Abstract
The article presents a milli-fluidic device and electronic extrusion system for fabricating alginate/carbon nanotube microfibers using calcium chloride as a crosslink agent. The device was designed, simulated, and prototyped, and successfully applied the mixture of alginate and carbon nanotube to generate microfibers in different concentrations and stepper motor speeds. The flow extrusion pump was used to pump fluid flow to the milli-fluidic device and crosslink calcium chloride to form sodium alginate-CNT microfibers. The microfibers were fabricated and characterized using techniques like FE-SEM, FTIR, Raman Spectroscopy, and XRD. The results showed the material’s amorphous crystallinity and the composite nature of the fibbers, consisting of alginate as the matrix and CNTs as the reinforcing, conductive filler. The invented technology successfully generated microfibers with sizes ranging from 60 to 100 μm and I-V measurements were tested. The study demonstrates the potential of the developed system for fabricating conductive microfibers with properties relevant to nerve tissue engineering. However, further biological validation is required to confirm their suitability for nerve repair applications. The findings have significant implications for the design of scaffolds in regenerative nerve therapies. These findings support the potential of the fabricated microfibers as conductive scaffolds for nerve tissue engineering, although further biological validation is required.
Similar content being viewed by others
Introduction
The need for advanced nerve tissue engineering solutions is evident in the face of the persistent challenges associated with peripheral nerve injuries. Traditional strategies for nerve repair have evolved to include more sophisticated approaches like nerve- guided catheters, which combine scaffolding and cells, and have shown potential in promoting significant functional recovery. Additionally, non-surgical treatments such as laser irradiation and traditional Chinese medicine have been explored for their neurogenerative effects. These advancements highlight the urgency and potential in developing new materials and techniques for nerve repair and regeneration1.
Carbon nanotubes have garnered attention in nerve regeneration research due to their unique properties. However, current strategies for peripheral nerve regeneration, including those involving CNTs, face challenges. Despite advancements in surgical reconstruction and postoperative rehabilitation, patients often experience lingering motor and sensory deficits. This underscores the need for novel therapeutic strategies and technologies that can effectively promote nerve regeneration and enhance functional outcomes2. The fabrication of the alginate and CNT has garnered significant attention in recent years due to highly stable nanomaterials that exhibit remarkable mechanical and electrical characteristics, including exceptional tensile strength and flexibility. Fabrication technology has witnessed remarkable advancements over the past few decades, enabling precise control and manipulation of materials at the micro- and nanoscale3. Milli-fluidics has emerged as a powerful platform for various biological studies due to its ability to mimic physiological conditions and its high-throughput capabilities4. This technique offers precise control over the diameter and cross- sectional shape of microfibers through the manipulation of microchannel device size, geometry, and flow rate ratio5. Milli-fluidics enables the continuous production of fibers with highly tunable geometries and mechanical properties6. Traditional microfiber fabrication methods, such as electrospinning, often face challenges in achieving consistent fiber diameter, morphology, and uniform distribution of nanomaterials7. Additionally, electrospinning may not be suitable for incorporating hydrophilic materials like alginate due to their tendency to clog the spinneret8. Numerous techniques have been suggested to produce carbon nanotube (CNT) microfibers. The three most established methods are: the direct growth of CNTs through chemical vapor deposition9,10,11; spinning CNT fibers from nanotube forests12,13, wet spinning from solutions containing CNTs and surfactants14,15.To address the limitations of traditional methods, this thesis proposes milli- fluidic approach for fabricating alginate and carbon nanotube (CNT) microfibers. This method utilizes a milli-fluidic device to control the flow of alginate and CNTs, enabling the production of microfibers with consistent diameter, morphology, and uniform CNT dispersion16. The milli-fluidic environment facilitates uniform dispersion of CNTs within the alginate matrix, preventing clumping and clogging. This uniform dispersion is crucial for achieving the desired properties of the microfibers, such as electrical conductivity and mechanical strength17.
The proposed milli-fluidic approach offers several advantages over traditional methods such as precise control over microfiber diameter and morphology, the microchannel dimensions and flow rates can be precisely controlled to achieve consistent microfiber characteristics18. This level of control is difficult to achieve with traditional methods, such as electrospinning, which can be susceptible to variations in fiber diameter and morphology due to factors such as environmental conditions and spinneret clogging19. The second advantage is the uniform CNT dispersion, the milli-fluidic environment facilitates uniform dispersion of CNTs within the alginate matrix, preventing agglomeration and clogging20. This uniform dispersion is essential for achieving the desired properties of microfibers, such as mechanical strength and electrical conductivity. Traditional methods, such as electrospinning, can be prone to CNT agglomeration, which can negatively impact the properties of the microfibers21,22,23,24. The third advantage is the scalability, the milli-fluidic device can be easily scaled up for large-scale production of microfibers. This scalability is important for commercial applications of microfibers, such as in tissue engineering and drug delivery. Traditional methods, such as electrospinning, can be time-consuming and difficult to scale up for large-scale production24– 26. An electronic extrusion system integrated with the milli- fluidic device to enable real-time monitoring and control of the fabrication process was proposed in27. This integration allows for precise control of fluid flow rates, chemical concentrations, and crosslinking durations, ensuring consistent microfiber properties28. The fabrication of microfibers using milli-fluidic technology enables precise control over the diameter and morphology of the microfibers, leading to consistent and tunable properties. This level of control is challenging to achieve with traditional methods29,30,31.
The alginate and CNT microfibers hold promise for various applications due to their unique properties, including biocompatibility, biodegradability, and electrical conductivity. These properties make them suitable for a range of applications in tissue engineering, biomedical devices, and environmental remediation32. In the field of tissue engineering, alginate and CNT microfibers can serve as scaffolds for nerve regeneration. These microfibers mimic the extracellular matrix (ECM), providing a supportive environment for neural cell growth and differentiation33. The biocompatibility and biodegradability of alginate allow it to be gradually degraded as the nerve tissue regenerates, while the electrical conductivity of CNTs promotes nerve cell adhesion and outgrowth34. Alginate and CNT microfibers can also be used for drug delivery applications, as they can be loaded with drugs and then implanted into the body, where they can slowly release the drugs in a controlled manner. This targeted drug delivery approach can reduce systemic side effects and improve drug efficacy35,36,37,38. In biomedical devices, alginate and CNT microfibers can be used to create implants, biosensors, and diagnostic tools39,40,41,42,43. For example, alginate and CNT microfibers can be used to fabricate neural electrodes for recording neural activity or stimulating nerve cells. The electrical conductivity of alginate and CNTs allows for efficient transfer of electrical signals between the electrodes and the nervous system, and CNT microfibers can also be used in environmental remediation applications44. In this study, the application proposed for the fabricated microfibers of alginate and carbon nanotube is nerve regulation because the carbon nanotube microfibers (CNT) will be used to repair damaged nervous system tissue. Damaged nerve tissues are reconnected by biopolymer, but these biopolymers are non-electrically conductive, so we need CNTs to connect the signals between the tissues, as CNTs are conductive materials. The electrical conductivity of CNTs can promote nerve cell adhesion and neurite outgrowth45. The mechanical strength and porosity of the CNT microfibers provide support for nerve cells and promote their growth and differentiation46,47,48,49. Hence, the researchers are continuing to explore the potential of CNT microfibers for nerve regulation applications, and there is great promise for these materials in the future. This study represents a comprehensive exploration of the development process, covering the design, simulation, fabrication, and characterization of the fabricated CNT microfibers. It delves into the intricacies of milli-fluidic technology, nanomaterials, and electronic control systems, with the goal of contributing to the advancement of materials science and related fields. The milli-fluidic device was designed using Google SketchUp software, simulated using COMSOL Multiphysics software, and then printed using a 3D printer and PDMS replication, while the electronic extrusion system was designed to control the flow of fluids and the addition of chemicals to the milli-fluidic device, including the crosslinking agent (CaCl2) to obtain the microfibers of alginate and CNT, which are then applied to the nerve regulation application.
Damaged neuron-cell communication and nerve degeneration are the prevalent manifestations of nerve injury50. The human brain consists of a vast assembly of meticulously arranged neurons, each executing special functions and facilitating communication with the body via the nervous system. Neurons, the primary nerve cells in the brain, transmit information through a combination of electrical and chemical signals throughout the nervous system51. Tissues in the central nervous system (CNS) are unique because they cannot be restored. Regeneration of neural tissue is hampered by its dynamic nature, difficulties in repairing the blood-brain barrier, and disruption of secondary tissue52. Therefore, it’s important that any method aiming to repair the central nervous system mainly focuses on copying injured axons, fixing nerve signals, and growing new neurons. When the central nervous system is damaged, it often causes astrocytosis, where numerous astrocytes are produced. At the same time, these reactive astrocytes can harm both nerve and non-nerve cells53. This makes it impossible to produce axons in neurons and restore nervous tissue in a balanced manner. Injuries during orthopedic surgery can also harm nerves. The reaction of nerves to severe injuries can lead to nerve function failure. First, problems with nerve function may manifest as coordination issues or difficulty recalling names. These issues can worsen if many neurons break down. Depending on the type of nervous system damage, restoring the activity of damaged nerves or treating damage from neurodegenerative disorders is a significant concern in the field of biomedicine54. Neuronal development involves creating, fixing, or substituting weak neurons and nerve structures. This includes generating new axons, synapses, neurons, glia, and myelin sheaths. For materials applied to nerve tissue, they need to conduct electricity to support cell production, break down naturally, and have bioactivity for delivering growth factors. Neurodegenerative disorders typically occur because the neural network is damaged, leading to brain disorders55. The combination of sodium alginate CNT has not been widely explored in the context of nerve regulation48,49,52. The advantages over existing materials, such as enhanced nerve cell adhesion and neurite outgrowth, along with the alginate and CNT microfibers exhibiting improved electrical conductivity compared to traditional biomaterials, promote nerve cell adhesion and neurite outgrowth. This enhanced conductivity is crucial for interfacing with the nervous system and facilitating nerve regeneration17,56,57.
In this study, we proposed a new method to produce CNT microfibres in which alginate and carbon nanotubes were combined and mixed to fabricate sodium alginate-CNT microfibres. We use the milli-fluidic device platform and the electronic extrusion system to carefully control the flow rate and concentration of the sodium alginate-CNT solutions. This allows us to produce uniform sodium alginate-CNT microfibres, which we can then use in nerve regulation system applications to repair nervous tissue damage. Damage nervous tissue that is reconnected by biopolymers, but these biopolymers are non-electrically conductive (insulators), which means they are not providing a high surface area for volume, and it’s not small enough. Therefore, we need the intervention of nanomaterials, specifically carbon nanotubes. These nano-conductive materials are used because, first, they are electrically conductive to provide signals to the cells, have a high surface area over a high surface volume, and are small enough to facilitate the diffusion of nutrients and growth factors. This allows for the restoration of electrical conductivity signals between the damaged nervous tissues. Secondly, the nano size of the nanotubes allows for the diffusion of nutrients and growth factors due to their porous nature, and thirdly, they do not integrate well with the cells. However, when combined with alginate, an FDA-approved non-conductive material, the biocompatibility of carbon nanotubes increased.
Design constraint and parameter
The study employs a structured research method to establish, design, develop, and characterize experiments, as illustrated in flow chart Fig. 1. It begins with an overview of the experimental framework, followed by detailed steps in the creation and assessment of the milli-fluidic devices and microfibres. The methodology includes the design of a 3D mold for millifluidic devices and the fabrication of a specialized millifluidic extrusion head. It also includes the simulation processes for milli-fluidics and the making of PDMS milli-fluidic devices, which shows how precise and efficient these steps need to be. We also discuss the development of an extrusion pump, which plays a pivotal role in the controlled delivery and application of materials in the experiments. A key focus is on the generation of microfibres composed of alginate and carbon nanotubes, which are essential for the study’s objective. The characterisation process employed a variety of analytical techniques, including FTIR, Raman spectroscopy, XRD, SEM, and I-V measurement. Each of these methods provides critical insights into the composition, structure, and properties of the synthesised microfibres. Lastly, the chapter details an experiment to apply these sodium alginate-CNT microfibers in simulating nerve fibers, which is central to understanding their potential applications in biomedical contexts. This approach exemplifies a comprehensive methodology that combines advanced fabrication techniques with detailed analytical assessments, aiming to contribute significantly to the field of nerve tissue regeneration research.
The establishment of experiments
The establishment of experiments. Table 1 consists of several key components, including a design Mold, Multiphysics COMSOL modelling, PDMS fabrication, the development of an extrusion pump, and various spectroscopy techniques, such as Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy, as well as X-ray diffraction and I-V measurements. We used the design mould to shape the final system into the desired shape, and the PDMS fabrication process created a flexible polymer material known as polydimethylsiloxane. The extrusion pump was used to deliver fluids to the sample being studied, while the various spectroscopy techniques were used to analyse the chemical composition and structure of the sample. We used X-ray diffraction to study the crystal structure of a material. All these components significantly contribute to the experimentation table, enhancing our understanding of the sample’s properties.
Design of the 3D mold for making milli-fluidic device
The methodology for designing the 3D Mold is shown in Fig. 2. The 3D mould design process began with the conceptualisation and planning of the design. This included determining the dimensions, shapes, and specific features of the Mold. We created it using Google Sketchup 2021, a 3D modelling software. This software enabled the creation of a detailed 3D model of the mould, enabling its viewing and manipulation from all perspectives. After completing the design, we exported it as an STL file, a commonly used file format for 3D printing. We then imported the STL file into the Ultimaker Cura software for slicing. The Ultimaker Cura software was used to generate G-code of the 3D model for printing the instructions that the 3D printer followed to build the object. The G-code contained information about the exact location and shape of each layer of mold, as well as the temperature and speed of the printer. The 3D printer received the G-code, interpreted the instructions, and proceeded to construct the object. This process took from 15 min to 20 min to complete. After the object finished printing, it was removed from the printer, and then post-processing was performed. This included cleaning, smoothing, and painting the surface of the mold. Finally, the process ended with the final 3D mould being ready for injection into the alginate and carbon nanotube.
Fabrication of a milli-fluidic extrusion head dimension
The device was fabricated using design specifications, which included a length of 50 mm, width of the channels 1 mm, height 50 mm, radius of the inlet and outlet 2.5 mm, radius of the interaction channel 6 mm, and overall device dimensions of 160 mm as shown in Fig. 3. Table 2 characterized and explained the technical parameters of the proposed milli-fluidic device.
Simulation of the milli-fluidic device
Modelling and simulation of the milli-fluidic device were carried out in COMSOL Multiphysics 5.6, which was run on a computer with the following configuration: Intel Core i5 CPU at 2.4 GHz, 8 GB of RAM, and Windows 10 64-bit. The two-phase flow model, set to a flow rate of 0.130 (kg/s), demonstrates that increasing the continuous phase velocity reduces the droplet size. Figure 4 shows the model geometry of a 3D milli-fluidic device. Prior to employing the modeling equation, the fluid characteristics were established with a density of 1000 kg/m³ and a dynamic viscosity of 1 Pa*s [244], and the boundary condition was configured to adhere to a no-slip condition. These fluid parameters align with the specifications outlined in the Handbook of Pharmaceutical Excipients [244]. The configuration of fluid parameters indicates the utilisation of water-based fluids in the simulation for mixing purposes.
The imposition of a no-slip boundary condition elucidates the hydrophobic nature of the material employed in the fabrication of the milli-fluidic device. In this study, hydrophobic polydimethylsiloxane (PDMS) was chosen as the material for milli-fluidic fabrication. The milli-fluidic device consists of a main channel with inlet and outlet. The inlet is located at the top and bottom of the device, and the outlet is located at the bottom right corner.
The dynamic viscosity was increased due to the presence of Sodium alginate (Alg) and CNTs. The given range of 998–1000 mPa.s used as a starting point for the Sodium alginate (Alg). The presence of CNTs would likely increase this value further, especially since the CNTs create a network structure within the fluid, which significantly increased the viscosity. The density was estimated 1000 kg/m³ because the increase of density due to dissolved alginate and suspended CNTs. It can be calculated by:
where Δρ accounts for the increased density due to dissolved alginate and suspended CNTs. Figure 5 shows the simulation process of milli-fluidic device. The milli-fluidic was designed based on the requirements to generate extrusion microfibers. The milli- fluidic device was designed in Google Sketch Up 2021and imported as *.dxf file format into COMSOL Multiphysics version”5.6 software. The design was based on the physics of creeping flow and diluted species movement, and a fine-sized element was chosen for the milli-fluidic device’s mesh network. To mimic highly fine meshing, the model was generated for fluid mixing after the meshing procedure was completed by setting inlet 1 to 0 mol/m3. Inlet was set to 0 mol/m3 as a reference point or a control for the fluid mixing simulation. It serves as a baseline for comparison with the inlet, which was set to 1 mol/m3. By having one inlet set to a known concentration, the simulation can accurately model and predict the mixing and distribution of the fluid in the system.
Additionally, setting the inlet to 0 mol/m3 allows the simulation to also study the transport, which is often used to understand the flow dynamics of the fluid. After the simulation was finished, the findings were shown for outlet as both quantitative values and a graphical representation of concentration gradients (mol/m3). Flow rates were ranging from 10 mg/s to 4500 mg/s with 50 mg/s were used to investigate the influence of flow rate. The flow characteristics inside the milli-fluidic device and how molecules within the fluids respond are characterized by two dimensionless numbers, Reynold’s and Péclet’s number. The Reynolds number was calculated by adding the Reynolds equation. Re = (ρ * U * L) / µ where: ρ is the fluid density (kg/m3), U was characteristic velocity (m/s), L is the characteristic length (m), µ is the dynamic viscosity (Pa·s), The simulation and evaluation along the milli-fluidic device layout’s boundary. The Péclet number of the milli-fluidic can be approximated by adding expressions of Péclet. The Péclet number is a dimensionless quantity that describes the relative magnitude of convective transport to diffusive transport in a fluid system. It is calculated using the following equation Pe = (U * L) / (D) where: U is the characteristic velocity (m/s), L is the characteristic length (m), D is the diffusion coefficient in (m2/s). Appendix B represents diffusion coefficient (D), velocity magnitude (V), and length of the pathway (L) as designed to the value of diffusion coefficient and velocity magnitude of flow in the milli-fluidic. It is obvious that the design met the two requirements for a passive milli-fluidic device by determining the values Re and Pe. The process of the simulation of milli-fluidic Table 3 shows the parameters used in Eq. 2.1 to get Reynolds number. In COMSOL Multiphysics version 5.6 the finely meshed milli-fluidic device design was shown in Fig. 5. The main purpose of the milli-fluidic device was to control the flow rate and, in turn, influence the size and characteristics of the extrusion microfibers generated within the device. The milli-fluidic system was designed to achieve this control by carefully simulating and manipulating the flow dynamics of the fluid within the device. To achieve this, the simulation considers parameters such as flow rate, fluid density, dynamic viscosity, and boundary conditions.
Fabrication of PDMS milli-fluidic device
Figure 6 shows the process of the fabrication of a milli-fluidic device using a PDMS cured using curing agent at a ratio of 10:1 began with the creation of a 3D printed mold. This mold served as the template was created using a 3D printer for making the PDMS milli-fluidic device. Next, a mixture of PDMS cured using curing agent was prepared and poured into the mold. The mixture was mixed in a specific ratio of 10:1 and were pasted the printed mold on the glass then put the PDMS on the whole mold. The PDMS was cured using curing agent and left polymerized at room temperature. The PDMS cured using curing agent was then kept at the vacuum chamber for 24 h to ensure there were no visible bubbles. The last step was putting the oven at 60 Ċ for 1 h to solidify the mold. If the mixture did not fill the mold correctly or if there were any defects, the process was repeated starting with the creation of a new 3D printed mold. However, if the mixture filled the mold correctly and there were no defects, the process moved on to the final mold step. In this step, the PDMS was cured and then removed from the 3D printed mold to form the final milli-fluidic device. Once completed, the final milli- fluidic device was ready for use.
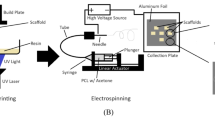
The development of the extruder pump
The stepper motor was controlled by the electronic extrusion system using an Arduino- Uno microcontroller. As a result, the linear slide method was used to drive the syringe into the microchannel when creating the electronic extrusion system. The hardware component of the electronic extrusion system was designed to continuously push and draw alginate from 5 ml syringes at different flow rates (5 to 10 ml/min). Figure 6 shows the overall schematic diagram of the connection of the extrusion system model to the milli-fluidic device for generating sodium alginate-CNT microfibers. Instead of a ball screw, a conventional lead screw was utilized in the extrusion system to minimize costs and meet the necessary conditions (performing fluid dispersion). The rotation of the lead screw served as a linear slide to push the attached 5 ml syringes from the syringe holder driving the linear slider, a 12 V unipolar stepper motor was coupled to the lead screw through a motor coupler. The BL-TB6600-V1.2 D412 motor driver was powered by a 12 V direct current (DC) adapter and a maximum current of 5 (A) for the electronic component. Additionally, the Arduino uno microcontroller received 12 V DC power.
As shown in Fig. 7, the motor driver serves as the connection between the control circuits and the motors. The microcontroller’s programming code was used to regulate the stepper motor’s speed and direction. The ENA (+/-) line is a vital element in motor control systems as it provides a means to activate or deactivate the motor driver, allowing for greater control over the motor’s operation and conserving power when the motor is not in use. The pulsed signals produced by the microcontroller are inputs that cause the stepper motor to move. (CLK-), (DIR-) and (5 V+) were connected to the 5 V power source as the direction’s positive pins. The negative pin of clock (CLK-) was connected to the Arduino Uno’s digital pin (3), the negative pin of direction (DIR-) was connected to the Arduino Uno’s D4, and the Arduino uno’s 5 V + was connected to 5 V + microcontroller. The motor driver’s alphabet pins were used to identify and connect the stepper motor’s input pins (A+, A-B+, and B-). The Arduino uno digital pins (7, 8, 9, 10, and 11) were connected to the buttons 1, 2, 3, and 4, respectively. Buttons 1, 2, 3, and 4 represent (100 rpm, 150 rpm, 200 rpm and reverses), respectively. The reset button, meanwhile, was simply wired to ground and the reset pin of the microcontroller. All the connections were constructed in fritzing software. Figure 8 shows the circuit diagram of the extrusion pump system. The system begins by presenting a menu interface and asking the user to enter the flow rate.
The system relies on an active “HIGH” signal, so we pressed a push button to start the extrusion procedure. We used the user-selected flow rate selection push button to regulate the infusion’s flow rate, the code to define each input pin, and the library to connect the Arduino Uno stepper motor driver. Upon receiving the “HIGH” signal from the switches, the Arduino Uno activated the step size based on the selected flow rate, thereby infusing liquid into the milli-fluidic device. Figure 9 displays the coding that determines the status of the reverse pushbutton when the user presses it. The program declares the set of variables, activates input/output pin D mode, and reads the states of the pushbuttons (Reverse, 100, 150, and 200 rpm). Programmable step sizes were available for each push button, enabling the stepper motor to rotate. The stepper motor’s step angle was set at 1.8° for each step. To complete a 360° rotation, 200 steps were required. Setting 1/8 micro steps allows the TB6600 stepper motor driver to set 1600 micro steps. A positive step size number allows for rotation, which infuses liquid into the milli-fluidic device in a clockwise direction. Meanwhile, a negative step size number causes an anticlockwise spin, which diffuses or reverses the direction of the syringe’s plunger. The rotation angle increases as the number of steps increases, causing the linear slider to advance and force the liquid into the milli-fluidic device. The user performs a specific function when they press a push button.
Generation of microfibers of alginate carbon-nanotubes
The stepper motor library in Arduino was already pre-written and simple codes were used in the Arduino software to control the stepper motor’s output. To determine the specific step sizes required for the motor to rotate, a calibration process was conducted by adjusting the step size 100, 150 and 200 rpm and observing the effect on the rotation speed or RPM. The rotation speed of the stepper motor was manually measured during this process. The amount of liquids purged in minutes was determined by the RPM of the stepper motor that rotated the lead screw in both clockwise and anticlockwise directions. It was important to calibrate the rotation speed value of the stepper motor according to the flow rates of the electronic extrusion system to ensure the proper amount of liquid was fed into the milli-fluidic device when the user selected the desired flow rate. The process began by choosing a flow rate of 100, 150 and 200 rpm and allowing the liquid to purge using a 5.0 ml syringe. The amount of liquid purged was then measured and recorded, along with its respective rotation speed value for the chosen flow rate. Two types of solutions were prepared for producing microfibers. First, the alginate in concentrations of (0.05 g/L, 0.06 g/L and 0.07 g/L) to 10 ml of distilled water and mixed for 10 min, and carbon nanotubes (0.05 g/L, 0.06 g/L and 0.07 g/L) concentrations were then added to the solution and mixed for an additional 10 min. The second solution was prepared by adding carbon nanotubes (0.05 g/L, 0.06 g/L and 0.07 g/L) to 10 ml of distilled water and mixing for 10 min as shown in Fig. 10. Then, calcium chloride was added to 10 ml of distilled water and mixed for an additional 10 min. The solutions, alginate and carbon nanotube were then extruded together through a syringe needle with a diameter of 0.5 mm and collected in a solution of calcium chloride per 10 ml distilled water. The formed microfibers were then washed with distilled water and dried in the oven at 60 degrees Celsius for 24 h. The properties of the microfibers were characterized by measuring their diameter, and conductivity. The diameter of the microfibers was measured using image-J software. The conductivity of the microfibers was measured using a 2-point probe measurement system for determining the I-V curve of the samples. on the properties of the microfibers.
Characterization of alginate -carbon nanotube microfiber
All samples used for characterization, including SEM, FTIR, Raman, XRD, and I–V measurements, were prepared in standard dimensions of 2.5 cm × 2.5 cm to ensure consistency and comparability across tests. To ensure a uniform dispersion of carbon nanotubes and prevent agglomeration, the CNTs were sonicated in distilled water for 20 min using an ultrasonic bath operating at 40 kHz before being mixed with the alginate solution. All quantitative data were analyzed using one-way ANOVA followed by Tukey’s post hoc test to determine statistical significance across experimental groups. A p-value of < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism 9 (GraphPad Software, CA, USA), and results are reported as mean ± standard deviation (SD) based on three independent replicates (n = 3).
Fourier transform infrared spectroscopy FTIR
The study used FTIR to analyze the structural properties of fabricated alginate microfibers made of carbon nanotubes and calcium chloride (Fig. 11(a)). The FTIR analysis was performed using a PerkinElmer Spectrum 100 spectrometer with a diamond ATR accessory. The sample was placed on an attenuated total reflectance window and irradiated with a mid-infrared beam. The absorption spectrum was recorded over a range of wavelengths. The FTIR spectra were used to determine the functional groups present in the alginate/CNT microfibers, including hydroxyl, carboxyl, and amine groups. The spectra also assessed the degree of crosslinking within the microfibers and any changes in structural properties after the addition of carbon nanotubes and calcium chloride.
Raman spectroscopy
Raman spectroscopy (Horiba Scientific Xplore Plus) in this context, is specifically used to observe vibrational modes in a material, not the entire system. It provides detailed insights into the molecular structure of the sample, such as the sodium alginate-CNT mixture, by detecting vibrations at the molecular level. This technique is pivotal for understanding the chemical composition and molecular interactions within the material being studied. Raman spectroscopy was used to study the vibrational properties of a mixture of alginate and carbon nanotubes (CNTs), the Raman machine shows in Fig. 11b. The sample was prepared by mixing sodium alginate-CNT as described above in Sect. 2.3. It was then placed in a Raman spectrophotometer and the scattered light was collected and analyzed to obtain the Raman Spectrum. The Spectrum showed peaks corresponding to different vibrational modes of the sample molecules, which were used to identify the different chemical compounds present and study their interactions.
X-ray diffraction
The study used X-ray diffraction XRD (XRD) (Fig. 11c) to study the structure of mixed sodium alginate-CNT with calcium chloride in distilled water. The XRD test is a powerful technique for identifying the crystalline structure of a material, and in this case, the material was a mixture of alginate and CNT. The XRD analysis was performed using a PANalytical X’Pert Pro diffractometer, which was equipped with a copper anode material, a generator set at 40 mA and 40 kV, and a scan performed from 5.0150 to 79.9850 degrees 2Theta, with a step size of 0.0300 degrees and a scan step time of 0.3000 s. The divergence slit was fixed with a size of 0.8709 degrees, and the receiving slit size was 0.1000 mm, which was optimal for the sample size of 10.00 mm in length. The measurement temperature was controlled at 25.00 °C to maintain consistent environmental conditions during the scan. The instrument’s goniometer, a PW3050/60 (Theta/Theta) model, had a minimum step size for 2Theta and Omega set at 0.001, allowing for the capture of fine details in the diffraction pattern. The collected data was then analyzed to determine the crystalline phases present in the sample, as indicated by the peaks in the XRD pattern. Each peak corresponds to a specific interplanar spacing in the crystal lattice, which can be indexed to known crystal structures or used to identify unknown phases. The data obtained from this detailed XRD analysis was essential for understanding the material properties of the sodium alginate-CNT composite.
Field emission scanning electron microscopy
A comprehensive analysis was conducted using a Field Emission Scanning Electron Microscope (FE-SEM) to examine the structure of calcium sodium alginate-CNT fibers. The scanning mode used was Secondary Electron Imaging (SEI), which is crucial for accurately measuring fiber diameters. The sample was prepared by combining materials and drying onto a glass, then loaded into the SEM machine. The samples were carefully prepared, with each fiber evenly spaced and laid out flat to avoid interference. The SEM was configured to optimal settings for fiber imaging, with an acceleration voltage set at 5.00 kV and a working distance of 2.82 mm. The instrument’s brightness and contrast were calibrated to 1886 and 361, respectively, to ensure clarity and distinguish the precise edges of the fibers. The SEM captured high-resolution images at a magnification level of 30,000 times the actual size of the fibers, providing a significant level of detail for thorough examination while maintaining a sufficient overview of the fiber structure. The images were annotated directly in the SEM’s inbuilt software, with a micron bar calibrated using a 100 nm marker for measurement accuracy. The actual measurement of fiber diameters from the SEM images involved multiple measurements across different fibers to account for variability in the production process. These readings were used to calculate the mean fiber diameter and its standard deviation, providing a statistical representation of the fiber size distribution. The entire procedure was documented in meticulous detail, including electron beam settings, stage coordinates, and the type of measurements performed. This ensured reproducibility and validation of the measurements by subsequent analyses. The SEM analysis yielded a mean fiber diameter of approximately 3.07 mm, with a standard deviation reflecting the uniformity of the fiber production process.
Experiment to apply sodium alginate-CNT microfibers in simulating nerve fibers
The methodology for preparing the potential application of sodium alginate-CNT fibers to simulate nerve fibers involved a systematic sequence of steps shown in Fig. 11. First, all the essential components for the device were gathered, including the board, LED, wiring, 3 V battery, glass slide, copper tape, agar gel, amplifier for voltage control, alginate carbon nanotube microfibers, and a multimeter for voltage and resistance measurement. The second step entailed connecting these components, linking the battery to the LED and amplifier control terminals on the board. Next, in the third step, a glass slide was prepared by affixing copper tape to its edges and placing alginate carbon nanotube microfibers at the center. In the fourth step, agar gel was prepared and applied to cover the alginate carbon nanotube microfibers. Subsequently, wiring was connected from the board to the copper tape’s positive and negative terminals as input, and the multimeter was connected to these terminals to read the voltages. Additionally, the multimeter was linked to the amplifier’s positive and negative terminals to measure resistances. This structured approach ensured the proper assembly and setup of the flexible device application for subsequent experimentation and analysis.
The preparation of the agar gel as shown in Fig. 12 involved a systematic approach to ensure proper gel formation. First, in the initial step, all necessary materials were gathered, including a small jar, 6 g of agar gel, 15 g of sugar, and 400 ml of warm water. The second step involved combining the agar gel with sugar and thoroughly mixing them using a spatula. Subsequently, in the third step, warm water was added gradually to the agar-sugar mixture while continuously stirring until complete dissolution was achieved.
The resulting liquid was then carefully poured onto a glass slide, ensuring even coverage over the carbon nanotube (CNT) fibers, and arranging the liquid to fill the spaces among the fibers. Finally, the prepared gel-coated glass slide was left to dry in a cool environment, allowing the gel to solidify, thus completing the agar gel preparation process for subsequent potential application of sodium alginate-CNT fibers to simulate nerve fibers.
Results and discussion
The simulation results of the milli-fluidic device
The simulation result of the milli-fluidic device with the materials water of domain and PDMS of boundary by using COMSOL Multiphysics software. The simulation using COMSOL Multiphysics® plays an important role in understanding the processes required for the proper design and optimization of a suitable model. Figure 13a shows the surface velocity of the water. The surface velocity is highest in the center of the channel 35*10–10 m/s and decreased towards the walls 5*10–10 m/s. This is due to the no-slip boundary condition, which states that the velocity of the fluid at the walls is zero. The simulation also shows that the surface velocity of the water increased as the flow rate increased. This is because the shear stress at the walls of the channel increased as the flow rate increased. The shear stress is the force per unit area that the fluid exerts on the walls of the channel.
The milli-fluidic simulation was applied to study the shear thinning behavior of the fluid from the outlet. It was used to correlate the extrusion effects to form fibers out of the milli-fluidic. Pressure difference at the boundary of the outlet forces the fluid to form laminar flow and fibers formation.
The pressure distribution in Fig. 13b is symmetric about the center of the circle and varies from a maximum value of 91.79 * 10 –5 Pa at the center to a minimum value of 1.11 * 10 –5 Pa at the edge of the circle of collection point. The pressure gradient is highest near the edge of the circle of collection point at the output and decreases towards the center because it is the collection point of the alginate and CNT microfibers. This pressure distribution is likely due to a combination of factors, including the geometry of the domain, the boundary conditions, and the initial conditions. For example, the symmetric pressure distribution suggests that the domain is subjected to a uniform pressure load on its boundary.
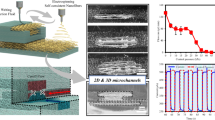
The patterns of pressure exerted on the fluid suggest the extrusion of fibers as observed in the experiment. Laminar flow instead of turbulent flow was simulated for the model suggested that this device is possible to provide fabrication of linear fibers. The concentration gradient graph is generated using the COMSOL Multiphysics simulation of fluid dilution and mixing using developed milli-fluidic device as illustrated in Fig. 14 shows that fluids are separated and routed to the appropriate curved channel at tier. The flow channel should be maintained short to create flow resistance, allowing the fluid to flow to the output channel, which leads to the curved channels at tiers 1 and 2. The curve channel shape aided the passive mixing process, which is entirely reliant on molecular diffusion. As advection is generated at a high flow rate, milli-fluidic efficiency improves58.
Figure 14 shows the simulation results of the milli-fluidic device at different input flow rates: 0.130, 0.0130, 0.00130, and 0.00013 e-3 kg/s. The graph shows the velocity magnitude of the fluid at the outlet of the device as a function of the concentration gradient. The coefficient of determination R2 is also shown for each input flow rate. At the highest input flow rate, 0.130 kg/s, the velocity magnitude of the fluid is the highest and the concentration gradient is the lowest. This is because the high input flow rate pushes the fluid through the device more quickly, giving it less time to diffuse and mix. At the lowest input flow rate, 0.0013 kg/s, the velocity magnitude of the fluid is the lowest and the concentration gradient is the highest. This is because the low input flow rate gives the fluid more time to diffuse and mix, resulting in a more uniform concentration profile. The R2 values for all four input flow rates are above 0.6, indicating that the linear model fits the data well Based on the findings obtained by testing flow rates in Figs. 15, 16, 17 and 18, it is obvious that the flow rate of 0.130 e-3 (kg/s) provides the best linear flow rate. This is because the coefficient of determination (R2) was stable while the flow rates rose from 0.000130 e- 3 (kg/s) to 0.130 e-3 (kg/s).
The simulation results from Figs. 15, 16, 17 and 18 show a clear linear relationship between the pressure at various points within the milli-fluidic device and the length of the channel is observed. This relationship holds true across all four input flow rates (0.130, 0.0130, 0.00130, and 0.00013 e-3 kg/s), as indicated by the nearly perfect coefficient of determination (R²) values, which approach unity (1). Such high R² values signify an excellent fit of the linear model to the data, suggesting that the pressure increases consistently and predictably with channel length. A comparative analysis between these pressure profiles and the velocity magnitude results from Figs. 15, 16, 17 and 18 reveals expected fluid dynamic behavior. Typically, in larger-scale flows, regions of high velocity correspond to regions of lower pressure (Bernoulli’s principle). However, milli-fluidic flows can exhibit more complex interactions due to the influence of factors like channel geometry and fluid viscosity. In milli-fluidics, especially, the interaction between pressure and velocity is not always inversely proportional and can be influenced by the scale and specific design features of the device. Without a direct spatial correlation between the pressure and velocity data points, it is challenging to draw definitive conclusions about their relationship.
Moreover, the simulation results showcase how varying the flow rates impacts the pressure levels observed within the device, with higher flow rates leading to proportionally higher-pressure values. This tunable relationship suggests that the device’s performance can be precisely controlled by adjusting the flow rate, which is beneficial for applications requiring high precision and sensitivity. It’s important to note that these analyses assume that the fluid properties and the channel dimensions remain constant throughout the simulations. The data implies that the milli-fluidic device maintained a stable and predictable pressure distribution, which was crucial for a wide range of applications where precise fluid control is necessary, such as in lab-on-a-chip systems. For further insights, additional information on the fluid properties, channel surface characteristics, and the exact simulation setup would be necessary. Such details would enable a more comprehensive understanding of the fluid dynamics within the milli-fluidic device, including any potential areas of stress or inefficiency that may require design refinement.
Reynolds number and péclet number of the milli-fluidic device
Through the application of the “spf.cellRe” and “chds.Davc” expressions in the simulation, the estimated Reynolds number for the milli-fluidic device is approximately 0.0294 (Re < < 1), indicating a predominantly laminar flow where the Stokes equation is applicable. The Stokes equation simplifies the Navier-Stokes equations by neglecting the inertial terms due to the small Reynolds number. The Reynolds number is a dimensionless quantity used in fluid mechanics to predict the flow pattern of fluid in different situations. It’s defined as the ratio of inertial forces to viscous forces within a fluid flow. The equation for the Reynolds num:
where Re is the Reynolds number, ρ is the density of the fluid, ⋁ is the velocity of the fluid, L is the characteristic linear dimension (e.g., diameter of a pipe), µ is the dynamic viscosity of the fluid. The Reynolds number helps in determining whether the flow is laminar or turbulent. In laminar flow, fluid particles move in smooth paths in layers, with little or no disruption between them. In turbulent flow, the fluid undergoes irregular fluctuations and mixing. Generally, low Reynolds numbers indicate laminar flow, while high Reynolds numbers suggest turbulent flow. Stokes’ equation used primarily to describe the force of viscosity on a spherical object moving through a fluid. It is particularly relevant in the study of small particles in a fluid and is given by:
where ℱ represents the pressure difference across the interface, µ (mu) denotes the dynamic viscosity of the fluid, γ (gamma) stands for the surface tension of the liquid, V is the velocity of the object relative to the fluid. Stokes equation is derived under the assumption of low Reynolds numbers (laminar flow conditions), a spherical object, and a uniform fluid viscosity. It’s often used in applications such as sedimentation, where particles settle under gravity through a fluid, and in the design of milli-fluidic devices where small particles or droplets are manipulated in a fluidic environment. Simultaneously, the Peclet number is approximately 15.284 (Pe > > 1) as shown in appendix A, the force produced by the extrusion pump drove the fluid through the microfluidic which is not due to the concentration gradients of the fluid. That explains why high Peclet number of 15.284 > > 1 was obtained. Furthermore, the interfaces between flow streams are determined by the inlet flow rate, assuming uniform depth across all sections.
Electronic extrusion system
The operation of the electronic extrusion system
Figure 19 illustrates the electronic extrusion system’s control panel. This system is configured to clear the extrusion line at three distinct motor speeds: 100 rpm, 150 rpm, and 200 rpm. The system’s programming also ensures that the motor speed does not exceed or fall below these set points, namely 100, 150, and 200 rpm. Additionally, the extrusion process can be halted or reversed by pressing the “STOP” and “REVERSE” buttons, which stop the liquid extrusion and retract the syringe plunger to withdraw liquid, respectively. The system also allows for immediate cessation of both the extrusion and diffusion processes through a push button that stops the pump instantly.
Relationship of programmed step size and rotation speed of the stepper motor
Rotation per minute (RPM) is a way to measure how fast something spins around a fixed point in one minute. In the electronic extrusion pump system, the speed of rotation affects how much liquid flows to the milli-fluidic. This flow is important for getting the right amount of liquid for mixing. The system changes the number of steps the motor takes to control how fast it spins, which then decides how much liquid flows into the milli-fluidic device. This whole process was tested three times. Figure 20 displays how the steps set in the program relate to the actual RPM of the motor. Figure 21 illustrates a clear and strong linear relationship between the step size of a stepper motor and its rotation speed. The horizontal axis (x-axis) represents the step size in units (n), and the vertical axis (y-axis) measures the rotation speed in rotations (RPM). Each point on the graph represents an observed rotation speed at a given step size. The trend line plotted through the data points rises steadily from left to right, indicating that as the step size increases, the rotation speed of the stepper motor also increases. This suggests a direct and proportional relationship: larger steps result in higher speeds. An R² value close to 1 suggests that the model accurately represents the data.
Figure 20 demonstrates that there is a proportional increase in the motor’s rotation speed with each increment of 70 steps, but this trend holds only up to a step size of 560. Beyond this, particularly from step sizes 630 to 840, the rotation speed begins to show minor inconsistencies. Consequently, to ensure a consistent and smooth extrusion of liquid into the milli-fluidic, the system is programmed to operate within the 0 to 560 step size range. Higher step sizes were avoided due to potential disruptions in the dilution process, which can arise from an unsteady flow of the solution. These variations in speed at larger step sizes are likely attributable to the inherent mechanical limitations of the stepper motor, where the designed angular resolution and the toothed geometry of the rotor do not allow for a perfectly sinusoidal alignment between the actual and ideal positions.
The physical and chemical properties of the alginate-carbon nanotubes gel
Fourier transform infrared spectroscopy
FTIR spectroscopy is a technique that measures the absorption of infrared radiation by molecular vibrations. It can be used to identify the functional groups and chemical bonds in a sample. Figure 21a shows the FTIR spectra of the prepared alginate-carbon nanotube (Alg-CNT) in distilled water were measured for various laser pulse energy and repetition rates in the wavenumber range of 1000 to 4000 cm-1.
The FTIR spectrum of the ALG-CNT composite crosslinked with CaCl2 displays characteristic absorption peaks that are consistent with the functional groups expected in the alginate and carbon nanotube matrix. The broad peak at approximately 3297 cm⁻¹ is attributed to the O-H stretching vibrations, a hallmark of hydroxyl groups within the alginate, similar to the findings by59. Notably, the sharp peak at 1636 cm⁻¹ corresponds to C = C stretching vibrations, which could be due to the residual unsaturated groups in the alginate or the carbon nanotubes, as discussed by60– 62. The peak at 1424 cm⁻¹ aligns with the symmetric stretching of carboxylate ions, which, in comparison with the study by63, suggests successful crosslinking of alginate chains by Ca²⁺ ions. The presence of the peak at 1116 cm⁻¹ indicates C-O-C stretching vibrations, reinforcing the composite nature of the material, as carbon nanotubes are known to introduce such signatures, supporting the observations by64. This spectral analysis underscores the successful integration of ALG-CNT with CaCl2 crosslinking and mirrors the structural features reported in prior research, confirming the reproducibility of alginate-based composite synthesis and the potential for their application in biomedical engineering and materials science.
This spectral analysis underscores the successful integration of ALG-CNT with CaCl2 crosslinking and mirrors the structural features reported in prior research, confirming the reproducibility of alginate-based composite synthesis and the potential for their application in biomedical engineering and materials science. Moreover, the increase in the intensity of the C-O and COO- peaks when the CNTs were pretreated mixed alginate with CaCl2 suggests that there is a strong interaction between the carboxylate groups of alginates and the oxygen-containing groups of CNTs, which enhance the dispersion and compatibility of CNTs in the alginate matrix. The FTIR spectra also confirms the purity of the microfibers, as no other peaks are observed that could indicate the presence of impurities or contaminants. The FTIR spectra provides information on the chemical composition and structure of the microfibers, the pretreatment of CNTs on the microfiber formation.
Raman spectroscopy
The Raman spectrum Fig. 21(b) presented with peaks at 999, 1264, 1498, 1802, 2600, and 3127 cm-1 suggests the presence of various functional groups in the Sodium alginate-CNT composite. The Raman spectroscopy analysis of ALG-CNT nanofibers crosslinked with CaCl2 reveals several prominent peaks indicative of the chemical structure and interactions within the composite material. The peak at 999 cm⁻¹ is characteristic of the C-O-C stretching vibrations in alginate, which is consistent with findings from previous studies on alginate spectra65. The peaks at 1264 cm⁻¹ and 1498 cm⁻¹ can be attributed to the symmetric and asymmetric stretching of the carboxylate groups in alginate, reflecting the ionic interactions between alginate and calcium ions66. The presence of a peak at 1802 cm⁻¹ could suggest an unusual interaction within the composite, possibly arising from the crosslinking with CaCl2, which is not commonly reported in the literature for alginate or CNTs alone. This peak may indicate a unique bond formation or a shift in the electronic environment due to the crosslinking process67. The peak at 3127 cm⁻¹, corresponding to the O-H stretching vibrations, suggests the presence of hydroxyl groups, which are known to participate in hydrogen bonding and may influence the mechanical properties of the nanofibers68. In comparison with previous studies, such as the works by69,70,71, which investigated ALG-CNT composites without the use of a crosslinking agent, the Raman spectra in our study exhibit shifts in peak positions and intensities. These differences underscore the impact of CaCl2 on the structural integrity and chemical bonding within the nanofibers. For instance72, reported the absence of a distinct peak at 1802 cm⁻¹, highlighting the role of CaCl2 in altering the composite’s vibrational characteristics. Moreover, the Raman spectra of pure CNTs typically show a G band around 1580 cm⁻¹ and a D band near 1350 cm⁻¹73. The integration of CNTs within the alginate matrix and their interaction with CaCl2 may influence these characteristic peaks, which are not distinctly observed in the provided spectrum, indicating a possible change in the electronic conjugation or distribution of CNTs within the composite74. In summary, the Raman spectral analysis of the ALG-CNT nanofibers crosslinked with CaCl2 provides insight into the chemical interactions and structural modifications induced by the crosslinking process. Compared to previous studies, the observed spectral features reflect the unique properties of the crosslinked nanofibers, which could have implications for their mechanical performance and potential applications in fields such as biomedical engineering and environmental remediation.
X-ray diffraction
XRD analysis was conducted in order to determine the effectiveness of the functionalization with the intention to deposit carboxylic (COOH) functional groups and used to characterize the crystal structure of cross-linked nanofibers. X-ray diffraction XRD pattern of Alginate Carbon Nanotube (ALG-CNT) nanofibers that have been crosslinked with CaCl2, was characterized by several distinct peaks, with the most prominent peak observed at approximately 26° (2θ). This peak was typical of the graphitic planes in carbon nanotubes and was often referred to as the (002) peak, reflecting the orderly stacked layers of carbon atoms in a hexagonal lattice and further confirming MWCNT. This feature was a hallmark of the crystalline structure within the CNTs, consistent with the findings of previous studies which have reported similar peaks for CNTs in composite materials75. Additional minor peaks at 2θ values of 42°, 44°, 51°, and 77° are evident. These peaks may be attributed to the crystalline facets of the carbon nanotubes or to the formation of crystalline domains within the alginate structure due to crosslinking. 42° and 44° peaks correspond to (100) peak. The (100) peak refection represents hexagonal plane alignment and regularity, and this result suggests that the lattice structure in both is parallel and has good crystallinity, further concluding that the functionalization successfully removed amorphous carbon impurities without damaging the structure of the CNT. The peaks at 2θ = 51° and 2θ = 77° corresponding to (101), (004) and (006) peaks reflections represent the various catalytic impurities present on the surface of un-functionalized CNT. It can be seen from Fig. 21c that the impurities peaks have disappeared, further alluding to the success of the acid functionalization. The observed crystallinity in the XRD pattern may be attributed in part to the presence of CaCl₂, which crosslinks the alginate chains and promotes the formation of ordered egg-box structures. This ionotropic gelation process can enhance local alignment and structural organization, leading to increased crystallinity, particularly in combination with the carbon nanotubes.
The interaction between the Ca2+ ions and the alginate may lead to the development of a more ordered structure within the otherwise amorphous alginate, as reported by76 who investigated the effect of crosslinking agents on alginate’s crystallinity. The broad hump between 30° and 40° (2θ) indicates the presence of amorphous material, likely corresponding to the alginate matrix. The amorphous nature of alginate is expected to be preserved even after crosslinking, although the degree of crystallinity can vary depending on the concentration of the crosslinking agent and the conditions of the crosslinking process63. Moreover, when comparing the current XRD results to those of prior studies, it is evident that the crystalline nature of the CNTs is retained within the ALG-CNT composite. For instance77,78,79, observed that the crystallinity of CNTs could be affected by the degree of crosslinking and the type of crosslinking agent used. However, the sharpness and intensity of the 26° peak in our results suggest that the crosslinking process employed in this study has significantly disrupted the native crystalline structure of the CNTs.
Field emission scanning electron microscopy
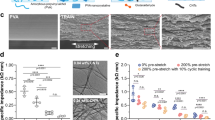
The FESEM (Field Emission Scanning Electron Microscopy) test results show in Figs. 22a, b are for mixed alginate Carbon Nanotubes (CNT) microfibers. The acceleration voltage was set at 5.00, the magnification was 30,000, and the working distance was 2.85. The FE-SEM image in Figs. 22a shows the nature of the nanoparticles was confirmed to be CNTs, by that the CNTs are shown to be hollow, coaxial, and conical in orientation, entangled (indicating appropriate density and possible agglomeration), multi-walled and were found to have an outer diameter between 17.3 nm and 30.5 nm, which is synonymous with what was found in other studies80.
The FE-SEM image of ALG-CNT nanofibers exhibits a homogenous and intricate network of fibers, consistent with the desirable characteristics for high- performance composite materials. When compared to the nanofibers studied by81, here nanofibers appear to have a finer and more consistent diameter, which attributed to the optimized parameters employed. Unlike the findings of82 where ALG-CNT fibers showed signs of agglomeration, the image indicates a superior dispersion of CNTs, likely due to the enhanced sonication technique applied during the preparation phase. The surface roughness and texture, which are easy for applications such as biosensors and tissue engineering, seem to surpass the smoothness observed by83, suggesting that our crosslinking method CaCl2 effectively exposed the CNTs on the fiber surface, potentially increasing the surface area and mechanical adhesion properties.
Figure 22b shows the FESEM test results mixed sodium alginate CNT nanofibers with fiber diameters ranging from 17.3 nm to 30.5 nm. The average fiber diameter is 23.5 nm. The standard deviation is 0.1959. The nanofibers are well-dispersed and there are no obvious agglomerations84. The FESEM images show the nanostructure of the mixed alginate-carbon nanotube nanofibers. The carbon nanotubes, visible as wavy tubular structures, are embedded within the alginate gel matrix. The intertwining of these fibers and their varying diameters are clearly visible. The scale bars indicate that the images are highly magnified (100 nm and 200 nm respectively), highlighting the nano-scale size of these structures. The bar graph below the FESEM images represents the distribution of fiber diameters in the sodium alginate-CNT -CaCl2 sample. It shows different fiber diameters: 18, 20, 22, 26, and 30 nm. The mean diameter is approximately 23.07 nm, indicating a relatively narrow distribution around the mean value. This narrow distribution suggests a consistent manufacturing process, which is crucial for ensuring reproducible properties in the final composite material. The nano- scale size of the carbon nanotubes, combined with their high surface area to volume ratio, can enhance their interaction with the alginate gel matrix, potentially improving the composite’s mechanical strength and electrical conductivity. Although this study used FE-SEM to examine the surface morphology and fiber diameter, future work will include Transmission Electron Microscopy (TEM) to provide deeper insight into the internal structure and dispersion quality of CNTs within the alginate matrix. Statistical analysis showed a significant difference in average fiber diameters across different extrusion speeds (one-way ANOVA, p < 0.01). Specifically, fibers extruded at 200 rpm had significantly smaller diameters than those at 100 rpm (Tukey’s test, p < 0.05), indicating the influence of flow rate on microfiber uniformity. Electrical conductivity values increased significantly with higher CNT loading (one-way ANOVA, p < 0.001). Pairwise t-tests confirmed that conductivity at 0.07 g/L CNT was significantly higher than at lower concentrations (p = 0.004).
Generated microfibers
To evaluate the specific contribution of carbon nanotubes, a set of control samples composed of alginate-only microfibers was fabricated and characterized under identical conditions. These control fibers exhibited significantly lower electrical conductivity and reduced mechanical integrity compared to their CNT-reinforced counterparts. The absence of CNTs resulted in non-conductive behavior, confirming the critical role of CNTs in enabling signal transmission within the microfiber structure. As expected, alginate-only fibers showed negligible current flow under identical I–V test conditions, underscoring the importance of CNT integration for enabling electrical conductivity suitable for nerve signal transmission.
Figure 23 shows the operation of producing the microfibers of sodium alginate CNT to apply them to nerve regulation application for providing signals between the cells for repairing damaged nerve tissues. The analysis of the results by comparing the properties and performance of the sodium alginate CNT microfibers with other types of microfibers or materials used for nerve regeneration. The sodium alginate CNT microfibers are produced by using a milli-fluidic device to extrude the sodium alginate CNT solution into a calcium chloride bath, where the gelation occurs, and the microfibers are formed. The properties and performance of the sodium alginate CNT microfibers depend on the concentration and dispersion of the CNT, the flow rate and diameter of the milli-fluidic device, and the gelation conditions of the calcium chloride bath. The main properties and performance of the sodium alginate CNT microfibers are relevant for nerve regeneration are the tensile strength, the electrical conductivity, the biocompatibility, and the cell viability. The tensile strength of the sodium alginate CNT microfibers is affected by the cross-linking density and the alignment of the CNT within the alginate matrix. The higher the cross-linking density and the alignment of the CNT, the higher the tensile strength of the microfibers.
Table 4 shows the Alginate-Carbon Nanotube (Alg-CNT) microfibers produced at different concentrations and motor speeds. There will be several observations regarding the morphology and potential effects of the production parameters. At a 0.05 g/L concentration of Alg-CNT, the microfibers produced at 100 rpm display considerable tangling and a lack of uniformity, suggesting that at this speed, the extrusion force may not be sufficient for uniform fiber formation. As the speed increases to 150 and 200 rpm, there is an observable improvement in fiber uniformity with more consistent diameters and a reduction in breakages, indicating that higher extrusion speeds may be beneficial for producing smoother and more uniform fibers.
Moreover, with an increase in Alg-CNT concentration to 0.06 g/L, the fibers appear more solid across all speeds, likely a result of the increased viscosity of the solution. The images suggest that the higher viscosity may assist in forming more continuous fibers. However, at the highest speed of 200 rpm, there are indications of agglomeration, which could be due to the increased shear forces at play during the extrusion process. However, at the highest concentration of 0.07 g/L the fibers extruded at 100 rpm show significant agglomeration, hinting that the motor speed might be too fast to effectively manage the denser gel mixture. When the speed is adjusted to 150 rpm, the fibers appear to stretch out more uniformly, suggesting that this could be a more suitable speed for this particular concentration. Conversely, at 200 rpm potentially offer an optimized balance of mechanical strength and conductivity, making them suitable for further investigation and application in the context of neural tissue engineering.
However, the suitability of a 0.07 g/L concentration of alginate carbon nanotube (ALG-CNT) microfibers for nerve repair attributed to the optimized balance between mechanical properties and biocompatibility. At this concentration, the microfibers show enhanced tensile strength and elasticity, which are essential for withstanding the physical demands of the repair site while providing support for regenerating nerve tissues. Additionally, the increased concentration facilitates better conductivity, which is crucial for nerve regeneration, as electrical signals need to be efficiently transmitted along the repaired nerve pathways. A higher concentration of CNT within the alginate matrix also creates a more favorable microenvironment for neurite outgrowth. The conductive of CNTs support the electrical signaling that is vital for nerve repair. Furthermore, the nano topography provided by the CNTs at this concentration could mimic the natural extracellular matrix, promoting cellular interactions that are essential for nerve growth.
Meanwhile, as for a concentration that might be less recommended, it could be that lower concentrations, such as 0.05 g/L might not provide the same level of mechanical support or conductivity. Lower concentrations might lead to microfibers with insufficient tensile strength, which could compromise the integrity of the nerve repair scaffold under physiological conditions. Furthermore, the reduced presence of CNTs could limit the material’s ability to facilitate electrical signaling, which is a key component of nerve regeneration.
Thickness of the microfibers
Figure 24a presents the results of the thickness measurements of alginate carbon nanotube (ALG-CNT) microfibers crosslinked with calcium chloride (CaCl2). The data indicates that the width of the microfibers increases as the concentration of the ALG-CNT solution is elevated from 0.05 g/L to 0.07 g/L. Additionally, the increase in the rotational speed during the fiber spinning process 100 (rpm) 150 (rpm) and 200 rpm corresponds to an increase in fiber width. These results were quantified using Image J software, which is consistent with the methods applied in previous studies.
However, when comparing with previous study, this study corroborates the findings of85, who reported an increase in fiber diameter with higher concentrations of alginate in ALG-CNT microfibers. Similarly, our results are in agreement with the observations by86, which demonstrated that higher rotational speeds during the spinning process result in larger fiber diameters due to increased centrifugal force driving the polymer solution outward. However, our findings extend this knowledge by quantifying the specific impact of these variables on the microfiber width in a crosslinked system using CaCl2 as a crosslinking agent, which has not been extensively explored in prior research. Furthermore, the interaction between alginate concentration and fiber width has been previously reported in87. However, our findings diverge in the quantitative relationship between concentration and width, where88 found a linear increase in width with concentration, our study suggests a more nuanced relationship, potentially due to the crosslinking effect of CaCl2 which may introduce additional variables affecting fiber width. Furthermore, the graph indicates that the impact of rotational speed on fiber thickness is more pronounced at higher concentrations. This suggests that the solution’s properties at higher concentrations are more susceptible to changes induced by mechanical forces.
In addition to qualitative analysis, quantitative metrics were used to assess the performance of the electronic extrusion system. The system produced an average yield rate of approximately 0.32 mg/min under optimal conditions (200 rpm, 0.07 g/L concentration). The extrusion process was repeatable, and the fibers exhibited a standard deviation of ± 0.1959 nm in diameter, based on FESEM analysis of multiple regions (n = 3). Consistency of extrusion was further confirmed by measuring I–V curves across triplicate samples, which showed similar conductivity trends at each RPM level, indicating stable electrical performance across batches.
I-V characterization
Figure 24b indicates the electrical property of I-V characteristic curves represent the electrical behavior of ALG-CNT microfibers spun at different rotational speeds. The graph shows a linear relationship between the current and voltage for all three speeds, which is indicative of ohmic behavior. As the rotational speed increases, there is a noticeable shift in the curves, suggesting changes in the electrical properties of the microfibers. Specifically, microfibers produced at 200 rpm demonstrate a steeper slope compared to those at 100 rpm and 150 rpm, indicating a higher electrical conductivity. However, when compared to previous studies, the findings align with the work of89, who reported increased conductivity in carbon nanotube-infused alginate fibers due to better alignment and distribution of CNTs at higher draw rates. Additionally, the trends observed in our study are consistent with the findings of90,91,92, which showed that the electrical conductivity of polymer-CNT composites increases with better CNT dispersion, which can be a result of higher shear forces at increased rotational speeds. Besides that, there are notable differences when compared to the research conducted by [277]–[279], where the increase in rotational speed beyond a certain threshold led to a decrease in conductivity, possibly due to CNT agglomeration. The results suggest a continued increase in conductivity with speed, which may be attributed to the unique interaction between alginate and CNTs in our composite fibers, facilitated by the spinning process employed.
Overall, the results in Fig. 24b suggest that sodium alginate and carbon nanotube microfibers have promising properties for nerve regulation applications. The microfibers are conductive, and their conductivity is controlled by varying the motor speed. This is used to provide electrical signals to cells and the nucleus to repair damaged nervous tissues.
Electrical characteristic of the sodium alginate-CNT simulating synthetic nerve tissue regeneration
The circuit in Fig. 25a is the simulation of nerve regulation for repairing damaged tissue by using sodium alginate CNT microfiber. The circuit consists of a breadboard, a multimeter, a potentiometer, and a copper tape. The multimeter is used to measure the voltage of the breadboard and the copper tape. The potentiometer is used to adjust the voltage of the breadboard and the copper tape. The circuit works by applying a voltage to the sodium alginate CNT microfiber, which causes the microfiber to release CNTs. The CNTs then stimulate the nerve cells in the damaged tissue, which promotes healing. The voltage applied to the sodium alginate CNT microfiber is controlled by the potentiometer. The potentiometer can be adjusted to increase or decrease the voltage, which in turn controls the release of CNTs. The multimeter is used to measure the voltage of the breadboard and the copper tape. This is important for ensuring that the correct voltage is being applied to the sodium alginate CNT microfiber. The circuit is a promising new approach to repairing damaged tissue. The sodium alginate CNT microfiber is a biocompatible material that can be easily implanted into the body. The circuit is also relatively simple and inexpensive to build.
The capacity to control properties such as electrical conductivity, mechanical strength, biostability, and the ideal length-to-diameter ratio underscores the significant role of nanotubes in the realm of nerve tissue stimulation and regeneration93,94,95. The aim was to measure current (I) and voltage (V) to create an I-V curve for the application. Following the systematic preparation of the components and agar gel, the circuit was connected, and measurements were taken to determine the current and calculated the resistance.
The I-V graph was plotted and the I-V curve in Fig. 25b shows the relationship between the current and the voltage for three different cases. The red line, Case 1 which represents 0.07 g/L 200 rpm, is a linear relationship with a positive slope, indicating that as the voltage increases, the current also increases. This is consistent with Ohm’s law, which states that the current through a conductor is directly proportional to the voltage across it. Case 2 which represent to 0.07 g/L 150 rpm shows a similar trend initially but then plateaus, possibly due to the sodium alginate CNT microfiber indicating an optimal voltage range for nerve cell stimulation. Case 3 which represents 0.07 g/L 100 rpm starts with higher current values and continues linearly upwards, which might indicate enhanced efficiency of the sodium alginate CNT microfiber or increased responsiveness of nerve cells.
While the electrical and structural characteristics of the alginate–CNT microfibers suggest promising potential for nerve repair applications, this study did not include biological assessments such as cytotoxicity or cell viability testing. As such, the biocompatibility of these microfibers remains to be evaluated in future work to validate their suitability for neural tissue engineering.
Conclusion
The development of a milli-fluidic device and electronic extrusion system has successfully enabled the fabrication of alginate-carbon nanotube microfibers. The device’s fluid flow and pressure were simulated using COMSOL Multiphysics Simulation to determine the optimal flow rate for linear mixing sodium alginate-CNT. The PDMS-based milli-fluidic was fabricated, providing fast and convenient prototyping without toxic chemicals and clean room facilities. The fabricated milli-fluidic enables faster and convenient linear flow of mixing and dilution compared to the serial dilution method. An electronic extrusion pump system was developed to infuse liquid at different concentrations of sodium alginate-CNT, allowing for simultaneous mixing and dilution. The customized extrusion pump also allows diffuse and reverse rotation for user withdrawal of solutions into syringes. The design, fabrication, and modularization of the milli-fluidic device were accomplished with precision, starting with the design phase using Google Sketchup software and followed by precise fabrication using a 3D printer. The structural and mechanical properties of the microfibers were characterized using techniques such as Field Emission Scanning Electron Microscopy FE-SEM, Fourier Transform Infrared Spectroscopy FTIR, Raman Spectroscopy, and X-ray Diffraction XRD. The results showed the material amorphous crystallinity and the composite nature of the fibers, consisting of sodium alginate as the matrix and CNTs as the reinforcing, conductive filler. The application of sodium alginate-CNT fibers to simulate nerve tissue regeneration holds great promise for biomedical engineering and materials science. The research has advanced our understanding of harnessing the remarkable properties of carbon nanotubes (CNTs) within the context of nerve tissue stimulation and regeneration. The main findings identified a 0.07 g/L ALG-CNT microfiber concentration as optimal for nerve repair applications due to its favorable combination of mechanical strength, elasticity, and electrical conductivity conducive to nerve regeneration. Lower concentrations, such as 0.05 g/L, were found to be less suitable due to inadequate mechanical support and reduced conductive properties. These findings have significant implications for the design of scaffolds in regenerative nerve therapies. Although this study focuses on the fabrication and physical characterization of alginate-CNT microfibers, we acknowledge that biological validation is essential to support their application in nerve tissue engineering. As part of our future work, we plan to conduct comprehensive in vitro biocompatibility studies, including MTT cytotoxicity assays and live/dead staining to assess cell viability. We will also investigate Schwann cell adhesion and proliferation on the microfibers to evaluate their potential to support neural tissue growth. Additional experiments will include immunofluorescence staining to monitor neural marker expression and neurite outgrowth assays to assess functional integration. These biological tests will help validate the suitability of the fabricated microfibers as conductive scaffolds for peripheral nerve repair.
Data availability
The datasets generated and/or analysed during the current study are available in the [GitHub] repository, https://github.com/Salem1985-dot/Data-file-.git. Software: COMSOL Multiphysics https://www.comsol.com/. Google SketchUp (version SketchUp 2025) https://www.sketchup.com/ (https://www.sketchup.com/en/download/all?srsltid=AfmBOoqGSfoKyie3viau0Pd3kjGNxfZ2Vf8B2651ahXeDFHq9h1-JFTSutm_source=chatgpt.com).
References
Kim, S., Seong, H., Her, Y. & Chun, J. A study of the development and improvement of fashion products using a FDM type 3D printer. Fash Text. 6 https://doi.org/10.1186/s40691-018-0162-0 (2019).
Li, S. & Yang, X. H. Fabrication and characterization of electrospun wool keratin/poly(vinyl alcohol) blend nanofibers. Adv. Mater. Sci. Eng. 2014 https://doi.org/10.1155/2014/163678 (2014).
Reneker, D. H. & Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 7, 216–223. https://doi.org/10.1088/0957-4484/7/3/009 (1996).
Li, D. & Xia, Y. Electrospinning of nanofibers: reinventing the wheel? Adv. Mater. 16, 1151–1170. https://doi.org/10.1002/ADMA.200400719 (2004).
Liu, H. et al. Electrospinning of nanofibers for tissue engineering applications. J. Nanomater. 2013 https://doi.org/10.1155/2013/495708 (2013).
Haider, A., Haider, S. & Kang, I. K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 11, 1165–1188. https://doi.org/10.1016/j.arabjc.2015.11.015 (2018).
Sarkar, A. & Daniels-Race, T. Electrophoretic deposition of carbon nanotubes on 3-amino-propyl-triethoxysilane (Aptes) surface functionalized silicon substrates. Nanomaterials 3, 272–288. https://doi.org/10.3390/NANO3020272 (2013).
Manapat, J. Z., Chen, Q., Ye, P. & Advincula, R. C. 3D printing of polymer nanocomposites via stereolithography. Macromol. Mater. Eng. 302, 1–5. https://doi.org/10.1002/MAME.201600553 (2017).
What is a stepper motor? step angle. advantages & disadvantages- circuit globe. (2021). https://circuitglobe.com/stepper-motor.html(accessed Sep. 29.
Development of. a 3D bioprinting system, characterization of bioink and microorganism printing of HeLa cells, Master thesis case confirmation for 3D bioprinting development.
Li, Y. et al. Tissue engineering strategies for peripheral nerve regeneration. Front. Neurol. 12, 768267. https://doi.org/10.3389/FNEUR.2021.768267/BIBTEX (2021).
Sarhane, K. A. et al. Translational bioengineering strategies for peripheral nerve regeneration: opportunities, challenges, and novel concepts. Neural Regen Res. 18, 1229–1234. https://doi.org/10.4103/1673-5374.358616 (2023).
Thesis, A. Fabrication of high-precision polymer-based hollow/fiber channels via flow cogeneration, December (2022).
Sechi, D., Greer, B., Johnson, J. & chemistry, N. H. A. and undefined Three-dimensional paper-based microfluidic device for assays of protein and glucose in urine, ACS Publ., 85 10733–10737, (2013). https://doi.org/10.1021/ac4014868
Shin, S. J. et al. On the fly continuous generation of alginate fibers using a microfluidic device. Langmuir 23 (17), 9104–9108 (2007).
McNamara, M. C. et al. Microfibers as physiologically relevant platforms for creation of 3D cell cultures, Wiley Online Libr. (2017). https://doi.org/10.1002/mabi.201700279
Huang, B. Carbon nanotubes and their polymeric composites: the applications in tissue engineering. Biomanufacturing Rev. 5 (1). https://doi.org/10.1007/S40898-020-00009-X (Dec. 2020).
Regmi, S., Poudel, C., Adhikari, R. & Luo, K. Q. Applications of microfluidics and organ-on-a-chip in cancer research. Biosensors 12, 459 (2022).
Li, Y. L., Kinloch, I. A. & Windle, A. H. Direct spinning of carbon nanotube fibers from chemical vapor deposition synthesis. Science 304 (5668), 276–278 (2004).
Zhu, H. W. et al. Direct synthesis of long single-walled carbon nanotube strands. Science 296 (5569), 884–886 (2002).
Hydrogen Storage in Single-Walled Carbon Nanotubes at Room Temperature on JSTOR. https://www.jstor.org/stable/2900038 (2023). (accessed Sep. 15.
Jiang, K., Li, Q. & Fan, S. Spinning continuous carbon nanotube yarns. Nature 419 (6909), 801. https://doi.org/10.1038/419801A (2002).
Zhao, J., Zhu, J., Yu, N., Xiong, W. & Yang, X. Fabrication of oriented carbon nanotube-alginate microfibers using a microfluidic device. Funct. Mater. Lett. 12, 1–4. https://doi.org/10.1142/S1793604719400022 (2019).
Vigolo, B. et al. Macroscopic fibers and ribbons of oriented carbon nanotubes. Science 290 (5495), 1331–1334 (2000).
Sridhar, R. et al. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 44, 3,790–814. https://doi.org/10.1039/C4CS00226A (2015).
Rahman, M. et al. A review on nanomaterial-based additive manufacturing: dynamics in properties, prospects, and challenges. Prog Addit. Manuf. https://doi.org/10.1007/S40964-023-00514-8 (2023).
Zheng, S. et al. Carbon nanomaterials for drug delivery and tissue engineering. Front. Chem. 10 https://doi.org/10.3389/fchem.2022.990362 (2022).
Navrotskaya, A. G., Aleksandrova, D. D., Krivoshapkina, E. F., Sillanpää, M. & Krivoshapkin, P. V. Hybrid materials based on carbon nanotubes and nanofibers for environmental applications. Front. Chem. 8 https://doi.org/10.3389/fchem.2020.00546 (2020).
Nag, A. & Mukhopadhyay, S. C. Fabrication and implementation of carbon nanotubes for piezoresistive-sensing applications: A review. J. Sci. Adv. Mater. Devices. 7 (1). https://doi.org/10.1016/j.jsamd.2021.100416 (Mar. 2022).
Zheng, S. et al. Carbon nanomaterials for drug delivery and tissue engineering, Front. Chem. 10 (2022). https://doi.org/10.3389/FCHEM.2022.990362
Nasir, A., Kausar, A. & Younus, A. A review on preparation, properties and applications of polymeric Nanoparticle-Based materials. Polym. - Plast. Technol. Eng. 54 (4), 325–341. https://doi.org/10.1080/03602559.2014.958780 (2015).
Liu, Y., Sun, L., Zhang, H., Shang, L. & Zhao, Y. Microfluidics for drug development: from synthesis to evaluation. Chem. Rev. 121, 13, 7468–7529. https://doi.org/10.1021/ACS.CHEMREV.0C01289 (2021).
Davoodi, P., Gill, E. L., Wang, W. & Huang, Y. Y. Advances and innovations in electrospinning technology. In Biomedical Applications of Electrospinning and Electrospraying 20211 (45–81 ). Woodhead Publishing.
M. Daniele, D. Boyd, … A. A.-A. healthcare, and undefined 2015, Microfluidic strategies for design and assembly of microfibers and nanofibers with tissue engineering and regenerative medicine applications, Wiley Online Libr., 4, 1,11–28,(2014), doi: 10.1002/adhm.201400144.
Dos Santos, D. M., Correa, D. S., Medeiros, E. S., Oliveira, J. E. & Mattoso, L. H. C. Advances in functional polymer nanofibers: from spinning fabrication techniques to recent biomedical applications. ACS Appl. Mater. Interfaces. 12, 41, 45673–45701. https://doi.org/10.1021/ACSAMI.0C12410 (2020).
Del, A. R. et al. An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: focusing on cartilage tissue engineering. J. Biol. Eng. 13 (1). https://doi.org/10.1186/S13036-019-0209-9 (Nov. 2019).
Nagarajan, S. et al. Nanofibrous scaffolds for tissue engineering application. Handb. Nanofibers. 665–691. https://doi.org/10.1007/978-3-319-53655-2_30 (2019).
Zieliński, A. & Majkowska-Marzec, B. Whether carbon nanotubes are capable, promising, and safe for their application in nervous system regeneration. some critical remarks and research strategies, Coatings 12, 1643, (2022). https://doi.org/10.3390/COATINGS12111643
Ahmad Raus, R., Wan, W. M. F., Nawawi & Nasaruddin, R. R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 16, 3, 280–306. https://doi.org/10.1016/j.ajps.2020.10.001 (2021).
Homaeigohar, S. & Boccaccini, A. R. Nature-Derived and synthetic additives to poly(ɛ-Caprolactone) nanofibrous systems for biomedicine; an updated overview. Front. Chem. 9 https://doi.org/10.3389/FCHEM.2021.809676 (2022).
Patra, J. K. et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16 (1). https://doi.org/10.1186/S12951-018-0392-8 (2018).
Zamani, M., Prabhakaran, M. P. & Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 8, 2997–3017. https://doi.org/10.2147/IJN.S43575 (2013).
Akgöl, S., Ulucan-Karnak, F., kuru, C. İ. & Kuşat, K. The usage of composite nanomaterials in biomedical engineering applications. Biotechnol. Bioeng. 118, 8, 2906–2922. https://doi.org/10.1002/BIT.27843 (2021).
Rasouli, R., Barhoum, A., Bechelany, M. & Dufresne, A. Nanofibers for biomedical and healthcare applications. Macromol. Biosci. 19 (2). https://doi.org/10.1002/MABI.201800256 ( 2019).
Wang, J. et al. Water purification and environmental remediation applications of carbonaceous nanofiber-based materials, Elsevier, Accessed: Dec. 24, [Online]. (2023). Available:https://www.sciencedirect.com/science/article/pii/S0959652621041901
Abidian, M. R., Corey, J. M., Kipke, D. R. & Martin, D. C. Conducting- polymer nanotubes improve electrical properties, mechanical adhesion, neural attachment and neurite outgrowth of neural electrodes. Small 6, 3, 421–429. https://doi.org/10.1002/SMLL.200901868 (2010).
Scholar, M. D. et al. Electrospun nanofibers for neural tissue engineering. Pubs Rsc Org. 2, 35–44. https://doi.org/10.1039/b9nr00243j (2010).
Bai, S. et al. Silk nanofiber hydrogels with tunable modulus to regulate nerve stem cell fate. J. Mater. Chem. B. 2 (38), 6590–6600 (2014).
Prabhakaran, M. P., Ghasemi-Mobarakeh, L., Jin, G. & Ramakrishna, S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 112 (5), 501–507 (2011).
Horner, P. J. & Gage, F. H. Regenerating the damaged central nervous system. Nature 407, 6807, 963–970. https://doi.org/10.1038/35039559 (2000).
Gu, X., Ding, F., Yang, Y. & Liu, J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 93, 2, 204–230. https://doi.org/10.1016/j.pneurobio.2010.11.002 (2011).
Duara, R. et al. Human brain glucose utilization and cognitive function in relation to age. Annals Neurology: Official J. Am. Neurol. Association Child. Neurol. Soc. 16 (6), 702–713 (1984).
Bedir, T., Ulag, S., Ustundag, C. B. & Gunduz, O. 3D Bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Engineering: C. 110, 110741 (2020).
Silver, J. & reviews neuroscience, J. M. N. and undefined Regeneration beyond the glial scar, nature.comJ Silver, JH MillerNature Rev. Neurosci. nature.com, 2004, (2004). https://doi.org/10.1038/nrn1326
Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 106, 54–69 (2020).
Farokhi, M. et al. Conductive biomaterials as substrates for neural stem cells differentiation towards neuronal lineage cells. Macromol. Biosci. 21 (1). https://doi.org/10.1002/MABI.202000123 (2021).
Uz, M. & Mallapragada, S. K. Conductive polymers and hydrogels for neural tissue engineering. J. Indian Inst. Sci. 99 (3), 489–510. https://doi.org/10.1007/S41745-019-00126-8 (2019).
Stroock, A. et al. Generation of solution and surface gradients using microfluidic systems. ACS Publ. 16, 22, 8311–8316. https://doi.org/10.1021/la000600b (2000).
Wang, K. et al. Electrodeposition of alginate with PEDOT/PSS coated MWCNTs to make an interpenetrating conducting hydrogel for neural interface. Compos. Interfaces. 26 (1), 27–40. https://doi.org/10.1080/09276440.2018.1465766 (2019).
Basavaraja, C., Kim, B. S. & Huh, D. S. Characterization and AC electrical conductivity for the dispersed composites containing alginate-multiwalled carbon nanotubes. Macromol. Res. 19 (3), 233–242. https://doi.org/10.1007/S13233-011-0314-1 (2011).
[248], B., Wei, J., Wang, Z., Chen & Chen, G. Carbon-nanotube-alginate composite modified electrode fabricated by in situ gelation for capillary electrophoresis. Chem. - Eur. J. 14, 31, 9779–9785. https://doi.org/10.1002/CHEM.200801124 (2008).
Meikhail, M. & Hezma, A. Of textiles, undefined coloration, and undefined 2018, characterization and physical studies of Poly (vinyl alcohol)/Sodium alginate/multiwall carbon nanotube biocomposites. Jtcps Journals Ekb Eg. 15 (1), 95–108. https://doi.org/10.21608/jtcps.2018.6289.1013 (2018).
Karkeh-Abadi, F., Saber-Samandari, S. & Saber-Samandari, S. The impact of functionalized CNT in the network of sodium alginate-based nanocomposite beads on the removal of Co (II) ions from aqueous solutions. J. Hazard. Mater. 312, 224–233 (2016).
Valiani, A. et al. Study of carbon nano-tubes effects on the chondrogenesis of human adipose derived stem cells in alginate scaffold. Int. J. Prev. Med. 5 (7), 825 (2014).
Campos-Vallette, M. M. et al. Characterization of sodium alginate and its block fractions by surface-enhanced Raman spectroscopy. Wiley Online Libr. 41 (7), 758–763. https://doi.org/10.1002/jrs.2517 (2010).
Jensen, H. M. et al. Rapid determination of alginate monomer composition using Raman spectroscopy and chemometrics. Researchgate Net. https://doi.org/10.1039/9781847558312-00543 (2015).
Llorens-Gámez, M. & Serrano-Aroca, Á. Low-cost advanced hydrogels of calcium alginate/carbon nanofibers with enhanced water diffusion and compression properties. Polymers 10 (4), 405 (2018).
Salomonsen, T., Jensen, H. M., Stenbæk, D. & Engelsen, S. B. Chemometric prediction of alginate monomer composition: A comparative spectroscopic study using IR, raman, NIR and NMR. Carbohydr. Polym. 72 (4), 730–739 (2008).
J. et al. Fabrication of oriented carbon nanotube-alginate microfibers using a microfluidic device. Funct. Mater. Lett. 12 (6). https://doi.org/10.1142/S1793604719400022 (2019).
Yildirim, E., Yin, X., Nair, K. & of Biomedical, W. S. J. and undefined Fabrication, characterization, and biocompatibility of single-walled carbon nanotube‐reinforced alginate composite scaffolds manufactured using freeform fabrication, Wiley Online Libr., 87, 2, 406–414, (2008). https://doi.org/10.1002/jbm.b.31118
F. Alvarez-Primo, S. A. Kumar, … F. M.-I. J. of, and undefined 2019, Fabrication of surfactant-dispersed HiPco single-walled carbon nanotube- based alginate hydrogel composites as cellular products, mdpi.com, 2016, doi: 10.1038/srep32456.
Jeyaseelan, A., Ghfar, A. A., Naushad, M. & Viswanathan, N. Design and synthesis of amine functionalized graphene oxide for enhanced fluoride removal. J. Environ. Chem. Eng. 9 (4), 105384 (2021).
Chegeni, M., Rozbahani, Z. S., Ghasemian, M. & Mehri, M. Synthesis and application of the calcium alginate/SWCNT-Gl as a bio-nanocomposite for the Curcumin delivery. Int. J. Biol. Macromol. 156, 504–513 (2020).
da Costa, J. S., de Souza, J. F., dos Santos, D. R. & Fajardo, A. R. Composite aerogels of alginate/poly (acrylamide)/carbon nanotubes with enhanced performance for cationic dyes adsorption. Mater. Sci. Engineering: B. 298, 116820 (2023).
Ashrafi, S. D., Safari, G. H., Sharafi, K., Kamani, H. & Jaafari, J. Adsorption of 4- nitrophenol on calcium alginate-multiwall carbon nanotube beads: modeling, kinetics, equilibriums and reusability studies. Int. J. Biol. Macromol. 185, 66–76 (2021).
Salesa, B., Martí, M., Frígols, B. & Polymers, Á. S. A. and undefined Carbon nanofibers in pure form and in calcium alginate composites films: New cost-effective antibacterial biomaterials against the life-threatening multidrug- resistant, mdpi.com, 2019, (2019). https://doi.org/10.3390/polym11030453
Shirazi, Y., Tofighy, M. A. & Mohammadi, T. Synthesis and characterization of carbon nanotubes/poly vinyl alcohol nanocomposite membranes for dehydration of isopropanol, J. Memb. Sci. 378 1–2, 551–561, (2011). https://doi.org/10.1016/J.MEMSCI.2011.05.047
Liu, X. et al. Ultra-light, high flexible and efficient CNTs/Ti3C2-sodium alginate foam for electromagnetic absorption application. J. Mater. Sci. Technol. 35, 12, 2859–2867. https://doi.org/10.1016/J.JMST.2019.07.019 (2019).
Lin, T., Wang, H., Wang, H. & Wang, X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 15, 9, 1375–1381. https://doi.org/10.1088/0957-4484/15/9/044 (2004).
Lin, T., Wang, H., Wang, H. & Wang, X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 15 (9), 1375–1381. https://doi.org/10.1088/0957-4484/15/9/044 (2004).
McNamara, M. C. et al. Enhancing the conductivity of cell-laden alginate microfibers with aqueous graphene for neural applications, Front. Mater. 7 (2020). https://doi.org/10.3389/FMATS.2020.00061/FULL
Homaeigohar, S., Tsai, T. Y., Young, T. H., Yang, H. J. & Ji, Y. R. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydr. Polym. 224, 115112 (2019).
Cramariuc, B. et al. Fiber diameter in electrospinning process. J. Electrostat. 71 (3), 189–198 (2013).
Yeung, R. A. & Kennedy, R. A. A comparison of selected physico-chemical properties of calcium alginate fibers produced using two different types of sodium alginate. J. Mech. Behav. Biomed. Mater. 90, 155–164 (2019).
Sim, S. et al. Neural probe integrated with Low-impedance electrodes implemented using vertically aligned carbon nanotubes for Three-dimensional mapping of neural signals. Sens. Actuators B. 9, 134124 (2023).
Mirmohammadi, S. A., Sadjadi, S. & Bahri-Laleh, N. Electrical and electromagnetic properties of CNT/polymer composites. In Carbon Nanotube-Reinforced Polymers. (233–258). (Elsevier, 2018).
Ma, P., Tang, B., Carbon, J. K. & undefined Effect of CNT decoration with silver nanoparticles on electrical conductivity of CNT-polymer composites. Elsevier https://doi.org/10.1016/j.carbon.2008.06.048 (2008).
Wang, Q., Dai, J., Li, W., Wei, Z. & Jiang, J. The effects of CNT alignment on electrical conductivity and mechanical properties of swnt/epoxy nanocomposites. Compos. Sci. Technol. 68 (7–8), 1644–1648 (2008).
Jayathilaka, W. A., Chinnappan, A. & Ramakrishna, S. A review of properties influencing the conductivity of cnt/cu composites and their applications in wearable/flexible electronics. J. Mater. Chem. C. 5 (36), 9209–9237 (2017).
Ruan, B. & research letters, A. J. N. and undefined Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions, Springer 7 (2012). https://doi.org/10.1186/1556-276X-7-127
Ke, K. et al. Evolution of agglomerate structure of carbon nanotubes in multi-walled carbon nanotubes/polymer composite melt: A rheo-electrical study. Compos. Part. B: Eng. 43 (8), 3281–3287 (2012).
Márquez-Lucero, A. et al. A method to evaluate the tensile strength and Stress–Strain relationship of carbon nanofibers, carbon nanotubes, and C-Chains. Small 1. 6, 640–644. https://doi.org/10.1002/SMLL.200400115 (2005).
Zhang, L. & Webster, T. J. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 4 (1), 66–80. https://doi.org/10.1016/J.NANTOD.2008.10.014 (2009).
Harrison, B. S. & Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 28 (2), 344–353. https://doi.org/10.1016/J.BIOMATERIALS.2006.07.044 (2007).
Tran, P. A., Zhang, L. & Webster, T. J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv Rev. 61, 12, 1097–1114. https://doi.org/10.1016/J.ADDR.2009.07.010 (2009).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Salman, A., Fhong, S.c., Salem, A.A. et al. Development of millifluidic device and electronic extrusion system for fabrication of sodium alginate/carbon nanotube microfibers. Sci Rep 15, 26192 (2025). https://doi.org/10.1038/s41598-025-12075-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12075-7