Abstract
The use of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) has been the primary vector control strategy in Sénégal since 2007, significantly reducing the malaria burden. However, the emergence insecticide resistance in malaria vectors threatens the effectiveness of these interventions. This study aimed to assess susceptibility, spatiotemporal variations, and the impact of IRS with bendiocarb and pirimiphos-methyl on allele frequencies and resistant genotypes of kdr and ace-1 in An. gambae s.l. in IRS and control zones in central Sénégal between 2013 and 2014. Larvae of An. gambiae s.l. were collected from four IRS and two control districts in central Sénégal. Emerging adult females were exposed to insecticide-impregnated papers containing pyrethroids, DDT, dieldrin, bendiocarb and pirimiphos-methyl over two years. Species identification and resistance markers analysis were performed using PCR, targeting kdr mutations (Vsgc-1014F and Vsgc-1014S) and ace-1 mutations. Susceptibility test revealed resistance to permethrin and deltamethrin in at least three of the four IRS districts, while control districts showed resistance or suspected resistance to these insecticides. Resistance to DDT and dieldrin was detected in all districts. However, An. gambiae s.l. populations from IRS districts remained susceptible to bendiocarb and pirimiphos-methyl. Molecular identification confirmed the presence of three species (An. arabiensis, An. gambiae s.s. and An. coluzzii) along with hybrids. An. arabiensis populations collected in IRS areas showed a significantly lower frequency of the 1014F allele than those in control areas.
Similar content being viewed by others
Introduction
Interventions to control and reduce the incidence of malaria globally, particularly in Africa south of the Sahara, have mainly been carried out by indoor residual spraying (IRS) and insecticide-treated nets 1. The latter have, up to now, been impregnated with pyrethroids, the main class recommended by the World Health Organization1. Chlorfenapyr, from the pyrrole class, recently received provisional approval from the expert group for the prequalification of products used in vector control as an insecticide for impregnating mosquito nets2.
Resistance of Anopheles gambiae complex (An. gambiae s.l.) vectors to pyrethroids is currently widespread3,4,5, and the intensity of this resistance has been reported in many countries6,7,8. From 2010 to 2018, resistance of vectors to at least one class of insecticides concerned 73 countries, 64 of which reported resistance to pyrethroids9. This spread of resistance has been attributed mainly to strong selection pressure from the combined use of insecticides of the same class in public health (insecticide-treated nets and indoor residual spraying) and agriculture10,11.
The impact of resistance to pyrethroids on the efficacy of insecticide-dependent control strategies has yielded mixed results in the studies carried out to date. While some studies have indicated a reduction in the protective effect of long-lasting insecticide-treated nets (LLINs) in areas of resistance in Benin12 and Burkina Faso13, others have found no discernible impact on malaria transmission in Benin14 and Malawi15. Nevertheless, several studies have underscored the threat posed by vector resistance to the effectiveness of control tools16,17,18. This threat arises from the reduced efficacy of insecticides against resistant strains, leading to continued transmission despite control efforts. To enhance the effectiveness of vector control measures, the WHO has recommended implementing several resistance management strategies19. This includes combining interventions using insecticides with different modes of action and rotating insecticides used in IRS programs19. Given the recent introduction of IRS in the central zone of Sénégal in 2013 and 2014, this study proposes evaluating the insecticide susceptibility of An. gambiae s.l. and assessing the impact of treatments on the allelic and genotypic frequencies of the Vsgc-1014F, Vsgc-1014S and G119S mutations in these vectors.
Results
Mortality rates of An. gambiae s.l.
Deltamethrin and permethrin
In 2013, a total of 2817 An. gambiae s.l. mosquitoes (1943 tested; 874 control) were used for susceptibility testing, compared with 3208 (2515 tested; 693 control) in 2014 across the districts. Additionally, in the control districts, 299 An. gambiae s.l. mosquitoes (195 tested; 104 control) were examined in 2013 and 287 (213 tested; 74 control) were examined in 2014 in Kaffrine. In Ndoffane, the numbers tested were 244 (208 tested; 36 control) in 2013 and 321 (214 tested; 107 control) in 2014, respectively. After the susceptibility tests, all anophelines were identified as An. gambiae s.l. No other anopheline species was observed.
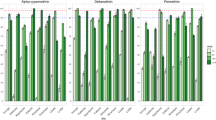
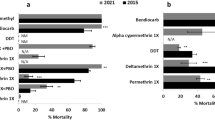
In 2013, susceptibility tested conducted in four IRS districts revealed that An. gambiae s.l. populations tested in Koumpentoum and Nioro were respectively susceptible to permethrin (100%) and deltamethrin (98%). However, in Koungheul district, suspected resistance to deltamethrin (95%) and permethrin (92%) was observed among An. gambiae s.l. populations, while resistance to pyrethroids was noted in Malem Hodar (deltamethrin: 78% and permethrin: 89%) and Nioro permethrin (70%) (Fig. 4).
In the control districts, in 2013, suspected resistance to deltamethrin (95.5%) was recorded in An. gambiae s.l populations in Ndoffane, while those in Kaffrine exhibited resistance to the same molecule; with a mortality rate of 88% (Fig. 1). Comparative analyses of deltamethrin mortality rates in An. gambiae s.l. population of transect 1 showed that although there was no statistically significant difference (Fisher’s exact test: OR: 2.6 95% CI: 0.75–11.5; P = 0.112), the susceptibility status of the Koungheul population to this pyrethroid was different from that of its Kaffrine control.
Similarly, on transect 2, despite the difference in phenotype status between populations tested in 2013, the mortality rates of deltamethrin between IRS area and control area were not statistically significant (Fisher’s exact test: OR: 2.6 95% CI: 0.38–25.5; P = 0.45). In 2014, An. gambiae s.l. populations in Koumpentoum and Nioro were resistant to deltamethrin and permethrin. Those tested in Malem Hodar and Koungheul showed suspected resistance to permethrin (95% and 96%), while mosquitoes exposed to deltamethrin were susceptible (100% at both sites) (Fig. 1).
For the control of transect 2 (Ndoffane), populations were resistant to all pyrethroids tested, whereas in Kaffrine, control of transect 1, their populations presented both a suspected resistance to permethrin (96%) and susceptibility to deltamethrin (98%) (Fig. 1).
In transect 1 of 2014, An. gambiae s.l. populations in Koungheul were susceptible to deltamethrin, whereas those in Nioro (transect 2) were resistant to both pyrethroids tested (deltamethrin and permethrin). The difference between both populations was statistically significant (χ2 = 13.184, df = 1, P < 0.01).
DDT and dieldrin
In IRS districts, susceptibility tests for DDT and dieldrin were carried out on 780 An. gambiae s.l. mosquitoes (488 tested; 292 control) in 2013, compared to 965 (656 tested; 309 control) in 2014.
For control districts, the number of An. gambiae s.l. used for DDT and dieldrin in 2013 was 283 (187 tested; 96 control) and 302 (205 tested; 97 control) for Kaffrine and Ndoffane districts, respectively. For 2014 tests, the corresponding values were 382 (254 tested; 128 control) and 313 (211 tested; 102 control) for Kaffrine and Ndoffane respectively.
Resistance of An. gambiae s.l. to DDT and dieldrin was recorded in all districts of transect 2, with mortality rates varying from 82 to 73% and 92% (Nioro district) and from 45 to 69% and 87% to 79% (control district) in 2013 and 2014, whereas for transect 1, only Malem Hodar populations were susceptible to dieldrin (98%, CI 95% 92–99.7) in 2013 (Fig. 2).
Bendiocarb and pirimiphos-methyl
Susceptibility tests for bendiocarb were conducted on a total of 528 of An. gambiae s.l. specimens (tested: 392; control: 136) in 2013 and 544 (tested: 420; control: 124) in 2014 in the IRS districts. For pirimiphos-methyl, the corresponding values were 493 (tested: 401; control: 92) for 2013 and 568 (tested: 461; control: 107) in 2014.
Exception for the control districts, where An. gambiae s.l. populations were resistant to bendiocarb in 2013 and suspected resistance in the Kaffrine control district in 2014, however all An. gambiae s.l. populations tested in IRS districts were susceptible to this insecticide (Fig. 3). Conversely, the total susceptibility of An. gambiae s.l. populations tested for pirimiphos-methyl was observed at all districts (both controls and IRS) in 2013 and 2014 (Fig. 3).
Species composition of the An. gambiae complex exposed to diagnostic doses by year and collection district
A total of 1180 adult An. gambiae s.l. specimens exposed to diagnostic doses of WHO-registered insecticides were identified by IMP-PCR (Table 1).
Molecular analyses of the An. gambiae complex revealed the presence of three species (An. arabiensis, An. gambiae s.s., and An. coluzzii), as well as hybrids (An. gambiae s.s./An. coluzzii). An. arabiensis was the predominant species with a mean proportion of 87.7% (95% CI 85.6–89.5), followed by An. gambiae s.s. 7.2% (95% CI 5.8–8.8) and An. coluzzii 4.9% (95% CI 3.7–6.3). Hybrids (An. gambiae s.s./An. coluzzii) accounted for just 0.2% of the total specimens identified (Table 1).
Furthermore, species identification revealed an annual variation in An. gambiae s.s. populations, with a proportion of 2.6% in 2013 rising to 10.5 in 2014). This difference in proportion was statistically significant (χ2 = 25.95, df = 1, P = 0.01). In the IRS districts and their corresponding controls, An. arabiensis was predominant across all locations. Nevertheless, An. gambiae s.s. demonstrated statistical significance only in Nioro, where it accounted for 30.9% compared to its control, where it represented only 0.06% of the 2014 total (Fisher’s exact test: OR: 48.4, 95% CI: 8.2–1947.5; P = 0.001) (Table 1).
Species composition of An. gambiae s.l. according by survival status, year and collection district
The species composition of An. gambiae complex varied according to status (Surviving and dead), year and collection district.
The analysis revealed the presence of three species, namely An. arabiensis, An. gambiae s.s. and An. coluzzii, as well as hybrids (An. gambiae s.s./An. coluzzii), in both surviving and dead specimens. Notably, An. arabiensis was predominantly found among surviving specimens of the populations in transect 2, constituting 67.9% in 2013 and 80.8% in 2014 (Table 2).
In 2014, An. coluzzii was more prevalent among dead specimens compared to surviving ones in the districts of Nioro, Koumpentoum and Kaffrine, with proportions of 66.7% versus 33.3%, 83.3% versus 16.7% and 100% versus 0%, respectively. Conversely, in Koungheul, the average proportion of this species was higher among surviving specimens (66.7% versus 33.3%; Fisher’s exact test: OR: 0.3, 95% CI: 0.05–6.0, P = 0.55) (Table 2). Notably, for these districts, there was no statistically significant difference in the mean proportions of the other two species (An. arabiensis, An. gambiae s.s.) between dead and surviving specimens (Nioro: Fisher’s exact test: OR: 3.73, 95% CI : 0.52–42.28; P = 0.189, Koumpentoum: Fisher’s exact test: OR: 4.94, 95% CI: 0.53–240.7; P = 0.21 and Kaffrine: Fisher’s exact test: OR: Inf, OR: inf, 95% CI:0.01-inf; P = 1).
For the districts of Koungheul and Kaffrine in 2013, similar proportions of An. coluzzii were observed in both surviving and dead specimens.
Species composition of the An. gambiae complex by susceptible phenotype, mortality status and insecticide in the districts of transect 1
Table 3 presents the mortality rates of An. arabiensis, An. coluzzii and An. gambiae s.s. according to dead phenotype, population status and insecticide. In the IRS districts with An. gambiae s.l. populations exhibiting resistance to pyrethroids (Malem Hodar in 2013, Koumpentoum and Malem Hodar in 2014) as well as DDT, the mortality rates of An. arabiensis were over 50%. Conversely, in Koungheul, where resistance to DDT was observed, and An. gambiae s.l. populations were susceptible to pyrethroids, An. arabiensis mortality rates exceeded 60% overall, irrespective of the year.
In Koumpentoum, where the status of An. gambiae s.l. populations changed between 2013 and 2014, a statistically significant difference in An. arabiensis mortality rates for permethrin was observed (Fisher’s exact test: OR: 0.0, 95% CI: 0.0–0.14; P = 0.00). Similarly, in the Nioro district, where phenotypic status remained consistent between the two years, significant differences in species mortality rates for permethrin (21.05 versus 4.5; Fisher’s exact test: OR: 0.18, 95% CI: 0.03–0.8; P = 0.016) and DDT (32.1 versus 10.0; Fisher’s exact test: OR: 0.24, 95% CI: 0.04–1.00; P = 0.03) were evident.
Regarding bendiocarb, mortality rates in An. coluzzii were 70% and 15% in 2013 and 2014, respectively, in Koungheul, whith similar outcomes observed for pirimiphos-methyl (Table 3).
Species composition of the An. gambiae complex by susceptible phenotype, mortality status and insecticide in the districts of transect 2
Mortality rates determined through molecular analysis revealed variations in the species composition in Nioro between 2013 and 2014. the An. gambiae s.s. population, the highest mortality rates were recorded in Nioro (deltamethrin: 60.0%, DDT: 60% and bendiocarb: 30%) in 2014 (Table 4).
In the Ndoffane district, only the 2014 An. arabiensis population was resistance to deltamethrin (24%, 95% CI: 9.4–45.1), permethrin (17.3, 95% CI: 8.2–30.3) and DDT (18.3, 95% CI: 8.8–32). Conversely, total susceptibility to bendiocarb and pirimiphos-methyl was observed in all control districts (Table 4).
Mutations and allele frequencies
Mutations and allele frequencies of Vgsc-1014F in An. gambiae s.l. in IRS and control districts
In IRS districts, a total of 415 An. gambiae s.l. (159 in 2013 and 256 in 2014) were analysed for the Vgsc-1014F mutation (Table 6). Both Vgsc-1014F and Vgsc-1014S mutations were detected in all adult mosquitoes reared from larval stages collected in IRS districts and control areas.
Among the specimens analyzed for the Vgsc-1014F, 371 were An. arabiensis (147 in 2013 and 224 in 2014), 14 were An. gambiae s.s. specimens (1 in 2013 and 13 in 2014), 30 were An. coluzzii specimens (11 in 2013 and 19 in 2014) and 2 were An. gambiae s.s./An. coluzzii hybrids.
The frequency of Vgsc-1014F allele varied across IRS districts and between years for An. arabiensis, An. gambiae s.s., and An. coluzzii. In An. arabiensis, mean frequencies were 0.18 and 0.34 in 2013, and decreased to 0.10 and 0.15 in 2014 in transects 1 and 2, respectively. For An. gambiae s.s., allele frequencies in 2014 were 0.77 and 0.13, while only one specimen was identified in 2013. The highest Vgsc-1014F frequencies for An. coluzzii were observed in 2014 (Table 5).
Additionally, a total of 272 An. arabiensis were examined in control districts (74 in 2013 and 198 in 2014). Furthermore, 3 An. coluzzii specimens were analysed (2 in 2013 and 1 in 2014). The allele frequencies of An. arabiensis were 0.14 in 2013 and 0.30 and 0.08 in 2014 for transects 1 and 2, respectively (Table 5).
The annual comparative analysis revealed that the mean allele frequencies of 1014F in An. arabiensis were significantly higher in Koungheul populations (0.28) compared to Kaffrine populations (0.14) in 2013 (χ2 = 8.5275, df = 1, P = 0.034). However, in 2014, the opposite trend was observed, with Koungheul exhibiting a lower frequency of 1014F (0.10) compared to Kaffrine (0.30) (χ2 = 17.05, df = 1, P = 0.01) (Table 5). Nevertheless, in 2014, there was no significant difference between the Vgsc-1014F allele frequencies of An. arabiensis populations in IRS districts (0.15) and those in control areas (0.08) (χ2 = 3.7829, df = 1, P = 0.051). In transect 1, the mean allele frequencies of 1014F recorded in An. arabienis in control areas and Koumpentoum were significantly different in 2013 (Fisher’s exact test: OR: 6.679, 95% CI: 1.55–60.452; P = 0.034) and 2014 (χ2 = 14.29, df = 1, P = 0.001).
Mutations and allele frequencies of Vgsc-1014S in An. gambiae s.l. in IRS and control districts
A total of 360 An. arabiensis (136 in 2013 and 224 in 2014), 7 An. gambiae s.s. (1 in 2013 and 6 in 2014), 25 An. coluzzii (10 in 2013 and 15 in 2014) and 1 An. gambiae s.s./An. coluzzii hybrid adult from larvae collected in IRS districts were analysed for the Vgsc-1014S.
The mean allele frequencies of An. arabiensis populations from the IRS districts were 0.40 in transect 1 and 0.48 in transect 2 in 2013, and 0.22 and 0.30 respectively in these two sites in 2014. Additionally, in 2014, An. gambiae s.s. was predominantly found in transect 2, whereas the highest allele frequencies were recorded in transect 1 (Table 5).
In control districts, the Vgsc-1014S mutation was characterized in 260 An. arabiensis (74 in 2013 and 186 in 2014), 1 An. gambiae s.s. and 1 An. coluzzii in 2014. Only An. arabiensis populations in these districts carried Vgsc-1014S, with mean frequencies of 0.51 in 2013 and 0.49 in 2014 in transect 1, and 0.26 in 2014 in transect 2. Moreover, no specimen of An. coluzzii collected along these two transects carried the Vgsc-1014S (Table 5).
Comparison between districts along the transect 1 revealed significantly higher frequencies of allele 1014S in An. arabiensis populations from the control district compared to those found in Koungheul district in 2013 (0.51 versus 0.32; χ2 = 8.476, df = 1, P = 0.035) and (0.49 versus 0.28; χ2 = 13.682, df = 1, P = 0.001) in 2014. Additionally, frequencies of the 1014S allele found in An. arabiensis from the control district were significantly higher than those Koumpentoum district (χ2 = 13.568, df = 1, P = 0.001) in 2013 and (χ2 = 23.297, df = 1, P = 0.001) in 2014 (Table 5). However, along transect 2, no significant difference in frequencies of allele 1014S was observed between these two An. arabiensis populations (IRS and control) in 2014 (χ2 = 0.818, df = 1, P = 0.365) (Table 5).
Mutation and allele frequency of ace-1R (G119S) in An. gambiae s.l. in IRS and control districts
A total of 464 An. arabiensis (182 in 2013 and 282 in 2014), 57 An. gambiae s.s. (2 in 2013 and 55 in 2014) and 36 An. coluzzii (19 in 2013 and 17 in 2014) were analysed (Table 5). In both years, the frequencies of 119S allele were consistently low in both transects, ranging from 0.01 to 0.05 in An. arabiensis and from 0 to 1 in An. gambiae s.s. In contrast, higher frequencies were observed in An. coluzzii, particularly in Koungheul in 2013 (Table 5).
Phenotype and temporal frequencies of the 1014F allele in An. arabiensis, An. gambiae s.s. and An. coluzzii from IRS and control districts
The results of the mutation research, according to the phenotype, showed that across all IRS districts, the average frequencies of allele 1014F were generally higher in surviving An. arabiensis specimens compared to those found dead after exposure to DDT and pyrethroids (Table 6).
In An. gambiae s.s. populations, the mean frequencies of allele 1014F did not show a consistent increase in surviving specimens. However, for An. coluzzii, the frequencies of this allele were consistently higher in surviving specimens, regardless of the year or district considered (Table 6).
In control districts, molecular identifications indicated that the instead of the analysed mosquitoes were An. arabiensis. A total of 223 specimens (222 An. arabiensis and 1 An. coluzzii), out of which 138 surviving and 86 dead specimens were characterised for Vgsc-1014F mutation. The mean frequencies of allele 1014F were consistently higher in surviving than in dead An. arabiensis specimens (Table 6). In Control of transect 1, these differences were statistically significant in 2013 (Fisher’s exact test: OR: 0.09, 95% CI: 0.097–0.484; P = 0.008) and in 2014 (χ2 = 7.49, df = 1, P = 0.062) (Table 5). Additionally, the same trend was observed in 2014 in the control of transect 2 (χ2 = 12.267, df = 1, P = 0.01).
Phenotype and temporal frequencies of the 1014S allele in An. arabiensis, An. gambiae s.s. and An. coluzzii from IRS districts
For the Vgsc-1014S mutation, a total of 243 surviving An. arabiensis specimens (76 in 2013 and 167 in 2014) and 186 dead specimens (84 in 2013 and 102 in 2014) were successfully analyzed in IRS areas. Overall, the results showed that the allele 1014S was significantly more prevalent in surviving compared to dead specimens after exposure to pyrethroids in both 2013 (χ2 = 14.94, df = 1, P < 0.01) and 2014 (χ2 = 25.43, df = 1, P < 0.001) (Table 7).
Out of a total of 44 An. gambiae s.s. specimens analyzed, the frequency of allele 1014S was not statistically significantly higher in surviving specimens compared to dead (Fisher’s exact test: OR: 1.28, 95% CI: 0.236–6.53; P = 0.731) in 2014 (Table 7). Regarding An. coluzzii, all 13 dead specimens were homozygous susceptible, with 1 in 2013 and 12 in 2014. A total of 207 An. arabiensis in the control districts, were molecularly characterized and only An. arabiensis carried the Vgsc-1014S mutation.
The allele frequencies of 1014S in surviving specimens were two or three times higher than those found in dead specimens in 2013. However, in 2014, the frequency of allele 1014S in alive specimens collected in the control area of transect 1 doubled that of dead specimens, with a statistically significant difference (χ2 = 7.693, df = 1, P = 0.05).
Genotypes resulting from the Vgsc-1014F and Vgsc-1014S mutations
The analysis of genotyping results for both kdr mutations (Vgsc-1014F and Vgsc-1014S) revealed that the proportions of homozygous resistant individuals (Phenylalanine-Phenylalanine) and SS (Serine-Serine) were significantly lower in An. arabiensis populations collected from the districts treated with bendiocarb and pirimiphos-methyl than in those collected from controls areas. This difference was statistically significant (χ2 = 10.205, df = 1, P = 0.01) (Table 8).
Regarding the status, the proportions of resistant homozygotes FF and resistant homozygotes SS were higher in surviving specimens compared to dead specimens, whereas in susceptible homozygotes LL (Leucine-Leucine) were more prevalent. Among surviving An. arabiensis specimens exposed to pyrethroids, some specimens carried the allele 1014F or 1014S in the heterozygous resistant state (FS) or in the heterozygous susceptible state (LF, LS) (Table 8).
Comparative analysis of resistant homozygotes FF and resistant homozygotes SS in An. arabiensis populations indicated that the latter were more abundant in surviving specimens from control districts than those from IRS districts. In 2014, the difference between the proportion of surviving resistant homozygotes FF collected in the control area IRS and those collected in IRS districts was not significant (P > 0.05). The observed difference was statistically significant in transect 2 (Fisher’s exact test: OR: 0.11, 95% CI: 0.02–0.36; P < 0.001), whereas no significant difference was evident in transect 1. Additionally, surviving resistant homozygotes SS collected in control districts were significantly higher than those collected in IRS districts (Fisher’s exact test: OR: 0.08, 95% CI: 0.02–0.68; P = 0.07), regardless of the transect.
Methods
Study area
The study took place during 2013 and 2014 in the Sudano-Sahelian zone of central Sénégal, specifically within the groundnut basin (Fig. 4). Both IRS-treated health districts and untreated control districts were included in the study design.
The primary economic activity in the region is farming, with a focus on staple crops such as millet, maize, sorghum. Additionally, off-season crops like watermelon, market gardening, and cotton are grown in certain near rivers areas.
In the study area, An. gambiae s.s., An. arabiensis and An. funestus are main malaria vectors, and Plasmodium falciparum being the primary parasite responsible for malaria cases20. Insecticide applications were conducted within the health districts of Malem Hodar, Koungheul, Koumpentoum and Nioro. Specifically, treatments were done only in villages located within health posts that exhibited a high incidence of malaria (more than 15 cases per 1000 inhabitants), identified as “hot spots”. Control districts selected for the of IRS-treated districts were Ndoffane (to control for Nioro) along transect 1 (Kaolack-Gambia), and Kaffrine, which served as a control for Malem Hodar, Koungheul, and Koumpentoum along transect 2 (Kaolack-Tambacounda) (Fig. 4).
Insecticide formulations used
In 2013, IRS with bendiocarb were administered in all villages 21, however during the the actual year only hotspots with a confirmed malaria incidence exceeding 15 cases per 100 individuals were sprayed with pirimiphos-methyl. Bendiocarb was the insecticide used for treatment in eligible habitations within these districts.
In 2014, there was a strategic shift to increase efficacy, moving from bendiocarb to pirimiphos-methyl (Actellic® 300, CS), which was implemented in hot spot areas only except Ndrame Ndimb, which is under the jurisdiction of the Thilagran health post in Nioro District. In Ndrame Ndimb, Bendiocarb (FICAM WG 10%) was left for spraying.
Detailed data regarding spraying operations and the outcomes of insecticide applications in the monitored villages are presented in Table 9.
Insecticide susceptibility usingS WHO tube test
Susceptibility test was carried out following the WHO standard protocol22 using unfed females aged 3–5 days old, which were obtained from larvae collected and reared in the field until emergence. Six insecticides belonging to four different families were tested: pyrethroids (0.05% deltamethrin and 0.75% permethrin), organochlorines (4% DDT and 4% dieldrin), organophosphates (1% pirimiphos-methyl), and carbamate (0.1% bendiocarb).
For each insecticide, a minimum of 100 females of An. gambiae s.l. were tested in four batches of 25 females per tube. Two additional batches of 25 females were used as controls. Females were exposed to impregnated papers for one hour, during which the Knock-Down (KD) effect was recorded at 10, 15, 20, 30, 40, 50, and 60 min for pyrethroids and DDT.
After the exposure period, the mosquitoes were observed at 27 °C ± 2 °C temperature and 75% ± 10% relative humidity. Mortality was recorded 24 h post-exposure, and the results were validated if control mortality was less than 5%. If control mortality fell between 5 and 20%, it was corrected using the Abbott formula23. If control mortality exceeded 20%, the test was discarded and repeated. Following susceptibility tests, mosquitoes were morphologically identified under a binocular loupe using the key of Gillies and De Meillon24 and subsamples were individually stored at room temperature in tubes containing silica gel. All mosquitoes that survived exposure and a sample of dead specimens were subjected to molecular analysis.
Molecular identification and detection of kdr and ace-1R mutations
Genomic DNA extraction was performed using the CTAB 2% (Cetyltrimethylammonium bromide) method. The extracted DNA from the mosquito was then suspended in 200 µl of ultrapure DNA/RNA-free water (Invitrogen, 10,977 035) per sample. Prior to PCR (Polymerase Chain Reaction), a 1/10 dilution was carried out.
In the laboratory, surviving females exposed to pyrethroids, along with a batch of dead individuals, were molecularly identified using IMP-PCR (intentional mismatch primer-PCR) according the method described by Wilkins et al25. Additionally, they were genotyped for kdr mutations (Vgsc-1014F and Vgsc-1014S) using the technique outlined by Huynh et al26. Similarly, the search for ace-1mutation (Insensitive Acetyl cholinesterase: G119S) was performed on subsamples (survivors/deaths) of individuals tested with different classes of insecticides (bendiocarb, pirimiphos-methyl and pyrethroids) using the method developed by Weill et al27 as adapted by MR4 of CDC Atlanta (MR4, 2014).
Data analysis
All data collected during the study were entered into Microsoft Office 2010 Excel. Subsequently, a database was constructed after recording the mortality 24 h post-exposure and conducting morphological identification under a binocular loupe.
Percentage homogeneity tests were performed with Pearson’s chi-square (χ2) or Fisher test to statistically compare proportions. Statistical analyses, confidence interval calculations, significance tests and graphs were performed with R software version 4.2.2. The interpretation of susceptibility within the An. gambiae s.l. complex was validated based on the WHO criteria22.
Discussion
The insecticide susceptibility of vector populations and the impact of bendiocarb and then pirimiphos-methyl treatments on the allele and genotype frequencies of kdr mutations (Vsgc-1014F and Vsgc-1014S) and ace-1 were investigated in the IRS districts and their corresponding controls. In this study, An. gambiae complex populations tested in both transects showed resistance to DDT. Additionally, there was a suspected resistance to dieldrin in An. gambiae s.l. populations observed across all studied districts along the transect 1 and along the transect 2. Such phenotypic resistance to these insecticides has been previously reported in various studies by Hamon et al28. Other studies carried out in the country or in neighboring countries have also reported vector resistance to DDT and dieldrin, such as studies in Sénégal29,30,31, the Gambia32 and Côte d’Ivoire33.
However, regarding pyrethroids, this study showed a suspected and confirmed resistance to permethrin and deltamethrin in most districts, lasting for at least one year. Permethrin resistance was particularly pronounced in the populations An. gambiae s.l. of transect 2. Nonetheless, vector susceptibility to permethrin or deltamethrin was documented in Koumpentoum, Koungheul and Nioro districts. This variability in susceptibility of An. gambiae s.l. populations has been reported in previous studies34,35 and appears to be influenced by factors such as seasonality. These results could be associated, on the one hand, to a variation in the specific composition observed in districts of transect 2, which often depends on rainfall or the vector control strategies implemented32, and on the other hand to a dynamic variation in ecosystems in which a single species is found36. Moreover, resistance to permethrin and deltamethrin was more pronounced in control districts compared to IRS districts. Conversely, An. gambiae s.l. populations tested were susceptible to both bendiocarb and pirimiphos-methyl in most study districts, except those from the controls, where resistance to bendiocarb was observed.
The results also unveiled the presence of three species within the An. gambiae complex, An. arabiensis, An. gambiae s.s. and An. coluzzii, with An. arabiensis being predominantly collected of in larval collections. These results confirm those obtained by Diouf et al.30 in other sites in Sénégal. Hybrids An. gambiae s.s./An. coluzzii were identified in Koumpentoum and Nioro, although in relatively small proportions like previously reported in south-eastern Sénégal31 and the Gambia32. These finding species were as well as detected of both surviving and dead specimens An. gambiae s.l. after exposure to insecticides. This could be explained by the fact that mutations in genes encoding binding targets of insecticides are not the only ones involved in vector resistance to insecticides: other mechanisms are also involved.
Mutations (Vgsc-1014F, Vgsc-1014S and G119S) were more commonly identified in An. arabiensis and An. gambiae s.s. across IRS and control districts. However, An. coluzzii only carried the G119S mutation, with frequencies being relatively high, exceeding 50%.
There were more frequencies of ace-1 mutation in the transect 1. Conversely, the study detected very low frequencies of ace-1R gene in An. arabiensis and An. gambiae s.s. populations.
This observation regarding the ace-1 mutation may be attributed to pre-zygotic barriers to gene flow between species and minimal insecticide usage in the area primarily for food farming purposes, resulting in limited selection pressure on larval populations of An. gambiae s.l. Similar findings have been reported in previous studies37,38. Additionally, the ace-1 mutation was exclusively found in An. coluzzii. Kdr mutations were present across all three species, whereas in hybrids, only the Vgsc-1014F was detected. The 1014F allele frequencies were relatively low in An. arabiensis from both IRS and control districts, whereas 1014S allele frequencies were substantially higher in control districts, consistent with previous studies39. Conversely, kdr mutations were more frequently observed in IRS districts in An. gambiae s.s. and An. coluzzii, although statistical comparison of allele frequencies constrained due to the small sample sizes.
In recent years, the involvement of kdr mutations in conferring phenotype resistance among An. gambiae complex vectors to insecticides has been subject to debate in sub-Saharan Africa. While the link between kdr mutations and insecticide resistance has been demonstrated in some regions such as Sénégal30,40,39 and Côte d’Ivoire41,40, it hasn’t been clearly demonstrated universally. However, the findings of this study suggest a significant association between kdr mutations (Vgsc-1014F and Vgsc-1014S) and phenotypic resistance of An. arabiensis populations to pyrethroids and DDT. In Koumpentoum, significant association between these kdr and phenotypic resistance was observed in An. coluzzii populations. These results are in line with previous research conducted in Sénégal31 and Benin41. Moreover, in An. coluzzii, although the presence of the 1014F allele was noted in dead specimens, its significant prevalence in alive specimens could elucidate the involvement of the Vgsc-1014F mutation in the phenotypic resistance of this species42
Overall, the results of the research on kdr mutations indicates that the implementation of IRS with bendiocarb and subsequently with pirimiphos-methyl appears to lead to a reduction in the frequencies of alleles 1014F and 1014S, as well as in the proportions of resistant genotype (FF and SS) within An. arabiensis populations in IRS-treated compared to control districts. Similar findings were reported in Uganda38.
This outcome suggests that the use of IRS, particularly with bendiocarb and pirimiphos-methyl, in areas where pyrethroid resistance is prevalent, could potentially restore susceptibility or reduce the presence of resistant genotype (FF and/or SS) specimens, there by maintaining the efficacy of pyrethroid-based control strategies.
Conclusion
This study highlights the impact of IRS using bendiocarb and pirimiphos-methyl on An. gambiae s.l. resistance dynamics in Sénégal. Although resistance to DDT and pyrethroids was widespread, increased susceptibility to these insecticides was observed in IRS-treated districts, suggesting a beneficial effect of these insecticides in mitigating resistance. As both coumpound target acetylcholinesterase, the relatively low frequency of ace-1 (119S) particularly in An. arabiensis may explain their continued efficacy. The decline in kdr allele frequencies and resistant genotypes in An. arabiensis further supports the value of rotating or combining insecticides with different targets. These findings underscore the importance of monitoring kdr and ace-1 markets to guide IRS strategies and sustain vector control effectiveness.
Data availability
All data generated or analyzed during this study are included in this published article.
References
WHO. World Malaria Report. Geneva. World Health Organization. (2016).
WHO. Conditions for deployment of mosquito nets treated with a pyrethroid and piperonyl butoxide. Geneva: World Health Organization. (2017).
Baffour-Awuah, S. et al. Insecticide resistance in malaria vectors in Kumasi, Ghana. Parasit. Vectors 9, 633 (2016).
Keïta, M. et al. Susceptibilité d’Anopheles gambiae sensu lato aux insecticides communément utilisés dans la lutte antivectorielle au Mali. Bull Soc Pathol Exotic. 109, 39–45 (2016).
Keita, K. et al. Species identification and resistance status of Anopheles gambiae s.l. (Diptera: Culicidae) mosquitoes in Guinea. J. Med. Entomol. 54, 677–681 (2017).
Awolola, T. S. et al. Pyrethroids resistance intensity and resistance mechanisms in Anopheles gambiae from malaria vector surveillance sites in Nigeria. PLoS ONE 13 (12), e0205230 (2018).
Pwalia, R. et al. High insecticide resistance intensity of Anopheles gambiae (s.l.) and low efficacy of pyrethroid LLINs in Accra, Ghana. Parasit. Vectors. 12, 299 (2019).
Wat’senga, F. et al. Intensity of pyrethroid resistance in Anopheles gambiae before and after a mass distribution of insecticide-treated nets in Kinshasa and in 11 provinces of the Democratic Republic of Congo. Malar. J. 19, 169 (2020).
WHO. World Malaria Report. Geneva. World Health Organization. (2019).
Mathias, D. K. et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malar. J. 10, 10 (2011).
Reid, M. C. & McKenzie, F. E. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar. J. 15, 107 (2016).
N’Guessan, R., Corbel, V., Akogbéto, M. & Rowland, M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area. Benin. Emerging. Infect. Dis. 13 (2), 199 (2007).
Toé, K. H. et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect Dis. 20 (10), 1691 (2014).
Sovi, A. et al. Impact of operational effectiveness of long-lasting insecticidal nets (LLINs) on malaria transmission in pyrethroid-resistant areas. Parasit. Vectors. 6, 319 (2013).
Lindblade, K. A. et al. A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi. Malar. J. 14, 31 (2015).
Ranson, H. et al. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control?. Trends. Parasitol. 27, 91–98 (2011).
Viana, M., Hughes, A., Matthiopoulos, J., Ranson, H. & Ferguson, H. M. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc. Natl. Acad. Sci. 113, 8975–8980 (2016).
Thomas, M. B. & Read, A. F. The threat (or not) of insecticide resistance for malaria control. Proc. Natl. Acad. Sci. 113, 8900–8902 (2016).
WHO. Global plan for insecticide resistance management in malaria vectors. Geneva. https://www.who.int/malaria/publications/atoz/gpirm/en/. (2012).
Trape, J. F. et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 51, 123–137 (1994).
Lo, C. et al. Evaluation of the residual efficacy of indoor residual spraying with bendiocarb (FICAM WP 80) in six health districts in Senegal. Malar. J. 18, 198 (2019).
World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. (2016).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Ecol. Entomol. 18, 265–267 (1925).
Gillies, M. T., De Meillon B., The Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region). In johannesburg: Publications of the South Africa Institute for Medical Research. 343 (1968).
Wilkins, E. E., Howell, P. I. & Benedict, M. Q. IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar. J. 5, 125 (2006).
Huynh LY, Sandve SR, Hannan LM, Van Ert M, Gimnig JE. Fitness costs of pyrethroid insecticide resistance in Anopheles gambiae. In: Annual Meeting of the Society for the Study of Evolution. Christchurch, New Zealand (2007).
Weill, M. et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect. Mol. Biol. 13, 1–7 (2004).
Hamon, J. & Garrett-Jones, C. La résistance aux insecticides chez des vecteurs majeurs du paludisme et son importance opérationnelle. Bull. OMS. 28, 1–24 (1963).
Niang, E. A., Konaté, L., Diallo, M., Faye, O. & Dia, I. Patterns of insecticide resistance and knock down resistance (kdr) in malaria vectors An. arabiensis, An. coluzzii and An. gambiae from sympatric areas in Senegal. Parasit. Vectors. 9, 71 (2016).
Thiaw, O. et al. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal treated nets universal coverage for 10 years. Malar. J. 17, 123 (2018).
Diouf, E. et al. Multiple insecticide resistance target sites in adult field strains of An. gambiae (s.l.) from southeastern Senegal. Parasit. Vectors 13, 567 (2020).
Opondo, K. O. et al. Status of insecticide resistance in Anopheles gambiae (s.l.) of The Gambia. Parasit. Vectors. 12, 287 (2019).
Camara, S. et al. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasit. Vectors. 11, 19 (2018).
Ranson, H. et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar. J. 8, 299 (2009).
Abdalla, H. et al. Insecticide resistance in Anopheles arabiensis in Sudan: temporal trends and underlying mechanisms. Parasit. Vectors. 7, 213 (2014).
Diabate, A. et al. Role of agricultural use of insecticides in resistance to pyrethroids in anopheles gambiae s.l. in Burkina Faso. Am. J. Trop. Med. Hyg. 67 (6), 617–622 (2002).
Amoudji, A. D. et al. Insecticide resistance profiles of Anopheles gambiae s.l. in Togo and genetic mechanisms involved, during 3-year survey: Is there any need for resistance management?. Malar. J. 18, 177 (2019).
Abeku, T. A. et al. Insecticide resistance patterns in Uganda and the effect of indoor residual spraying with bendiocarb on kdr L1014S frequencies in Anopheles gambiae s.s.. Malar. J. 16, 156 (2017).
Gueye, O. K. et al. Insecticide Resistance Profiling of Anopheles coluzzii and Anopheles gambiae Populations in the Southern Senegal: Role of Target Sites and Metabolic Resistance Mechanisms. Genes 11, 1403 (2020).
Ahoua, A. L. et al. Status of pyrethroid resistance in Anopheles gambiae s.s. M form prior to the scaling up of long-lasting insecticidal nets (LLINs) in Adzopé, Eastern Côte d’Ivoire. Parasit. Vectors. 5, 289 (2012).
Djègbè, I. et al. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar. J. 10, 61 (2011).
Edi, C. A. V. et al. Long-term trends in Anopheles gambiae insecticide resistance in Côte d’Ivoire. Parasit. Vectors. 7, 500 (2014).
Acknowledgements
We sincerely thank the PMI (President’s Malaria Initiative) and the National Malaria Control Programme in Senegal.
Funding
This study was supported by PMI (President’s Malaria Initiative) monitoring activities in Senegal.
Author information
Authors and Affiliations
Contributions
E.D., L.K., O.F and I.D. conceived and designed the study; E.D., M.W.S., M.D.S., A.N., A.K. and O.N collected the samples. ED., M.W.S. performed the laboratory analyses; E.D., L.K, I.D and E.A.N analyzed the data, wrote the manuscript with contributions from E.D., L.K. and I.D. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional Information
The original online version of this Article was revised: The original version of this article was published with an outdated version of Figures 1, 2 and 4, and Figure 5 contained a display error on panel D. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Diouf, E.H., Senghor, M.W., Sy, M.D. et al. The impacts of indoor residual spraying with bendiocarb and pirimiphos-methyl on allelic frequencies of kdr and ace-1 mutations in central Senegal. Sci Rep 15, 31239 (2025). https://doi.org/10.1038/s41598-025-12052-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12052-0