Abstract
Theileriosis, babesiosis, anaplasmosis, and ehrlichiosis are the most important constraints to livestock production in Karamoja region, North-eastern Uganda. However, there are no large-scale studies on the prevalence and seasonal variation of tick-borne haemoparasites that are needed to design and implement tick-borne disease control programs. We collected 7080 blood samples from cattle across four districts of north-eastern Uganda during the dry (November 2022 to February 2023) and wet (July to August 2023) seasons. These samples were screened for the most important tick-borne haemoparasites (TBH) by conventional PCR, followed by capillary sequencing of representative PCR amplicons. There was no statistically significant difference [p > 0.05] in the overall prevalence of infection with at least one of the screened TBHs during the wet [39.0%; CI 7.3–40.6] and dry seasons [39.2%: CI 37.6–40.9]. Prevalence of the individual TBHs during the dry season were:—Babesia bigemina 11.8% (CI 10.8–12.9), Babesia bovis 11.8% (CI 10.8–12.9), Anaplasma marginale 9.2% (CI 8.2–10.2), Ehrlichia ruminantium 5.1% (CI 4.4–5.8) and Theileria parva 1.3% (CI 1.0–1.8). Prevalence of individual TBHs during the dry season were:—T. parva 22.6% (CI 21.3–24), A. marginale 13.6% (CI 12.5–14.8), B. bigemina 12.7% (CI 11.6–13.8), E. ruminantium 1.4% (CI 1.1–1.9) and B. bovis 0.3% (CI 0.1–0.5). Geospatial location, increasing age, sex, overnight stay in cattle kraals, and cattle breeds were significant predictors of infection with different TBHs during either season. Co-infection with the individual TBHs ranged between 0.14–2.74% and 0–1.64% during the dry and wet seasons respectively. In both seasons, the co-infection rate with all five TBHs was 0.03% (CI 0.0–0.16). Phylogenetic analyses of the representative TBH sequences revealed high level of conservation within the targeted genes of the samples in this study and those within the East Africa region that were retrieved from the GenBank. This study demonstrate high level of infection/co-infection with different TBHs in both dry and wet seasons indicating that ticks and tick-borne diseases are a major impediment to livestock production in Karamoja region. This shows the need of having a ticks and tick-borne disease control program. Moreover, B. bovis was detected for the first time in this region.
Similar content being viewed by others
Introduction
The impact of ticks and tick-borne diseases (TTBDs) on the socioeconomics of pastoral communities across the tropical climate is enormous1. This has been driven by the endemicity of both ticks and tick-borne haemoparasites (TBH), establishing a relationship whose impact on the cattle hosts is unknown. Karamoja region in Uganda has a high cattle ixodid tick species diversity, and abundance2. In the past 8 years, various studies have indicated a continued rise in the numbers of tick species infesting cattle in the region. Between 2013 and 2020, fifteen ixodid tick species were reported to infest cattle from North-eastern Uganda3,4,5. The most recent of these tick surveys revealed that over 85% of the cattle in the region are infested with as many as fifteen; Rhipicephalus, Amblyomma and Hyalomma species. The most common species included R. appendiculatus, A. variegatum, A. lepidum, R. evertsi and R. decoloratus2. Rhipicephalus microplus was also detected for the first time infesting cattle in the region6. Most of the common tick species detected are known vectors of important TBHs.
The most important tick-borne diseases (TBDs) in Karamoja region are East Coast fever (ECF), anaplasmosis, babesiosis and ehrlichiosis7. These diseases are caused by obligate intracellular protozoan and rickettsial microorganisms within the genera; Theileria, Anaplasma, Babesia, and Ehrlichia respectively. They are maintained in the environment by multiplying in tick vectors and animal reservoirs. These haemoparasites often cause clinical disease in susceptible animals8,9. East Coast fever, caused by the protozoan Theileria parva, is a lymphoproliferative disease. East Coast fever is associated with the highest socioeconomic impact to cattle producing households in Uganda10. Bovine babesiosis is caused by B. bigemina and B. bovis11. Babesia bigemina is endemic in Uganda10 while, a recent survey indicated that B. bovis is present in north-eastern Uganda12. Bovine anaplasmosis, caused by the bacterium A. marginale13, is endemic in Uganda, while heartwater, which is a potentially zoonotic disease14, is caused by the bacterium E. ruminantium15.
TBDs lead to huge economic losses16, and the outcome of these losses is greatest among small-scale resource poor communities like those in north-eastern Uganda (Karimojong pastoralists)1, who depend majorly on livestock for their livelihoods. Indeed, Karimojong pastoralist ranked ECF and anaplasmosis as the most important constraints to cattle production because they are associated with high morbidity, mortality, and treatment costs7. The direct losses due to TBDs are as a result of net reduction in production, loss of weight, livestock fatalities, costs of diagnosis, treatment, and tick control1. In Uganda, the annual losses due to TBDs were estimated to be United States Dollars (USD) 1.1 billion17.
The prevalence and distribution of TBHs are affected by multiple factors, including the presence of a wide range of susceptible hosts, competent tick vectors, livestock grazing systems, and the variation in vector dynamics due to climate and tick-habitat changes. Other risk factors for infection of cattle with TBHs include the breed, age, agro-ecological zone18, distribution of ticks, level of tick infection with TBHs, and cattle tick infestation rate19. Cattle breed influences their inherent resistance to ticks and TBDs20. Karamoja region presents with a wide range of susceptible hosts, competent tick vectors, extensive livestock management system, and variations in tick habitats along the entire 270 km2 pastoral landscape. These factors holistically contribute to the epidemiology of TBDs. However, the effect of these factors on the level of occurrence of TBDs is not uniform in all areas. Therefore, identifying and quantifying the risk of various factors contributing to TBD occurrence in specific endemic settings is important in the design of cost-effective control strategies. Persistent (carrier state) TBH infections and co-infections regularly occur in areas of high endemicity like Karamoja region. TBH co-infections can influence the severity of disease, alter the clinical signs and symptoms, and complicate diagnosis and treatment thereby exacerbating the threat to animal health worldwide21. The risk of being co-infected with TBH after a single tick bite depends on both the prevalence of co-infections in the ticks and in the hosts that they feed on22. Previous studies reported high tick and cattle TBH infection rates in the region19,23.
Karamoja region, which is remote, rural, and semi-arid, is inhabited by the Karimojong pastoralists. Cattle, largely of the indigenous zebu breed (Bos indicus), are the main livestock type kept in the region24. The region is home to 16.7% of the national total cattle herd of 14,500,00024. The cattle and their herders move regularly across the region to exploit the seasonal variation in the availability of pasture and water for their livestock25. This movement coupled with other risk factors promote the proliferation of ticks and increases contact amongst cattle from different locations which increases the likelihood of tick infestations and outbreaks of TBDs18.
There is limited data available on the TBH occurrence and co-infection status in cattle herds from north-eastern Uganda. To inform future sustainable ticks and tick-borne disease [TTBD] control strategies, it is imperative to conduct regular ticks and tick-borne haemoparasite [TTBH] surveys. These surveys are essential in providing up-to-date data that are useful in guiding the formulation and implementation of TTBD control programs. We thus conducted this study to determine the prevalence, co-infection status as well the seasonal variation of the most important TBHs in Karamoja region .Additionally, we explored the predictors of cattle infection with different TBH.
Methods
Study area
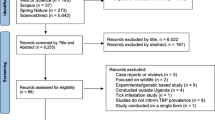
Uganda is stratified into five administrative levels (districts, counties/municipalities, sub counties/town councils, parishes/wards, and villages/cells) and a collection of districts in a continuous geographical block often occupied by a homogeneous group of people make a region. Karamoja region, located in northeastern Uganda, comprises of nine districts. The region is bordered by South Sudan to the north, Kenya to the east, the sub-regions of Acholi and Teso to the west, and Bugisu to the south. The area is semi-arid, characterized by a unimodal rainfall (700–1000 mm) pattern with poor distribution and reliability. The wet season spans from April to September with scanty rains in June, a main peak in July/August and a minor peak in May, followed by an intense dry season typically with strong winds from October to April, with January as the driest month. Daily temperatures average 30–35 °C25. This study was undertaken in 59 cattle rearing villages in four districts (Amudat, Kaabong, Karenga, and Moroto). The four districts were purposively selected based on the knowledge of their high cattle density (Fig. 1).
Map of Karamoja region showing study districts and sites. Red pins represent villages from where blood specimens were collected. This map was generated in ArcMap 10.7 software using open-source shape files (https://data.humdata.org/dataset/uganda-administrative-boundaries-admin-1-admin-3) and village Geographic Information System [GIS] coordinates taken at the time of animal sampling.
Study design and sample size determination
This was repeated cross-sectional study conducted in the dry (November 2022 to February 2023) and the wet (July to August 2023) seasons. Cluster sampling26 implemented in C Survey version 2.027 was used to calculate the number of villages needed to satisfy the set precision. The list of villages (clusters) in the four study districts herein after called the sampling frame was obtained from the national bureau of statistics online database <https://data.humdata.org/dataset/uganda-administrative-boundaries-admin-1-admin-3> . TBH infections are typically associated with clustering into epidemiological units. Hence the sample size parameters were set at; 30% anticipated prevalence19, 5% as the precision of the sample estimate, Interclass Correlation Coefficient (ICC) of 0.1528, and a design effect of 2.1719. Fifty-nine (59) clusters [and 60 animals per cluster] in four districts of Amudat, Kaabong, Karenga and Moroto were adequate to estimate the prevalence of TBHs with this set precision29. Hence, 3540 cattle per season were screened for different TBHs. The cattle included in this survey were randomly selected from herds, irrespective of gender, breed, or age.
Blood sample collection
Blood samples were collected from the middle ear vein. Briefly, a 25-gauge needle was used to prick the ear vein and a capillary tube (Glass Micro-Haematocrit, Thomas Scientific, Swedesboro, USA) used to transfer about 125 µl of blood onto the Flinders Technology Associates [FTA] cards (Whatman, Whatman International Ltd, Maidstone, England). The blood samples were allowed to air-dry on the FTA cards30, sequentially labelled and packed in FTA pouches with a silica gel desiccant (Sigma Aldrich, Co., Life sciences, USA) prior to transportation to the Molecular Biology Laboratory (MOBILA) at the College of Veterinary Medicine, Animal Resources and Biosecurity (COVAB) for analysis. Additional information about the samples (village name and GPS position, animal sex, age, breed, and date of sampling) was recorded on a separate data capture form.
DNA extraction from FTA cards
DNA was extracted from FTA card and eluted in Chelex®100 resin (Sigma Aldrich, Co., Life sciences, USA) according to a previously described protocol30. Briefly, five 3-mm discs were punched out from each FTA card blood sample using a Harris 3.0-mm MicroPunch (GE Healthcare, Chicago, Illinois, USA) and discharged into 1.5 ml microcentrifuge tubes. To avoid carryover contamination between samples, 10 discs were punched from unused filter paper after each sample. For negative control, five 3 mm discs were also punched from unused filter paper. The five 3 mm discs from the FTA card blood samples and or unused filter paper for negative control were initially washed twice in 1.0 ml FTA Purification Reagent (GE Healthcare, Chicago, Illinois, USA) by rocking the tubes on an orbital shaker (Orbit™ 1000 Multipurpose Digital shaker—Labnet, Edison, NJ, USA) at 250 rpm for 15 min, and thereafter rinsed twice with 1.0 ml TE − 1 buffer (10 mM Tris–HCl, 0.1 mM EDTA, pH 8.0) for 15 min. Thereafter, the discs were dried at 37 °C for 30 min in an incubator (Heldoph, Schwabach, Germany). DNA was eluted from the discs in 100 μl of 5% w/v Chelex/cell culture water at 90 °C for 30 min in a thermocycler (My cycler, Biorad, USA).
PCR detection of tick-borne pathogens
Molecular detection of Theileria, Anaplasma, Babesia, and Ehrlichia species was completed in two steps. Initially, all the DNA eluents were subjected to Genus-specific PCRs using primers for specific amplification and detection of the V4 hypervariable region of the 18S rDNA gene for Theileria and Babesia species8 and the V1 hypervariable region of the 16S rDNA gene for Anaplasma and Ehrlichia species31. Thereafter, species-specific PCRs were performed on samples that were positive from the genus-specific PCRs, with primers that target specific genes for T. parva, A. marginale, B. bovis, B. bigemina, and E. ruminantium. In all the PCRs, the reaction volume was 12.5 μl consisting of 6.25 μl of 2 X Dream Taq PCR master mixes (Thermo Scientific), 0.25 μl primers, and 5 μl of DNA template. The used PCR methods, respective genera and species-specific primers, their target genes, expected PCR product sizes, and thermocycling conditions are presented in Table 1 . We used previously positive samples by PCR and capillary sequencing as positive controls namely; T. parva (MK673339), A. marginale (KY522981), B. bigemina (MG426198), B. bovis (OL361842), and E. ruminantium (MK371028)23,32,33 and tissue culture water as negative control. PCR products were resolved in 2% agarose (Biotium, Inc., USA) gels containing 4 μl of GelRed™ nucleic acid stain (Biotium Inc, 46,117 Landing Pkwy, Fremont, CA), and visualised on a GelDoc 2000 (Bio-Rad) transilluminator and gel documentation system.
Sequencing and phylogenetic analysis of tick-borne pathogens
PCR products selected from 55 TBH samples with strongly positive PCR reactions spread across the study districts were purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and Sanger sequenced in both forward and reverse directions at an accredited commercial sequencing laboratory (Inqaba Biotechnical Industries (Pty) Limited (Pretoria, South Africa)) using the ABI 3500XL Genetic Analyzer platform. Each sample was tested and only sequenced after passing their internal quality control systems. The obtained nucleotide sequences were edited and aligned using MUSCLE v.3.8.3.134 within MEGA software version 10 using the default settings. These sequences were queried by comparing them with the reference samples in National Center for Biotechnology Information (NCBI) using the BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to infer the identity of each TBH. The identity of each sequence was assigned to the best hit of the TBH species sequences returned with highest identity score (over 90%) and most significant E-value (closest to 0.0). Sequences that did not contain internal ambiguous bases were carefully curated and submitted to the GenBank. Thereafter, sequences obtained from this study, and those downloaded from the GenBank were compiled and aligned using MUSCLE in default settings. The evolutionary models from the sequence data were reconstructed for B. bigemina RAP-1a gene, B. bovis SBP-2 gene, T. parva p104 gene, and A. marginale MSP-4 gene, using the maximum likelihood model with 1000 bootstrap replications. To evaluate the evolutionary divergence of the queried sequences and those from GenBank, pairwise p-distance estimation and comparison were completed using MEGA 10 software35 left at default settings for each sequence.
Data analysis
Descriptive statistics [prevalence, Odds ratios-ORs, and their 95% Confidence intervals-CIs] were computed in R-4.1.2 software using the epiR packages36,37. Potential predictors of testing positive for the TBH to be included in the multivariable regression model were screened using the performance package applied to a linear regression model containing all collected predictors. Predictors with variance inflation factor (VIF) greater than or equal to 5 were removed from the model and hence we maintained a model with acceptable multicollinearity. Generalized linear logistic regression models were fitted using the glm function in R software to identify the important predictors of testing positive for different TBHs. The goodness of fit was tested using standard model performance diagnostic procedures; R-Squared (R2) and Akaike information criterion (AIC); where models with higher R2 values and lower AIC were considered as the best fit models. The ORs and their CIs for the intercepts and predictors were determined at the 95% CI.
The difference in percentage prevalence estimates between the dry and wet season sampling events (termed: delta [δ] prevalence) for each of the TBHs detected was computed. The delta [δ] prevalence captures both directionality and magnitude of the difference where negative values indicate percentage reduction whereas positive values indicate percentage increase at the wet season collection. A simple linear regression model with “district” as the only predictor (with Moroto set as the reference) was fit to test the directionality (decrease or increase) and statistical significance of the delta [δ]. P values < 0.05 suggested evidence of statistical association between delta prevalence from other districts in relation to Moroto district.
ArcGIS v 10.8 (spatial analyst extension) software was used to map prevalence estimates in different villages.
Results
Overall detection of TBHs in the two sampling events
A total of 3540 cattle were screened at each of the sampling events during the dry and wet seasons. Of these, 1387 (39.2%, 95% CI 37.6–40.9) and 1,380 (39.0%, 95% CI 37.3–40.6%) were positive for at least one of the five TBHs, during the dry and wet seasons respectively. There was no statistically significant difference [p > 0.05] in TBH prevalence during the dry and wet season sampling events. District level prevalence of infection with at least one of the TBHs varied widely between 36.4–44.7% and 21.7–55.7% in the wet and dry seasons respectively. During the wet season, Amudat (55.7%; CI 50.8–60.5 n = 420) and Kaabong (21.7%; CI 19.6–23.8 n = 1500) had the highest and the lowest prevalence of infection with at least one of the TBHs respectively. During the dry season, Moroto (44.7%; CI 41.8–47.7; n = 1440) and Amudat (34.3%; CI 29.8–39; n = 420) had the highest and the lowest TBH prevalence of infection with at least one of the TBHs, respectively.
The prevalence of individual TBHs during the dry season were: B. bigemina 11.8% (CI 10.8–12.9), B. bovis 11.8% (CI 10.8–12.9), A. marginale 9.2% (CI 8.2–10.2), E. ruminantium 5.1% (CI 4.4–5.8) and T. parva 1.3% (CI 1.0–1.8). In the wet season, the prevalence’s of individual TBHs were: T. parva 22.6% (CI 21.3–24), A. marginale 13.6% (CI 12.5–14.8), B. bigemina 12.7% (CI 11.6–13.8), E. ruminantium 1.4% (CI 1.1–1.9) and B. bovis 0.3% (CI 0.1–0.5) (Fig. 2 and Table 2).
Prevalence rates of TBH within Districts
The prevalence of infection with individual TBHs within districts varied in the two seasons. In the dry season, the ranges across the study districts were: A. marginale 6.7–11.2%, E. ruminantium 2.3–7.1%, T. parva 0.1–6.0%, B. bovis 7.7–16% and B. bigemina 3.3–39%, while in the wet season, the ranges across the study districts were; A. marginale 3.1–27.9%, E. ruminantium 0.5–2.4%, T. parva 16.7–25.2%, B. bovis 0.0–0.4% and B. bigemina 6.1–18.3% (Fig. 3 and Table 2).
At sub county level, the prevalence of infection with any TBH varied widely from 8.3–54% and 12.5–50.8% in the dry and wet seasons respectively (Tables 3 and 4; Figs. 4 and 5). Similarly, the prevalence of individual TBHs varied greatly (Tables 3 and 4; Figs. 4 and 5). In both seasons, all study herds (59/59; 100%) were positive for at least one of the five TBHs. Herd-level prevalence varied from 3.3–76.7% and 18.3–75% in the dry and wet seasons, respectively (Additional file 1 and 2).
A heatmap of animal-level prevalence of different TBHs summarized by sub-county: Presents the number of animals that were positive for Theileria and Babesia species [18S positive], Anaplasma | Ehrichia species (16S positive) as well as individual TBHs as a proportion of total number of animals tested; per sub county in the dry season sampling event.
A heatmap of animal-level prevalence of different TBHs summarized by sub-county: Presents the number of animals that were positive for the Theileria and Babesia species [18S positive], Anaplasma | Ehrichia species (16S positive) as well as individual TBH as a proportion of total number of animals tested; per sub county for the wet season sampling event.
Difference in prevalence
The difference in percentage prevalence estimates [δ] between the dry and wet season sampling events varied, with some of the TBHs having statistically significant [δ] values. There was an increase in the T. parva prevalence across all districts in the wet season as compared to the dry season; however, this was not statistically significant. For A. marginale, a statistically significant reduction in prevalence was seen in the wet season as compared to the dry season in the districts of Amudat and Kaabong. Babesia bigemina had a statistically significant net reduction in prevalence in Moroto and Amudat districts and net increase in Karenga and Kaabong districts in the wet season as compared to the dry season. Babesia bovis prevalence reduced in Kaabong district in the wet season as compared to the dry season. For E. ruminantium, a statistically significant reduction in prevalence in the wet season as compared to the dry season was seen in Kaabong district (Fig. 6 and Additional file 3).
Co-infection analysis
Co-infections were seen in the blood samples taken in both the dry and wet seasons. The co-occurrence of B. bovis and B. bigemina (2.74%; CI 2.23–3.33) was the most frequent and the least was for E. ruminantium and T. parva (0.14%; CI 0.05–0.33) in the dry season. On the other hand, co-occurrence of A. marginale and E. ruminantium (1.64%; CI 1.25–2.11) was the most frequent while that of B. bovis and E. ruminantium was the least recorded in the wet season (0%; CI 0–0.1) (Table 5).
In the dry season, the overall prevalence of co-infections in descending order were B. bovis and B. bigemina at 2.74% (CI 2.23–3.33) followed by A. marginale and E. ruminantium at 1.64% (CI 1.25–2.11), A. marginale and B. bigemina, and B. bovis and A. marginale all at 1.21% (CI 0.88–1.63). Co-occurrences involving T. parva were the least prevalent and were recorded in only two study districts. While in the wet season, the overall prevalence of the most frequent co-infections in descending order were; A. marginale and E. ruminantium at 1.64% (CI 1.25–2.11), followed by A. marginale and B. bigemina at 1.21% (CI 0.88–1.63), A. marginale and T. parva at 0.14% (CI 0.05–0.33), and E. ruminantium and T. parva at 0.14% (CI 0.05–0.33). Co-occurrences involving B. bovis were the least prevalent and were not recorded in all the study districts (Table 5).
Predictors of cattle testing positive for TBH
Geospatial location, increasing age, sex, overnight stay in cattle kraals and cattle breeds were significant predictors for testing positive for at least one of the TBH except E. ruminantium in both sampling events.
In the dry season, location was a significant predictor of positivity in all the TBH screened. The odds of detecting B. bovis were higher in Kaabong compared to Karenga; however, location was not statistically significant for Moroto and Amudat compared to Karenga. For B. bigemina, the odds of detection were high in Moroto and Amudat compared to Karenga. For T. parva, the odds of detection were high in Amudat, Moroto and Kaabong compared to Karenga district. Location was not a good predictor of E. ruminantium and A. marginale except for Kaabong district (E. ruminantium) and Moroto (A. marginale). Generally, increasing age was associated with increasing odds of infection with A. marginale.
In the wet season, the odds of detecting B. bigemina were higher in Amudat, Kaabong and Karenga compared to Moroto district, while those of A. marginale were lower for the same districts. Location was not a good predictor of T. parva positivity although the odds were higher for Kaabong compared to Moroto. Generally, increasing age was associated with increasing odds of infection with B. bigemina, and A. Marginale. There were no statistically significant predictors of infection with E. ruminantium probably due to its low prevalence (Additional file 4).
Phylogenetic analysis
The phylogenetic analyses were based on the maximum likelihood method using nucleotide sequences from the Anaplasma marginale msp4, Babesia bigemina RAP-1a, Theileria parva p104 and Babesia bovis SBP2 genes. Sequence information of the 43 TBH from this study were deposited in the GenBank and given the following accession numbers:—B. bovis PQ449057-PQ449064; B. bigemina PQ412622-PQ412637; A. marginale PQ488867-PQ488874; T. parva PQ449065-PQ449075. Sequences of A. marginale grouped into two clusters (Fig. 7) and they clustered with international strains with limited branching. Isolates PQ488871 and PQ488867 clustered closely with other globally published sequences [A. marginale Clade 1]. Isolate PQ488871 clustered with a published sequence from cattle in Italy (AY702920.1) while isolates PQ488867, PQ488868, PQ488869, PQ488870, PQ488872, PQ488873 and PQ488874 clustered with published sequences from cattle in USA (AY010252.1) [A. marginale Clade 2].
Babesia bigemina sequences grouped into two clusters (Fig. 8) highlighting regional clustering with some intermixing of isolates. Isolates PQ412623, PQ412629, PQ412630, PQ412622, PQ412627, PQ412624, PQ412625 and PQ412628 clustered closely with isolates from cattle in Tanzania, Uganda, Turkey and India [B. bigemina Clade 1], while isolates PQ412636, PQ412626, PQ412632, PQ412637, PQ412633, PQ412631, PQ412634 and PQ412635 clustered closely with each other [B. bigemina Clade 2]. Babesia bovis sequences grouped into one cluster (Fig. 9). Sequences (PQ449057, PQ449059, PQ449061, and PQ449063) and the isolate XM_001610444.2 from Babesia bovis T2Bo were a highly conserved group exhibiting zero nucleotide differences [B. bovis Clade 1]. Sequence PQ449060 differed when compared to the conserved group, placing it within a moderately distinct lineage.
Theileria parva sequences grouped into 4 clusters (Fig. 10). Five isolates (PQ449072, PQ449073, PQ449074, PQ449067, PQ449070) clustered with published p104 sequences from cattle in Tanga region, Tanzania [T. parva Clade 1]. One isolate (PQ449069) clustered with other p104 sequences from cattle in Zanzibar Island [T. parva Clade 2] while PQ449075 clustered with p104 sequence from Mityana District, Uganda [T. parva Clade 3]. Sequences PQ449065, PQ449066, PQ449071 and PQ449068 clustered closely with each other [T. parva clade 4].
Discussion
More than 39% of the tested cattle were positive for at least one of the TBHs, and all the 59 village cattle herds in both seasons were positive for at least one of the TBHs. These results are consistent with previous findings in Uganda10,32,38 and show that regardless of the season, infections with T. parva, A. marginale, and B. bigemina are the most dominant. The TBH prevalence data showed spatial clustering; with a few sub counties or villages accounting for most of the TBH infections because TBHs are known to cluster alongside their vector-competent tick populations39.
The high TBH infection rates could be due to the high tick species diversity and cattle tick infestation rates in the region2,5, moreover, the ticks are infected with a wide diversity of TBH23. Most of the dominant tick species reported in the region are the vector-competent ticks of these TBHs2. Although the cattle tick infestation rates in the wet and dry seasons were not statistically different, the abundance of the vector-competent ticks of these TBHs was noted to be significantly higher during the wet season2. This could explain the similarity in the overall TBH positivity rate in the two seasons and the slightly higher wet season prevalence’s of some TBHs. The warmer temperature, high humidity and denser vegetation promoted by the wet season favor tick biology and reproduction and increases the chance of a tick finding a host.
The observed lack of a statistically significant difference in the overall TBH prevalence in the two seasons could be explained by these factors; (1) Karimojong pastoralists have a poor culture of tick control within their cattle herds, due to a variety of factors like; high cost of veterinary inputs, high poverty levels, inadequate supply of veterinary inputs, and low coverage by veterinary personnel, (2) The movement of cattle especially during the dry season to the dry-season green belts leads to the commingling of cattle from various districts, bringing with them a variety of ticks and TBHs, (3) The long distances moved by cattle in the dry season coupled with the scarcity of pastures negatively affect their nutrition status. This weakens their immune system and makes them more susceptible to TBHs, (4) Furthermore, the dry season green belts attract other animals including a variety of wildlife. This increases the number of potential hosts for the ticks which supports the completion of their lifecycles, and (5) The green belts provide favorable microclimates for the proliferation of ticks despite the harsh dry season weather in other parts of the region.
The low prevalence of E. ruminantium in both seasons is consistent with previous studies from other parts of Uganda, including 5.1% in Mbarara district40, 1.7% in Kotido and Moroto districts19, 3.1% in Serere district12, and 0.5% in Kasese district41, and even Africa, including 0.1% in Nigeria42, 0.6% in Ethiopia43, and 0.4% in Kenya44. Despite the low prevalence detected, high proportions of cattle in the region were infested with Amblyomma variegatum (39% wet & 72% dry season) and Amblyomma lepidum (48% wet & 34% dry season)2, the main tick vectors for E. ruminantium in Uganda. The high proportion of cattle infested with A. variegatum in the dry season could explain the higher dry season prevalence of E. ruminantium. Ehrlichia ruminantium-infective ticks present a highly virulent heartwater challenge to cattle which could lead to high case fatalities and hence a low E. ruminantium prevalence in the cattle population45. Molecular techniques such as polymerase chain reaction (PCR) and real-time PCR have high detection rates of E. ruminantium in cattle showing clinical signs compared to asymptomatic carriers46. In its lifecycle, after an infected tick bite, E. ruminantium initially replicates in reticulo-endothelial cells within the lymph nodes47. It has been postulated that, onset of clinical signs coincides with movement of organisms from the lymph to the bloodstream48,49.
In this study 11.8% and 12.7% of the cattle were positive for B. bigemina in the dry and wet season respectively. Previous studies in the region reported a prevalence of 16% for Babesia species using microscopy39 and 5% for B. bigemina using reverse line bloat hybridization-RLB19. However, the prevalence is consistent with findings from other parts of Uganda, including 8.7% in Kasese district50, 17.2% and 10% in central and eastern Uganda respectively51. The higher average tick counts per infested cow of R. decoloratus and R. microplus in the wet season compared to the dry season2 could explain the slightly higher prevalence in the wet season. On the other hand, 11.8% and 0.3% of the cattle were positive for B. bovis in the dry and wet season. This pathogen was recorded in 2022 for the first time in Uganda12. Its vector-competent tick, R. microplus was also recently reported for the first time in Karamoja region6. Babesia bovis does not persist in an infective form in the ticks beyond the larval stage52 which makes the larval stage the main transmission stage. This probably explains the low prevalence in the wet season despite the high tick challenge. Similarly, heat stimulation prior to attachment (37 °C for 3 days and 30 °C for 8 days), enables the transmission of B. bovis to the cow immediately the larvae attach53. These conditions for heat stimulation can be achieved during the dry season in Karamoja.
The proportion of cattle infected with A. marginale (9.2% and 13.6% in dry and wet seasons respectively) was much lower than that reported in Kotido and Moroto districts previously using RLB (73.8%). In other parts of Uganda, there was inconsistency with reports from previous studies, for example, 22% and 3% in central and eastern regions51, 19.2% in Kasese district50, and 3.7% in Mbarara district40. Most cattle had high infestation rates with the key A. marginale vector-competent ticks like R. decoloratus, R. microplus, and R. evertsi evertsi2. Furthermore, hematophagous arthropods such as tsetse flies, Stomoxys calcitrans, tabanids and mosquitoes were abundant in the grazing areas and may facilitate transmission of the pathogen.
The low prevalence of T. parva in the dry season is similar to previous studies conducted during the dry season in other parts of Uganda, including, 3.3% by RLB in Moroto and Kotido districts (3.3%)19, 5.3% by p104-based PCR in Tororo district54, but much lower than 24% by RLB in Mbarara38. The wet season prevalence was similar those observed in other parts of Uganda during the wet seasons, including, 27.9% by PCR in Kasese district50, 61.5% and 26.5% by PCR in central and eastern Uganda51, 18% by p104-PCR in some parts of Karamoja55. High temperatures (28–33 °C) slow or stop the rate of completion of the lifecycle of R. appendiculatus and that of the development of T. parva within the tick56,57. Such temperatures also lead to a low percentage of ticks getting infected after a blood meal from an infected host, and cause a reduction in the number of tick salivary gland acini infected with T. parva in adult R. appendiculatus57. These effects on the vector can combine to reduce the T. parva load in R. appendiculatus, and hence reduce the transmission rate of T. parva by ticks to cattle in the dry season.
Multiple TBH associations were identified. The exact cause of the variations in co-infections cannot be clearly accounted for by this study. However various studies have attributed such variations to climate and vegetation that affect vector dynamics, infection of vectors with multiple TBH, TBH endemicity, and changes in host dynamics25,58. The overall occurrence of co-infections was higher in the dry season samples compared to the wet season. The high prevalence and co-infection of TBH in cattle19 and co-infection of tick vectors with TBH23, high tick infestation and co-infestation on cattle2 and other factors affecting host-vector dynamics could be driving up the TBH co-infection rates in cattle. Co-infections with any pathogens normally complicate the outcome of the disease condition21. Therefore, in areas where co-infections are thought to occur, combined therapies and or vaccines should be encouraged. The high proportion of cattle co-infested with the vector-competent ticks of B. bigemina and B. bovis in the dry season2 could be responsible for both the high prevalence and co-infection rates of these two TBH in that season. While in the wet season, A. marginale and E. ruminantium co-infection was the most prevalent. The low prevalence of E. ruminantium could explain the low level of co-infections amongst these two TBH in both seasons. Furthermore, the low E. ruminantium and T. parva co-infection rates could be suggestive of a situation of competitive exclusion within the host.
Geospatial location, increasing age, sex, overnight stay in cattle kraals and cattle breeds were significant predictors for testing positive with TBH. Location was a significant predictor of positivity in all the TBH screened. This could be due to the inherent variation in the population of various vector-competent ticks. Tick vector clustering usually occurs in areas with the least vector control practices and or with factors that precipitate vector abundance [favourable microclimatic conditions] or vector-host interactions [transhumance and rustling]3,39. Older cattle were associated with a high risk of infection with B. bigemina, A. marginale and T. parva. Inverse age resistance to infection with the three TBH persists up to 24 months and diminishes thereafter3,39. Cattle kept in large ‘protected kraals’ had higher risk of testing positive for the TBH. The commingling of many animals from different areas in the protected kraal promotes tick proliferation and the transmission of TBH. There was a slightly higher risk of males and neutered cattle being positive to A. marginale compared to female cattle probably due likelihood of male animals roaming the landscape in search of mates and therefore most likely to encounter tick bites.
The clustering of the Karamoja sequences with those from Kenya and Tanzania bears several consequences. The genetic identity (0–5 nucleotide differences) of parasite genes between countries implies that cross-border dissemination is rather recent and active. For instance, the B. bigemina RAP-1α gene exhibited complete identity between Karamoja isolates and the ones from Uganda and Tanzania, showing evidence of a shared transmission cycle. Also, T. parva p104 sequences from Karamoja cattle form a clade with Tanzanian cattle isolates. On the contrary, Kenyan T. parva cattle isolates are slightly more divergent (6–12 nucleotide), suggesting separate transmission foci or older separation. Usually minimal sequence differences (especially where pairwise distance = 0) serve as markers indicating transboundary displacement. These findings suggest that there is active and probably much more increased transboundary movement of livestock carrying with them across the boarders an array of TBH and tick species. Such findings highlight the importance of regional coordination of the surveillance and control efforts of ticks and TBH.
Despite the limitation of the cross-sectional design of this study, the twofold wet and dry season surveys revealed a high prevalence of infection and co-infections with TBH. Regulation of livestock movement and tick control measures must be stepped up so as to minimize the rates of infection and transmission of TBH in the cattle herds. Livestock farmers should be sensitized on the appropriate control strategies they should employ to minimize the impact of TBH infections in their cattle herds.
Conclusion
This study demonstrated high level of infection/co-infection with different TBHs in both dry and wet seasons indicating that ticks and tick-borne diseases are a major impediment to livestock production in Karamoja region. It is therefore necessary to have a tick and tick-borne disease control program to reduce losses associated with TTBDs. Moreover, B. bovis was detected for the first time in this region signaling impending outbreaks of babesiosis in this region given that R. microplus [efficient transmitter of B. bovis] has recently been reported in this region.
Data availability
The datasets generated and/or analyses during the current study are available from the corresponding author and the sequences from the NCBI GenBank, https://www.ncbi.nlm.nih.gov/genbank/, under the accession numbers:- B. bovis PQ449057-PQ449064; B. bigemina PQ412622-PQ412637; A. marginale PQ488867-PQ488874 and T. parva PQ449065-PQ449075.
Abbreviations
- 18S rRNA:
-
18S Ribosomal RNA gene
- 16S rRNA:
-
16S ribosomal RNA gene
- DNA:
-
Deoxyribonucleic acid
- RNA:
-
Ribonucleic acid
- TBHs:
-
Tick-borne haemoparasites
- GPS:
-
Geographical positioning system
- TTBDs:
-
Ticks and tick-borne disease
- NARO:
-
National Agricultural Research Organization
References
Minjauw, B. & McLeod, A. Tick-borne diseases and poverty: The impact of ticks and tick-borne diseases on the livelihoods of small-scale and marginal livestock owners in India and Eastern and Southern Africa. (2003).
Etiang, P. et al. Distribution and prevalence of ixodid tick species (Acari: Ixodidae) infesting cattle in Karamoja region of northeastern Uganda. BMC Vet. Res. 20, 1–13. https://doi.org/10.1186/S12917-023-03802-1/FIGURES/4 (2024).
Byaruhanga, C., Collins, N. E., Knobel, D., Kabasa, W. & Oosthuizen, M. C. Endemic status of tick-borne infections and tick species diversity among transhumant zebu cattle in Karamoja Region, Uganda: Support for control approaches. Vet. Parasitol. Reg. Stud. Rep. 1–2, 21–30. https://doi.org/10.1016/j.vprsr.2015.11.001 (2015).
Akure, P. C. Tick Species Composition and Associated Haemoparasites of Cattle in a Semi-arid Area of Karamoja (University of Pretoria, 2019).
Balinandi, S. et al. Morphological and molecular identification of ixodid tick species (Acari: Ixodidae) infesting cattle in Uganda. Parasitol. Res. 119, 2411–2420. https://doi.org/10.1007/S00436-020-06742-Z/FIGURES/2 (2020).
Etiang, P. et al. Identification and distribution of Rhipicephalus microplus in selected high-cattle density districts in Uganda: Signaling future demand for novel tick control approaches. BMC Veterinary Res. 20, 1– 12 https://doi.org/10.1186/S12917-024-03979-Z (2024).
Byaruhanga, C., Oosthuizen, M. C., Collins, N. E. & Knobel, D. Using participatory epidemiology to investigate management options and relative importance of tick-borne diseases amongst transhumant zebu cattle in Karamoja Region, Uganda. Prev. Vet. Med. 122, 287–297. https://doi.org/10.1016/J.PREVETMED.2015.10.011 (2015).
Gubbels, J. M. et al. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 37, 1782–1789 (1999).
Dumler, J. S. Anaplasma and Ehrlichia infection. Ann. N. Y. Acad. Sci. 1063, 361–373. https://doi.org/10.1196/ANNALS.1355.069 (2005).
Kasaija, P. D., Estrada-Peña, A., Contreras, M., Kirunda, H. & de la Fuente, J. Cattle ticks and tick-borne diseases: A review of Uganda’s situation. Ticks Tick-Borne Dis. 12, 101756. https://doi.org/10.1016/J.TTBDIS.2021.101756 (2021).
Bock, R., Jackson, L., de Vos, A. & Jorgensen, W. Babesiosis of cattle. Parasitology 129 (Suppl), S247–S269 (2004).
Heylen, D. J. A. et al. Tick-borne pathogens and body condition of cattle in smallholder rural livestock production systems in East and West Africa. Parasit. Vectors. 16, 1–12. https://doi.org/10.1186/S13071-023-05709-0/FIGURES/3 (2023).
Kocan, K. M., de la Fuente, J., Blouin, E. F., Coetzee, J. F. & Ewing, S. A. The natural history of Anaplasma marginale. Vet. Parasitol. 167, 95–107. https://doi.org/10.1016/j.vetpar.2009.09.012 (2010).
Allsopp, M. T., Louw, M. & Meyer, E. C. Ehrlichia ruminantium: An emerging human pathogen? Annals of the New York. Acad. Sci. 1063, 358–360. https://doi.org/10.1196/annals.1355.060 (2005).
Cowdry, E. V. Studies on the etiology of heartwater 1. Observation of a rickettsia, Rickettsia ruminantium (n. sp.), in the tissues of infected animals. J. Exp. Med. 42, 231–252 (1925).
Ocaido, M., Otim, C. P. & Kakaire, D. Impact of major diseases and vectors in smallholder cattle production systems in different agro-ecological zones and farming systems in Uganda. Crops 56, 51 (2009).
de la Fuente, J. et al. Towards a multidisciplinary approach to improve cattle health and production in Uganda. Vaccines 7, 165 https://doi.org/10.3390/VACCINES7040165 (2019).
Gachohi, J., Skilton, R., Hansen, F., Ngumi, P. & Kitala, P. Epidemiology of East Coast fever (Theileria parva infection) in Kenya: Past, present and the future. Parasit. Vectors. 5, 1. https://doi.org/10.1186/1756-3305-5-194 (2012).
Byaruhanga, C. et al. Molecular investigation of tick-borne haemoparasite infections among transhumant zebu cattle in Karamoja Region, Uganda. Vet. Parasitol. Reg. Stud. Rep. 3–4, 27–35. https://doi.org/10.1016/j.vprsr.2016.06.004 (2016).
Jonsson, N. N., Piper, E. K. & Constantinoiu, C. C. Host resistance in cattle to infestation with the cattle tick Rhipicephalus microplus. Parasite Immunol. 36, 553–559. https://doi.org/10.1111/PIM.12140 (2014).
Krause, P. J. et al. Concurrent lyme disease and babesiosis: Evidence for increased severity and duration of illness. JAMA 275, 1657–1660. https://doi.org/10.1001/JAMA.1996.03530450047031 (1996).
Belongia, E. A. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. (Larchmont NY). 2, 265–273. https://doi.org/10.1089/153036602321653851 (2002).
Byaruhanga, C. et al. Molecular detection and characterisation of protozoan and rickettsial pathogens in ticks from cattle in the pastoral area of Karamoja, Uganda. Ticks Tick-Borne Dis. 12, 101709. https://doi.org/10.1016/J.TTBDIS.2021.101709 (2021).
UBOS UB of S. National Livestock Census 2021 Main report. Kampala: (2024).
Egeru, A. et al. Spatio-temporal dynamics of forage and land cover changes in Karamoja sub-region. Uganda Pastoralism. 4, 6. https://doi.org/10.1186/2041-7136-4-6 (2014).
Hayes, R. J. & Bennett, S. Simple sample size calculation for cluster-randomized trials. Int. J. Epidemiol. 28, 319 (1999).
Farid, M., Frerichs, R. & Csurvey Software (2007). http://www.ph.ucla.edu/epi/csurvey.html (Accessed 13 April 2016) 2007.
Muhanguzi, D. et al. Improvements on restricted insecticide application protocol for control of human and animal African Trypanosomiasis in eastern Uganda. PLoS Negl. Trop. Dis. 8, e3284. https://doi.org/10.1371/journal.pntd.0003284 (2014).
Cochran, W. G. Cochran_1977_Sampling_Techniques__Third_Edition.pdf 1977:448.
Ahmed, H. A., MacLeod, E. T., Hide, G., Welburn, S. C. & Picozzi, K. The best practice for preparation of samples from FTA®cards for diagnosis of blood borne infections using African trypanosomes as a model system. Parasit. Vectors. 4, 68. https://doi.org/10.1186/1756-3305-4-68 (2011).
Bekker, C. P. J., de Vos, S., Taoufik, A., Sparagano, O. A. E. & Jongejan, F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 89, 223–238 (2002).
Tayebwa, D. S. et al. Molecular epidemiology of Babesia species, Theileria parva, and Anaplasma marginale infecting cattle and the tick control malpractices in Central and Eastern Uganda. Ticks Tick-Borne Dis. 9, 1475–1483. https://doi.org/10.1016/j.ttbdis.2018.06.012 (2018).
Byaruhanga, C. et al. Molecular detection and phylogenetic analysis of Anaplasma marginale and Anaplasma centrale amongst transhumant cattle in north-eastern Uganda. Ticks Tick-Borne Dis. 9, 580–588. https://doi.org/10.1016/j.ttbdis.2018.01.012 (2018).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. https://doi.org/10.1093/nar/gkh340 (2004).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Fox, J. & Weiberg, S. An R companion to applied regression: Appendices. Robust. Regres. R. 2014, 1–17 (2014).
Harrell, J. F. Hmisc: Harrell Miscellaneous. R Package Version. 30–12, 1–397 (2006).
Muhanguzi, D., Matovu, E. & Waiswa, C. Prevalence and characterization of Theileria and Babesia species in cattle under. Different Husb. Syst. Western Uganda. 2, 51–58 (2010).
Lolli, C. et al. Infections and risk factors for livestock with species of Anaplasma, Babesia and Brucella under semi-nomadic rearing in Karamoja Region, Uganda. Trop. Anim. Health Prod. https://doi.org/10.1007/s11250-016-1005-x (2016).
Muhanguzi, D., Ikwap, K., Picozzi, K. & Waiswa, C. Molecular characterization of Anaplasma and Ehrlichia species in different cattle breeds and age groups in Mbarara district (Western Uganda). Int. J. Anim. Vet. Adv. 2, 76–88 (2010). http://maxwellsci.com/print/ijava/v2-76-88.pdf (accessed October 15, 2024).
Tumwebaze, M. A. et al. First molecular detection of Babesia ovis, Theileria spp., Anaplasma spp., and Ehrlichia ruminantium in Goats from Western Uganda. Pathogens 9, 895 https://doi.org/10.3390/PATHOGENS9110895 (2020).
Lorusso, V. et al. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasit. Vectors. 9, 1–13. https://doi.org/10.1186/S13071-016-1504-7/FIGURES/4 (2016).
Teshale, S., Geysen, D., Ameni, G., Dorny, P. & Berkvens, D. Survey of Anaplasma phagocytophilum and Anaplasma sp. Omatjenne infection in cattle in Africa with special reference to Ethiopia. Parasit. Vectors. 11, 1–10. https://doi.org/10.1186/S13071-018-2633-Y/TABLES/5 (2018).
Njiiri, N. E. et al. The epidemiology of tick-borne haemoparasites as determined by the reverse line blot hybridization assay in an intensively studied cohort of calves in western Kenya. Vet. Parasitol. 210, 69–76. https://doi.org/10.1016/J.VETPAR.2015.02.020 (2015).
Pretorius, A. et al. A heterologous prime/boost immunisation strategy protects against virulent E. ruminantium Welgevonden needle challenge but not against tick challenge. Vaccine 26, 4363–4371. https://doi.org/10.1016/J.VACCINE.2008.06.006 (2008).
Steyn, H. C., Pretorius, A., McCrindle, C. M. E., Steinmann, C. M. L. & Van Kleef, M. A quantitative real-time PCR assay for Ehrlichia ruminantium using pCS20. Vet. Microbiol. 131, 258–265. https://doi.org/10.1016/j.vetmic.2008.04.002 (2008).
Allsopp, B. A., Bezuidenhout, J. D. & Prozesky, L. Heartwater. In: (eds Coetzer, J. A. W. & Tustin, R. C.) Infectious Diseases of Livestock. second ed., Cape Town: ABC; 507–535. (2005).
Prozesky, L., Du Plessis, J. L. & Heartwater The development and life cycle of Cowdria ruminantium in the vertebrate host, ticks and cultured endothelial cells. Onderstepoort J. Vet. Res. 54, 193–196 (1987).
Du Plessis, J. L. Pathogenesis of heartwater. I. Cowdria ruminantium in the lymph nodes of domestic ruminants. Onderstepoort J. Vet. Res. 37, 89–95 (1970).
Byamukama, B. et al. Molecular detection of selected tick-borne pathogens infecting cattle at the wildlife–livestock interface of Queen Elizabeth National Park in Kasese District, Uganda. Ticks Tick-Borne Dis. 12, 101772. https://doi.org/10.1016/J.TTBDIS.2021.101772 (2021).
Tayebwa, D. S. et al. Molecular epidemiology of Babesia species, Theileria parva, and Anaplasma marginale infecting cattle and the tick control malpractices in Central and Eastern Uganda. Ticks and Tick-Borne Dis. https://doi.org/10.1016/j.ttbdis.2018.06.012 (2018).
Mahoney, D. F., Mirre, G. B. A note on the transmission of Babesia bovis (syn. B. argentina) by the one-host tick, Boophilus microplus. Res. Vet. Sci. 26, 253–254 (1979).
Dalgliesh, R. J. & Stewart, N. P. Some effects of time, temperature and feeding on infection rates with Babesia bovis and Babesia bigemina in Boophilus microplus larvae. Int. J. Parasitol. 12, 323–326 (1982).
Muhanguzi, D. et al. Prevalence and spatial distribution of Theileria parva in cattle under crop-livestock farming systems in Tororo District, Eastern Uganda. Parasit. Vectors. 7, 91. https://doi.org/10.1186/1756-3305-7-91 (2014).
Kabi, F., Masembe, C., Muwanika, V., Kirunda, H. & Negrini, R. Geographic distribution of non-clinical Theileria parva infection among indigenous cattle populations in contrasting agro-ecological zones of Uganda: Implications for control strategies. Parasit. Vectors. 7, 414. https://doi.org/10.1186/1756-3305-7-414 (2014).
Branagan, D. The developmental periods of the Ixodid tick Rhipicephalus appendiculatus Neum. under laboratory conditions. Bull. Entomol. Res. 63, 155. https://doi.org/10.1017/S0007485300050951 (1973).
Young, A. S. & Leitch, B. L. Epidemiology of East Coast fever: Some effects of temperature on the development of Theileria parva in the tick vector, Rhipicephalus appendiculatus. Parasitology 83, 199–211. https://doi.org/10.1017/S0031182000050162 (1981).
Norval, R. A. et al. Theileria parva: Influence of vector, parasite and host relationships on the epidemiology of theileriosis in southern Africa. Parasitology 102 Pt 3, 347–356 (1991).
Acknowledgements
We are very grateful to Daniel Hudner who provided some insights during the design and implementation of the study that generated the results presented in this manuscript. We also acknowledge different field and laboratory personnel: Mutoniwase Gloria, Kesiime Christine, Kanyike Fred, Magambo Phillip Kimuda, Kaziro Michael, Anole Moses, Lomong Phillip, Eladu Fredrick, Nalumansi Betty, Lonyiko Ibrahim, Nabong Agnes, Lokiru Paul, Jeremy Lomonyang, Awas Walter, Okino Moses, Logwe John Branda, Ogwang Emmanuel, Cheptoyek Levi, and Lotiang Eliyah who helped with sample collection and analysis. We also acknowledge the cattle owners and herdsmen of the villages where this study was carried out for offering their cattle and helping with their restraint.
Funding
This work was funded by Mercy Corps Uganda [collaborative research agreement: UGO1/MRT 0883/APOLOU/21 to DM] with proceeds from their five-year USAID funded food and nutrition security activity [Apolou] in Karenga, Kaabong, Kotido and Moroto Districts and their USAID/DFID-funded project aimed at Increasing Public and Private Investment in Animal Health Systems to Strengthen Productive Assets and Veterinary Governance for Improved Resilience in Karamoja (RCF) to inform livestock disease surveillance and response approaches and policies in the Karamoja sub-region. Additional partial funding was received [to PE] from the Germany Academic Exchange Service (DAAD). The contents of this Manuscript are the responsibility of Authors and do not necessarily reflect the views of DAAD, USAID or the United States or United Kingdom Governments. The funding agencies [DAAD, USAID, DFID and Mercy Corps Uganda] played no role whatsoever in the design, analysis and reporting of the study. However, MK who worked with Mercy corps when this study was designed and implemented approved the study designs by DM, PE & RT.
Author information
Authors and Affiliations
Contributions
PE, RT, and DM conceived and designed the study. PE, MK, HW, SA, MKa, SA, HA and DM collected blood samples and performed molecular analysis. JN, SA, HA, DM and PE did data analysis. PE, DM, RT, JN, CB, WA, SB and MNM drafted and critically reviewed this manuscript. DM, RT, PE and KKM sourced funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Makerere University School of Veterinary Medicine and Animal Resources Institutional Animal care and Use committee [Reference number: SVARREC/32/2019] and the Uganda National Council of Science and Technology [Reference number: A 616]. We declare that all the methods used during cattle restraint, blood collection, preservation, and molecular analyses were performed in accordance with the relevant guidelines and regulations as provided by the Uganda National Council of Science and Technology < https://www.uncst.go.ug/details.php?option=smenu&id=13&Downloads.html > . Written Informed consent was obtained from the cattle owners before collection of cattle blood samples. This study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org/arrive-guidelines).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Etiang, P., Kamusiime, M., Wamala, H. et al. Prevalence and seasonal variation of tick-borne haemoparasites in cattle from north-eastern Uganda. Sci Rep 15, 30328 (2025). https://doi.org/10.1038/s41598-025-12164-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12164-7
Keywords
This article is cited by
-
Cartographie par IA des données sur les cultures des petits exploitants, des lacs peu étudiés, des tiques sur le bétail, des risques liés au COVID long et de l’hypertension
Nature Africa (2025)
-
AI mapping of smallholder crop data, under-studied lakes, ticks on cattle, long COVID risks, and hypertension
Nature Africa (2025)