CH4 flow dynamics in coal, governed primarily by adsorption, desorption, and seepage processes, are critical for determining gas extraction efficiency. Coal seam deformation under varying stress conditions further significantly impacts CH4 flow. Utilizing a self-developed coal solid-gas coupling test apparatus, this study conducted simultaneous measurements of CH4 flow and coal deformation under different axial-to-radial stress ratios. The temporal relationship between CH4 flow and coal deformation was analyzed, establishing a quantitative correlation between the two. The influence of stress on both phenomena was examined. A model incorporating residual strain was developed to evaluate coal strain throughout the entire CH4 flow process. Results demonstrated that both CH4 flow and coal deformation exhibit Langmuir-like relationships with time. Similarly, a Langmuir-like relationship was observed between the amount of CH4 adsorbed and coal deformation during adsorption. Within the experimental stress range, an increase in the axial-to-radial stress ratio inhibited CH4 flow, consequently reducing coal deformation. Volumetric strain exhibited greater sensitivity to changes in the stress ratio compared to radial or axial strain. Residual strain was identified in coal during both CH4 adsorption and desorption, with its prominence inversely related to the axial-to-radial stress ratio. The model, accounting for residual strain alongside isothermal flow and deformation characteristics, accurately represented the temporal evolution of coal deformation during CH4 flow. This research provides a theoretical foundation for enhancing the efficacy of gas extraction.
Similar content being viewed by others
Introduction
China possesses abundant coal resources alongside substantial coalbed methane (CBM) reserves1. As shallow coal resources diminish, mining operations are progressively shifting towards deeper deposits. This transition is accompanied by increasingly complex geological conditions at depth, including elevated in-situ stress, gas pressure, and temperature, which have intensified gas disasters, compromising both safety and mining efficiency2. Efficient CBM extraction constitutes a fundamental approach to mitigating such disasters. The gas flow process, encompassing adsorption, desorption, and seepage, is intrinsically linked to coal deformation (swelling/shrinking), significantly impacting extraction effectiveness3,4. Consequently, establishing optimal CBM extraction parameters necessitates a thorough understanding of the coupled characteristics of gas flow and coal deformation throughout the entire extraction process.
Gas adsorption and desorption in coal constitute a thermodynamically reversible process. Consequently, the associated coal swelling (during adsorption) and shrinkage (during desorption) are fundamentally reversible phenomena. Significant research has investigated CBM sorption characteristics and the resulting coal deformation. Baran et al.5 experimentally demonstrated anisotropic coal deformation during gas adsorption and desorption under varying gas pressures, establishing a corresponding deformation model consistent with empirical data. Similarly, Ren6, Zhang7 and Nie8 analyzed deformation patterns induced by gas adsorption-desorption and developed mathematical models relating sorption to coal strain. Complementary studies by Tang9, Lin10 and Bergen11 examined the temporal dynamics of gas adsorption in shale/coal and associated anisotropic deformation behavior. In contrast, Liu’s experimental work12,13 under multiple gas pressures revealed a linear relationship between adsorbed gas volume and coal deformation magnitude. Ma14, Liang15, and Wei16 experimentally quantified the influence of effective stress on coal deformation throughout complete gas adsorption-desorption. Complementing this empirical work, George17 and Espinoza3 developed theoretical models elucidating the mechanistic relationship between gas desorption dynamics and coal deformation.
Similarly, coal experiences deformation during gas seepage processes. Wei18, Chen19, and Li20 conducted stress-seepage experiments demonstrating that external stress exerts significant control over coal permeability, which exhibits strong coupling with coal deformation. Complementing this research, Jiang21 and Li22 quantified deformation behavior during gas seepage under varying temperatures. Their findings reveal that elevated temperatures amplify pore and fracture deformation, consequently driving substantial permeability evolution in coal matrices.
In summary, existing studies frequently overlook coal deformation dynamics throughout the entire CH4 flow process. During gas extraction, effective stress within the coal seam continuously evolves due to declining gas pressure. Furthermore, mining-induced stresses at the working face create highly dynamic loading conditions, characterized by complex axial/radial stress evolution23. Consequently, CH4 flow in coal represents a tightly coupled multi-physics phenomenon involving stress, seepage, and thermal fields. Despite this interdependence, current research rarely integrates stress-seepage coupling when analyzing CH4 adsorption/desorption behavior under varying stress states.
To address this gap, the authors employed a self-developed coal solid-gas coupling test apparatus to conduct CH4 flow experiments under controlled multi-axial stress states. This approach enabled quantitative characterization of coal deformation dynamics throughout the complete CH4 flow cycle (adsorption, desorption, and seepage). Based on these observations, a novel strain model incorporating CH4 flow-induced deformation mechanisms was formulated to enhance CBM extraction efficiency.
Experimental protocols
The collection and Preparation of coal sample
Coal samples were obtained from a newly exposed seam in the Qinshui Basin, Shanxi Province, China. Following immediate on-site sealing, specimens were transported to the laboratory for processing. Cylindrical cores (Φ50 mm × 100 mm) were prepared using a diamond core drill, with all surface precision-ground to achieve parallelism and smoothness. To ensure experimental reproducibility, specimens underwent rigorous selection based on diameter, height, weight, wave velocity, and compressive strength, et al. Ten specimens meeting these criteria were ultimately selected and surface-degreased using anhydrous ethanol prior to testing.
Strain gauges (25 mm gauge length) were mounted at symmetrical mid-height positions on opposing sample faces to measure axial and radial deformation, respectively. Prior to testing, specimens were radially encapsulated in heat-shrink sleeves and subjected to vacuum dehydration. This involved placing samples in a vacuum oven at 70 °C for over 24 h until mass stabilization (< 0.1% variation over 1 h). Figure 1 illustrated this preparation sequence.
Experimental equipment
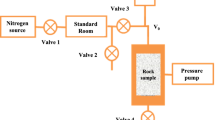
A self-developed coal solid-gas coupling test apparatus (Fig. 2) was employed for this investigation. The system integrates five critical functions of triaxial stress application via independent radial/axial hydraulic pumps, vacuum processing capabilities, CH₄ flow characterization, full-field strain monitoring, automated data acquisition24. CH4 adsorption characteristics were quantified using the high-pressure volumetric method, while triaxial strain measurements were obtained through positioned resistance strain gauges. Crucially, the apparatus enables simultaneous monitoring of CH4 transport dynamics and coal deformation throughout testing cycles.
Experimental procedure and protocol
CH4 isothermal adsorption experiments followed Chinese National Standard GB/T 19,560 − 2008 (High-pressure isothermal adsorption testing of coal), while CH4 seepage experiments complied with Coal Industry Standard MT/T 223–1990 (Method for determination of coal and rock permeability)7. According to the working front stress state in the gas pre-extraction stage25, this paper utilized the control variable method to analyze the gas flow and coal deformation characteristics under different stress loading ratio, and the details of specific experimental condition settings were shown in Table 1. Figure 3 documented the experimental workflow and specific testing conditions.
The adsorption capacity of CH4 in coal can be calculated using equation of state.
Where, V0 was the reference tank volume (in cm3). P0 and Z0 was the gas pressure (in MPa) and compression coefficient in the tank prior to adsorption, respectively. Vc was the total volume of the coal pores and pipeline (in cm3). Va was the CH4 adsorption amount (in cm3). Pt and Zt was the gas pressure (in MPa) and compression coefficient in the reference tank at time t, respectively. The pipeline volume was 13.75 cm3.
The steady-state method was used to measure coal permeability under varying stress conditions, and the flow was found to conform to Darcy’s law26.
Where, K was the coal permeability (in m2). µ was the gas viscosity coefficient (in Pa∙s). q was the gas flow (in cm3/s). Pa was the atmospheric pressure (in MPa). L was the height of coal (in cm). A was the cross-sectional area of coal sample (in cm2). P1 and P2 were the gas pressure in the inlet and outlet respectively (in MPa).
The test of coal porosity
The gas expansion method was employed to determine coal porosity under varying confining stresses, following the experimental procedure outlined in Fig. 2. The experimental procedure commenced by loading the coal sample into the triaxial stress chamber and applying confining stress via valves V4 and V6. Helium was then introduced into the reference tank by opening valve V1 and adjusting pressure reducer V7 until a stable pressure of 1 MPa was achieved. Following pressure stabilization, valve V1 was closed and the initial reference tank pressure (P0) was recorded. Gas injection into the coal sample was initiated by opening valve V2. The equilibrium pressure (P1) in the reference tank was recorded after stabilization of both the reference tank and outlet pressures. All pressure measurements adhered to Boyle’s law.
The coal porosity ϕ at different levels of stress can be obtained using the following Eq.
Where, Vv and V was the volume of pore and coal (in cm3), respectively.
Figure 4 illustrated the evolution of coal porosity under varying stress conditions. Increasing radial/axial stress ratios induced progressive compaction of native pores and fractures, resulting in systematically reduced porosity.
Timeliness analysis of gas flow and coal strain
Gas adsorption and coal strain
Figure 5 presented the temporal evolution of CH4 adsorption capacity and sorption kinetics, while Fig. 6 quantified induced coal strain, where negative values represented shirking and positive values represented swelling. Both CH4 adsorption and coal deformation kinetics followed Langmuir-type behavior6,27, as confirmed by nonlinear regression of experimental data (Table 2). The governing equations were as follow.
Where, n = x, j, s. n = x represented the process of CH4 adsorption, n = j represented the process of CH4 desorption, n = s represented the process of CH4 seepage. Qn was CH4 amount (in cm3/g). an was the ultimate amount (in cm3/g). bn was the constant associated with time (in 10−3/s). εn was the strain of coal within tn (in 10−2). An was the ultimate strain of coal (in 10−2). Bn was the strain constant associated with time (in 10−3/s). tn was time (in 103s).
Where, εx−X was the radial strain of coal during adsorption process, 10−2. εx−Y was the axial strain, 10−2. εx−V was the volumetric strain, 10−2.
CH4 adsorption exhibited three stages of kinetics with adsorption time: an initial rapid uptake, followed by a decelerated increase, culminating in equilibrium10,28. Coal strain demonstrated congruent evolution, with volumetric strain exceeding radial strain, which surpassed axial strain. Under low-pressure conditions, elevated CH4 concentration gradients and limited molecular mean free paths within the coal matrix29 promoted surface diffusion dominance in micropores9. This mechanism drove accelerated adsorption and concurrent strain accumulation.
As adsorption progressed, increasing mean pore pressure reduced CH₄ concentration gradients within the coal matrix while extending molecular mean free path. These changes diminished diffusive transport, causing adsorption kinetics and induced strain to asymptotically approach equilibrium. Under triaxial stress conditions, hydrostatic pore pressure exerted equal radial and axial loading. However, axial expansion is suppressed relative to radial expansion due to the high confining stresses along the axial direction.
Gas desorption and coal strain
Figure 7 quantified the temporal evolution of CH4 desorption capacity, kinetics, and velocity, while Fig. 8 documented corresponding coal strain. Both gas desorption and coal strain followed Langmuir-type decay patterns. Experimental data were fitted using nonlinear regression, with parameters tabulated in Table 3.
Where, εj−X was the radial strain of coal during desorption process, 10−2. εj−Y was the axial strain, 10−2. εj−V was the volumetric strain, 10−2.
CH4 desorption kinetics and associated strain evolution exhibited temporal symmetry with adsorption processes. During initial desorption, steep pressure gradients between the coal matrix and external environment drove rapid gas release via pore desorption and fracture seepage. This abrupt pressure decline induced instantaneous matrix contraction, manifesting as transient compressive strain. As pore pressure equilibrated with atmospheric boundary conditions (0.1 MPa), seepage fluxes decayed asymptotically. Consequently, both desorption rates and strain magnitudes approached equilibrium.
The desorption rate (defined as the rate of desorption amount to equilibrium adsorption amount) and velocity exhibited kinetics congruent with cumulative desorption. Volumetric strain dominated the deformation response during desorption, manifesting as pronounced matrix contraction. Due to higher axial confinement stress, axial strain remained suppressed relative to radial direction throughout both adsorption (swelling) and desorption (shirking).
Gas seepage and coal strain
Figure 9 characterized gas seepage dynamics and concurrent coal strain evolution under incremental confining stresses. During CH4 seepage, coal deformation exhibited Langmuir-type kinetics congruent with and yet magnitude-constrained relative to adsorption-phase strain. Nonlinear regression confirmed Langmuir behavior for seepage-induced strain, with fitting parameters documented in Table 4.
Where, εs−X was the radial strain of coal during seepage process, 10−2. εs−Y was the axial strain, 10−2. εs−V was the volumetric strain, 10−2.
During CH4 seepage, gas transport encompassed concurrent adsorption and desorption processes. Upon achieving steady-state flow, adsorption-induced swelling dominated over desorption-induced shirking, resulting in volumetric shrinking of coal matrix. This differential strain response originated from greater adsorption site occupancy versus limited desorption coverage under equivalent pressure conditions.
Effect of axial/radial stress ratio on the coal strain
Effect of axial/radial stress ratio on the coal strain during CH4 adsorption
Adsorption-induced pore pressure elevation and surface interactions caused omnidirectional coal swelling. Figure 10 demonstrated the Langmuir-type correlation between volumetric strain and CH4 adsorption capacity across tested stress states. Nonlinear regression confirmed superior Langmuir model fidelity, with fitting parameters tabulated in Table 5.
Where, ηn and γn were the fitting parameters during CH4 adsorption/desorption process, and the units of the two parameters were 10−2 and g/cm3, respectively.
Both CH4 equilibrium adsorption amount and coal strain decreased with increasing axial/radial stress ratio (σ%). At σ% = 1.5, the equilibrium adsorption amount of CH4 was 3.42 cm3/g, and the corresponding axial, radial and volumetric strain of coal were 1.32 × 10−2, 0.18 × 10−2 and 2.84 × 10−2, respectively. At σ% = 3.5, the equilibrium adsorption amount of CH4 was 2.84 cm3/g, and the corresponding axial, radial and volumetric strain of coal were 1.06 × 10−2, 0.12 × 10−2 and 2.26 × 10−2, respectively. This represented reductions of 16.96% (adsorption), 19.70% (εx−X), 33.33% (εx−Y), and 20.42% (εx−V) respectively with increased stress anisotropy.
These reductions originated from two stress-dependent mechanisms. (1) Lower stress ratios preserved greater micropore surface area, providing enhanced adsorption site density and pronounced matrix swelling. Increasing axial stress induced progressive micropore closure, reducing accessible adsorption sites and constraining strain development. (2) Elevated stress anisotropy impeded CH4 diffusion through constricted pore networks, reducing the Knudsen diffusion coefficient. Concurrently, diminished gas-matrix collision frequency lowered adsorption kinetics, further suppressing CH4 uptake and associated swelling.
Coal strain anisotropy emerged from the coupled effects of fracture pattern, anisotropic stress states, and pore pressure. Linear regression analyses (Fig. 11) quantified directional strain sensitivity to adsorption under increasing stress anisotropy. Volumetric strain exhibited the strongest adsorption dependence, followed by radial and axial strains, confirming progressive mechanical constraint along the highly stressed axial direction.
Effect of axial/radial stress ratio on the coal strain during CH4 desorption
Coal exhibited shirking deformation due to gas desorption. The relationship between coal strain and gas desorption amount was presented in Fig. 12, with corresponding fitting results detailed in Table 6.
At σ% = 1.5, the CH4 desorption amount was 2.87 cm³/g with a desorption rate of 83.93%. The corresponding axial, radial, and volumetric strains of the coal were 1.03 × 10⁻², 0.14 × 10⁻², and 2.18 × 10⁻², respectively. When σ% increased to 3.5, the equilibrium CH4 desorption amount decreased by 19.50% to 2.31 cm³/g, while the desorption rate declined by 3.0–81.41%. Concurrently, the axial, radial, and volumetric strains decreased to 0.91 × 10⁻², 0.06 × 10⁻², and 1.88 × 10⁻², which were reduced by 11.65%, 57.14% and 13.76%, respectively.
These reductions originated from two mechanisms, ① Elevated stress diminished coal matrix surface energy, suppressing CH4 desorption and consequent shrinkage16. ② Compression of micropores and fractures under external stress restricted CH4 desorption/seepage pathways, thereby inhibiting shrinkage strain and rate development. Linear regression analysis of stress-dependent strain data (Fig. 13) confirmed consistent stress sensitivity patterns between desorption and adsorption processes.”
Effect of axial stress/radial stress ratio on the coal deformation during CH4 seepage
Coal exhibited non-elastic behavior30, resulting in stress-dependent permeability variations. As shown in Fig. 14, coal strain and permeability evolved with increasing stress ratios. At σ% = 1.5, permeability measured 4.53 × 10⁻¹⁷ m², accompanied by axial, radial, and volumetric strains of 0.84 × 10⁻², 0.12 × 10⁻², and 1.81 × 10⁻², respectively. When σ% increased to 3.5, permeability declined by 53.86% to 2.09 × 10⁻¹⁷ m², while strains decreased to 0.37 × 10−2, 0.05 × 10−2 and 0.79 × 10−2, which were reduced by 55.95%, 58.33% and 56.36%, respectively. This systematic reduction indicated the experimental coal transitions through compression and elastic deformation stages under elevated stress31.
During gas seepage in coal, deformation manifested through dual-scale mechanisms15: macroscopically as volumetric strain variation, and microscopically as pore-fracture-matrix reorganization. Assuming constant seepage parameters and pore pressure during initial conditions, coal deformation primarily results from synergistic effects of increasing axial stress and methane advection. As seepage progresses, compression of pores and fractures inhibits CH₄ flow, reducing both flow velocity and internal pressure per unit time. This process macroscopically reduces permeability while simultaneously inducing matrix swelling. Linear regression analysis (Fig. 14) revealed strain sensitivity followed the hierarchy: volumetric > radial > axial strain.
Discussion
Time-dependent analysis of coal strain during CH4 flow revealed stress-ratio-dependent deformation patterns (Fig. 15). Using adsorption-desorption kinetic equations, a residual strain model capturing hysteresis effects in coal matrices was derived.
Where, Δε was the residual coal strain.
Residual strain
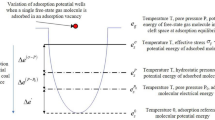
Residual strain generation during gas adsorption-desorption represents a critical manifestation of the complex coal-gas interactions, governed primarily by elastic deformation, pore structure evolution, and adsorption-desorption hysteresis.
As a heterogeneous and non-ideal elastic medium, coal exhibits inherent strength variations. During adsorption-induced swelling, localized plastic deformation may occur in high-stress regions. During subsequent desorption-shrinkage, the stress state within these regions cannot fully recover to its initial condition due to constraints imposed by surrounding elastic zones32. Furthermore, while matrix swelling may close primary fractures, the complete reopening of fractures during shrinkage is impeded by frictional resistance and debris infilling. This irreversibility manifests macroscopically as residual strain.
The swelling-shrinking forces generated during gas adsorption and desorption continuously modify the microstructure of coal. Irreversible deformation of pores and fractures directly contributes to residual strain development33. Under cyclic force loading, weak pore walls undergo compaction or permanent dilation, altering pore morphology and volume. Concurrently, stress concentrations during adsorption-swelling can exceed the strength of coal, generating new micro-fractures and propagating existing ones34. Debris production from fracture wall abrasion further modifies fracture geometry through infilling or roughness alteration. These competing mechanisms—fracture propagation (increasing void volume and contributing to residual expansion strain) versus pore collapse and debris occlusion (reducing effective porosity and enhancing shrinkage)—collectively determine the evolution of pore and fracture and the magnitude of residual volumetric strain.
Adsorption-desorption hysteresis constitutes another primary factor in residual strain. The prevalence of ink-bottle shaped pores (characterized by narrow necks and wide bodies) in coal impedes desorption through capillary condensation effects at pore throats35. Additionally, strong interactions between CH4 molecules and the coal surface necessitate higher energy barriers for desorption, resulting in retained gas. This retained gas sustains matrix support and swelling pressure, preventing the coal from fully reverting to its initial state before adsorption.
Permeability evolution
Coal permeability dynamics are fundamentally governed by micropore architecture and its response to multiphysical processes during gas extraction. Disruption of in-situ stress equilibrium initiates critical restructuring of internal pores and fractures through stress redistribution36. Porosity and permeability—key parameters in stress-seepage coupling—evolve under triaxial constraints of external stress, pore pressure, and coal mechanical properties37.
During elastic deformation under increasing effective stress, coal undergoes sequential compaction phases. Initial fracture closure and matrix compression reduce pore volume and specific surface area, diminishing equilibrium gas adsorption capacity16. This microstructural collapse impairs CH₄ flow through three concurrent mechanisms: (1) seepage pathway constriction, (2) pore pressure gradient reduction, and (3) increased flow resistance, culminating in macroscopic permeability decline38.
Concurrently, CH4 adsorption-desorption induces matrix swelling/shrinking, directly modulating permeability through interdependent stress mechanisms: adsorbed CH4 generates compressive stress causing reverting elastic matrix deformation, while free-phase gas exerts swelling stress triggering unrecoverable plastic deformation through structural displacement39. This prevents full restoration of pre-adsorption pore-fracture geometry. Residual gas further provides structural reinforcement and sustained swelling pressure, with resulting irreversible strain impairing subsequent gas seepage.
During CBM extraction, thermo-hydro-mechanical (THM) coupling continuously modifies pore-fracture network architecture, governing gas transport through a dynamically coupled system integrating matrix deformation, skeletal restructuring, and gas flow40. Elucidation of stress-dependent deformation and transport mechanisms provides critical foundations for optimizing CBM extraction parameters and advancing multiphysics coupling theory, facilitating precision methane recovery and geohazard mitigation.
Conclusion
(1) CH4 adsorption and desorption in coal exhibited three stages of rapid-increase, slow-increase, and plateau stages. Both adsorbed/desorbed gas amount decreased with increasing axial/radial stress ratio. Higher stress ratios further reduced CH₄ adsorption/desorption rates compared to lower ratios.
(2) CH4 flow induced variable magnitudes of coal swelling/shrinking. During initial flow stages, volumetric strain increased rapidly before equilibrating as gas transport stabilized.
(3) Temporal evolution of both gas flux and coal strain followed Langmuir-type kinetics. Adsorption/desorption amount similarly exhibited Langmuir relationships with coal strain. Increasing stress promoted linear decay of coal strain.
(4) Irreversible residual strain developed during CH4 adsorption-desorption, exhibiting inverse correlation with axial/radial stress ratios. The established model incorporated this deformation hysteresis through a time-dependent constitutive framework with residual strain quantification across all process stages.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Liu, J. F., Fokker, P. A., Peach, C. J. & Spiers, C. J. Applied stress reduces swelling of coal induced by adsorption of water. Geomech. Energy Environ. 16, 45–63 (2018).
Fairhurst, C. Some challenges of deep mining. Eng 3 (4), 527–537 (2017).
Espinoza, D. N., Pereira, J. M., Vandamme, M., Dangla, P. & Vidal-Gilbert, S. Desorption-induced shear failure of coal bed seams during gas depletion. Int. J. Coal Geol. 137, 142–151 (2015).
Mohammad, A. A., Tao, C., Gerhard, S., Espinoza, D. N. & Sanjeev, P. Subsidence associated with dewatering and gas extraction from coal seams: contribution of desorption-induced coal shrinkage. J. Hydrol. 637, 131355 (2024).
Baran, P., Czerw, K., Czuma, N. & Zarbska, K. Development of temperature-induced strains in coal-CH4 and coal-CO2 system. Adsorpt. Sci. Technol. 37 (1–2), 24–33 (2019).
Ren, J. G. et al. CO2 adsorption/desorption, induced deformation behavior, and permeability characteristics of different rank coals: application for CO2-enhanced coalbed methane recover. Energy Fuels. 36 (11), 5709–5722 (2022).
Zhang, X. D. et al. CO2 and N2 adsorption/desorption effects and thermodynamic characteristics in confined coal. J. Pet. Sci. Eng. 6, 109166 (2021).
Nie, B. S., Hu, S. T., Li, X. C., Zhai, S. R. & Meng, J. Q. Experimental study of deformation rules during the process of gas adsorption and desorption in briquette coal. Int. J. Min. Reclam. Environ. 28 (5), 277–286 (2014).
Tang, J. R., Wang, X. C., Lu, Y. Y., Ao, X. & Han, S. B. Experimental study on time effect and deformation anisotropy of shale and coal under CO2. J. China Coal Soc. 43 (8), 2288–2295 (2018).
Lin, H. F. et al. Experimental study on the influence of gas pressure on CH4 adsorption-desorption-seepage and deformation characteristics of coal in the whole process under triaxial stress. Fuel 333, 126513 (2023).
Bergen, F. V., Spiers, C., Floor, G. & Bots, P. Strain development in unconfined coals exposed to CO2, CH4 and ar: effect of moisture. Int. J. Coal Geol. 77 (1–2), 43–53 (2009).
Liu, Y. B. et al. Experimental study of swelling deformation effect of coal induced by gas adsorption. Chin. J. Rock. Mech. Eng. 29 (12), 2484–2491 (2010).
Liu, Y. B. Test study on deformation characteristics of extra-soft coal in high-pressure absorption process. Min. Saf. Environ. Prot. 43 (5), 1–4 (2016).
Ma, H. F. et al. Experimental study on desorption characteristics of coalbed methane under variable loading and temperature in deep and high geothermal mine. Adv. Civ. Eng. 8878125 (2020). (2020).
Liang, B., Jia, L. F., Sun, W. J. & Jiang, Y. Experimental on the law of coal deformation and permeability under desorption and seepage. J. China Univ. Min. Technol. 47 (5), 935–941 (2018).
Wei, J. P., Wen, Z. H., Yuan, Y. W., Wang, Q. & Si, L. L. Study on influence of stress on desorption characteristics of coal containing gas. Coal Sci. Technol. 49 (5), 35–43 (2021).
George, J. S. & Barakat, M. A. The change in effective stress associated with shrinkage from gas desorption in coal. Int. J. Coal Geol. 45 (2), 105–113 (2001).
Wei, M. Y. et al. Quantitative study on coal permeability evolution with consideration of shear dilation. J. Nat. Gas Sci. Eng. 36 (S1), 1199–1207 (2016).
Chen, M., Masum, S., Sadasivam, S. & Thomas, H. Modelling anisotropic adsorption-induced coal swelling and stress-dependent anisotropic permeability. Int. J. Rock. Mech. Min. Sci. 153, 105107 (2022).
Li, J. H., Li, B. B., Cheng, Q. Y. & Gao, Z. L. Characterization of anisotropic coal permeability with the effect of sorption-induced deformation and stress. Fuel 309, 122089 (2022).
Jiang, C. Z. et al. Controlling effects of differential swelling index on evolution of coal permeability. J. Rock. Mech. Geotech. Eng. 12, 461–472 (2020).
Li, X. C., Yan, X. P. & Kang, Y. L. Effect of temperature on the permeability of gas adsorbed coal under triaxial stress conditions. J. Geophys. Eng. 15 (2), 386–396 (2018).
Yang, W. et al. Tunnelling outburst potential affected by mechanical properties of coal seam. Tunn. Undergr. Space Technol. 83, 99–112 (2019).
Li, L. et al. Permeability characteristics of CH4, CO2, and N2 during the whole process of adsorption in coal with accumulated pressure under triaxial stresses. J. Pet. Sci. Eng. 213, 110380 (2022).
Xie, H. P., Gao, F. & Ju, Y. Research and development of rock mechanics in deep ground engineering. Chin. J. Rock. Mech. Eng. 34 (11), 2161–2178 (2015).
Zhang, L., Chen, S., Zhang, C., Fang, X. Q. & Li, S. The characterization of bituminous coal microstructure and permeability by liquid nitrogen fracturing based on µCT technology. Fuel 262, 116635 (2022).
Meng, Y. & Li, Z. P. Experimental comparisons of gas adsorption, sorption induced strain, diffusivity and permeability for low and high rank coals. Fuel 234, 914–923 (2018).
Long, H. et al. Adsorption and diffusion characteristics of CH4, CO2, and N2 in micropores and mesopores of bituminous coal: molecular dynamics. Fuel 292, 120268 (2021).
Zhou, Y. B. et al. Influence of coal deformation on the Knudsen number of gas flow in coal seams. Energy 233, 121161 (2021).
Zhao, Y. X. et al. Failure mechanisms in coal: dependence on strain rate and microstructure. J. Geophys. Res. Solid Earth. 119 (9), 6924–6935 (2014).
Li, B. B. et al. Experimental study on damage and the permeability evolution process of methane-containing coal under different temperature conditions. J. Pet. Sci. Eng. 184, 106509 (2020).
Geng, W. L. et al. Influence of long-term CH4 and CO2 treatment on the pore structure and mechanical strength characteristics of Baijiao coal. Energy 242, 122986 (2022).
Zhang, L., Tan, T. J., Wang, E. Y., Liu, Y. B. & Chen, D. Evolutionary characteristics and correlations between deformation energy and strain in anthracite coal during stress wave-induced catastrophes. Eng. Geol. 347, 107931 (2025).
Sun, Z. D. et al. Experimental study on the fracture behavior of sandstone after ScCO2-water-rock interaction. J. Nat. Gas Sci. Eng. 68, 102904 (2019).
Wang, T. et al. Pore structure of deep coal of different ranks and its effect on coalbed methane adsorption. Int. J. Hydrogen Energy. 59, 144–158 (2024).
Zhang, X. M., Li, Y. R. & Yang, D. T. Repeated uniaxial compression test of permeable friction courses mixtures under seepage field and stress field coupling. Adv. Mater. Sci. Eng. 2943138 (2022). (2022).
Schwartz, B., Huffman, K., Thornton, D. & Elsworth, D. The effects of mineral distribution, pore geometry, and pore density on permeability evolution in gas shales. Fuel 257, 116005 (2019).
Jia, L. D. et al. Study on the evolution mechanism of coal permeability during gas production and coal mining. Chin. J. Rock. Mech. Eng. 41 (1), 132–146 (2022).
Lu, P., Shen, Z. W., Zhu, G. W. & Fang, E. C. The effective stress and mechanical deformation and damage characteristics of gas-filled coal. J. China Univ. Sci. Technol. 6, 55–62 (2001).
Shu, Y., Sang, S. X., Jin, Y. & Zheng, H. M. Multi-scale reservoir response characteristics of coalbed methane development through stress relief via horizontal well cavity completion in tectonically deformed coals. Fuel 381, 133600 (2025). (Part C).
Funding
for this study was supported by the National Key Research and Development Program of China (No. 2023YFC3009004), the China Postdoctoral Science Foundation (No. 2023MD744247), the Shaanxi Provincial Department of Science and Technology (No. 2024JC-YBQN-0401), the Education Department of Shaanxi Province (No. 23JP093), and the Shaanxi Province Postdoctoral Science Foundation (No. 2023BSHEDZZ298).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Hang Long: Writing, Formal analysis, Investigation, Writing-Original draft, Funding acquisition. Hai-fei Lin: Conceptualization, Methodology, Investigation, Resources, Review & Editing. Dong-min Ma and Chen Chen: Visualization, Investigation. Shu-gang Li and Yang Bai: Investigation, Data Curation, Writing-Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Long, H., Lin, Hf., Ma, Dm. et al. Experimental study of gas flow and coal deformation at different levels of axial/radial stress ratio. Sci Rep 15, 26345 (2025). https://doi.org/10.1038/s41598-025-12133-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12133-0