Abstract
Percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS) undergoing hemodialysis (HD) remains challenging, with limited long-term outcome data. We investigated the long-term prognosis of ACS due to de novo coronary artery lesions and stent thrombosis (ST) in patients with and without HD. We analyzed 187 patients with ACS from the Osaka Cardiovascular Conference Long ST registry, a retrospective, multicenter registry of definite ST, and 1,856 patients with ACS due to de novo coronary artery lesions at Kansai Rosai Hospital. Patients were grouped by HD status and ACS etiology (de novo- and ST-ACS). The primary outcome was the 6-year incidence of major adverse cardiac events (MACE) defined as a composite of cardiac death, non-fatal myocardial infarction, target vessel revascularization, and subsequent ST. The 6-year MACE rate was highest in ST-ACS with HD, followed by de novo-ACS with HD, ST-ACS without HD, and de novo-ACS without HD (82.1 vs. 62.5 vs. 38.3 vs. 24.2%, respectively, p < 0.001). Multivariate analysis identified HD (hazard ratio [HR]: 2.50, 95% confidence interval [CI]: 1.89–3.32, p < 0.001) and ST-ACS (HR: 1.69, 95% CI: 1.17–2.45, p = 0.006) as independent MACE predictors. The long-term prognoses following ACS are unfavorable in patients on HD, particularly those with ST-ACS.
Similar content being viewed by others
Introduction
Patients who undergo hemodialysis (HD) have been reported to have a high risk of cardiovascular diseases, with approximately 50% of them experiencing coronary artery disease1. In addition, > 50% of deaths due to a known cause in patients on HD are attributed to cardiovascular diseases1,2. Acute coronary syndrome (ACS) is a critical clinical event associated with high in-hospital mortality rates and poor long-term survival3,4. Recently, the proportion of patients on HD undergoing percutaneous coronary intervention (PCI) has increased with advancements in stent performance and PCI techniques. However, PCI in patients undergoing HD remains challenging because of the high rates of restenosis and stent thrombosis (ST) following PCI5,6. Particularly, coronary revascularization in patients on HD presenting with ACS is associated with several unresolved issues. First, although the rate of mid-term mortality after ACS due to de novo coronary artery lesions in patients on HD has improved, the mortality rate remains as high as 40% at 1 year post-ACS occurrence, and reports on the long-term prognosis are limited7,8,9,10. Furthermore, ST-related ACS is a critical PCI complication that can cause sudden death and is associated with poor prognosis. Patients on HD are at particularly high risk, with HD being recognized as a strong predictor of ST11,12,13. However, no studies have examined the long-term prognosis of ACS due to ST in patients on HD.
In this study, we aimed to investigate the long-term prognosis following ACS due to de novo coronary artery lesions and ST in patients on HD compared with non-HD patients.
Methods
Study population
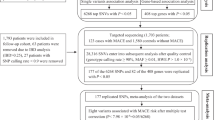
This study included pooled patients with ACS from two cohorts. The first cohort included 187 patients with ACS due to definite ST from the Osaka Cardiovascular Conference Long ST registry, a retrospective, multicenter registry, collected between January 2008 and December 201714. The patients in this cohort had angiographically confirmed ST based on the Academic Research Consortium definition15. Details of this study have been previously reported14. The second cohort consisted of patients with ACS with de novo coronary artery lesions from the Kansai Rosai Hospital institutional registry. After excluding 14 patients who underwent coronary artery bypass grafting (CABG) for ACS and 24 who did not undergo PCI, the registry enrolled 1,856 patients with ACS due to de novo coronary artery lesions who underwent primary PCI between March 2011 and June 2023. We compared the clinical outcomes among the following four groups: patients on HD with ACS due to ST (ST-ACS with HD), non-HD patients with ACS due to ST (ST-ACS without HD), patients on HD with ACS due to de novo coronary artery lesions (de novo-ACS with HD), and non-HD patients with ACS due to de novo coronary artery lesions (de novo-ACS without HD) (Figure. 1).
This study was conducted in accordance with the Declaration of Helsinki, and each center obtained ethical approval from the relevant Institutional Review Board. Because this study was observational, with no intervention, invasiveness, or use of human biological specimens, the requirement for written informed consent from patients was waived following the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The relevant study information was made publicly available, and individuals were given the opportunity to refuse the inclusion of their data.
Interventional procedures
Patients with ACS who had significant stenosis or occlusion on initial coronary angiography and underwent PCI were eligible for inclusion. Standard PCI and post-PCI management, including anti-platelet therapy were performed. Intravenous heparin (5000 IU), oral aspirin (200 mg), prasugrel (20 mg), and clopidogrel (300 mg) were administered before PCI. After PCI, all patients received prasugrel (3.75 mg) or clopidogrel (75 mg) once daily in addition to aspirin (100 mg) for the optimal duration according to relevant guidelines16,17.
Outcome measures
The primary outcome measure was major adverse cardiac events (MACE), defined as a composite of cardiac death (CD), non-fatal myocardial infarction (MI), target vessel revascularization (TVR), and subsequent ST. Secondary outcomes were other clinical outcomes, including all-cause death, CD, non-fatal MI, TVR, and definite ST.
Definitions
Acute coronary syndrome (ACS) was defined as the presence of high-risk unstable angina, a non-ST elevation myocardial infarction (MI) (NSTEMI), or ST-elevation MI (STEMI). STEMI was diagnosed in the presence of either a new ST elevation at the J point in at least 2 contiguous leads ≥ 2 mm (0.2mV) in men or ≥ 1.5 mm (0.15mV) in women in leads V2–3 and/or ≥ 1 mm (0.1mV) in other contiguous chest leads or the limb leads, a presumed new Q wave, or a left bundle branch block on the qualifying 12-lead ECG. NSTEMI and unstable angina were defined as the absence of ST segment elevation criteria with the presence and absence of significant cardiac enzyme elevation, which was 2-fold higher than the upper limit of the normal range18,19. Target vessel revascularization (TVR) was defined as any clinically indicated repeat percutaneous coronary intervention (PCI) for the target vessel, bypass surgery for restenosis in the target vessel, or any other complications in the target vessel18. Revascularization was considered clinically indicated if angiography at follow-up revealed a percent diameter stenosis of ≥ 50% and if one of the following was present: a positive history of recurrent angina pectoris, possibly associated with the target vessel; objective signs of ischemia at rest or during an exercise test, possibly associated with the target vessel; and abnormal results of any invasive functional diagnostic test20. ST was defined according to the Academic Research Consortium definition21.
Statistical analyses
Data on baseline characteristics were presented as frequencies and percentages for discrete variables and as medians (interquartile ranges) for continuous variables. Continuous variables with and without homogeneity of variance were analyzed using the Student’s and Welch’s t-tests, respectively. Categorical variables were analyzed using Fisher’s exact test for 2 × 2 comparisons. For more than 2 × 2 comparisons, nominal and ordinal variables were analyzed using the chi-square and Mann–Whitney U tests, respectively. Cumulative incidence rates were assessed using the Kaplan–Meier method, and the rates were reported as estimate ± standard error. Between-group differences were assessed using the log-rank test, and subsequently adjusted with Holm correction for multiple comparisons. The associations of MACE with baseline clinical, lesion, and procedural variables were first analyzed using a univariate Cox proportional hazards regression analysis. Subsequently, a multivariate Cox proportional hazards regression analysis was performed to adjust for confounders and identify the risk factors for MACE while adjusting for covariates including age, sex, body mass index, left ventricular ejection fraction, hypertension, dyslipidemia, diabetes mellitus, hemodialysis, family history, history of PCI, history of CABG, history of MI, congestive heart failure, stroke, atrial fibrillation, type of ACS, lesion location, bifurcation, moderate-severe calcification, The American College of Cardiology (ACC) and the American Heart Association (AHA) (ACC/AHA) classification, approach site, use of aspiration, use of distal protection, imaging guided PCI, use of 2nd or 3rd generation drug-eluting stents (DES), final thrombolysis in MI (TIMI) flow, ST-ACS. Clinical factors that are known to affect outcomes and that achieved p < 0.05 in the univariate analysis were included in the multivariate Cox regression analysis. Statistical analyses were performed using the SPSS software (version 28.0; IBM Corp., Armonk, NY, USA).
Results
Baseline patient characteristics
Table 1 presents the patients’ baseline characteristics. Regarding the baseline differences in patient characteristics between patients on HD and non-HD patients, the prevalence of diabetes mellitus was significantly higher in the ST-ACS and de novo-ACS with HD groups compared with the respective without HD groups (82 vs. 39%, p < 0.001; 61 vs. 33%, p < 0.001). A significantly lower body mass index (21.4 vs. 23.5 kg/m2, p < 0.001) and ejection fraction (56 vs. 60%, p = 0.007), and a significantly higher prevalence of hypertension (83 vs. 70%, p < 0.001), history of PCI (47 vs. 21%, p < 0.001), history of CABG (9 vs. 2%, p < 0.001), history of MI (17 vs. 10%, p = 0.001), congestive heart failure (16 vs. 8%, p < 0.001), and stroke (11 vs. 6%, p = 0.003) were observed in the de novo-ACS with HD group only. Patients in the ST-ACS with HD group were significantly younger (63 vs. 70 years, p = 0.017) compared with those in ST-ACS without HD group. Regarding the baseline difference in patient characteristics between the ST- and de novo-ACS groups, patients in the ST-ACS group were significantly younger (63 vs. 73 years, p = 0.004; 70 vs. 73 years, p = 0.049). Prevalence of a history of MI was higher in the ST-ACS with and without HD groups than in the de novo-ACS with and without HD group (35 vs. 17%, p = 0.048; 23 vs. 10%, p = 0.001), whereas proportion of men (85 vs. 76%, p < 0.001), history of PCI (39 vs. 21%, p < 0.001), and history of CABG (4 vs. 2%, p = 0.029) was significantly higher in the ST-ACS without HD group only.
Baseline lesion and procedural characteristics
Table 2 presents the baseline lesion and procedural characteristics. Regarding the baseline differences between patients with and without HD, the frequency of calcification (74 vs. 17%, p < 0.001; 54 vs. 15%, p < 0.001) and the femoral approach (88 vs. 61%, p = 0.040; 80 vs. 35%, p < 0.001) was higher in the ST-ACS and de novo-ACS with HD groups, whereas the frequency of use of distal protection (0 vs. 21%, p = 0.037; 2 vs. 14%, p < 0.001) was higher in the ST-ACS and de novo-ACS without HD groups. Regarding the baseline differences between the ST-ACS and de novo-ACS groups, the frequency of aspiration (77 vs. 29%, p < 0.001; 73 vs. 58%, p < 0.001) was higher in the ST-ACS with and without HD groups than in the de novo-ACS with and without groups. The frequency of the femoral approach (61 vs. 32%, p < 0.001) and distal protection (21 vs. 14%, p = 0.021) was higher in the ST-ACS group, whereas that of imaging-guided PCI (91 vs. 99%, p < 0.001) was higher in the de novo-ACS without HD group only.
Clinical outcomes after ACS due to de novo coronary artery lesions and stent thrombosis in HD and non-HD patients
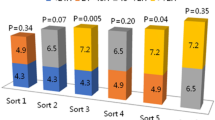
As shown in Fig. 2, the 6-year MACE rate was the highest in the ST-ACS with HD group, followed by the de novo-ACS with HD, ST-ACS without HD, and de novo-ACS without HD groups, and significantly different after Holm correction (82.1 vs. 62.5 vs. 38.3 vs. 24.2%; hazard ratio [HR] for ST-ACS with HD vs. de novo-ACS with HD: 2.32, 95% confidence interval [CI]: 1.24–4.35, p = 0.009; HR for ST-ACS without HD vs. de novo-ACS without HD: 1.99, 95% CI: 1.49–2.68, p < 0.001; HR for ST-ACS with HD vs. ST-ACS without HD: 3.44, 95% CI: 1.78–6.65, p < 0.001; HR for de novo-ACS with HD vs. de novo-ACS without HD: 3.13, 95% CI: 2.47–3.97, p < 0.001). Supplementary Table S1 shows the secondary outcome measures in the four groups. The 6-year all-cause death rate was higher among patients on HD than among non-HD patients (p < 0.001) but was similar between the ST- and de novo-ACS with HD groups (71.1 vs. 70.8%, p = 0.309) and between the ST- and de novo-ACS without HD groups (23.2 vs. 20.6%, p = 0.579). Although the 6-year CD rate tended to be higher in the ST-ACS with HD group than in the de novo-ACS with HD group (53.9 vs. 34.2%, p = 0.038), the difference did not reach statistical significance after Holm correction. The 6-year non-fatal MI, TVR, and recurrent ST rates were significantly higher in the ST-ACS without HD group than in the de novo-ACS without HD group (7.6 vs. 3.1%, p < 0.001; 28.5 vs. 15.2%, p < 0.001; 7.1 vs. 0.6%, p < 0.001). The detailed causes of death and clinical outcomes are shown in Fig. 3 and Supplementary Figure S1. Regarding the cause of death, arrhythmia and sudden death as CD, and infection and sepsis as non-CD were the leading causes of death in the ST-ACS and de novo-ACS with HD groups compared with the ST-ACS and de novo-ACS without HD groups. Conversely, cancer was a leading cause of non-CD in patients without HD compared with those on HD.
Study flow chart. Clinical outcomes were compared among 17 patients on HD with ACS due to ST (ST-ACS with HD), 170 non-HD patients with ACS due to ST (ST-ACS without HD), 246 patients on HD with ACS due to de novo coronary artery lesions (de novo-ACS with HD), and 1,610 non-HD patients with ACS due to de novo coronary artery lesions (de novo-ACS without HD). ACS, acute coronary syndrome; HD, hemodialysis; MACE, major adverse cardiac events; PCI, percutaneous coronary intervention; ST, stent thrombosis.
The 6-year cumulative incidence of MACE. The 6-year MACE rate was significantly higher in the ST-ACS with HD group, followed by the de novo-ACS with HD, ST-ACS without HD, and de novo-ACS without HD groups, indicating worse outcomes in patients on HD with ACS due to ST. *Statistically significant at p = 0.05 after Holm’s correction for multiple comparisons. ACS, acute coronary syndrome; CI, confidence interval; HD, hemodialysis; HR, hazard ratio; MACE, major adverse cardiac events; ST, stent thrombosis.
Causes of death in each group. Arrhythmia and sudden death were the most common causes of cardiac death, whereas infection and sepsis were the leading causes of non-cardiac deaths in the ST- and de novo-ACS with HD groups compared with the ST- and de novo-ACS without HD groups, respectively. In contrast, cancer was a more frequent cause of non-cardiac death in non-HD patients compared with those on HD. ACS, acute coronary syndrome; HD, hemodialysis; ST, stent thrombosis.
Factors associated with MACE
The associations of MACE with baseline clinical, lesion, and procedural variables were analyzed using Cox proportional hazards regression analysis (Table 3). After multivariate analysis, HD (HR: 2.50, 95% CI: 1.89–3.32, p < 0.001), ST-ACS (HR: 1.69, 95% CI: 1.17–2.45, p = 0.006), left ventricular ejection fraction, diabetes mellitus (HR: 1.36, 95% CI: 1.09–1.71, p = 0.007), history of MI (HR: 1.56, 95% CI: 1.11–2.18, p = 0.011), and femoral approach (HR: 1.60, 95% CI: 1.26–2.03, p < 0.001) were positive independent predictors of MACE after ACS, whereas imaging-guided PCI and left ventricular ejection fraction (HR: 0.98, 95% CI: 0.98–0.99, p < 0.001) (HR: 0.40, 95% CI: 0.18–0.87, p = 0.021) were negatively associated with MACE after ACS.
Discussion
Summary of the present study
In the present study, we found that long-term prognosis after ACS was unfavorable in patients on HD, particularly those with ACS due to ST, compared with those with ACS due to de novo coronary artery lesions. The major findings were the following: (1) among patients on HD with ACS due to ST, 80% experienced cardiac events, and 70% died within 6 years; (2) among patients on HD with ACS due to de novo coronary artery lesions, 60% experienced cardiac events, and 70% died within 6 years; and (3) in both patients on HD and non-HD patients, the long-term prognosis after ACS due to ST was worse than that after ACS due to de novo coronary artery lesions.
Long-term prognosis after ACS in patients on HD
There are few reports on the long-term prognosis of ACS due to de novo coronary artery lesions in patients on HD. Shroff et al.9 reported 1- and 2-year mortality rates of 50 and 65.1% in 2008, whereas Szummer et al.10 reported a 1-year mortality rate of 41.2% in 2013. The 1- and 2-year mortality rates observed in our study were slightly better (37.5 and 43.1%, respectively), possibly due to the implementation of primary PCI in all cases and improvements in technology as well as instrumentation between 2011 and 2023, when the cases were enrolled. Recent reports on long-term prognosis are lacking; however, a 5-year mortality rate of 89.9% was reported in 19983. Despite advancements in PCI techniques and medications, we found that the long-term prognosis remains poor, with a 6-year mortality rate of 70%.
Furthermore, ACS due to ST is a critical event, and HD has been reported to be a risk factor for ST22. However, few studies have focused on the mid- and long-term prognoses of ACS due to ST in patients on HD. Van Werkum et al.6 reported MACE rates of 23.6, 25.2, and 27.9% at 1, 2, and 3 years after the first ST in the overall population, similar to the results observed for non-HD patients with ACS due to ST in the present study (21.8, 30.0, and 35.5%, respectively). Moreover, patients with ACS due to ST have been reported to have a poor long-term prognosis14, despite their relatively low average age of 63 years, with 8 of 10 patients on HD experiencing cardiac events within 6 years and 7 of 10 patients on HD dying, indicating particularly poor prognosis.
One of the reasons for the poor prognosis after ACS due to ST and de novo coronary artery lesions in patients on HD is the higher incidence of arrhythmia or sudden death. Cardiac arrest or arrhythmia in patients on HD accounts for 33% of all deaths, and a higher rate of sudden CD is observed among patients on HD than among non-HD patients1. In the present study, the risk was even higher after ACS, with arrhythmia or sudden death being the most common cause of CD among patients on HD, as shown in Fig. 3. Another reason is the increased susceptibility of patients on HD to infections. The mortality rate from sepsis and pulmonary infections is much higher in patients on HD than in non-HD patients23,24. Infection or sepsis was the leading cause of non-CD among patients on HD (Fig. 3).
Comparison of long-term prognosis after ACS due to ST and de novo coronary artery lesions
The short-term prognosis of ST-ACS has been reported to be worse than that of de novo-ACS, mainly due to differences in underlying comorbidities25. Herein, the long-term prognosis in both the ST-ACS with and without HD groups was also worse than that in the de novo-ACS with and without HD groups. In addition to the differences in comorbidities, ST-ACS often results in suboptimal angiographic outcomes because repeat treatment after post-stent placement requires balloon-based treatment, including angioplasty using a plain balloon or drug-coated balloon, which results in lower rates of achieving thrombolysis in MI (TIMI) grade 3 flow and a higher degree of residual stenosis, as evidenced by the lower TIMI 3 rates observed after ST-ACS (Table 2). However, even after adjusting for these confounding factors in the multivariate analysis, ST-ACS remained a significant risk factor for MACE. TVR and recurrent ST has been associated with MACE rates11, and the high rates of TVR and recurrent ST in non-HD patients may contribute to the higher rates of MACE in ST-ACS. Among patients on HD, although the incidence of all-cause death was similar between the ST-ACS and de novo-ACS, the prevalence and incidence of CD tended to be higher in the ST-ACS, resulting in a higher rate of MACE. This difference in the KM curve is mainly driven by early events, specifically those occurring within the first year. Additionally, causes of death in ST-ACS with HD group more frequently include AMI, arrhythmias and sudden death compared with those in de novo-ACS. Indeed, it has been reported that in-hospital events are more frequent in patients with ST-ACS than in those with de novo-ACS25, suggesting that the impact of ACS event on mortality is greater in ST-ACS patients on HD than that in the de novo-ACS patients on HD.
Clinical implications
Results of the present study demonstrate that the prognosis of patients on HD is poor after the occurrence of ST- or de novo-ACS. In a recently published trial involving patients with advanced chronic kidney disease, initial invasive treatment did not reduce the risk of death or non-fatal MI compared to initial conservative treatment26. Therefore, it is necessary to evaluate functional ischemia and avoid unnecessary stent placement. Furthermore, better long-term outcomes have been reported with CABG compared with PCI27. Therefore, selecting staged CABG after ensuring TIMI flow in the acute phase or opting for emergent CABG when required should be considered as viable therapeutic approach. In addition, efforts should be made to prevent sudden death using an implantable cardioverter-defibrillator (ICD) after ACS treatment. The usefulness of ICDs in patients on HD has been reported28, and subcutaneous ICDs may be effective because patients on HD have a high risk of infection29.
Limitations
This study has some limitations. First, the current study combined the 2 cohorts with different inclusion periods. This discrepancy could introduce selection bias related to data completeness and procedural background. However, in order to minimize potential bias, identical inclusion and exclusion criteria were applied to both cohorts. Moreover, the median follow-up duration was comparable between the two groups (median follow-up duration: 1054 days in ST-ACS group vs. 1009 days in de novo-ACS group, p = 0.558). Since those studies were conducted at high-volume centers located in a metropolitan area, the risk of bias due to region or institutional factors is considered low. Additionally, in the de novo-ACS group, only 2% of patients were treated with BMS or 1 st DES, suggesting that the differences in stent performance after 2017, when the inclusion periods differed, likely had minimal impact on clinical outcomes. ST in patients on HD is an extremely rare event; therefore, in order to preserve statistical power, we prioritized retaining an adequate number of HD patients over matching the inclusion periods. Therefore, there was heterogeneity in the baseline demographic and procedural variables among the four study groups. However, each cohort adhered to the same inclusion and exclusion criteria, and included consecutive cases. In addition, the proportion of patients on HD in this study (approximately 10%) was similar to that in other ST databases13, representing the real-world clinical setting after ST occurrence. Second, there were differences in the rate of ACS types (STEMI, NSTEMI, or unstable angina) among the four groups. The rate of STEMI as a clinical presentation of ACS has been reported to be as low as 10–15% among patients on HD9,10, compared with that among non-HD patients (> 50%)19,30. These rates were similar to those in the present study, and multivariate analysis revealed that STEMI and NSTEMI did not affect the MACE (Table 3). Third, some background data were unavailable. The etiology and duration of HD were not recorded. Data on post-intervention medications were also limited, although evidence of specific medications in patients on HD remains lacking. Further, the morphological causes of ST or TVR were not considered, although the lesion severity was evaluated using angiography.
Conclusions
Long-term prognosis after ACS is unfavorable in patients on HD, particularly those with ACS due to ST.
Data availability
The deidentified participant data will be shared on a request basis. Please directly contact the corresponding author to request data sharing.
References
United States Renal Data System (Natl Inst. of Health – Natl Inst. of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. USRDS Annual Data Report: epidemiology of kidney disease in the United States. (2022).
Cheung, A. K. et al. Cardiac diseases in maintenance Hemodialysis patients: results of the HEMO study. Kidney Int. 65, 2380–2389 (2004).
Herzog, C. A., Ma, J. Z. & Collins, A. J. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl. J. Med. 339, 799–805 (1998).
McManus, D. D. et al. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 124, 40–47 (2011).
Lee, J. M. et al. Chronic kidney disease in the second-generation drug-eluting stent era: pooled analysis of the Korean multicenter Drug-Eluting stent registry. JACC Cardiovasc. Interv. 9, 2097–2109 (2016).
van Werkum, J. W. et al. Long-term clinical outcome after a first angiographically confirmed coronary stent thrombosis: an analysis of 431 cases. Circulation 119, 828–834 (2009).
Fox, C. S. et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National cardiovascular data acute coronary treatment and intervention outcomes network registry. Circulation 121, 357–365 (2010).
Bagai, A. et al. Temporal trends in utilization of cardiac therapies and outcomes for myocardial infarction by degree of chronic kidney disease: a report from the NCDR chest pain-MI registry. J. Am. Heart Assoc. 7, e010394 (2018).
Shroff, G. R., Li, S. & Herzog, C. A. Trends in mortality following acute myocardial infarction among dialysis patients in the united States over 15 years. J. Am. Heart Assoc. 4, e002460 (2015).
Szummer, K. et al. Treatments and mortality trends in cases with and without dialysis who have an acute myocardial infarction: an 18-year nationwide experience. Circ. Cardiovasc. Qual. Outcomes. 12, e005879 (2019).
Armstrong, E. J. et al. Predictors and outcomes of recurrent stent thrombosis: results from a multicenter registry. JACC Cardiovasc. Interv. 7, 1105–1113 (2014).
Kimura, T. et al. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: observations from the registry of stent thrombosis for review and Reevaluation (RESTART). Circulation 122, 52–61 (2010).
Kuramitsu, S. et al. Risk factors and long-term clinical outcomes of second-generation drug-eluting stent thrombosis. Circ. Cardiovasc. Interv. 12, e007822 (2019).
Ishihara, T. et al. Long-term outcomes and clinical predictors of mortality following occurrence of stent thrombosis. J. Am. Heart Assoc. 11, e023276 (2022).
Kim, J. S. et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc. Interv. 6, 369–376 (2013).
JCS Joint Working Group. Guidelines for secondary prevention of myocardial infarction (JCS 2011). Circ. J. 77, 231–248 (2013).
Kimura, K. et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ. J. 83, 1085–1196 (2019).
Thygesen, K. et al. Third universal definition of myocardial infarction. Circulation 126, 2020–2035 (2012).
Ishihara, M. et al. Long-term outcomes of non-ST-Elevation myocardial infarction without creatine kinase elevation- the J-MINUET study. Circ. J. 81, 958–965 (2017).
Cutlip, D. E. et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 115, 2344–2351 (2007).
Garcia-Garcia, H. M. et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation 137, 2635–2650 (2018).
Kimura, T. et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation 119, 987–995 (2009).
Sarnak, M. J. & Jaber, B. L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 58, 1758–1764 (2000).
Sarnak, M. J. & Jaber, B. L. Pulmonary infectious mortality among patients with end-stage renal disease. Chest 120, 1883–1887 (2001).
Belle, L. et al. Do patients with drug-eluting stent thrombosis have a similar prognosis to patients presenting with st-elevation myocardial infarction of de Novo lesions? J. Interv Cardiol. 24, 320–325 (2011).
Bangalore, S. et al. Management of coronary disease in patients with advanced kidney disease. N Engl. J. Med. 382, 1608–1618 (2020).
Marui, A. et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with end-stage renal disease requiring dialysis (5-year outcomes of the CREDO-Kyoto PCI/CABG registry Cohort-2). Am. J. Cardiol. 114, 555–561 (2014).
Herzog, C. A. et al. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 68, 818–825 (2005).
Koman, E., Gupta, A., Subzposh, F., Saltzman, H. & Kutalek, S. P. Outcomes of subcutaneous implantable cardioverter-defibrillator implantation in patients on Hemodialysis. J. Interv Card Electrophysiol. 45, 219–223 (2016).
Daida, H. et al. Management and two-year long-term clinical outcome of acute coronary syndrome in japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ. J. 77, 934–943 (2013).
Acknowledgements
We thank the staff who offered continuous support and constant encouragement.
Author information
Authors and Affiliations
Contributions
All authors have participated in the work and have reviewed and agree with the content of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nakao, S., Ishihara, T., Okada, K. et al. Long-term prognosis after acute coronary syndrome due to de novo coronary artery lesions and stent thrombosis in patients on hemodialysis. Sci Rep 15, 26063 (2025). https://doi.org/10.1038/s41598-025-11968-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11968-x