Abstract
Currently, no consensus exists on optimal treatment for post-endoscopic submucosal dissection (ESD) gastric ulcer. This study aims to compare rebamipide and tegoprazan combination therapy with tegoprazan monotherapy for post-ESD ulcer healing. Between May 2022 and September 2023, 140 patients (141 lesions) who underwent ESD for gastric epithelial neoplasms at five tertiary hospitals were randomly assigned to tegoprazan monotherapy or combination therapy groups. The size, stage and healing quality of the post-ESD ulcers were assessed at weeks 4 and 8. Modified intention-to-treat (mITT) and per-protocol analyses included 132 and 121 lesions, respectively. The ulcer healing rate at week 4 was significantly higher in the combination therapy group than in the tegoprazan monotherapy group (96.4% vs. 93.6%, P = 0.02 in mITT). Combination therapy significantly predicted higher-than-average ulcer healing at week 4 (odds ratio 2.28, 95% confidence interval 1.04–5.02, P = 0.04). The proportion of flat ulcer scar at week 8 was significantly higher in the combination group than in the tegoprazan monotherapy group (73.8% vs. 48.4%, P = 0.007). Combination treatment with rebamipide and tegoprazan led to faster ulcer healing and the development of high-quality post-ESD scars compared to tegoprazan monotherapy.
Similar content being viewed by others
Introduction
The global adoption of endoscopic submucosal dissection (ESD) for gastric neoplasms has positioned it as a preferred method due to its advantages, including rapid recovery, short hospital stay, and cost-effectiveness1. Moreover, as well as a high rate of en bloc resection and curative resection regardless of the size and location of lesions, along with comparable long-term clinical outcomes to surgical resection, making it the preferred treatment for early gastric cancer or precancerous lesions2,3,4. However, ESD inevitably induces large or deep ulcers, leading to potential complications such as bleeding and perforation5,6.
The management of post-ESD ulcers mirrors that of peptic ulcer disease, necessitating the suppression of gastric acid secretion and maintenance of a high pH to prevent bleeding and facilitate mucosal healing. Although proton-pump inhibitors (PPIs) are potent acid-suppressive agents commonly used for post-ESD ulcer healing, studies suggest that PPI monotherapy may not suffice for post-ESD ulcers7. Mucoprotective agents act by reducing oxygen radicals, increasing blood flow, and promoting the production of protective prostaglandins in ulcer mucosa, thereby accelerating ulcer healing. Previous research has specifically highlighted the benefits of combining rebamipide with PPIs for post-ESD ulcer healing and bleeding prevention compared to PPI monotherapy8,9,10,11,12,13.
Recently, potassium-competitive acid blockers (P-CABs) have gained popularity due to their faster onset and longer duration of action than PPIs, along with non-inferior efficacy14,15. However, while P-CABs have demonstrated superiority in preventing post-ESD ulcer bleeding compared to PPIs, their efficacy in post-ESD ulcer healing remains debated16,17,18,19,20,21. Moreover, it is unclear whether combining rebamipide with P-CABs enhances ulcer healing compared to P-CAB monotherapy. Tegoprazan, a novel P-CAB approved in South Korea in 2018, is widely prescribed for gastroesophageal reflux disease, peptic ulcer disease, and Helicobacter pylori (H. pylori) eradication22. In this study, we aim to compare the effects of combining rebamipide with tegoprazan versus tegoprazan monotherapy on post-ESD ulcer.
Results
Clinical characteristics of study patients
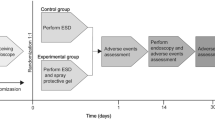
A total of 140 patients (141 lesions) were randomly allocated to either the combination therapy group (70 lesions) or the tegoprazan monotherapy group (71 lesions). Among them, nine patients dropped out, resulting in 131 patients (132 lesions) being eligible for mITT analysis. During the study period, 11 patients were excluded due to < 80% drug compliance, leaving 120 patients (121 lesions) for the PP analysis. A detailed flowchart of the study is shown in Fig. 1.
Representative images for quality of post-ESD ulcer scar at 8 weeks after ESD: flat scar (a), intermediate scar (b), and nodular scar (c). Flat scars were defined as lesions with smooth mucosal healing without any elevation; nodular scars exhibited one or more elevated nodular components at the ulcer base or margin; intermediate scars were characterized by ambiguous features that did not fully meet the criteria for either flat or nodular types. ESD, endoscopic submucosal dissection.
The baseline characteristics of the study population are shown in Table 1. Patient demographics, including age, sex, body mass index (BMI), and underlying conditions such as diabetes and hypertension, were comparable between the two groups. Additionally, there were no significant differences in the endoscopic factors (histopathology, lesion location, tumor with fibrosis, long lesion diameter, specimen size, and depth of submucosal invasion) or procedure-related factors (en bloc resection rate, complete resection rate, and procedure time). H. pylori infection and gastrointestinal symptoms did not significantly differ between the two groups.
Post-ESD ulcer healing at 4 weeks and 8 weeks after ESD
Table 2 presents the healing rates and stages of post-ESD ulcers at 4 and 8 weeks post-ESD. In the mITT analysis, although the initial post-ESD ulcer area was significantly larger in the combination therapy group than in the tegoprazan monotherapy group (1064.9 mm2 vs. 889.7 mm2, P = 0.05), the post-ESD ulcer area decreased more significantly in the combination therapy group at week 4 (1020.3 mm2 vs. 838.3 mm2, P = 0.03). The mean healing rate at week 4 (96.4% vs. 93.6%, P = 0.02) was significantly higher in the combination therapy group than in tegoprazan monotherapy group in the mITT analysis. These results were consistent in PP analysis (1017.1 mm2 vs. 838.4 mm2, P = 0.04 and 96.2% vs. 93.6%, P = 0.05). Although three patients in the tegoprazan monotherapy group remained in the active stage, there was no significant difference in the proportion of the post-ESD ulcer stage at week 4 between the two groups in both the mITT and PP analyses.
At 8 weeks post-ESD, although the post-ESD ulcer area decreased more in the combination therapy group than in tegoprazan monotherapy group (1071.6 mm2 vs. 886.4 mm2, P = 0.04 in mITT analysis; 1073.3 mm2 vs. 886.3 mm2, P = 0.05 in PP analysis), the mean healing rate was comparable between the two groups. The post-ESD ulcer in scar stage in the combination therapy group at week 8 was higher than that in tegoprazan monotherapy group, although statistical significance was not reached (88.5% vs. 85.9%, P = 0.79 in mITT analysis; 88.7% vs. 85.2%, P = 0.78 in PP analysis).
Predictors for a higher rate of post-ESD ulcer healing at 4 weeks after ESD
To identify independent predictors of a higher rate of post-ESD ulcer healing compared to the mean healing rate (94.9% at week 4), logistic regression analysis was conducted (Table 3). In the multivariate analysis, male sex (odds ratio [OR] 0.40, 95% confidence interval [CI] 0.16–0.95, P = 0.04) and the presence of diabetes (OR 0.27, 95% CI 0.09–0.81, P = 0.02) were identified as significant negative predictors. Additionally, the combination therapy of rebamipide and tegoprazan was identified as a significant positive predictor for a higher rate of ulcer healing at week 4 (OR 2.28, 95% CI 1.04–5.02, P = 0.04).
Post-ESD delayed bleeding, spontaneous bleeding and clinical symptoms
The post-ESD delayed bleeding was observed in one patients in combination treatment group during the follow-up period (1.4% vs. 0%, P = 0.50). On follow-up endoscopy, spontaneous bleeding at week 4 was significantly lower in the combination therapy group than in the tegoprazan monotherapy group, both in mITT (6.2% vs. 36.8%, P < 0.001) and PP analyses (3.6% vs. 36.9%, P < 0.001). However, a significant difference in spontaneous bleeding was no longer observed at week 8 in both mITT and PP analyses (Table 2).
The clinical symptom scores of the combination therapy and tegoprazan monotherapy groups were not significantly different at baseline (1.79 vs. 1.97, P = 0.70) (Table 1). This was consistently observed at week 4 (0.86 vs. 1.00, P = 0.59 and 0.96 vs. 1.00, P = 0.90) and week 8 (0.59 vs. 0.78, P = 0.62 and 0.66 vs. 0.79, P = 0.57) in both mITT and PP analysis (Table 2).
Quality of ulcer healing at 8 weeks after ESD
Assessment of the quality of ulcer healing at week 8, conducted by two independent reviewers, revealed a significantly higher prevalence of flat-type post-ESD ulcers and ulcer scars in the combination therapy group compared to the tegoprazan monotherapy group, irrespective of the reviewer (Table 4). The Cohen’s kappa coefficient, which determined the agreement between the two endoscopists on the quality of ulcer healing, was 0.862 at week 8.
Safety assessment
During the study period, five cases of TEAE were reported by five patients, and there were no serious adverse events except one post-ESD delayed bleeding in either group. Two cases of TEAE (2.8%) were reported in the tegoprazan monotherapy group, consisting of headache and abdominal distension. The other two cases of TEAE (2.9%) reported in the combination group were lumbar sprain and finger fracture; however, investigators did not consider these events to be related to the study drug. There was no statistically significant between the two groups (P < 0.999).
Discussion
This multicenter, randomized controlled trial evaluated the effectiveness of combining rebamipide, a mucoprotective agent, with P-CAB for post-ESD ulcer healing. In this study, combination therapy was associated with a higher rate of ulcer healing at week 4 and resulted in a higher prevalence of flat-type post-ESD ulcers and ulcer scars at 8 weeks after ESD. These results suggest that the combination of rebamipide and P-CAB contributes to rapid post-ESD ulcer healing and the production of high-quality post-ESD scars.
Although controlling gastric pH using PPIs and P-CABs is mainstay for post-ESD ulcer healing, there is currently no consensus on the optimal treatment duration or drug regimen. Previous studies have demonstrated the efficacy of combining rebamipide with PPI for post-ESD ulcer healing compared to PPI alone at weeks 4 or 87,8,9,10,11. However, until now, the effect of rebamipide in combination with P-CAB on post-ESD ulcer healing has rarely been evaluated. In present study, combination therapy showed a high rate of post-ESD ulcer healing at week 4 (> 96%) and was an independent predictive of a high ulcer healing rate. These findings suggest that rebamipide has the potential to stimulate post-ESD ulcer healing. And, this aligns with previous study demonstrating the superiority of rebamipide and PPI combination treatment on 4-week ESD-induced ulcer healing8. The faster ulcer shrinkage effect of rebamipide may be attributed to its ability to promote mucosal regeneration through multiple mechanisms. It restores Sonic Hedgehog signaling, which is essential for epithelial cell proliferation, and enhances prostaglandin E₂ production via COX-2 upregulation, thereby increasing mucus secretion and improving mucosal blood flow23,24,25. Additionally, rebamipide stimulates the expression of vascular endothelial growth factor and other angiogenic factors, promoting neovascularization and tissue remodeling26. These coordinated actions contribute to accelerated ulcer healing and may improve scar quality after ESD.
In contrast, the combination of rebamipide and tegoprazan was not superior in changing the post-ESD ulcer stage. In our earlier study, the scar stage of post-ESD ulcers at week 4 was significantly higher in the rebamipide combination therapy group than in the PPI monotherapy group8. Although the healing process of post-ESD ulcers differs somewhat from that of peptic ulcer disease, ulcer size is the most crucial factor affecting post-ESD ulcer healing27. In this study, despite a larger ulcer size in the combination therapy group, no patients remained in the active stage at 4 weeks after ESD, unlike the tegoprazan monotherapy group, in which three patients had active stage ulcers. The scar stage in the combination group at week 8 was higher than that in the monotherapy group, although the difference was not statistically significant. Additionally, while the superiority of P-CABs in post-ESD ulcer healing remains controversial, they are considered more potent and effective. In this study, only P-CAB therapy resulted in a high rate of post-ESD ulcer healing at 4 and 8 weeks after ESD. Compared with our previous study, the tegoprazan monotherapy group showed a higher healing rate of post-ESD ulcers than the PPI monotherapy group8. These factors may have contributed to these outcomes.
Delayed post-ESD bleeding is the most common and serious complication of ESD. The most effective strategy for preventing bleeding from an artificial ulcer after ESD involves promoting rapid recovery from mechanical and artificial gastric mucosal wounds. Previous studies have demonstrated that vonoprazan, a P-CAB, effectively prevents post-ESD ulcer bleeding, attributed to its potent and long-lasting gastric acid suppression7,16. Although the low incidence of delayed bleeding in this study limited the ability to directly evaluate the effect of rebamipide combination therapy on post-ESD bleeding, the observed modest improvement in ulcer healing at week 4 suggests potential clinical relevance. Given that most post-ESD ulcer bleeding occurs within the first two weeks, this early enhancement in mucosal healing may indicate that rebamipide plays a beneficial role in reducing delayed bleeding through accelerated tissue repair29. Further large-scale studies are warranted to more clearly elucidate the potential preventive effects of combination therapy on post-ESD ulcer bleeding.
The reported rates of locally recurrent EGC after ESD range from 0.7 to 3.7%28. Therefore, follow-up surveillance endoscopy is periodically performed to confirm the healing of post-ESD ulcers and detect the local recurrences. Although most post-ESD ulcers typically displayed a flat scar, irregular scars with abnormally nodular features were presented in 0.15–13.3% of patients, raising clinical dilemmas that can be confused with tumor recurrence29,30. Previous studies identified factors affecting the type of scar, reporting that antral tumor location, small size of the post-ESD ulcer, and PPI treatment predict nodular-type post-ESD scars, while H. pylori infection may be associated with a flat scar30,31,32,33. In this study, the proportion of flat-type ulcer scar at week 8 was significantly higher in the combination group than tegoprazan monotherapy group, suggesting that combination treatment could reduce the midjudgment of non-flat post-ESD scars as tumor recurrence and subsequently prevent unnessesary biospies for confirmation. Additionally, rebamipide additive treatment could be beneficial for patients with risk factors for non-flat post-ESD ulcer scar development, such as H. pylori-negative or antrally located gastric neoplasms.
This study had several limitations that should be considered in its interpretation. First, despite its prospective, randomized design, the sample size was relatively small, and there could be potential hidden confounding factors that were not identified. In addition, the open labelled design of the study could have influenced the assessment of study outcomes such as ulcer scar evaluation, introducing potential biases including performance or assessment bias. Second, because this study was conducted only in the Korean population, the findings may not be directly applicable to Western patients, who could have different rates of H. pylori infection and different degrees of atrophic gastritis in the Asian population. Third, only one case of post-ESD bleeding after administration of the study medication was observed in the present study, which limited our ability to evaluate the effect of combination therapy on post-ESD bleeding. In our study, 116 patients who underwent second-look endoscopy, and among them, 9 patients required endoscopic hemostasis: 8.5% (5/59) in the tegoprazan monotherapy group and 7.0% (4/57) in the combination therapy group. Although second-look endoscopic hemostasis did not influence ulcer healing outcomes, it may have contributed to the low incidence of post-ESD ulcer bleeding observed in this study, along with the relatively small sample size.
In conclusion, the combination treatment with tegoprazan and rebamipide resulted in rapid post-ESD ulcer healing and improved ulcer scar quality. This combination therapy may offer benefits, particularly in patients with a high risk of bleeding, and reduce the risk of misclassification of post-ESD tumor recurrences. Further studies with larger patient cohorts, especially those with a higher risk of post-ESD bleeding, are needed to comprehensively evaluate the efficacy of rebamipide and P-CAB combination treatment on post-ESD outcomes.
Methods
Study design
This prospective, open-label, randomized, multicenter study enrolled patients requiring ESD for the treatment of gastric epithelial neoplasms between May 2022 and September 2023 at five Hallym University Affiliated Hospitals: Chuncheon Sacred Heart Hospital, Dongtan Sacred Heart Hospital, Hallym University Sacred Heart Hospital, Kangdong Sacred Heart Hospital, and Kangnam Sacred Heart Hospital. This clinical trial was prospectively registered with the Clinical Research Information Service (CRIS), a primary registry of the WHO International Clinical Trials Registry Platform, under the identifier KCT0007063 on 08/03/2022. The enrolled patients were randomly assigned to either the tegoprazan monotherapy group or the rebamipide and tegoprazan combination therapy group for 8 weeks. Follow-up endoscopy was performed at 4 ± 1 weeks and 8 ± 1 weeks after ESD to assess the stage and size of post-ESD ulcers and the quality of ulcer healing. This study was conducted in accordance with the Declaration of Helsinki and relevant institutional guidelines and regulations. All experimental protocols were reviewed and approved by the Institutional Review Boards of the participating Hospitals (IRB No. 2022-03-006, 2022-02-009, 2022-02-018, 2021-12-011, HKS 2022-06-018). Written informed consent was obtained from all participants prior to study enrollment.
Study population
Patients meeting the following criteria were included: (1) aged between 19 and 85 years, (2) histologically proven gastric adenoma or carcinoma eligible for ESD, and (3) willingness to participate in the study with voluntary signing of a written informed consent. Patients meeting any of the following criteria were excluded: (1) known hypersensitivity to any component of rebamipide or tegoprazan, (2) abnormal values in laboratory tests: serum total bilirubin or creatinine > 1.5 times the upper limit of normal, serum alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and blood urea nitrogen (BUN) > 2 times the upper limit of normal, (3) history of malignancy other than gastric cancer within the last 5 years, (4) history of the following diseases: Zollinger-Ellison syndrome, Barrett’s esophagus, primary esophageal motility abnormality, esophageal strictures, pancreatitis, and malabsorption, (5) history of major surgery that may affect gastric acid secretion, (6) inability to discontinue drugs affecting the healing of post-ESD ulcer (warfarin, clopidogrel, direct oral anticoagulant, steroid, nonsteroidal anti-inflammatory drugs, anticholinergics, prostaglandin analogs, sucralfate, and aspirin) until the end of the clinical trial, (7) severe organ failure (such as liver dysfunction, and renal dysfunction), (8) pregnant and/or lactating women, and (9) patients deemed unsuitable for participation in this study by researchers.
Randomization and follow-up
Eligible patients undergoing ESD were screened to determine their eligibility based on inclusion and exclusion criteria. ESD procedures were conducted by experienced endoscopists following a standardized protocol8. Afterward, pantoprazole was administered intravenously twice daily from the day of the procedure (day 0) to the day of discharge (day 2). A second-look endoscopy was performed one or two days after ESD at the discretion of the physician and endoscopic hemostasis was conducted if necessary. Upon discharge, patients were randomly assigned to either the tegoprazan monotherapy group (tegoprazan 50 mg once daily for 8 weeks) or the rebamipide and tegoprazan combination therapy group (rebamipide 100 mg three times daily and tegoprazan 50 mg once daily for 8 weeks) using a random number chart.
Follow-up endoscopy was scheduled at 4 ± 1 weeks and 8 ± 1 weeks post-ESD to assess ulcer healing and stage. Drug compliance and adverse events were monitored during the follow-up endoscopy. H. pylori infection was assessed before ESD by histological examination, rapid urease test, and/or C13-urea breath test, with positivity defined as at least one positive result. H. pylori-positive patients received eradication treatment at the end of the study. Additionally, all patients completed the modified Korean form of the Gastrointestinal Symptom Rating Scale (KGSRS) at enrollment, 4 ± 1 weeks, and 8 ± 1 weeks to evaluate changes in clinical symptoms after ESD34,35.
Study outcomes and safety assessment
The primary outcome was the ulcer healing rate at 4 weeks post-ESD. The ulcer healing rate was defined as [(initial ulcer area—ulcer area at week 4 or 8) × 100/initial ulcer area]. Ulcer areas were calculated by multiplying the maximal diameter by the largest diameter perpendicular to the maximal diameter, measured using the biopsy forceps. The secondary outcomes were the ulcer healing rate at 8 weeks, change in ulcer stage at 4 and 8 weeks post-ESD, delayed post-ESD bleeding, quality of ulcer healing on follow-up endoscopic examination, and KGSRS during the follow-up period. The ulcer stage was assessed based on the classification of Sakita and Fukutomi, categorized as active (A1, A2), healing (H1, H2), and scarring (S1, S2) stages. Post-ESD delayed bleeding was defined as the occurrence of active hemorrhage in the post-ESD ulcer from day 2 to day 56 that meets one of the following criteria: (i) obvious bleeding signs such as hematemesis, melena, or hematochezia requiring endoscopic intervention, (ii) hypovolemic shock (systolic blood pressure ≤ 90mm Hg or pulse rate ≥ 110/min) with bleeding symptoms, (iii) hemoglobin (Hb) decrease > 2g/dl, or hematocrit decrease > 6% and Hb < 10 g/dl, (iv) identification of active hemorrhage, exposed vessels, or blood clot on endoscopic examination after ESD. Spontaneous bleeding was defined as minor oozing observed in post-ESD ulcers observed after air inflation during follow-up endoscopy, which was self-limited and did not require any hemostatic intervention. Two endoscopic experts (H.L. and Y.J. Y.) assessed the quality of ulcer healing, categorizing it as flat, nodular, or intermediate lesions, as previously described (Fig. 2)8. Any discrepancies between the two reviewers were interpreted as intermediate in the final assessment. To minimize assessment bias, the reviewers were blinded to treatment assignment and assessed only anonymized pre- and post-ESD endoscopic images, with no access to additional clinical data. The total score for each parameter in the KGSRS ranges from 0 to 39, with a higher score suggesting more severe gastrointestinal symptoms.
All safety assessments, including the frequency and severity of adverse events, clinical laboratory evaluations, vital sign measurements, and physical examination findings, were monitored throughout the study. Treatment-emergent adverse events (TEAE) were defined as those that occurred during treatment. Serious adverse events were defined as adverse events that could cause death, life-threatening adverse events, hospitalization, disability, or congenital anomalies, and were coded by system organ class and preferred term using MedDRA. Safety assessments included monitoring adverse events, clinical laboratory evaluations, and vital signs throughout the study.
Sample size and statistical analysis
To estimate the sample size, we referenced real-world effectiveness rates of similar drugs. Vonopranzan monotherapy exhibited a healing rate of 77.6% post-ESD gastric ulcers, considered equivalent to tegoprazan alone19. Meanwhile, combination therapy with vonopranzan and rebamipide demonstrated a healing rate of 90.7%, equivalent to tegoprazan and rebamipide36. This calculation resulted in a Cohen’s effect size of approximately 0.9616,19. The optimal range of participants for an exploratory study, ensuring an overall power of 80% or 90% and an effect size between 0.5 and 0.8, was deemed suitable for the evaluation. Thus, we planned to enroll a total of 140 participants, with 70 in each group, considering a 10% dropout rate. If a patient had more than one gastric neoplasm for ESD, each lesion was considered an independent observation for the analysis.
For the modified intention-to-treat (mITT) analysis, we included all patients who received at least one dose of the study drug and underwent follow-up endoscopy to assess the primary or secondary outcomes. Patients who did not undergo endoscopic follow-up and therefore had no available outcome data were excluded from the mITT analysis. For example, such exclusions occurred in cases where patients withdrew consent, experienced serious adverse events, or underwent surgery due to non-curative resection before any follow-up endoscopy was performed. For the per-protocol (PP) analysis, patients who completed the assigned follow-up endoscopic examination and achieved more than 80% drug compliance were included.
A chi-square test or Fisher’s exact test was used for categorical variables, and an independent sample t-test or Mann–Whitney U test was used for continuous variables. The kappa statistic was used to determine the agreement of the endoscopists in evaluating the quality of ulcer healing. Multivariate logistic regression analysis was employed to identify the independent factors associated with a high ulcer healing rate and ulcer scar quality. A significance level of 0.05 was used for all statistical analyses. All statistical analyses were performed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA).
Data availability
The data sets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
References
Gotoda, T., Yamamoto, H. & Soetikno, R. M. Endoscopic submucosal dissection of early gastric cancer. J. Gastroenterol. 41, 929–942. https://doi.org/10.1007/s00535-006-1954-3 (2006).
Liu, Q., Ding, L., Qiu, X. & Meng, F. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: A systematic review and meta-analysis. Int. J. Surg. (London, England) 73, 28–41. https://doi.org/10.1016/j.ijsu.2019.11.027 (2020).
Kim, Y. I. et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy 47, 293–301. https://doi.org/10.1055/s-0034-1391284 (2015).
Pyo, J. H. et al. Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: A non-inferiority-matched cohort study. Am. J. Gastroenterol. 111, 240–249. https://doi.org/10.1038/ajg.2015.427 (2016).
Kosaka, T. et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: A single-center retrospective study. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 26, 183–191. https://doi.org/10.1111/den.12099 (2014).
Suzuki, H. et al. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: “Real-world evidence” in Japan. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 31, 30–39. https://doi.org/10.1111/den.13246 (2019).
Gao, H. et al. Comparison of efficacy of pharmacological therapies for gastric endoscopic submucosal dissection-induced ulcers: A systematic review and network meta-analysis. Expert Rev. Gastroenterol. Hepatol. 14, 207–220. https://doi.org/10.1080/17474124.2020.1731304 (2020).
Shin, W. G. et al. Can rebamipide and proton pump inhibitor combination therapy promote the healing of endoscopic submucosal dissection-induced ulcers? A randomized, prospective, multicenter study. Gastrointest. Endosc. 75, 739–747. https://doi.org/10.1016/j.gie.2011.11.004 (2012).
Kato, T. et al. Clinical trial: rebamipide promotes gastric ulcer healing by proton pump inhibitor after endoscopic submucosal dissection–A randomized controlled study. J. Gastroenterol. 45, 285–290. https://doi.org/10.1007/s00535-009-0157-0 (2010).
Araki, H. et al. Combination of proton pump inhibitor and rebamipide, a free radical scavenger, promotes artificial ulcer healing after endoscopic submucosal dissection with dissection size > 40 mm. J. Clin. Biochem. Nutr. 51, 185–188. https://doi.org/10.3164/jcbn.12-14 (2012).
Fujiwara, S. et al. A randomized controlled trial of rebamipide plus rabeprazole for the healing of artificial ulcers after endoscopic submucosal dissection. J. Gastroenterol. 46, 595–602. https://doi.org/10.1007/s00535-011-0372-3 (2011).
Asakuma, Y. et al. Comparison of an ecabet sodium and proton pump inhibitor (PPI) combination therapy with PPI alone in the treatment of endoscopic submucosal dissection (ESD)–induced ulcers in early gastric cancer: Prospective randomized study. Hepatogastroenterology 56, 1270–1273 (2009).
Inaba, T. et al. Basal protrusion of ulcers induced by endoscopic submucosal dissection (ESD) during treatment with proton pump inhibitors, and the suppressive effects of polaprezinc. Hepatogastroenterology 57, 678–684 (2010).
Scarpignato, C. et al. Pharmacologic treatment of GERD: Where we are now, and where are we going?. Ann. N. Y. Acad. Sci. 1482, 193–212. https://doi.org/10.1111/nyas.14473 (2020).
Marabotto, E. et al. Vonoprazan fumarate for the treatment of gastric ulcers: A short review on emerging data. Clin. Exp. Gastroenterol. 13, 99–104. https://doi.org/10.2147/ceg.s228352 (2020).
Shiratori, Y. et al. Vonoprazan versus proton pump inhibitors for postendoscopic submucosal dissection bleeding in the stomach: A multicenter population-based comparative study. Gastrointest. Endosc. 95, 72-79.e73. https://doi.org/10.1016/j.gie.2021.06.032 (2022).
Toya, Y. et al. Protective effect of proton pump inhibitors and potassium competitive acid blockers against post-gastric endoscopic submucosal dissection bleeding: A single-center, propensity score-matched analysis. Scand. J. Gastroenterol. 56, 199–204. https://doi.org/10.1080/00365521.2020.1862906 (2021).
Kawai, D. et al. Vonoprazan versus lansoprazole in the treatment of artificial gastric ulcers after endoscopic submucossal dissection: A randomized, open-label trial. BMC Gastroenterol. 21, 236. https://doi.org/10.1186/s12876-021-01822-5 (2021).
Liu, C. et al. The efficacy of vonoprazan for management of post-endoscopic submucosal dissection ulcers compared with proton pump inhibitors: A meta-analysis. J. Dig. Dis. 20, 503–511. https://doi.org/10.1111/1751-2980.12813 (2019).
Kim, E. H., Park, S. W., Nam, E., Lee, J. G. & Park, C. H. Comparative efficacy of various anti-ulcer medications after gastric endoscopic submucosal dissection: A systematic review and network meta-analysis. Surg. Endosc. 33, 1271–1283. https://doi.org/10.1007/s00464-018-6409-4 (2019).
Kang, H., Kim, B. J., Choi, G. & Kim, J. G. Vonoprazan versus proton pump inhibitors for the management of gastric endoscopic submucosal dissection-induced artificial ulcer: A systematic review with meta-analysis. Medicine 98, e15860. https://doi.org/10.1097/md.0000000000015860 (2019).
Lee, K. J. et al. Randomised phase 3 trial: Tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment. Pharmacol. Ther. 49, 864–872. https://doi.org/10.1111/apt.15185 (2019).
Nishizawa, T. et al. Rebamipide-promoted restoration of gastric mucosal sonic hedgehog expression after early Helicobacter pylori eradication. Digestion 79, 259–262. https://doi.org/10.1159/000213241 (2009).
Kleine, A., Kluge, S. & Peskar, B. M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig. Dis. Sci. 38, 1441–1449. https://doi.org/10.1007/bf01308601 (1993).
Sun, W. H. et al. Induction of cyclooxygenase-2 in rat gastric mucosa by rebamipide, a mucoprotective agent. J. Pharmacol. Exp. Ther. 295, 447–452 (2000).
Tarnawski, A. S., Chai, J., Pai, R. & Chiou, S. K. Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: A key to its ulcer healing action?. Dig. Dis. Sci. 49, 202–209. https://doi.org/10.1023/b:ddas.0000017439.60943.5c (2004).
Kakushima, N. et al. Histopathologic characteristics of gastric ulcers created by endoscopic submucosal dissection. Endoscopy 38, 412–415. https://doi.org/10.1055/s-2006-925166 (2006).
Ryu, D. G. et al. Local recurrence after endoscopic submucosal dissection of early gastric cancer. J. Clin. Med. https://doi.org/10.3390/jcm12052018 (2023).
Arantes, V. et al. Polypoid nodule scar after gastric endoscopic submucosal dissection: Results from a multicenter study. Endosc. Int. Open 06, 1198–1203 (2018).
Cheon, Y. K. et al. A clinical study of protruding lesions that arise at the scar of an endoscopic mucosal resection for an early gastric carcinoma and a gastric flat adenoma. J. Korean Gastric Cancer Assoc. 1, 55–59 (2001).
Kim, I. S. et al. Comparisons of the mucosal healing process between flat and protruded type after endoscopic submucosal dissection for gastric neoplasms. Digestion 99, 219–226. https://doi.org/10.1159/000491592 (2019).
Cheon, J. H. et al. Helicobacter pylori eradication therapy may facilitate gastric ulcer healing after endoscopic mucosal resection: A prospective randomized study. Helicobacter 13, 564–571. https://doi.org/10.1111/j.1523-5378.2008.00647.x (2008).
Nakamura, T., Tsukamoto, Y., Yamanaka, T. & Hahashi, S. A study on the whitish protrusion apperaing in the base of peptic ulcer during the administration of proton pump inhibitor. Gastroenterol. Endosc. 34, 1548–1555 (1992).
Kwon, S., Jung, H.-K., Hong, J. H. & Park, H. S. Diagnostic validity of the Korean gastrointestinal symptom rating scale (KGSRS) in the assessment of gastro-esophageal reflux disease. emj 31, 73–80. https://doi.org/10.12771/emj.2008.31.2.73 (2008).
Svedlund, J., Sjödin, I. & Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 33, 129–134 (1988).
Ichida, T., Ueyama, S., Eto, T., Kusano, F. & Sakai, Y. Randomized controlled trial comparing the effects of vonoprazan plus rebamipide and esomeprazole plus rebamipide on gastric ulcer healing induced by endoscopic submucosal dissection. Int. Med. (Tokyo, Japan) 58, 159–166. https://doi.org/10.2169/internalmedicine.1146-18 (2019).
Acknowledgements
This research received financial supported by Korea Otsuka Pharmaceutical Co., Ltd. and was funded by the National Research Foundation of Korea (NRF) grant of the Korea government (MSIT) (no. 2022R1G1A10125421322182102840103 to Hyun Lim).
Author information
Authors and Affiliations
Contributions
Study conception and design: Hyun Lim, Woon Geon Shin Data Generation, collection, assembly, analysis and/or interpretation: Young Joo Yang, Jae Gon Lee, Chang Kyo Oh, Yu Jin Kim, Seung In Seo, Hyun Lim, Sang Pyo Lee, Tae Wan Kim, Chang Seok Bang, Woon Geon Shin, Jin Bae Kim, Hyun Joo Jang, Gwang Ho Baik, Sea Hyub Kae Manuscript drafting and revision: Young Joo Yang, Hyun Lim Approval of the final manuscript: Hyun Lim.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y.J., Lim, H., Lee, J.G. et al. Comparison of rebamipide and tegoprazan combination therapy and tegoprazan monotherapy for ESD induced gastric ulcers a randomized multicenter study. Sci Rep 15, 27127 (2025). https://doi.org/10.1038/s41598-025-11964-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11964-1