Abstract
Salinity is one of the most challenging environmental factors limiting plant development and yield by interfering with key physiological and metabolic activities. In this study, we explored how applying spermine externally could help Plantago major L. cope with salt stress. A detailed analysis was carried out, examining changes in plant growth, water balance, biochemical composition, enzyme activity, gene expression, and metabolite accumulation. Plants were grown under four different conditions: untreated control, salinity stress alone, spermine treatment alone, and salinity combined with spermine. Exposure to salt significantly hindered shoot and root growth, decreased chlorophyll levels and water retention, and increased oxidative stress and the buildup of certain stress-related compounds. However, the addition of spermine, especially in the combined salinity and spermine treatment, helped alleviate these negative effects. It improved water content, preserved chlorophyll, lowered damage markers like malondialdehyde, and boosted the plant’s antioxidant system. On a molecular level, genes such as phenylalanine ammonia-lyase (PAL), caffeic acid O-methyltransferase (COMT), and 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (DAHPS)—involved in the production of protective secondary metabolites—showed altered expression patterns in response to stress and spermine. These changes were mirrored in the increased levels of compounds like phenylalanine, caffeic acid, and rosmarinic acid. Statistical analyses, including principal component analysis and clustering, highlighted the salinity plus spermine group as having the most favorable overall response. Together, the results suggest that spermine strengthens Plantago major’s ability to withstand salinity by triggering a broad range of protective mechanisms and may serve as a valuable tool for improving plant resilience in salt-affected soils.
Similar content being viewed by others
Introduction
Salinity is among the most widespread abiotic stresses that hinder agricultural productivity globally, especially in arid and semi-arid regions where improper irrigation, elevated evaporation rates, and inadequate drainage accelerate soil salinization1. High levels of soluble salts—particularly sodium chloride—not only interfere with water absorption in plants but also disturb ionic balance, trigger oxidative damage, and disrupt core physiological and metabolic functions2. Consequently, salt-stressed plants commonly show signs of reduced growth, chlorotic leaves, membrane injury, and lowered reproductive efficiency3. To survive these conditions, plants activate a network of defense strategies that include osmotic adjustment, maintenance of ion equilibrium, activation of antioxidant enzymes, and enhanced production of secondary metabolites with protective roles4.
Among the various approaches to counteract salt-induced damage, the use of exogenous polyamines has gained growing interest5. Polyamines such as putrescine, spermidine, and spermine are small, polycationic compounds that participate in numerous plant processes related to growth and stress adaptation6. Spermine, in particular, has demonstrated promising effects in alleviating salt stress through several mechanisms, including membrane and DNA stabilization, reactive oxygen species (ROS) scavenging, enhancement of antioxidant defenses, modulation of ion transport, and regulation of stress-responsive genes7. While its beneficial effects have been documented in a variety of crops, the specific physiological and molecular roles of spermine in medicinal and stress-tolerant species such as Plantago major have not been fully explored.
Plantago major L. (broadleaf plantain) is an herbaceous perennial widely valued for its medicinal properties and its notable resilience to environmental stresses8. This species is known to accumulate a range of secondary metabolites—including phenolic acids, flavonoids, and iridoid glycosides—especially under adverse conditions, making it a suitable model for studying biochemical and molecular responses to stress9. Previous studies have reported the activation of the phenylpropanoid and shikimate pathways under abiotic stress in Plantago species, yet little information is available on how exogenously applied polyamines, particularly spermine, affect these pathways under saline conditions10.
This study investigated whether spermine can improve salinity tolerance in Plantago major by examining its effects across a broad spectrum of traits, including growth performance, physiological adjustments, oxidative stress markers, and gene expression profiles. We focused on genes involved in the biosynthesis of key secondary metabolites, such as phenylalanine ammonia-lyase (PAL), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), caffeic acid O-methyltransferase (COMT), epoxidase, and 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (DAHPS), and assessed how their expression correlates with metabolite accumulation under different treatments. By integrating morphophysiological, biochemical, and molecular data, this work aimed to clarify the complex role of spermine in mediating stress responses and to contribute knowledge that could be applied in sustainable crop management strategies, particularly in saline-prone environments.
Materials and methods
Plant material and growth conditions
Seeds of Plantago major L. were procured from Pakan Bazr Co. (Isfahan, Iran). Surface sterilization was performed using 1% sodium hypochlorite solution for 3 min, followed by repeated rinsing with sterile distilled water. Sterilized seeds were sown in 15 cm plastic pots containing a sterilized substrate composed of loam soil, sand, and peat moss in a 2:1:1 (v/v) ratio. The pots were maintained under controlled growth chamber conditions: 25 ± 2 °C, 60–70% relative humidity, a 16 h light/8 h dark photoperiod, and a light intensity of 250 µmol m⁻² s⁻¹.
Experimental treatments and design
The experiment was conducted in a completely randomized design (CRD) with four treatment groups and three biological replicates per treatment, totaling 12 experimental units. Each replicate consisted of an individual pot containing one healthy Plantago major seedling. The four treatments were: Control (C): Plants irrigated with standard Hoagland nutrient solution, Salt stress (S): Plants subjected to salinity stress by gradually increasing NaCl concentration in the nutrient solution (25 mM increments every two days until reaching 100 mM), Spermine treatment (Spm): Plants treated with 0.5 mM spermine via foliar spray twice a week. Combined treatment (S + Spm): Plants exposed to both salinity stress (100 mM NaCl) and 0.5 mM spermine application. Treatments began two weeks after germination and continued for 21 days. The pots were randomly arranged in a growth chamber under controlled environmental conditions (25 ± 2 °C, 60–70% relative humidity, 16 h light/8 h dark photoperiod, and ~ 250 µmol m⁻² s⁻¹ light intensity) and were periodically re-randomized to minimize positional effects.
Morphological measurements
At the end of the treatment period, shoot and root lengths (cm), leaf number, and total leaf area (cm²) were measured using a digital caliper or ImageJ software. Shoot and root fresh weights (g) were recorded immediately after harvest. Dry weights were obtained after oven-drying at 70 °C for 48 h. The root-to-shoot ratio was calculated based on dry weights.
Water status and membrane integrity
To evaluate water status and membrane stability, relative water content (RWC) and electrolyte leakage (EL) were assessed. For RWC, fresh weight (FW), turgid weight (TW), and dry weight (DW) were obtained from leaf discs, and RWC was calculated using the formula: RWC (%) = [(FW − DW)/(TW − DW)] × 100. EL was determined by soaking leaf discs in deionized water for 24 h at room temperature to measure initial conductivity (C1), then autoclaving to obtain final conductivity (C2). EL was expressed as a percentage: (C1/C2) × 100.
Photosynthetic pigments and stomatal conductance
Photosynthetic pigments were extracted from fresh leaf tissue using 80% acetone. The contents of chlorophyll a, chlorophyll b, and total carotenoids were quantified spectrophotometrically following by Sudhakar, et al.11. Stomatal conductance and transpiration were optionally measured using a portable porometer on selected samples.
Biochemical and antioxidant parameters
Biochemical markers associated with stress responses were evaluated. Proline content was determined using the method of Bates, et al.12. Briefly, 0.5 g of fresh leaf tissue was homogenized in 10 mL of 3% (w/v) sulfosalicylic acid and centrifuged at 10,000 × g for 10 min. Two milliliters of the supernatant were mixed with 2 mL of acid ninhydrin (1.25 g ninhydrin in 30 mL glacial acetic acid and 20 mL 6 M phosphoric acid) and 2 mL of glacial acetic acid. The mixture was incubated at 100 °C for 1 h and then cooled on ice. The chromophore was extracted with 4 mL of toluene, and absorbance was read at 520 nm using a UV–Vis spectrophotometer. Proline concentration was expressed as µmol g⁻¹ fresh weight (FW), using a standard curve prepared with L-proline. Soluble sugars were quantified using the phenol–sulfuric acid method13. Approximately 0.5 g of fresh leaf tissue was extracted with 10 mL of 80% ethanol at 80 °C for 30 min. The extract was centrifuged, and 1 mL of the supernatant was reacted with 1 mL of 5% phenol and 5 mL of concentrated sulfuric acid. After 30 min, absorbance was measured at 490 nm. Total soluble sugar content was calculated using a glucose standard curve and expressed as mg g⁻¹ FW. Glycine betaine was determined according to the Grieve and Grattan14 protocol. Fresh tissue (0.5 g) was homogenized in 10 mL deionized water and incubated in a water bath at 60 °C for 2 h. After centrifugation, 1 mL of the extract was mixed with 1 mL of 2 N HCl and 0.5 mL of potassium tri-iodide solution. After cooling on ice for 90 min, 2 mL of ice-cold water and 5 mL of 1,2-dichloroethane were added. The lower organic phase was collected, and absorbance was recorded at 365 nm. Results were expressed as µmol g⁻¹ FW. Lipid peroxidation was assessed by quantifying MDA following the Heath and Packer15 method. Leaf tissue (0.5 g) was homogenized in 5 mL of 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 10,000 × g for 10 min. One milliliter of the supernatant was mixed with 4 mL of 0.5% thiobarbituric acid (TBA) in 20% TCA, incubated at 95 °C for 30 min, and quickly cooled on ice. The mixture was centrifuged, and absorbance of the supernatant was measured at 532 nm and 600 nm (for non-specific absorbance). MDA content was calculated using an extinction coefficient of 155 mM⁻¹ cm⁻¹ and expressed as nmol g⁻¹ FW. Ascorbic acid was measured using the redox titration method of Mukherjee and Choudhuri16. Briefly, 1 g of fresh tissue was homogenized in 5 mL of 6% TCA and filtered. One milliliter of the extract was titrated with 2,6-dichlorophenolindophenol dye until a light pink color persisted for 15 s. Ascorbic acid concentration was expressed as mg g⁻¹ FW.
Antioxidant enzyme activity
To assess enzymatic antioxidant activity, the levels of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and glutathione reductase (GR) were determined. Enzyme extracts were prepared by grinding 0.5 g of fresh leaf material in ice-cold 50 mM phosphate buffer (pH 7.0) with 1% polyvinylpyrrolidone, followed by centrifugation at 15,000 rpm for 15 min at 4 °C. SOD activity was based on the inhibition of nitroblue tetrazolium (NBT) reduction. CAT activity was measured by tracking H₂O₂ breakdown at 240 nm, while POD activity was assessed via guaiacol oxidation at 470 nm. APX activity was evaluated by monitoring ascorbate oxidation at 290 nm, and GR activity by tracking NADPH oxidation at 340 nm. Total soluble protein in extracts was quantified using the Bradford method.
Gene expression analysis
Gene expression was quantified via qRT-PCR. Total RNA was extracted from frozen leaves using TRIzol reagent, treated with DNase I, and assessed for quality via agarose gel electrophoresis and spectrophotometry. cDNA synthesis was performed using 1 µg of RNA and the RevertAid cDNA synthesis kit. Primers for PAL, HMGR, COMT, epoxidase, DAHPS, and the housekeeping gene actin were designed using Primer3 software (Table S1). qRT-PCR was performed with SYBR Green on a Rotor-Gene Q system under standard cycling conditions. Gene expression levels were normalized to actin and calculated using the 2–ΔΔCt method.
.
Secondary metabolite and hormonal profiling
Metabolite profiling focused on quantifying key secondary metabolites and hormonal markers. Methanolic extracts of leaf tissue were analyzed by HPLC using a C18 reverse-phase column. Rosmarinic, ferulic, caffeic, and p-coumaric acids were detected at 280 and 320 nm, using external calibration curves. Shikimic acid and aromatic amino acids (phenylalanine, tryptophan, and tyrosine) were measured post-derivatization with o-phthalaldehyde using fluorescence detection. ABA levels were determined by ELISA (Abbexa, UK) and validated through LC-MS/MS. Endogenous polyamines were extracted and quantified after derivatization with dansyl chloride as per Flores and Galston17followed by HPLC detection.
Statistical analysis
All experimental data were analyzed using SPSS software (version 25.0). One-way analysis of variance (ANOVA) was employed to assess significant differences among treatments for all measured traits. Prior to ANOVA, the Shapiro–Wilk test was used to confirm the normality of data distribution, and Levene’s test was performed to verify the homogeneity of variances. Only datasets that satisfied both assumptions were included in the ANOVA analysis. Where assumptions were violated, appropriate data transformations (e.g., log or square root) were applied before analysis. Post hoc comparisons among treatment means were conducted using Tukey’s Honestly Significant Difference (HSD) test at a significance level of p ≤ 0.05. All data are presented as mean ± standard error (SE) of three biological replicates unless otherwise noted. Figures and visualizations including bar charts, PCA plots, and heatmaps were created using GraphPad Prism 9 and R software to illustrate trends and treatment effects.
Results
Morphological responses of Plantago major to salinity and spermine treatments
Salinity stress significantly impaired vegetative growth in Plantago major, reducing shoot length (SL), root length (RL), leaf area (LA), and biomass (Fig. 1). However, the exogenous application of spermine (Spm) consistently alleviated these adverse effects. Under salinity stress alone (S), SL and RL declined by approximately 30–33%, while the combined salinity plus spermine treatment (S + Spm) restored these values by over 30% relative to salt-stressed plants, approaching but not fully reaching the levels of the control (C) group. Similarly, leaf number (LN) and LA decreased under salt stress but were substantially recovered in the S + Spm treatment, indicating that Spm supported both leaf expansion and shoot architectural integrity. Biomass accumulation reflected similar trends. Salinity caused a 40–45% reduction in both shoot fresh weight (SFW) and root fresh weight (RFW), as well as in shoot dry weight (SDW) and root dry weight (RDW). In contrast, Spm application—particularly in the S + Spm group—enhanced biomass by nearly 50% compared to salinity-only plants. Despite these gains, plants treated with Spm under saline conditions did not fully regain control-level biomass. Notably, the root-to-shoot dry weight ratio (R/S ratio) remained relatively constant across treatments, suggesting that spermine induced a balanced, systemic growth response rather than preferentially promoting root or shoot biomass allocation.
Effects of salinity and exogenous spermine on morphological traits of Plantago major. Measured traits include shoot length (A), root length (B), number of leaves per plant (C), leaf area (D), shoot fresh weight (E), root fresh weight (F), shoot dry weight (G), root dry weight (H), and root-to-shoot dry weight ratio (I). Plants were subjected to four treatments: control (C), salinity stress (S, 100 mM NaCl), spermine application (Spm, 0.5 mM), and combined salinity and spermine (S + Spm). Values represent means ± SE (n = 3).
Physiological responses to salinity and spermine treatments
Salinity substantially disrupted water relations and membrane integrity, as reflected by reduced relative water content (RWC) and increased electrolyte leakage (EL) (Fig. 2). The salinity plus spermine treatment (S + Spm) significantly mitigated these effects, with RWC recovering by 20% and EL declining by 43% compared to salt-stressed plants. Spermine (Spm) alone also improved these parameters under normal conditions, indicating its basal role in stabilizing water balance and membrane function. Photosynthetic pigment levels declined sharply under salinity—chlorophyll a (Chl a) and chlorophyll b (Chl b) by 40–50%, and carotenoids (Car) by 37.5%. Spm, especially in the S + Spm treatment, promoted notable pigment recovery, with increases of up to 67% in Chl b and 40% in Car relative to salt stress. Although pigment levels in S + Spm plants did not fully reach those of the control (C), the partial restoration highlights Spm’s protective effect on the photosynthetic apparatus. Stomatal conductance (gs) followed a similar trend, with a 40% decline under salinity and a 42% recovery in the S + Spm group, supporting the notion of improved gas exchange and water-use efficiency under spermine co-treatment.
Influence of salinity and exogenous spermine on physiological parameters of Plantago major. Assessed traits include relative water content (RWC) (A), electrolyte leakage (EL) (B), chlorophyll a (C), chlorophyll b (D), carotenoids (E), and stomatal conductance (F). Treatments include control (C), salinity stress (S, 100 mM NaCl), spermine application (Spm, 0.5 mM), and combined salinity plus spermine (S + Spm).
Biochemical responses to salinity and spermine treatments
Salinity induced marked biochemical changes associated with osmotic adjustment and oxidative damage. Proline, glycine betaine (GB), and soluble sugars (SS)—key osmoprotectants—were significantly elevated under salinity (Fig. 3). However, co-treatment with spermine (Spm) moderated their accumulation, indicating a balanced osmotic response without excessive metabolite buildup. Notably, proline content in salinity plus spermine treatment (S + Spm) plants was 36% lower than in salt-stressed (S) plants but still 75% higher than the control (C), suggesting improved osmotic regulation with reduced metabolic cost. Lipid peroxidation, as indicated by malondialdehyde (MDA) content, increased by 140% under salinity but was reduced by 42% with Spm co-treatment. This reduction in oxidative damage was accompanied by partial recovery of ascorbic acid (AsA) (40% increase over salt stress) and moderate increases in total phenolics (TP) and flavonoids (FLA). Together, these changes reflect an enhanced non-enzymatic antioxidant capacity in Spm-treated plants. Thus, Spm modulated both osmotic and oxidative stress responses, contributing to physiological stabilization under saline conditions.
Effects of salinity and spermine application on biochemical markers in Plantago major. Measured parameters include proline content (A), total soluble sugars (B), glycine betaine (C), malondialdehyde (MDA) content (D), ascorbic acid (E), total phenolic content (F) and Flavonoids (G). Treatments: control (C), salinity (S, 100 mM NaCl), spermine (Spm, 0.5 mM), and salinity plus spermine (S + Spm).
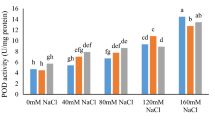
Antioxidant enzyme activities in response to salinity and spermine treatments
Salinity stress triggered substantial activation of the antioxidant enzyme system in Plantago major, as shown by elevated activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and glutathione reductase (GR) (Fig. 4). This reflects the plant’s attempt to detoxify excess reactive oxygen species (ROS) generated under saline conditions. While spermine (Spm) alone modestly enhanced these enzyme activities (6–10% increases), the combined salinity plus spermine (S + Spm) treatment exhibited intermediate activity levels—significantly higher than the control (C) but lower than salt-only (S) plants. For instance, SOD and CAT activities increased by 30–33% in S + Spm-treated plants compared to C, but remained 7–11% below S levels. This moderated activation suggests that Spm alleviates oxidative pressure, reducing the need for excessive enzymatic defense. Enzyme patterns across POD, APX, and GR showed similar trends, reinforcing Spm’s role in fine-tuning the antioxidant system rather than hyperactivating it. Such moderation likely reflects lower ROS production due to improved membrane stability and osmotic balance in the presence of Spm.
The observed enhancement of antioxidant enzyme activities—particularly SOD, CAT, POD, APX, and GR—in S plants clearly indicates a strong oxidative defense response. However, in the S + Spm treatment, enzyme activities were elevated relative to the C but consistently lower than those observed in S plants. This pattern suggests that exogenous Spm attenuated excessive oxidative signaling while sustaining a sufficient antioxidant baseline to mitigate ROS accumulation. Importantly, this biochemical moderation coincided with substantial recovery of growth parameters, including shoot and root length, biomass, and leaf area. For instance, GR and APX activities in the S + Spm group were significantly lower than in the S group, yet shoot biomass was 50% higher. These trends imply that the protective antioxidant response was more efficient rather than merely amplified, allowing energy and carbon resources to be redirected from stress mitigation back toward growth processes.
Activity of enzymatic antioxidants in Plantago major leaves under different treatments. Enzymes include superoxide dismutase (SOD) (A), catalase (CAT) (B), peroxidase (POD) (C), ascorbate peroxidase (APX) (D), and glutathione reductase (GR) (E). Treatments: control (C), salinity (S, 100 mM NaCl), spermine (Spm, 0.5 mM), and salinity plus spermine (S + Spm). Data represent means ± SE (n = 3).
Gene expression responses of stress-related pathways
The transcriptional responses of five key genes involved in secondary metabolism and hormonal signaling—phenylalanine ammonia lyase (PAL), epoxidase, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), caffeic acid O-methyltransferase (COMT), and 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase (DAHPS)—demonstrated consistent salinity-induced upregulation (Fig. 5). PAL and COMT, which are central to the phenylpropanoid pathway, showed the most pronounced responses, with 2.0- and 2.2-fold increases, respectively. Interestingly, spermine (Spm) application alone caused mild upregulation across all genes (1.1–1.4-fold), suggesting a priming effect. The combined salinity plus spermine (S + Spm) treatment led to intermediate expression levels—higher than the control (C) but 12–25% lower than salinity-only (S) plants—pointing to Spm’s regulatory influence on gene expression. This pattern was particularly notable for COMT and DAHPS, where the dampened transcription aligned with a moderated accumulation of ferulic acid and shikimic acid, respectively. These findings support the idea that Spm not only suppresses excessive stress responses but also fine-tunes gene activity to maintain metabolic balance under salt stress.
Relative expression levels of key stress- and metabolism-related genes in Plantago major under salinity and spermine treatments. Analyzed genes include phenylalanine ammonia lyase (PAL) (A), epoxidase (B), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) (C), caffeic acid O-methyltransferase (COMT) (D), and 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (DAHPS) (E). Expression levels were normalized to a housekeeping gene and presented as fold changes relative to the control. Data are means ± SE (n = 3).
Changes in metabolite profiles under salinity and spermine treatments
Quantification of key secondary metabolites revealed that salinity induced an upregulation across all measured compounds, including phenylalanine, caffeic acid, ferulic acid, rosmarinic acid, shikimic acid, and abscisic acid (ABA) (Fig. 6). However, spermine (Spm) co-treatment moderated this surge. In the combined salinity plus spermine (S + Spm) group, metabolite levels were elevated relative to the control (C) but consistently 8–15% lower than those in salinity-only (S) plants, paralleling the gene expression data. For instance, phenylalanine and caffeic acid levels increased by 50–60% under salinity but were reduced by 12.5% in S + Spm-treated plants. Rosmarinic acid and shikimic acid followed similar patterns. Ferulic acid accumulation, which is closely tied to caffeic acid O-methyltransferase (COMT) expression, showed a 14% reduction in the S + Spm group compared to salt-only plants, indicating Spm’s role in moderating metabolic flux. ABA levels also decreased by 25% under S + Spm treatment compared to salt stress, possibly due to reduced epoxidase expression. These results suggest that Spm fine-tunes the trade-off between defense metabolite accumulation and growth maintenance, enabling a more efficient stress adaptation strategy.
Accumulation of key metabolites associated with phenylpropanoid, shikimate, hormonal, and polyamine pathways in Plantago major under control, salinity (S), spermine (Spm), and combined (S + Spm) treatments. Metabolites include phenylalanine (A), caffeic acid (B), ferulic acid (C), rosmarinic acid (D), shikimic acid (E), abscisic acid (ABA) (F), and spermine content (G). Values represent means ± SE (n = 3). Different letters indicate statistically significant differences at p < 0.05 based on Tukey’s HSD test.
Hierarchical clustering and PCA analysis of traits and treatments
To visualize the global treatment effects on Plantago major, hierarchical clustering and PCA were employed using all measured traits. The heatmap analysis (Fig. 7) revealed two major treatment clusters: (i) Control and Spm, associated with robust growth, pigment stability, and low stress marker accumulation; and (ii) Salinity and S + Spm, characterized by elevated stress indicators and antioxidant responses. Notably, the S + Spm treatment positioned itself between the salt-only and control-like groups, signifying a partial recovery from stress.
Trait-level clustering showed three major functional categories: (1) Growth and water-related traits, (2) oxidative and osmotic stress markers, and (3) metabolic and gene expression traits. The S + Spm group displayed partial restoration of growth-related traits and moderate induction of stress responses—supporting the interpretation that spermine alleviates salinity damage without fully resetting the plant to control-like physiology. PCA further supported this pattern. The first two principal components (PC1 = 90%, PC2 = 9.1%) explained 99.1% of the overall variance (Fig. 8). Stress-related variables, such as proline, MDA, glycine betaine, and ABA, were strongly associated with the salinity group along PC1, whereas growth and pigment traits grouped with the control and Spm treatments on the negative axis. The S + Spm group again occupied an intermediate position, integrating features of both stress response and partial recovery. Notably, secondary metabolites and associated gene expression vectors (e.g., PAL, COMT, DAHPS) contributed prominently to the separation of this group, underscoring the role of metabolic flexibility in mediating spermine-driven tolerance.
Hierarchical clustering heatmap illustrating the global response of Plantago major to salinity (S), spermine (Spm), and their combined treatment (S + Spm), compared to control (C). The heatmap was generated using Z-score normalized data from all measured morphological, physiological, biochemical, enzymatic, molecular, and metabolic traits. Rows represent traits, and columns represent treatments. Clustering highlights distinct response modules and separates treatments based on phenotypic and metabolic similarities. Color intensity reflects relative trait expression levels.
Correlation analysis of morphological, physiological, biochemical, and metabolic traits
To elucidate the functional relationships among diverse traits modulated by salinity and spermine treatments in Plantago major, comprehensive correlation analyses were performed. These analyses provide a systems-level view of how various morphological, physiological, biochemical, and metabolic parameters interact under different treatment conditions. The Pearson correlation heatmap (Fig. 9a) revealed strong positive associations among vegetative growth traits, such as shoot and root length, leaf area, and fresh/dry biomass, underscoring their tight integration as indicators of plant vigor. Stomatal conductance and chlorophyll content also clustered with these growth traits, highlighting their shared contribution to photosynthetic efficiency and overall productivity. These parameters showed strong inverse correlations with classical stress indicators—including MDA (r = − 0.89 with shoot length; − 0.91 with dry biomass) and electrolyte leakage (EL)—reinforcing the physiological cost of oxidative and membrane damage under salt stress.
Stress-related osmolytes (proline and glycine betaine) displayed a robust mutual correlation (r = 0.93), and both correlated positively with MDA and ABA. This suggests that their upregulation is not only part of an osmotic adjustment strategy but also reflects systemic stress signaling. Interestingly, proline also showed negative associations with growth-related traits and pigment levels, supporting the idea that its overaccumulation—while protective—may coincide with growth suppression. Secondary metabolites such as total phenolics and flavonoids correlated positively with both osmoprotectants and antioxidant enzymes, suggesting coordinated induction of non-enzymatic defenses. Ascorbic acid displayed a somewhat distinct behavior, aligning more closely with RWC and chlorophyll b, indicating a central role in water balance and photoprotection.
On a molecular level, gene–metabolite correlations (Fig. 9b) illuminated key regulatory connections between transcriptional changes and biochemical outputs. PAL expression strongly correlated with phenylalanine (r = 0.98), caffeic acid (r = 0.94), and rosmarinic acid (r = 0.89), consistent with its gatekeeper role in the phenylpropanoid pathway. Similarly, DAHPS expression was positively linked to shikimic acid (r = 0.95) and rosmarinic acid, supporting its upstream control of aromatic amino acid biosynthesis and its integration into stress-induced secondary metabolism. COMT and HMGR expression correlated well with ferulic acid and caffeic acid, confirming their involvement in lignin biosynthesis and broader metabolic restructuring. Epoxidase and HMGR also showed moderate positive associations with ABA, linking transcriptional regulation to hormonal modulation under stress. Notably, spermine content itself exhibited strong positive correlations with DAHPS and COMT expression, suggesting that exogenous spermine may directly influence transcriptional regulation of protective biosynthetic pathways. These intercorrelations emphasize the tightly coupled nature of growth, stress response, and secondary metabolism. The data support a model where salinity disrupts physiological and biochemical homeostasis, triggering extensive compensatory responses. Spermine application appears to modulate these responses—limiting excessive accumulation of stress markers while maintaining sufficient defensive readiness. In this way, spermine reconfigures the internal trait network to favor metabolic balance and resilience.
(a) Heatmap showing Pearson correlation coefficients among morphological, physiological, and biochemical traits in Plantago major under various treatments. Positive correlations are shown in blue, negative correlations in red, with intensity indicating strength (r-values). Strong positive associations were observed among growth traits, while stress markers such as MDA and proline negatively correlated with photosynthetic and biomass parameters, highlighting the antagonistic interplay between growth and stress responses. (b) Heatmap of correlation analysis between gene expression levels (PAL, COMT, DAHPS, HMGR, Epoxidase) and corresponding metabolites (e.g., phenylalanine, caffeic acid, ferulic acid, rosmarinic acid, shikimic acid, ABA, spermine) in Plantago major. The analysis reveals strong gene–metabolite relationships, supporting transcriptional regulation of stress-induced secondary metabolic pathways. Color scale represents Pearson correlation coefficients (r), with positive and negative values indicating direct or inverse associations.
Radar chart-based comparative evaluation of treatments
To obtain a comprehensive visual overview of how Plantago major responded to salinity and spermine treatments across multiple biological levels, we constructed a radar chart incorporating all normalized traits—spanning morphology, physiology, biochemistry, enzymatic antioxidant activity, gene expression, and metabolite accumulation (Fig. 10). This approach enabled direct comparison of traits with varying scales and units, offering a multidimensional evaluation of treatment effects. Salinity-stressed plants (S) presented a distinct profile characterized by exaggerated activation of stress defense traits—most notably osmolyte accumulation (proline, glycine betaine), oxidative markers (MDA), abscisic acid (ABA), and enzymatic antioxidants (SOD, CAT, POD, APX, GR). However, this activation came at a significant cost to vegetative vigor and photosynthetic function. Shoot and root growth, fresh and dry biomass, chlorophyll pigments, stomatal conductance, and relative water content were all markedly suppressed, reflecting the classical growth–defense trade-off observed under abiotic stress conditions. In contrast, the spermine-only treatment (Spm) showed a profile that closely resembled the control group (C), with consistently high values across growth and physiological parameters. This indicates that spermine supports baseline metabolic activity and may precondition plants for improved stress resilience even in the absence of external stress. Interestingly, the Spm group also showed modest elevations in phenolic content, antioxidant enzyme activity, and gene expression (e.g., PAL, DAHPS), suggesting a priming effect that could enhance preparedness for potential stress exposure.
The most biologically favorable and strategically optimized profile emerged from the combined treatment (S + Spm). This group occupied a distinctive intermediate yet balanced space on the radar chart. Growth-related traits such as shoot/root length, biomass, and leaf area were substantially improved over the salinity-only group and approached control levels, reflecting effective mitigation of growth inhibition. Concurrently, stress defense traits including antioxidant enzyme activity, osmoprotectants, and secondary metabolite accumulation remained moderately elevated—sufficient to support defense, yet not excessively activated to compromise energy allocation. This integrated performance suggests that spermine application under salinity promotes a “controlled activation” model of stress response—where defense pathways are engaged to a degree that mitigates cellular damage without fully diverting resources away from growth and photosynthesis. Notably, gene expression and metabolite levels associated with the phenylpropanoid and shikimate pathways (e.g., PAL, COMT, DAHPS; phenylalanine, caffeic acid, rosmarinic acid) were well-represented in the S + Spm profile. This further reinforces the hypothesis that spermine modulates secondary metabolism and stress signaling in a dosage-sensitive and pathway-specific manner. Altogether, the radar chart provides a clear, integrated visualization of how exogenous spermine restructures plant responses at multiple levels—supporting the concept of spermine as a strategic biostimulant that harmonizes defense activation with developmental maintenance under salinity stress. The superior positioning of the S + Spm treatment across diverse trait clusters underscores its potential agronomic relevance for improving stress tolerance in medicinal and stress-adaptive species such as Plantago major.
Integrated radar chart summarizing normalized values (0–1 scale) of all measured traits in Plantago major across four treatments: control (C), salinity (S), spermine (Spm), and salinity + spermine (S + Spm). The circular plot visualizes multidimensional treatment performance, with S + Spm showing the most balanced response—combining stress tolerance (e.g., higher antioxidant activity and osmolyte accumulation) with improved growth, pigment retention, and metabolic adjustment. This chart highlights the comprehensive mitigating effect of spermine under salt stress.
Collectively, the data underscore a tightly coordinated response across morphological, physiological, biochemical, and molecular levels. The partial restoration of growth in S + Spm-treated plants was not due to the suppression of stress responses, but rather to their strategic modulation. Spermine fine-tuned the expression of genes involved in antioxidant and secondary metabolism, adjusted the accumulation of osmoprotectants and stress hormones, and restrained ROS production through a more economical deployment of enzymatic antioxidants. This metabolic rebalancing enabled the preservation of photosynthetic efficiency and turgor, thereby supporting continued biomass accumulation. These interlinked processes—revealed through clustering, PCA, and correlation analyses—highlight spermine’s central role in reestablishing metabolic flexibility and optimizing the growth–defense trade-off under salinity stress.
Discussion
Salinity stress is a major abiotic constraint that severely limits plant growth and productivity by inducing osmotic imbalance, ionic toxicity, and oxidative stress, ultimately disrupting key metabolic processes18,19. In this study, we investigated the protective role of exogenously applied spermine, a polyamine known for its stress-mitigating properties, in Plantago major under saline conditions. Through a comprehensive integration of morphological, physiological, biochemical, enzymatic, molecular, and metabolomic data, we demonstrated that spermine orchestrates a multifaceted response that significantly alleviates salinity-induced damage. Morphologically, salinity resulted in pronounced reductions in shoot and root length, leaf area, and biomass accumulation, in line with previous studies attributing these effects to impaired water uptake and ion toxicity20. Remarkably, spermine—especially under the combined salinity and spermine (S + Spm) treatment—partially restored these traits. The observed recovery suggests that spermine contributes to sustained turgor and cell expansion, possibly through osmotic regulation and modulation of hormonal balance21. At the physiological level, salinity reduced relative water content (RWC), pigment concentrations, and stomatal conductance, while markedly increasing electrolyte leakage (EL), an indicator of membrane destabilization22,23. Spermine application mitigated these effects, significantly improving RWC and pigment levels, and reducing EL24,25. These findings suggest that spermine stabilizes cellular membranes and protects chloroplast integrity, consistent with its reported roles in other plant systems26. The enhanced stomatal conductance observed under Spm and S + Spm treatments also points to improved gas exchange efficiency, essential for sustained photosynthesis under stress27.
Biochemically, salinity triggered the accumulation of osmolytes such as proline and glycine betaine, and increased MDA levels, reflecting osmotic imbalance and lipid peroxidation28. Spermine treatment moderated the accumulation of these compounds, lowering MDA content by over 40% in the S + Spm group and partially reducing osmolyte levels29. In parallel, the levels of ascorbic acid, total phenolics, and flavonoids were elevated in spermine-treated plants, indicating a stronger non-enzymatic antioxidant capacity30. These compounds not only scavenge ROS but also act as signaling molecules in stress adaptation31. Enzymatically, salinity strongly upregulated activities of SOD, CAT, POD, APX, and GR, reflecting the plant’s effort to detoxify ROS32. In S + Spm-treated plants, enzyme activities remained elevated compared to the control but were significantly lower than those in salt-only treatments33. This controlled activation implies that spermine moderates oxidative stress while conserving metabolic resources, thereby enabling effective redox regulation without unnecessary energetic expense34.
At the transcriptional level, genes involved in secondary metabolism (PAL, COMT, DAHPS, HMGR, and Epoxidase) were markedly upregulated under salt stress35. These genes play central roles in the biosynthesis of defense-related metabolites, including phenylpropanoids, terpenoids, and abscisic acid36. In the S + Spm group, gene expression was moderated—higher than control but significantly reduced compared to salt-only treatments—indicating spermine-induced transcriptional rebalancing37. This pattern was closely reflected in metabolite levels: compounds such as phenylalanine, caffeic acid, ferulic acid, rosmarinic acid, and shikimic acid showed similar moderated accumulation in the S + Spm group29. These trends indicate that spermine fine-tunes metabolic fluxes, maintaining defense without excessive resource diversion.
Multivariate analyses reinforced this interpretation. Principal component analysis (PCA) showed clear separation among treatments, with PC1 and PC2 capturing major trait variance. While salt-stressed plants clustered with high levels of MDA, ABA, and osmolytes, the S + Spm group occupied an intermediate position, combining improved growth with balanced stress responses. Key contributors to separation included antioxidant enzyme activity, pigment levels, and secondary metabolite content21. The radar chart further illustrated the integrated response under S + Spm treatment, which displayed the most balanced trait distribution of all treatments—indicating effective metabolic coordination38. Correlation analysis supported these findings by revealing strong positive associations between growth parameters and chlorophyll content, and negative correlations with EL and MDA. Additionally, osmoprotectants, antioxidant enzymes, and stress-related gene expression formed tightly connected clusters, suggesting linked regulatory pathways. Notably, strong gene–metabolite associations—such as between PAL and phenolic compounds—highlight the transcriptional regulation underlying metabolic adjustments35. In summary, our results show that spermine enhances salinity tolerance in Plantago major through a highly coordinated mechanism involving improved growth, stabilized membranes, optimized osmotic and redox status, and transcriptional–metabolic balance. Spermine-treated plants exhibit not merely reduced stress symptoms, but a restructured stress phenotype characterized by resilience, metabolic economy, and growth restoration. These insights contribute to the understanding of polyamines as central regulators of stress adaptation and may inform strategies for enhancing abiotic stress tolerance in medicinal and non-model crops.
Conclusion
This study provides robust evidence that exogenous application of spermine significantly enhances the salinity tolerance of Plantago major by orchestrating a complex network of physiological, biochemical, and molecular responses. Under saline conditions, P. major exhibited pronounced growth inhibition, pigment degradation, membrane destabilization, and oxidative stress—hallmarks of impaired cellular function due to ionic and osmotic imbalances. However, foliar supplementation with spermine, particularly in the S + Spm treatment, effectively mitigated these deleterious effects. Improvements in relative water content, membrane integrity, and photosynthetic pigment retention, alongside reductions in malondialdehyde accumulation and electrolyte leakage, underscore spermine’s role in maintaining cellular homeostasis. Beyond physiological improvements, spermine elicited significant modulation of antioxidant defense systems and secondary metabolism. The coordinated enhancement of both enzymatic (SOD, CAT, POD, APX, GR) and non-enzymatic (ascorbic acid, phenolics, flavonoids) antioxidant components contributed to oxidative stress mitigation. At the molecular level, spermine altered the expression of key genes in the phenylpropanoid and shikimate pathways, which was mirrored by the accumulation of protective metabolites such as caffeic acid, rosmarinic acid, and shikimic acid. These findings suggest that spermine not only primes defense mechanisms but also reconfigures metabolic pathways to enhance stress resilience. Multivariate analyses—including PCA, hierarchical clustering, and radar visualization—clearly distinguished the S + Spm treatment as a unique and optimized response, balancing growth recovery with stress adaptation. The integration of these datasets highlights spermine’s dual function as both a growth regulator and a metabolic modulator. In conclusion, spermine emerges as a promising, environmentally friendly biostimulant capable of improving salinity tolerance in P. major, with potential applications in the cultivation of medicinal and aromatic plants in salt-affected soils. Future research integrating transcriptomic and proteomic analyses, coupled with field trials, will be essential to validate and harness spermine’s full potential for sustainable crop management under saline conditions.
Data availability
Data used during the preparation of this manuscript is available within the article.
References
Hussain, S. et al. Salinity stress in arid and semi-arid climates: effects and management in field crops. in Climate Change and Agriculture (ed. Hussain, S.), (IntechOpen, (2019).
El Sabagh, A. et al. Consequences of salinity stress on the quality of crops and its mitigation strategies for sustainable crop production: an outlook of arid and semi-arid regions. in Environment, Climate, Plant and Vegetation Growth (eds. Fahad, S. et al.), 503–533 (Springer, (2020).
Hameed, A. et al. Effects of salinity stress on Chloroplast structure and function. Cells 10, 2023 (2021).
Hasanuzzaman, M. et al. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 22, 9326 (2021).
Raziq, A. et al. Exogenous spermidine modulates polyamine metabolism and improves stress responsive mechanisms to protect tomato seedlings against salt stress. Plant Physiol. Biochem. 187, 1–10 (2022).
Blázquez, M. A. & Polyamines Their role in plant development and stress. Annual Rev. Plant. Biology 75, 95–117 (2024).
Zhang, Y. et al. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 17, 2876–2890 (2021).
Keivani, M., Mehregan, I. & Albach, D. C. Evaluating morphological diversity among Plantago major L. populations and influence of ecological variables. Biologia 76, 1127–1139 (2021).
Davkova, I. et al. Insights from UHPLC-Orbitrap MS analysis of Plantago major as a potential herbal drug in wound healing. in The 12th Conference on Medicinal and Aromatic Plants of Southeast European Countries (eds. Ekeroğlu, N. et al.), 17–19 October 2024, İzmir-Türkiye, 58 (2024).
Siadeni, E. R., Kumleh, H. H. & Rezadoost, M. H. Secondary metabolites induction in plantain (Plantago major L.) via abiotic stresses in liquid medium. Plant. Cell. Tissue Organ. Cult. (PCTOC). 154, 493–505 (2023).
Sudhakar, P., Latha, P. & Reddy, P. Phenotyping crop plants for physiological and biochemical traitsAcademic Press, (2016).
Bates, L. S., Waldren, R. & Teare, I. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207 (1973).
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Grieve, C. & Grattan, S. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant. Soil. 70, 303–307 (1983).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198 (1968).
Mukherjee, S. & Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 58, 166–170 (1983).
Flores, H. E. & Galston, A. W. Polyamines and plant stress: activation of Putrescine biosynthesis by osmotic shock. Science 217, 1259–1261 (1982).
Khalifani, S. et al. Advanced computational approaches for predicting sunflower yield: insights from ANN, ANFIS, and GEP in normal and salinity stress environments. PLOS ONE. 20, e0319331 (2025).
Kordrostami, M. & Mafakheri, M. Consequences of water stress and salinity on plants/crops: physiobiochemical and molecular mitigation approaches. in Handbook of Plant and Crop Physiology (ed. Pessarakli, M.), 789–814 (CRC, (2021).
Peng, Y. et al. Revisiting the role of light signaling in plant responses to salt stress. Hortic. Res. 12, uhae262 (2025).
Hasan, M. M. et al. Spermine: its emerging role in regulating drought stress responses in plants. Cells 10, 261 (2021).
da Silva, T. I. et al. Spermine reduces the harmful effects of drought stress in Tropaeolum majus. Sci. Hort. 304, 111339 (2022).
Singh, P. et al. Silicon supplementation alleviates the salinity stress in wheat plants by enhancing the plant water status, photosynthetic pigments, proline content and antioxidant enzyme activities. Plants 11, 2525 (2022).
Alijani, S., Raji, M. R., Bistgani, Z. E., Ehtesham Nia, A. & Farajpour, M. Mitigation of salinity stress in Yarrow (Achillea millefolium L.) plants through spermidine application. PLOS ONE. 19, e0304831 (2024).
Talaat, N. B., Mahmoud, A. W. M. & Hanafy, A. M. Co-application of Salicylic acid and spermine alleviates salt stress toxicity in wheat: growth, nutrient acquisition, osmolytes accumulation, and antioxidant response. Acta Physiol. Plant. 45, 1 (2023).
Alcázar, R. et al. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249 (2010).
He, X., Hao, J., Fan, S., Liu, C. & Han, Y. Role of spermidine in photosynthesis and polyamine metabolism in lettuce seedlings under high-temperature stress. Plants 11, 1385 (2022).
Hossain, A. et al. Emerging roles of osmoprotectant glycine betaine against salt-induced oxidative stress in plants: A major outlook of maize (Zea mays L.). in Frontiers in Plant-Soil Interaction: Molecular Insights Into Plant Adaptation (eds. Tariq, A. & Hakeem, K.R.), 567–587 (Academic, (2021).
Wang, W., Kang, W., Shi, S. & Liu, L. Physiological and metabolomic analyses reveal the mechanism by which exogenous spermine improves drought resistance in alfalfa leaves (Medicago sativa L). Front. Plant Sci. 15, 1466493 (2024).
Heydari, F., Raji, M. R., Nejad, A. R., Aalifar, M. & Mumivand, H. Spermine mitigates the adverse effects of water deficit by strengthening antioxidant enzymes and anthocyanin pathway-related gene expressions. Ind. Crops Prod. 200, 116910 (2023).
Rao, M. & Zheng, B. The role of polyphenols in abiotic stress tolerance and their antioxidant properties to scavenge reactive oxygen species and free radicals. Antioxidants 14, 74 (2025).
van Janse, H. C. & Limami, A. M. Van Den ende, W. Spermine and spermidine priming against Botrytis cinerea modulates ROS dynamics and metabolism in Arabidopsis. Biomolecules 11, 223 (2021).
Korbas, A., Kubiś, J., Rybus-Zając, M. & Chadzinikolau, T. Spermidine modify antioxidant activity in cucumber exposed to salinity stress. Agronomy 12, 1554 (2022).
Yadav, P., Ansari, M. W. & Tuteja, N. Role of chemical additives in plant salinity stress mitigation. in Global Climate Change and Plant Stress Management (eds. Ansari, M.W., Singh, A.K & Narendra, T.), 371–381 (Wiley, (2023).
Qari, S. H. & Tarbiyyah, I. The genetic regulation of secondary metabolic pathways in response to salinity and drought as abiotic stresses. Appl. Sci. 11, 6668 (2021).
Kordrostami, M., Sanjarian, F., Shahbazi, S. & Ghasemi-Soloklui, A. A. Exploring low-dose gamma radiation effects on monoterpene biosynthesis in Thymus vulgaris: insights into plant defense mechanisms. Environ. Sci. Pollut. Res. 31, 32842–32862 (2024).
Kolesnikov, Y. S. et al. Polyamines metabolism and their biological role in plant cells: what do we really know? Phytochem. Rev. 23, 997–1026 (2024).
Soroori, S., Danaee, E., Hemmati, K. & Ladan Moghadam, A. Effect of spermidine foliar application on some morphophysiological traits and secondary metabolites of marigold (Calendula officinalis L.) under drought stress. J. Plant. Environ. Physiol. 17, 108–125 (2022).
Funding
This work has received no funding.
Author information
Authors and Affiliations
Contributions
MMH, and MK: conceived and designed the project; MMH, MK, FG, and MR: analyzed, wrote, revised and proofread the manuscript. All authors contributed to the article and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study adhered to all relevant institutional, national, and international guidelines and legislation in Iran. Plant materials were collected from the Pakan Bazr Company with permission from the appropriate institutional authority. The plant species were formally identified by Dr. Mojtaba Kordrostami, and voucher specimens have been deposited in Herbarium of The Graduate University of Advanced Technology under deposition number [Voucher Number (505)]. No special permissions were required for sample collection beyond institutional approval, and all plant materials used in the study complied with Iranian regulations and legislation. This study did not involve clinical trials or human/animal participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hossain, M.M., Kordrostami, M., Goto, F. et al. Spermine treatment improves salinity tolerance in Plantago major by altering growth parameters, biochemical profiles and gene expression. Sci Rep 15, 25762 (2025). https://doi.org/10.1038/s41598-025-11903-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11903-0