al) was dried in an oven under the vacuum at 373 K for about 12 h.
Synthesis of [Cho][Glu]
Choline-based ionic liquid ([Cho][Glu]) was synthesized via neutralization reaction. Choline hydroxide ([Cho][OH]) was prepared from an aqueous solution of choline chloride ([Cho][Cl]) using an anion exchange resin. To exchange bromide anion with hydroxide, an aqueous solution of choline chloride was placed on the top of the glass column filled with ion exchange resin. To confirm the absence of bromide ions in the synthesized [Cho][OH], silver nitrate was added to aqueous solution of choline chloride. The absence of any precipitate indicate that no bromide was present. Then the [Cho][OH] concentration in this solution was obtained using the titration with HCl 0.1 M as a titrant. Amino acids of glutamic acid [Glu] (1.2 equivalents) was dissolved in the prepared aqueous solution of choline hydroxide was slowly added to excess equimolar aqueous in a 100 ml round bottomed flask equipped with a reflux condenser and magnetic stirrer under reflux for 72 h at room temperature34,35. A rotary evaporator was used to removal of water at 352 K for 8 h. The excess amino acid was then precipitated by adding absolute ethanol. After water removal at 352 K for 10 h in a rotary evaporator, the product was repeatedly washed with ethanol to get rid of any remaining amino acid. Finally, the product was vacuumed for five hours at 338 K to eliminate the ethanol. The water amount of [Cho][Glu] in mass fraction was measured using a Karl-Fischer titrator (720-KSS Metrohm).The supplementary (Figs. S1) contains the 1H NMR spectra of [Cho][Glu]. For [Cho][Glu], integrated hydrogen peaks showed a purity of ≥ 98%.
[Cho][Glu] impregnation in AC/MOF composite
To employing the vacuum impregnation process, 5 mL of the IL solution (1 M) was used to impregnation [Cho][Glu] on the 0.2 g AC/MOF composite. The resulting slurry was then dried for 24 h at 378 K in an oven.
Membrane preparation
To prepare of PVC-based MMMs with various filler loding ((wAC/MOF = 5, 10, 15%, and w IL@AC/MOF = 15%)), at first 0.1 g PVC was dissolved in 5 mL THF. Then desired amount of the AC/MOF or IL@AC/MOF composite was dispersed in 5 mL THF under sonication and stirred conditions. After that, PVC solution was added to the AC/MOF or IL@AC/MOF solution and stirred. Then the obtained solution was casted on a flat glass plate and slowly evaporated at room temperature for 48 h. Finally, an oven was used to dry the created MMMs for 24 h at 333 K (Fig. 1). MMM thickness was calculated using MMM mass and density. The flotation method was used to measure the density of MMMs in an acetone/chloroform combination. After modifying the composition to keep the MMMs suspended throughout the experiment, the solution’s density was measured using a density analyzer (DSA-5000, Anton Paar). In this study, the thickness of all membranes was about 0.004 cm.
Characterization analysis
FESEM images and EDX spectra were investigated using a scanning electron microscope (SEM) (TESCAN-MIRA3, Germany) to analyze the morphology and surface properties of the AC/MOF composite, AC/MOF/PVC, and IL@AC/MOF/PVC. The existence of functional group and chemical bonds were identified using the ATR-FTIR spectroscopy. ATR-FTIR spectra were recorded using a spectrometer (Bruker Tensor 27) and 1HNMR spectra were obtained using 1HNMR (Bruker Av-400). The TGA curve was recorded using a TGA instrument (TGA-DAT, PerkinElmer Pyris Diamond) in N2 atmosphere with rate of 10 °C/min. X-ray diffraction (XRD) analysis (SHIMADZU, Labs XRD-6100) was applied to determine particle size and crystalline structure. Nitrogen adsorption–desorption isotherms were measured at 77 K using a gas adsorption analyzer (BEL BELSORP MINI II, Japan). Prior to the measurements, the samples were degassed under vacuum at a heating rate of 10 ºC/min up to 120 ºC and then maintained for 10 h.
Gas permeability measurement
A constant-pressure/variable-volume approach was used to assess the CO2/N2 permeability of MMMs, and all samples were measured at various pressures and temperatures. The authors have previously discussed the module performance of apparatus in detail36,37,38,39. Using the Eq. (1), the CO2/N2 permeability (P) of MMMs was determined under steady-state conditions40:
where A is the effective membrane area (cm2), l is the membrane thickness (cm), dV/dt is the permeation rate at steady–state conditions (cm3(STP)/s), and Δp is difference pressure of gas on both side of membrane (cm Hg)40. The membranes gas permeability was reported in the barrier unit. Membrane ideal selectivity for two gases as defined as Eq. (2):
The diffusion coefficient (D) was calculated using the lag time method by Eq. (3)41,42:
where l is the membrane thickness and θ is the lag time which defined as the time–intercept of a linear fit of the steady–state permeate. The lag time method was performed for the calculation of diffusivity of the slow diffusing gases43,44. The apparent solubility coefficient (S) can be computed using parameters of P and D by Eq. (3):
The permeation of gases via a membrane is investigated using the diffusion (D) and solubility coefficients (S). In contrast to the solubility coefficient is reciprocal link with the thermodynamic factor, the diffusion coefficient is correlated with the size of the penetrant molecule (kinetic factor)43.
Results and discussion
Characterization
XRD analysis
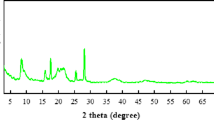
The crystalline structure of the synthesized activated carbon (AC), AC/MOF, IL@AC/MOF composites, was investigated using X-ray diffraction (XRD) analysis. The XRD pattern of the samples is shown in Fig. 2. In the XRD pattern of AC, the diffractogram exhibits two broad diffraction peaks centered at approximately 2θ = 24.18° and 43.28°, which correspond to the diffraction planes (002) and (100), respectively. These broad and weak peaks indicate a predominantly amorphous structure with a low degree of graphitization. The (002) reflection is attributed to the stacking of aromatic layers, while the (100) reflection is related to the in-plane structural ordering of carbon atoms. The broadness of the peaks suggests the presence of disordered carbon structures and a high degree of porosity.
The XRD patterns for the AC/MOF and IL@AC/MOF composites demonstrate the main diffraction peaks of the MOF (NH2-MIL53(Al)) at 2θ = 9.48O, 12.55O, 17.92O, 22.980, 25.12O, and 27.02O for the AC/NH₂-MIL-53(Al) composite, the XRD pattern retains the key diffraction peaks of NH₂-MIL-53(Al), indicating that the MOF crystalline framework remains intact after incorporation with activated carbon. Additionally, a broad peak around 2θ ≈ 23°, characteristic of the (002) plane of carbon, is observed, confirming the presence of activated carbon. The intensity of this peak is relatively low due to the dominance of the MOF structure in the composite, yet its presence suggests good dispersion of AC within the MOF matrix. Upon incorporation of activated carbon (AC), a noticeable broadening and slight decrease in peak intensity is observed, indicating a reduction in overall crystallinity of the composite. This phenomenon is likely due to the amorphous nature of activated carbon, which disrupts the periodic long-range order of the MOF crystals. Furthermore, the interfacial interaction between AC and the MOF domains may induce structural strain or lattice distortions.
In the case of the IL@AC/NH₂-MIL-53(Al) composite, the main diffraction peaks corresponding to NH₂-MIL-53(Al) are still discernible, although slightly broadened and with reduced intensity. This change can be attributed to the incorporation of ionic liquid, which may lead to partial pore filling or slight structural disorder within the MOF framework. The broad halo centered around 2θ ≈ 23° remains visible, confirming the persistence of amorphous AC in the structure. The absence of new crystalline peaks indicates that the IL is well-embedded within the porous network without forming a separate crystalline phase. The XRD pattern of the AC/MOF/PVC(wAC/MOF = 15%), shows characteristic diffraction peaks corresponding to both the MOF structure and the amorphous nature of the PVC matrix. The broad hump centered around 2θ = 20°–25° is indicative of the semi-crystalline nature of PVC. The peaks attributed to activated carbon are less intense and broad, appearing at around 23° and 43°, due to its amorphous structure and lower crystallinity. The successful incorporation of AC and MOF into the PVC matrix without significant phase transformation is confirmed by the preservation of characteristic peaks.
The XRD pattern shows a slight decrease in the intensity of the MOF-related peaks, indicating partial confinement of the ionic liquid (IL) within the MOF pores and/or interaction with the surface of activated carbon (AC). The characteristic broad halo of PVC remains visible, while a further reduction in crystallinity is observed, as evidenced by the decreased intensity and slight peak broadening. This can be attributed to the amorphous nature of IL, which disrupts the ordered arrangement of the MOF and PVC chains. The absence of new crystalline peaks suggests a good dispersion of IL within the composite and no formation of separate crystalline phases.
The observed changes in crystallinity can be attributed to several factors, including the synthesis conditions (e.g., solvothermal temperature and duration), mass ratio of AC to MOF precursor, and post-synthetic treatments such as vacuum-assisted drying or ionic liquid impregnation. Despite these changes, the persistence of major MOF peaks indicates that the crystalline framework of NH₂-MIL-53(Al) remains largely intact within the hybrid structure. The partial loss of crystallinity is not necessarily detrimental; in fact, it may improve gas transport pathways and provide additional interfacial sites beneficial for gas adsorption and diffusion, particularly for CO₂ separation.
BET analysis
The nitrogen (N2) adsorption and desorption isotherm for the AC, AC/MOF composite, and IL@AC/ MOF hybrid materials are shown in Fig. 3 The key textural parameters such as pore volume, specific surface area, and pore diameter are summarized in Table 1. According to the IUPAC classification, the N2 adsorption/desorption isotherm of AC demonstrates a Type I behavior with minor hysteresis. Both AC/NH2-MIL-53(Al) composite, and IL@AC/ MOF hybrid materials exhibited Type IV isotherms with a distinct H3 hysteresis loop, indicating the presence of mesoporous structures (see Fig. 3). This showed that biomass AC is identified as the microporous materials with BET surface areas up to 1243.57 m2 g−1and total pore volumes up to 1.18 cm3.g−1 (see Table 1). After the incorporation of ionic liquid into the AC/MOF matrix, a noticeable decrease in BET surface area and pore volume was observed. The surface area of IL@AC/MOF decreased to 744.23 m2 g−1, while the total pore volume dropped to 0.68 cm3 g−1, suggesting that IL molecules partially occupied the pores or covered the surface area of the composite material that causes the blockage of pores with IL.
FT-IR spectra
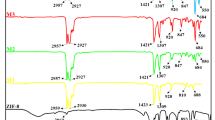
The ATR-FTIR spectra of AC, AC/MOF, IL@AC/MOF, PVC, AC/MOF/PVC(wAC/MOF = 5, 10, and 15%), and IL@AC/MOF/PVC composites (wAC/MOF = 15%) are presented in Fig. 4. The symmetric and asymmetric stretching vibrations of the PVC C–H bonds are observed in the 2900–3000 cm−1 range, as shown in Fig. 4a. The C–H bending vibrations adjacent to the chlorine atom are identified at 1430 and 1251 cm−1, confirming the structural integrity of PVC in the composite. However, slight shifts in the peak positions and intensities of the C–H bands suggest weak van der Waals or dipole–dipole interactions between PVC chains and other polar species in the composite, particularly IL and MOF41. In Fig. 4b, the intense peak was observed at 1481 cm−1 indicating the C=C aromatic stretching bond in the graphite-like polyaromatic structure of the AC. The C=O stretching vibration is observed at 1629 cm−1. Stretching vibrations of C–C and C=C bonds are present at peaks of 1036 and 1630 cm−1, respectively.
In the AC/MOF spectrum (Fig. 4c), the characteristic broad hydroxyl (O–H) stretching band around 3450 cm−1 is diminished, indicating hydrogen bonding interactions between hydroxyl groups of AC and the MOF framework. This is further supported by the flattening of the O–H peak, implying successful integration and interaction of AC with the MOF matrix. The symmetric and asymmetric N–H stretching vibrations from the amino terephthalic acid linker, observed at 3350–3480 cm−1, respectively, show slight peak broadening and overlap, indicating possible hydrogen bonding or electrostatic interactions with IL. N–H bending (scissoring) is observed near 1620 cm−145. In all MMM-containing spectra (e–h), the –NH₂ peaks become broader and slightly shifted, suggesting hydrogen bonding and coordination interactions between the NH₂ groups of MIL-53(Al) and the PVC matrix or IL components.
Furthermore, the sharp signal at 702 cm−1 and the faint peak at 621 cm−1 correspond to the C–H bending vibrations of aromatic rings, attributed to in-plane and out-of-plane bending, respectively. The stretching vibrations of C=O and C=C in the amino terephthalic acid moiety of the MOFs are responsible for the peaks at 1629 cm−1 and 1566 cm−1, respectively. The distinctive signal at 1425 cm−1 represents the carboxylic acid group of coordinated aluminum and free amino terephthalic acid42. The peak at 1251 cm−1 is attributed to C–N bending, while the aromatic C–H in-plane bending is evident at 965 cm−1. A separate band at 771 cm−1 aligns with previous findings for MIL-5344. Additionally, signals at 1562 cm−1 in the ATR-FTIR spectra are associated with the amino acid –NH group, which overlaps primarily with the C = O stretching of the COO⁻ group, indicating ionic interactions between the glutamate moiety and the framework. Overall, the FT-IR analysis confirms the successful synthesis of the IL@AC/MOF/PVC composite. The FTIR results suggest that multiple non-covalent interactions, including hydrogen bonding, π–π stacking, electrostatic interactions, and possible coordination bonding, contribute to the stability and compatibility of the composite membrane materials.
Scanning electron microscopy
FESEM and EDX mapping analyses were performed to examine the morphology and elemental distribution of the synthesized samples. Figures 5 and 6 display the FESEM images of activated carbon (AC), the AC/MOF composite, and cross-sectional views of the AC/NH₂-MIL-53(Al)/PVC membranes containing various filler loadings (wAC/MOF = 5,10, and 15%), as well as the IL@AC/NH₂-MIL-53(Al)/PVC composite with a filler loading of (wIL@AC/MOF = 15%). As shown in Fig. 5, the AC sample exhibits a porous structure, while the well-defined, three-dimensional hexahedral morphology observed for the MOF indicates successful crystallization with good structural regularity. The cross-sectional FESEM images of the MMMs demonstrate that the filler is well-embedded within the PVC matrix, with no significant agglomeration, suggesting good interfacial compatibility.
The FESEM and EDX mapping results of the AC/NH2-MIL-53(Al)/PVC composite confirm a homogeneous distribution of the filler particles throughout the membrane, indicating effective dispersion within the IL matrix. Following IL impregnation, a thin layer of ionic liquid is observed coating the outer surface of the AC/NH2-MIL-53(Al) particles, which is attributed to surface coating by the cholinium amino acid IL ([Cho][AA]). Although direct visualization of the ionic liquid layer via FESEM is limited due to its amorphous and possibly non-conductive nature, the smoother particle surfaces and reduced porosity compared to unmodified composites indicate successful IL loading. This surface modification is likely due to favorable interactions between the functional groups of the MOF and the ionic liquid, further improving the compatibility of the filler with the polymer phase. This observation is further supported by EDX elemental mapping, which reveals a noticeable increase in the relative intensity of N and Cl atoms, consistent with the presence of the ionic liquid. Additionally, cholinium-based ILs can form hydrogen bonds and electrostatic interactions with amine-functionalized MOFs, facilitating the formation of a stable coating layer.
Thermogravimetric analysis
TGA analysis was used to evaluate the thermal stability of membranes. The TGA thermograms for the AC,AC/MOF, IL@AC/MOF/PVC composite, PVC, AC/MOF/PVC, and, IL@AC/MOF/PVC MMMs are shown in Fig. 7. The respective weight loss for AC is 98%. The weight loss data of AC/MOF includes characteristic weight loss stages from 100 to 180 °C loss due to adsorbed water and from 300 to 350 °C loss from MIL-53 framework degradation as well as from 350 to 600 °C loss from organic linkers and AC degradation (Fig. 7a). According to Fig. 7b, TGA thermogram of PVC indicate that degradation proceeds in two stages. From 150 to 280 °C elimination of HCl takes place and chlorine is completely removed from the polymer. The crosslink and thermal cracking of PVC’s backbone takes place in the second stage (350–530 °C). The AC/MOF/PVC and, IL@AC/MOF/PVC MMMs have an TGA thermogram like PVC due to contain 85% them PVC (Fig. 7b). The loss of HCl from PVC is responsible for the weight loss that was noticed at temperatures between 150 and 280 °C. The breakdown of the PVC’s backbone is the cause of the weight loss that is seen at temperatures between 350 and 500 °C. The oxidation of AC is linked to the weight loss that is seen at temperature 400 °C. In the case of IL@AC/MOF, the observed weight loss at a temperature range of 300 to 400 °C is pointed to the destruction of ionic liquid. Additionally, the breakdown of the MOF linkers is linked to the weight loss seen at temperatures between 450 and 600 ̊C. In conclusion, the TGA analysis reveals that the composites are stable up to 200 °C.
Gas separation performance
We surveyed gas permeabilities and selectivities of the PVC, AC/MOF/PVC MMMs, and IL@AC/MOF/PVC MMMs with different AC/MOF and IL@AC/MOF loadings at temperatures of 288.15, 298.15, 308.15, and 318.15 K and pressures of up to 4 bar, single-component gas penetration was detected. The membrane was placed under vacuum at 353 K for four hours before the permeability of CO2 and N2 gases tests. To ensure complete desorption of the prior permeate gas, the prepared membranes degassed for a while prior to switching the feed gas. Figure 8 showed the permeability of pure CO2 and N2 gases as well as the CO2/N2 permeation ideal selectivity of PVC, AC/MOF/PVC with varying mass percent of (wAC/MOF = 5, 10, 15%), and IL@AC/MOF/PVC MMMs (wIL@ACMOF = 15%) at 298.1 5 K and 3 bar. Table 2 shows the diffusion coefficients, solubility, and permeability of IL@AC/MOF/PVC at various temperatures and pressure 3 bar for CO2 and N2. CO2 and N2 permeabilities of pure PVC membrane at temperature of 298.15 K and pressure of 3 bar were 98.60 and 3.95 Barrer, respectively and CO2/N2 ideal selectivity was computed as 24.96. The CO2 and N2 permeabilities for AC/MOF/PVC MMMs with 10 wt.% AC/MOF loading were found to be 597.57 and 22.41 Barrer, respectively, resulting in a CO2/N2 ideal selectivity of 26.66. These outcomes demonstrate that the addition of AC/MOF at a filler loading of 10 wt.% increased the CO2 permeability of membrane and enhanced CO2/N2 ideal selectivity.
As the AC/MOF loading was increased to 15 wt.%, the permeability of CO2 and N2 was further increased to 746.97 and 27.52 Barrer, respectively. Consequently, AC/MOF/PVC MMMs with 15 wt.% AC/MOF loading showed 27.14 CO2/N2 ideal selectivity. CO2 permeabilities of AC/MOF/PVC MMMs with 10 and 15 wt.% AC/MOF loadings were 6.1 and 7.6 times more than the pure PVC membrane. Additionally, the CO2/N2 selectivity of AC/MOF/PVC membranes with 10 and 15 wt.% AC/MOF loadings was 1.06 and 1.1 times greater than the selectivity of the pure PVC membrane, respectively. These findings indicate that the filler was considerable improved permeability but was slightly improved selectivity performance of MMM. However in practical gas separation moreover selectivity, high permeability has important role in efficiency of membrane too.
The CO2/N2 separation performance of AC/MOF/PVC MMM were capaired with that of the other MOF/Polymer MMMs in literature. For instance, the polymer MMM loaded with 15 wt% NH2-MIL-53(Al) /cellulose acetate (CA) showed the maximum CO2 permeability and CO2/N2 selectivity of 52.6 and 23.4, respectively46. Therefore, the permeability of the AC/MOF/PVC MMM that we synthesized in this study is 14 times more than that of the NH2-MIL-53(Al)/CA MMM. In addition, comparsion the separation performance of PES/MIL-53(Al)@MWCNT MMM containing 5 wt% of MIL-53(Al)@MWCNT which had a CO2 permeability of 183 Barrer47 shows that CO2 permeability of AC/MOF/PVC MMM having 15 wt.% AC/MOF loading is 4 times higher. According to the results, the CO2 permeabilities of AC/MOF/PVC MMMs reported in this study are better than those reported in the literature. The strong CO2 affinity of the open Al+3 sites of the AC/MOF framework48,49 and the improved free volume in the PVC matrix owing to increased AC/MOF loading are the reasons for the MMM’s noticeable improvement in CO2 permeability upon the addition of AC/MOF. The incorporation of IL@AC/MOF composite as a filler significantly enhanced the gas permeability of the PVC membrane. At a loading of 15 wt.% IL@AC/MOF and under operating conditions of 298.15 K and 3 bar, the measured CO₂ and N₂ permeabilities for the IL@AC/MOF/PVC MMM were 122.5 and 38.81 Barrer, respectively. This resulted in a CO2/N2 ideal selectivity of 31.56. Compared to the pure PVC membrane, the CO2 permeability increased by a factor of 12.4, while the CO2/N2 ideal selectivity improved by approximately 1.3 times. Although the increase in CO2/N2 selectivity was moderate (1.3 times), the IL@AC/MOF/PVC membrane exhibited a remarkable enhancement in CO₂ permeability, reaching 122.5 Barrer more than 12 times higher than the pure PVC membrane. This positions the membrane as a high-permeability system, ideal for industrial scenarios where process throughput and operational efficiency are prioritized. In such cases, membranes with higher permeance can reduce equipment size, energy costs, and capital expenditure, particularly when combined with acceptable selectivity levels. Thus, the present membrane holds significant promise for practical applications where permeation-driven economics outweigh marginal gains in selectivity.
These significant improvements in gas separation performance can be primarily attributed to the high porosity and strong CO₂ affinity of the IL@AC/MOF filler. The increased porosity facilitates the diffusion of CO2 molecules through the membrane by providing additional transport channels, while the chemical affinity between CO2 and the ionic liquid-functionalized filler promotes higher gas solubility and selectivity. The cholinium amino acid ionic liquids used in this study possess functional groups (e.g., hydroxyl, amino, and carboxyl groups) capable of forming strong hydrogen bonds and acid–base interactions with CO2 molecules. These interactions improve the chemical affinity and solubility of CO2 within the membrane matrix . The polar nature of ILs and their high CO2-philicity lead to preferential sorption of CO2 over N2, thereby enhancing selectivity. Moreover, a slight reduction in the pore volume of the filler, likely caused by IL incorporation, may enhance the molecular sieving effect, contributing further to the observed performance enhancement. In addition, the high-porosity MOF component acts as an effective support for the ionic liquid, increasing the interfacial contact area between CO₂ and IL, thereby improving CO2 uptake and transport across the membrane. The disparity in permeability between CO2 and N2 can be explained by their different physicochemical properties: CO₂ has a smaller kinetic diameter (3.3 Å) compared to N₂ (3.64 Å)50, allowing it to diffuse more readily through the membrane. Furthermore, N₂ exhibits a weaker interaction with the AC/MOF framework, leading to its lower solubility and diffusion rate. As gas solubility generally increases with gas condensability, CO2 being more condensable than N₂ demonstrates higher solubility in the IL-containing membrane system. At the same time, gas diffusivity increases with decreasing penetrant size, and since permeability is a product of solubility and diffusivity, the inverse relationship between molecular size and permeability further explains the superior performance of the MMMs toward CO2 separation. The time lag technique51 was used to calculate the solubility (S) and diffusion (D) coefficients of the MMMs. As summarized in Tables 2 and 3, MMMs exhibited higher diffusion coefficients than the pure PVC membrane. This increase is attributed to the porous nature of the AC/MOF filler, which facilitates gas transport by creating additional diffusion pathways. Additionally, the solubility coefficient of CO2 in the MMMs was substantially higher, owing to the chemical affinity between CO2 and the IL@AC/MOF hybrid filler. The presence of the amino acid-based ionic liquid contributes further to this enhancement by increasing the CO2-polymer interactions. This is especially effective for CO2, whose quadrupolar nature and greater condensability compared to N2 make it more soluble in polar media47.
Effect of feed pressure
In order to examine how feed pressure affects separation properties, permeability of the N2 and CO2 gases in the MMMs were measured at different pressures and constant temperatures (Table 3). The collected data demonstrate that the penetration of nitrogen and carbon dioxide decreased as feed pressure increased from 2 to 4 bars. According to the dual-mode transport and sorption hypothesis, gas solubility and membrane compaction are the main reasons for this phenomena47. Compaction processes reduce the free volume of membrane because of a decrease in membrane permeability. Glassy polymers like PVC make a low degree of penetranta “microvoid environment” accessible for sorption and permeation. Low permeability is induced by a reduction in the number of accessible permeation pathways when high penetrant concentrations are saturating the microvoids47. Figure 9 shows the selectivity of membranes against pressure. The rise in feed pressure led to an increase in the CO2/N2 selectivity. The fact that solubility is pressure-dependent may be the cause of this phenomenon. Compared to N2, CO2 solubility has a higher-pressure dependency. On the other hand, at high feed pressures, the solubility phenomena in permeation takes precedence over diffusion.
Effect of temperature
It is evident that as the temperature rises from 288.15 to 318.15 K, the gas penetration in the MMMs rises. The enhanced mobility of the penetrant gas molecules in the MMMs at higher temperatures may be the origin of this effect, as it rises the diffusion rate via the MMMs. Furthermore, the high mobility of the polymer chain and the high flexibility of the polymer membrane are both influenced by rising temperatures.Temperature dependency of the permeability, diffusion coefficient and solubility can be represents by the Arrhenius equation as Eqs. (5) and (6).52:
Pre-exponential factors are revealed by P0 , D0 and S0; the gas constant is denoted by R; and the activation energy of permeation and diffusion processes is shown by EP , ED and ΔHS, respectively. The slopes of ln (P), ln (D) and ln (S) via 1/T were used to compute EP , ED and ΔHs, respectively. Table 4. lists the expected activation energies for diffusion and permeation. The structure of mixed matrix membranes, the sort of polymer, and the size of the penetrant effect the permeation activation energy. The structure and free volumes of the mixed matrix membranes are the cause of the activation energy fluctuation53. The gas solubility enthalpy in MMMs is tabulated in Table 4. Since ED is positive and ΔHs is negative. |ΔHs| are smaller than ED the behavior of which is consistent with the findings of glassy polymers in the literature38. Additionally, EP is positive, and the mixed matrix membranes’ gas permeability increases as the temperature rises. The free volume of the MMMs is responsible for both positive EP and ED. As the membrane-free volume increased with temperature, the EP and ED values decreased54. A larger activation energy suggests a high-temperature dependence of the permeation.
Robeson upper bound analysis
The Robeson Upper Bound is a widely accepted benchmark used to evaluate the trade-off between permeability and selectivity in gas separation membranes. Figure 10 presents the CO2/N2 selectivity versus CO2 permeability of the AC/MOF/PVC MMMs and IL@AC/MOF/PVC MMMs plotted against the 2008 Robeson Upper Bound50. As shown, the embadation AC/MOF and IL@AC/MOF in to the PVC matrix is developed membrane performance. The performance of IL@AC/MOF/PVC MMM is especially approached to Robeson Upper Bound which demonstrate excellent separation efficiency. To further contextualize the performance, a comparative analysis was carried out with membrane materials reported in the literature. Table 5 summarizes CO2/N2 selectivity of our membranes alongside representative materials from the literature, as shown in Fig. 10. This comparison highlights the potential of our materials in practical gas separation applications.
Conclusions
In this work, AC/MOF and IL@AC/MOF composites, were synthesized and embedded in PVC matrix as filler to improve their permeability and gas separation capabilities. AC/MOF/PVC and IL@AC/MOF/PVC MMMs were characterized with FTIR, TGA, FESEM and EDX-Map. The loding of AC/MOF and IL@AC/MOF filler in to the PVC matrix develope the PVC membrane performance. The performance of IL@AC/MOF/PVC MMM is especially approached to Robeson Upper Bound which demonstrate this MMM can be useful gas separation applications.
Data availability
Data availability: The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Krishnan, A. et al. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 18, 2031–2054 (2020).
Feng, S. et al. Hydrothermal stable ZIF-67 nanosheets via morphology regulation strategy to construct mixed-matrix membrane for gas separation. J. Membr. Sci. 593, 117404 (2020).
Lee, C. S., Moon, J., Park, J. T. & Kim, J. H. Engineering CO2-philic pathway via grafting poly(ethylene glycol) on graphene oxide for mixed matrix membranes with high CO2 permeance. Chem. Eng. J. 453, 139818 (2023).
Dong, G., Li, H. & Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. 1(15), 4610–4630 (2013).
Lei, L. et al. Taming structure and modulating carbon dioxide (CO2) adsorption isosteric heat of nickel-based metal organic framework (MOF-74 (Ni)) for remarkable CO2 capture. J. Colloid Interface Sci. 15(612), 132–145 (2022).
Tara, N. et al. Simultaneous increase in CO2 permeability and selectivity by BIT-72 and modified BIT-72 based mixed matrix membranes. Chem. Eng. Res. Des. 178, 136–147 (2021).
Qian, Q. et al. MOF-based membranes for gas separations. Chem. Rev. 120(16), 8161–8266 (2020).
Xu, S., Huang, H., Guo, X., Qiao, Z. & Zhong, C. Highly selective gas transport channels in mixed matrix membranes fabricated by using water-stable Cu-BTC. Separ. Purif. Technol. 257, 117979 (2021).
Tien-Binh, N., Vinh-Thang, H., Chen, X. Y., Rodrigue, D. & Kaliaguine, S. Crosslinked MOF-polymer to enhance gas separation of mixed matrix membranes. J. Membr. Sci. 520, 941–950 (2016).
Bushell, A. F. et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 427, 48–62 (2013).
Wang, Y. et al. Preparation of HKUST-1/PEI mixed-matrix membranes: Adsorptiondiffusion coupling control of small gas molecules. J. Membr. Sci. 643, 120070 (2022).
Thür, R. et al. Bipyridine-based UiO-67 as novel filler in mixed-matrix membranes for CO2-selective gas separation. J. Membr. Sci. 576, 78–87 (2019).
Tien-Binh, N., Rodrigue, D. & Kaliaguine, S. In-situ cross interface linking of PIM-1 polymer and UiO-66-NH2 for outstanding gas separation and physical aging control. J. Membr. Sci. 548, 429–438 (2018).
Ahmad, M. Z. et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 15(558), 64–77 (2018).
Wu, T., Prasetya, N. & Li, K. Recent advances in aluminium-based metal-organic frameworks (MOF) and its membrane applications. J. Membr. Sci. 615, 118493 (2020).
Buddin, M. S. & Ahmad, A. L. A review on metal-organic frameworks as filler in mixed matrix membrane: Recent strategies to surpass upper bound for CO2 separation. J. CO2 Utilization. 51, 101616 (2021).
Kinik, F. P., Uzun, A. & Keskin, S. Ionic liquid/metal–organic framework composites: From synthesis to applications. Chemsuschem 10(14), 2842–2863 (2017).
Durak, O. et al. Composites of porous materials with ionic liquids: Synthesis, characterization, applications, and beyond. Microporous Mesoporous Mater. 1(332), 111703 (2022).
Zeeshan, M. et al. Core–shell type ionic liquid/metal organic framework composite: An exceptionally high CO2/CH4 selectivity. J. Am. Chem. Soc. 140(32), 10113–10116 (2018).
Zeeshan, M., Keskin, S. & Uzun, A. Enhancing CO2/CH4 and CO2/N2 separation performances of ZIF-8 by post-synthesis modification with [BMIM][SCN]. Polyhedron 155, 485–492 (2018).
Polat, H. M., Zeeshan, M., Uzun, A. & Keskin, S. Unlocking CO2 separation performance of ionic liquid/CuBTC composites: Combining experiments with molecular simulations. Chem. Eng. J. 373, 1179–1189 (2019).
Zeeshan, M. et al. Influence of anion size and electronic structure on the gas separation performance of ionic liquid/ZIF-8 composites. Microporous Mesoporous Mater. 306, 110446 (2020).
Kinik, F. P. et al. [BMIM][PF6] incorporation doubles CO2 selectivity of ZIF-8: Elucidation of interactions and their consequenceson performance. ACS Appl. Mater. Interfaces 8(45), 30992–31005 (2016).
Koyuturk, B., Altintas, C., Kinik, F. P., Keskin, S. & Uzun, A. Improving gas separation performance of ZIF-8 by [BMIM][BF4] incorporation: Interactions and their onsequences on performance. J. Phys. Chem. C 121(19), 10370–10381 (2017).
Chen, W. et al. PIM-based mixedmatrix membranes containing MOF-801/ionic liquid nanocomposites for enhanced CO2 separation performance. J. Membr. Sci. 636, 119581 (2021).
Ban, Y. et al. Confinement of ionic liquids in nanocages: Tailoring the molecular sieving roperties of ZIF-8 for membrane-based CO2 capture. Angew Chem. Int. Ed. Engl. 54, 15483–15487 (2015).
Ferreira, I. C. et al. Cr-based MOF/IL composites as fillers in mixed matrix membranes for CO2 separation. Separ. Purif. Technol. 276, 119303 (2021).
Habib, N. et al. Development of highly permeable and selective mixed matrix membranes based on Pebax® 1657 and NOTT-300 for CO2 capture. Separ. Purif. Technol. 234, 116101 (2020).
Yu, Y., Zhang, C., Fan, J., Liu, D. & Meng, J. A mixed matrix membrane for enhanced CO2/N2 separation via aligning hierarchical porous zeolite with a polyethersulfone based comb-like polymer. J. Taiwan Inst. Chem. Eng. 1(132), 104132 (2022).
Kayal, S. & Chakraborty, A. Activated carbon (type Maxsorb-III) and MIL-101(Cr) metal organic framework based composite adsorbent for higher CH4 storage and CO2 capture. Chem. Eng. J. 334, 780–788 (2018).
Noorani, N., Mehrdad, A. & Fatehi-mollayousef, A. Novel approaches to improving gas separation: Ionic liquids coated coke/NH2-UiO-66-based mixed matrix membranes for CO2/N2 separation. J. Mol. Struct. 1322, 140479 (2025).
Noorani, N., Mehrdad, A. & Shamszadeh, P. PVC-based mixed-matrixmembranes based on IL@AC/NH2-MIL-101nanocomposites for improved CO2 separation performance. Sci. Rep. 14, 23843 (2024).
Qi, L., Jiang, H., Lin, T., Chang, X. & Jiang, B. Fabrication of MIL-53(Al) based composites from biomass activated carbon (AC) for efficient p-nitrophenol adsorption from aqueous solution. Taiwan Inst. Chem. 127, 220–227 (2021).
Noorani, N. & Mehrdad, A. Experimental and theoretical study of CO2 sorption in biocompatible and biodegradable cholinium-based ionic liquids. Sep. Purif. Technol. 254, 117609 (2021).
Noorani, N. & Mehrdad, A. Cholinium-amino acid ionic liquids as biocompatible agents for carbon dioxide absorption. J. Mol. Liq. 357, 119078 (2022).
Noorani, N., Mehrdad, A. & Chakhmaghi, F. Thermodynamic study on carbon dioxide and methane permeability in polyvinylchloride/ionic liquid blends. Chem. Thermodyn. 145, 106094 (2020).
Mehrdad, A. & Noorani, N. Permeability behavior of polyvinyl chloride-ionic liquid ionomer for CO2/CH4 separation. Sep. Purif. Technol. 226, 138–145 (2019).
Noorani, N. & Mehrdad, A. Adsorption, permeation, and DFT studies of PVC/PVIm blends for separation of CO2/CH4. J. Mol. Liq. 292, 111410 (2019).
Noorani, N. & Mehrdad, A. Modification of PVC with 1-vinylimidazole for CO2/CH4 separation: sorption, permeation and DFT studies. Phys. Chem. Res. 8, 689–703 (2020).
Galiatsatou, P., Kanellopoulos, N. K. & Petropoulos, J. H. Characterization of the transport properties of membranes of uncertain macroscopic structural homogeneity. J. Membr. Sci. 280, 634–642 (2006).
Basu, S., Cano-Odena, A. & Vankelecom, I. F. J. Asymmetric matrimid®/[Cu3(BTC)2] mixed-matrix membranes for gas separations. J. Membr. Sci. 362, 478–487 (2010).
Adams, R., Carson, C., Ward, J., Tannenbaum, R. & Koros, W. Metal organic framework mixed matrix membranes for gas separations. Microporous Mesoporous Mater. 131, 13–20 (2010).
Perez, E. V., Balkus, K. J. Jr., Ferraris, J. P. & Musselman, I. H. Mixed-matrix membranes containing MOF-5 for gas separations. J. Membr. Sci. 328, 165–173 (2009).
Arenas, E., Bucio, E., Burillo, G. & Lopez, G. P. Radiation grafting of N-iso-propylacrylamide on to poly(vinylchloride) catheters by gamma irradiation. Polym. Bull. 58, 401–409 (2007).
Chong, S., Wang, T., Cheng, L., Lv, H. & Ji, M. Metal-organic framework MIL-101-NH2 supported acetatebased butylimidazolium ionic liquid as a highly efficient eterogeneous catalyst for the synthesis of 3-aryl-2-oxazolidinones. Langmuir 35(2), 495–503 (2019).
Reinecke, H. & Mijangos, C. PVC modification with pyridine groups. Synthesis, characterization and transformation to ionomers. Macromol. Chem. Phys. 199(10), 2199–2204 (1998).
Rostami, M. S. & Khodaei, M. M. Effect of incorporated hybrid MIL-53(Al) and MWCNT into PES membrane for CO2/CH4 and CO2/N2 separation. Fuel 356, 129598 (2024).
Stavitski, E. et al. Complexity behind CO2 capture on NH2-MIL-53(Al). Langmuir 27(7), 3970–3976 (2011).
Bourrelly, S. et al. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 127(39), 13519–13521 (2005).
Robeson, L. M. The upper bound revisited. J. Membr. Sci. 320, 390–400 (2008).
Seoane, B., Téllez, C., Coronas, J. & Staudt, C. NH2-MIL-53(Al) andNH2-MIL-101(Al) insulfur-containing copolyimide mixed matrix membranes for gas separation. Sep. Purif. Technol. 111, 72–81 (2013).
Mubashir, M., Yeong, Y. F., Lau, K. K., Chew, T. L. & Norwahyu, J. Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53 (Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 30(199), 140–151 (2018).
Guo, Z. et al. Ionic liquid tuning nanocage size of MOFs through a two-step adsorption/infiltration strategy for enhanced gas screening of mixed-matrix membranes. J. Membr. Sci. 15(605), 118101 (2020).
Car, A., Stropnik, C., Yave, W. & Peinemann, K.-V. PEG modified poly(amide-bethylene oxide) membranes for CO2 separation. J. Membr. Sci. 307, 88–95 (2008).
Ma, C. & Urban, J. J. Enhanced CO2 capture and hydrogen purification via hydroxy metal-organic framework/polyimide mixed matrix membranes. Chemsuschem 12, 4405–4411 (2019).
Q. Qian, Polymer-Metal–Organic Framework (MOF) Mixed-Matrix Membranes for Gas Separation Applications. (2021)
Fan, D., Ozcan, A., Ramsahye, N. A., Maurin, G. & Semino, R. Putting forward NUS-8-CO2H/PIM-1 as a mixed matrix membrane for CO2 capture. ACS Appl. Mater. Interfaces 14, 16820–16829 (2022).
Author information
Authors and Affiliations
Contributions
Narmin Noorani: Data duration, Writing-Original draft preparation, Visualization, Methodology, Investigation, Synthesis. Abbas Mehrdad: Conceptualization, Methodology, Validation, Writing-Reviewing and Editing. Alireza Fatehi-mollayousef: Methodology,Data duration . Aligholi Niaei: Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Noorani, N., Mehrdad, A., Fatehi-mollayousef, A. et al. The advancements in mixed matrix membranes containing MOFs and ionic liquids for CO2/N2 separation. Sci Rep 15, 26271 (2025). https://doi.org/10.1038/s41598-025-11724-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11724-1