Abstract
Fundamental treatments for autoimmune gastritis (AIG) have not yet been established; thus, analyzing AIG pathogenesis in detail to obtain useful information for prognosis prediction and treatment is crucial. This study explored bacteria involved in AIG pathogenesis by focusing on the gastric microbiota composition. Gastric biopsy tissues were collected endoscopically from the gastric corpus and antrum of patients with AIG and chronic gastritis. Total DNA was extracted from gastric biopsy specimens and used for 16S rRNA gene amplicon sequencing analysis. Principal coordinate analysis of diversity using the weighted UniFrac distance revealed that following Helicobacter pylori eradication, the gastric bacterial composition of patients with AIG differed significantly from that of patients with chronic gastritis, exhibiting decreased Shannon index, Pielou’s evenness, and Simpson index of alpha diversity. The gastric microbiota of patients with AIG was characterized by an increased abundance of the genus Streptococcus and a reduced abundance of the genus Prevotella compared with that of patients with chronic gastritis.
Similar content being viewed by others
Introduction
Chronic gastritis is classified into two types: type A and type B, caused by Helicobacter pylori infection1. Type A gastritis is characterized by atrophic changes in the fundus gland region of the stomach, primarily in the gastric corpus. It is an organ-specific, immune-mediated disease, also known as autoimmune gastritis (AIG), characterized by the destruction of the gastric parietal lobe, which results in the loss of intrinsic factors and decreased acid production. In contrast, type B gastritis is induced by H. pylori infection and characterized by atrophic changes that extend from the pyloric antrum to the gastric corpus.
In Japan, where the H. pylori infection rate is high, most cases of gastric mucosal atrophy are caused by H. pylori infection. The decline in the incidence of H. pylori infection and the widespread use of H. pylori-targeted medications have brought attention to AIG, which was considered a rare disease in earlier years. Another reason AIG did not receive much attention is that it was initially thought to be common among older women in Northern Europe1. According to a recent study by Notsu et al.2, the frequency of AIG in Japan was 0.49% (0.65% in women and 0.40% in men) across 6793 endoscopically performed examinations. Furthermore, a multicenter study by Terao et al.3 reported the prevalence of AIG in Japan to be 0.49% (43 of 8716 cases). These studies indicated that AIG is common in Japan and not only in Northern Europe. However, overseas studies reported that the prevalence of AIG ranges from 2.3% to > 10%4, and may further increase with the widespread use of diagnostic methods5.
When H. pylori eradication is successful, the inflammatory response to H. pylori disappears, gastric mucosal atrophy6 and parietal cell function improve in patients with gastritis, and reduced gastric acid secretion is recovered7. In contrast, AIG pathogenesis is attributed to parietal cell loss by antigastric parietal cell antibodies, resulting in the decreased secretion of gastric acid (hydrochloric acid and gastric intrinsic factor)4; hence, little improvement is expected from the eradication treatment. The cause and detailed mechanism of production of these autoantibodies remain unclear; consequently, no treatment has been established for AIG, and no improvement in atrophy or gastric acid secretion can be attained. Hence, analyzing AIG pathogenesis in detail to obtain useful information on its prognosis, prediction, and treatment is crucial. In this study, we focused on the composition of the gastric microbiota in patients with AIG and aimed to identify the gastric bacteria involved in AIG pathogenesis, their relationship with carcinogenesis, and their potential role in improving the condition.
Several studies have reported the involvement of H. pylori infection in the pathogenesis of autoimmune diseases8. The anti-H. pylori antibodies have also been suggested to act as autoantibodies in AIG. For instance, the β subunit of urease in H. pylori is highly homologous to the β subunit of ATPase in gastric parietal cells, thus leading to an autoimmune reaction9. Therefore, comparing the microbiota of patients with AIG with that in patients with H. pylori infection is important. However, patients with current H. pylori infection have an extremely high abundance of H. pylori in their gastric microbiota, significantly differing from that of patients without H. pylori infection. Therefore, the study participants were patients with atrophic gastritis who had a history of H. pylori infection but were no longer infected with H. pylori after undergoing eradication treatment. However, because of the similarities between type B gastritis and AIG, H. pylori eradication therapy is sometimes mistakenly administered to patients with AIG in Japan. Some patients with AIG may have also been infected with H. pylori. Therefore, in this study, we examined patients with AIG who had received eradication therapy 1 year prior regardless of whether they were currently infected with H. pylori, and patients with chronic atrophic gastritis (type B) who had previously been infected with H. pylori and received eradication therapy as control cases.
We examined the gastric microbiota of both patient groups to determine the differences in the composition following H. pylori eradication therapy. This study aimed to elucidate any microecological differences in the gastric microbiota of both groups and investigated the presence of bacteria that may be a risk factor for the development of H. pylori-negative gastric cancer.
Results
Characteristics of patients with AIG
We enrolled patients with atrophic gastritis who visited the Kyorin University Hospital for endoscopy between November 2018 and November 30 2024. We collected gastric biopsy specimens from 25 patients diagnosed with AIG and 26 patients with atrophic gastritis who had previously been infected with H. pylori and had received eradication therapy 1 year later. All patients provided informed consent before participating in this study. Specimens positive for H. pylori in 16S rRNA gene amplicon sequencing analysis or other diagnostic tests nd two samples with insufficient PCR amplification were excluded. Finally, we evaluated 20 patients with AIG and 19 with atrophic gastritis (control) (Fig. 1).
The characteristic findings in patients with AIG are shown in Tables 1 and 2. All patients with AIG had parietal cell or intrinsic factor antibodies in their sera, with 17 of the 20 cases being associated with corpus-dominant advanced atrophy, based on endoscopic examination. Endoscopic findings included a significantly higher percentage of corpus-dominant atrophy in patients with AIG (85.7%) than in those with non-AIG (0%) (P < 0.001). Notably, serum gastrin levels were high in these patients. In addition, serum anti-H. pylori IgG were present in one (case A15) and seven (cases A2, A9, A12, A13, A16, A19, and A20) of the 20 patients with AIG at high and low levels, respectively (Table 2). In the case of AIG and atrophic gastritis, H. pylori infection was assessed as negative at the time of sample collection based on both endoscopic findings and 16S metagenomic amplification sequencing.
Comparison of characteristics between patients with AIG and those with atrophic gastritis
The backgrounds of patients with AIG, control patients with atrophic gastritis, and the last eradication regimens administered to each patient are shown in Table 2. The mean age of patients with AIG was 67.8 ± 7.2 years and did not differ from that of control patients (60.8 ± 9.15 years). The male-to-female ratio in patients with AIG was 16:4 compared with 11:8 in the control group. Among patients with AIG, 19 were diagnosed as having an atrophic grade of O-3 with or without corpus-dominant atrophy, whereas only one patient (case A2) was diagnosed with O-2. Ten patients in the control group had closed-type atrophic gastritis (C-2 and 3). The period from the start of the eradication therapy to specimen collection was calculated for the patients who received eradication therapy. We noted a significant difference between the two groups, with 1 year for the AIG group and 3.5 ± 1.7 years for the control group (P < 0.01). The average UBT (urea breath test) values in the AIG group (14.1 ± 26.4‰) were significantly higher than those in the control group (0.9 ± 0.7‰) (P < 0.01).
The chi-square test showed that the number of times patients received eradication therapy was significantly higher in the AIG than in the control group.
Gastric microbiota in patients with AIG and those with atrophic gastritis
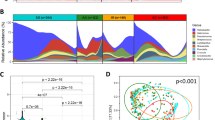
We found that the gastric microbiota in the corpus and antrum of patients with AIG consisted mainly of five phyla: Firmicutes, Proteobacteria, Bacteroidota, Fusobacteria, and Actinobacteria (Fig. 2 A). The abundance of Bacteroidota in the gastric antrum and corpus of patients with AIG was significantly lower than that in the gastric corpus and antrum of control patients (P < 0.05 and P < 0.01). The abundance of Proteobacteria in the gastric corpus of patients with AIG was significantly higher than that in the gastric corpus and antrum of controls (P < 0.01) while the abundance of Proteobacteria in the gastric antrum of patients with AIG was significantly higher than that in the gastric antrum of controls (P < 0.05).
The phylum Firmicutes mainly comprised the genus Streptococcus, the abundance of which in patients with AIG was higher than that in control patients (corpus and antrum, P < 0.05). The phylum Bacteroidetes comprised the genera Prevotella, Prevotella 7, Porphyromonas, Alloprevotella, and Capnocytophaga (Fig. 2B). The abundance of the genus Prevotella in the gastric corpus of patients with AIG was significantly lower than that in the gastric antrum of control patients (P < 0.05).
Alpha diversity of gastric microbiota in patients with AIG and those with atrophic gastritis
Regarding the alpha diversity of the gastric microbiota, the Shannon, Pielou’s evenness, and Simpson indices (Fig. 3A–C) in both the gastric corpus and antrum of patients with AIG were significantly lower compared with those in the control group. In addition, no significant difference of Chao-1 existed between the control and AIG groups (Fig. 3D).
Alpha diversity according to gastric bacterial compositions in patients with autoimmune gastritis and those with chronic gastritis after Helicobacter pylori eradication. Chao-1 (A), Shannon (B), Pielou’s evenness (C), and Simpson (D) indices are represented in samples from each group of patients. *Statistically significant (**: P < 0.01, *: P < 0.05).
Beta diversity of gastric microbiota in patients with AIG and those with atrophic gastritis
We calculated beta diversity using both unweighted and weighted UniFrac phylogenetic distance metrics and visualized it using principal coordinate analysis (PCoA) plots. The microbiota composition in the gastric corpus (Fig. 4A, B) and antrum (Fig. 5A, B) of patients with AIG differed significantly from that in patients with chronic gastritis according to PERMANOVA weighted UniFrac distances (Fig. 4B; q = 0.00062; Fig. 5B; q = 0.00004). We observed no significant difference in unweighted UniFrac distances between AIG and atrophic gastritis both the gastric corpus and antrum (Figs. 4A and 5A).
Principal coordinate analysis (PCoA) plots of unweighted (A) and weighted UniFrac distances (B) in which gastric corpus samples were colored according to clinical outcome (blue: AIG, orange: atrophic gastritis). The percentage of diversity captured by each coordinate is shown. PERMANOVA, analysis of similarity.
Principal coordinate analysis (PCoA) plots of (A) unweighted and (B) weighted UniFrac distances in which gastric antrum samples were colored according to clinical outcome (blue: AIG, orange: atrophic gastritis). The percentage of diversity captured by each coordinate is shown. PERMANOVA, analysis of similarity.
Association of specific microbiota taxa with AIG and atrophic gastritis according to Microbiome Multivariable Association with Linear Models 2 (MaAsLin 2)
To identify the most relevant taxa responsible for the differences in clinical diagnoses between the two groups, we performed Microbiome Multivariable Association with Linear Models 2 of microbiota in the gastric corpus and antrum. We detected the genera Haemophilus, Streptococcus, and Bergeyella in the gastric corpus of patients with AIG, whereas we identified the significant higher presence of the genera Cutibacterium, Centipeda, and Candidatus_Saccharimonas in the gastric corpus of control patients with atrophic gastritis (Supplementary Fig. 1). In the gastric antrum of patients with AIG, we detected the genus Streptococcus, whereas we identified the significant presence of the genera Selenomonas and Candidatus_Saccharimonas in the gastric body of control patients with atrophic gastritis (Supplementary Fig. 2).
When analyzing the time since eradication treatment (Year) using MaAsLin 2 as a linear model, we identified several significant temporal correlations. The orders Micrococcales and genera Acidiphilium and MN_122.2a (Fig. 6A and B) demonstrated positive correlations with time, whereas the genus Granulicatella exhibited a significant negative correlation over the same period.
Discussion
The environment of the normal stomach is acidic, which is considered unfavorable for bacterial growth. However, studies using molecular analyses have revealed that bacteria can colonize acidic gastric environments10. According to several studies11,12, the gastric microbiota of healthy individuals comprise five main phyla: Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Fusobacteria. Owing to the loss of acid-secreting gastric wall cells, the gastric environment in AIG is hypoacidic (hypochlorhydria). Because of this unique intragastric environment, the gastric microbiota composition in patients with AIG was assumed to differ from that of normal acid-secreting stomach in healthy individuals. However, in the present study, the results of the microbiota analysis showed that the same five phyla were predominant in AIG. Using cytology brush samples, a population-based study reported the gastric microbiota composition of patients with and without gastrointestinal symptoms13. The phyla present in the stomach of patients without gastrointestinal symptoms were Firmicutes (42%), Bacteroidetes (24%), Proteobacteria (17%), Actinobacteria (7%), and Fusobacteria (6%)13, consistent with our findings. These results suggested that the bacterial microbiota composition of the control group receiving eradication therapy resembled that of healthy individuals despite the residual condition of gastritis.
The abundance of Bacteroidota in the gastric microbiota of patients with AIG was significantly lower than that in patients with atrophic gastritis, whereas the abundance of Proteobacteria in the gastric corpus of patients with AIG was significantly higher than that in the gastric corpus and antrum of patients with atrophic gastritis. When we calculated the relative proportions of the Proteobacteria and Bacteroidetes phyla (P/B ratio) for each group, the average P/B ratio in patients with AIG was significantly higher than that in the atrophic gastritis group (data not shown). These findings suggested that despite receiving eradication therapy, patients with AIG may fail to re-establish a normal gastric microbiota composition probably owing to their inability to recover normal acid secretion.
Several studies13,14,15 have shown that H. pylori infection decreases the alpha diversity of gastric microbiota, suggesting a predominance of H. pylori over other microbes. H. pylori interacts with other gastrointestinal microbes, negatively correlating with the alpha diversity of gastric microbiota16. Accordingly, successful eradication of H. pylori results in an increase in the number of operational taxonomic units (OTUs) and Chao-1 index. Therefore, we assumed that the alpha diversity of the microbiota in patients with AIG and control patients with atrophic gastritis without H. pylori infection may be high; however, whether it returned to the same level as that in healthy individuals was unclear. The median OTUs in the present study ranged at 100–200 in both groups, whereas previous studies reported > 400 OTUs in the gastric microbiota of healthy individuals16. Therefore, in terms of alpha diversity, the stomachs of both the AIG and control groups were in a different state than that of healthy individuals possibly owing to the preceding eradication treatment. Yang et al.17 also compared patients that underwent eradication treatment (four-drug combination: omeprazole, tetracycline, metronidazole, and bismuth), dividing them into those who were successfully treated for eradication of H. pylori and those who were not. Patients in whom H. pylori was successfully eradicated showed higher values of alpha diversity than those for whom treatment was unsuccessful; however, different results were observed regarding beta diversity17.
In cases of atrophic gastritis, gastric acid secretion is unlikely to be fully restored, even after the eradication of H. pylori. A comparison of the gastric microbiota between patients with H. pylori-induced atrophic gastritis, AIG, and PPI (proton pump inhibitor) use has been reported18. Gastric biopsies from patients with AIG and those using PPI showed greater bacterial diversity than those of patients with H. pylori-induced atrophic gastritis.
In this comparative study between AIG and atrophic gastritis, the Shannon, Pielou’s evenness, and Simpson indices of the gastric microbiota in patients with AIG were significantly lower than those of the gastric microbiota in control patients with atrophic gastritis. The average Chao-1 of the gastric microbiota in patients with AIG did not differ from that in control patients with atrophic gastritis. This feature was not found in the control group; AIG may be associated with higher fasting serum gastrin concentrations (Table 1). Lower Shannon and Simpson indices indicated that the microbial community has lower diversity in patients with AIG than in control patients. A decrease in Pielou’s evenness was detected when the species were not equally distributed. Therefore, some bacterial characteristics associated with AIG may strongly influence the composition and affect the alpha diversity of the gastric bacterial microbiota. The correlation between the abundance of the genus genus _Sphingobium and serum gastrin levels in the gastric antrum microbiota (p = 0.000212, q = 0.031637), whereas no such correlation was detected in the gastric corpus microbiota of patients with AIG. In subjects not infected with H. pylori, an increase with age in the Proteobacteria was reported, specifically in the genera Enhydrobacter, Comamonadaceae, and Sphingobium19. The role of genus Sphingobium the human stomach and its relationship with gastrin have not yet been reported, and further analysis is needed.
In a previous study comparing the gastric microbiota between patients with AIG and non-AIG patients, Tsuboi et al.20 reported that the abundances of the genus Streptococcus and other genera, such as Selenomonas, Granulicatella, and Bacillus spp., were significantly higher in patients with AIG than in the control group, which was a successful H. pylori eradication group; however, 10 of 14 (71.43%) patients in the AIG group had also undergone eradication therapy. Therefore, this comparison was similar to the one in our study, which analyzed the gastric microbiota of uninfected and previously H. pylori infected patients who were currently negative for H. pylori. Further similarities included the higher abundances of two genera, Streptococcus and Granulicatella, in the gastric antrum and the higher abundance of the genus Streptococcus in the gastric corpus of patients with AIG. The genera Streptococcus and Granulicatella have also been associated with the emergence and persistence of gastric atrophy and intestinal metaplasia21. Granulicatella is a gram-positive facultative anaerobe associated with root canal infection22. The relative abundance of Streptococcaceae in the gastric microbiota of PPI-treated patients was significantly higher than that in untreated patients. The abundance of the genus Streptococcus was also significantly increased with PPI treatment independently of H. pylori infection23. These results suggested that the absence of H. pylori in the stomach and decreased gastric acid secretion may be responsible for the increased abundance of the genus Streptococcus.
The relationship between the incidence of gastric cancer and H. pylori infection is strong; however, the relationship between the incidence of gastric cancer and AIG remains unclear. The long-term gastric use of PPIs for > 3 years after H. pylori eradication increased the incidence of noncardiac carcinoma by 2.4-fold, with dysbiosis due to decreased gastric acid secretion potentially contributing to the development of gastric cancer24. The gastric microbiota of patients with gastric cancer is characterized by an increased presence of bacterial genera, including intestinal commensal bacteria and oral genera Streptococcus and Prevotella25,26. A meta-analysis revealed that opportunistic pathogens such as Fusobacterium, Parvimonas, Veillonella, Prevotella, and Peptostreptococcus were enriched, whereas commensals Bifidobacterium, Bacillus, and Blautia were depleted in patients with gastric cancer compared with those in patients with superficial gastritis27. However, in this study, the genus Streptococcus was more prevalent in AIG, whereas the genus Prevotella was more prevalent in atrophic gastritis controls. Animal experiments have revealed that Streptococcus anginosus induces carcinogenesis28; hence, examining the level of species carried by individuals is warranted in future studies.
Potassium-competitive acid blockers (PCABs) have been increasingly used to treat upper gastrointestinal disorders, replacing PPIs. However, PCAB use has been associated with an increased risk of gastric cancer among H. pylori-eradicated patients, with duration/dose response effects29. Long-term users of PPIs and PCABs were excluded from participating in the present study.
In the future, increasing the number of cases and comparing them with users of PPIs and PCABs will be necessary.
Recently, Rugge et al.30 reported that compared with the general population, corpus-restricted inflammation/atrophy in patients with AIG did not increase the risk of gastric cancer. They performed a long-term follow-up histological study to clarify the natural history, histological phenotypes, and associated cancer risk in patients with AIG who consistently tested H. pylori-negative30. Over the long term, AIG consistently featured oxyntic-predominant-mononuclear inflammation. No excess risk of gastric or other malignancies was found over a cumulative follow-up time of 10 541 person-years, except for (marginally significant) thyroid cancer30. Despite the conflicting opinions31 about the conclusions of that study, in an environment where H. pylori is absent but gastric acid secretion is reduced, the risk of gastric cancer could theoretically increase if the stomach is populated by bacteria linked to gastric cancer.
The proton pump of parietal cells is targeted by antiparietal cell antibodies. In addition, antigastric intrinsic factor antibodies have also been observed. The mechanism of autoantibody production involves the response of T-cells to parietal cell antigens damaged by H. pylori infection32. The pathogenic mechanism by which the similarities between bacterial and host molecules induce the production of autoreactive antibodies in the host is observed in many autoimmune diseases. In our study, although we did not show whether patients with AIG had ever been infected with H. pylori, six of the 15 patients had serum anti-H. pylori IgG antibodies, the antibody titers of two of which were particularly high (Table 2). Patients with AIG cannot properly absorb vitamin B12 from the small intestine, which increases their risk of pernicious anemia and peripheral neuropathy. Therefore, analyzing AIG pathogenesis in detail to obtain useful information for its prognosis, prediction, and treatment is crucial.
In this study, two gastric biopsy specimens were collected from each patient, one from the corpus and one from the antrum. We hypothesized that the gastric microbiota composition may change depending on the site of the stomach because patients with AIG are characterized by a state of inverse gastric atrophy. However, differences in the composition of gastric microbiota by site were not clear. PERMANOVA analysis using weighted and unweighted Unifrac distances to compare the beta diversity of the gastric microbiota in the corpus and antrum of the stomach showed no significant differences. We thus assumed that the atrophic severity observed in AIG may have spread over the entire stomach of patients with AIG.
This study had some limitations. First, the AIG sample size was small. Second, the period after eradication therapy in the control atrophic gastritis group was significantly longer than that in the AIG group. Third, the atrophic severity in AIG may have been greater than that in the control group. These factors may have affected the gastric microbiota composition and should be further explored in future studies with a larger number of cases.
In conclusion, the gastric bacterial composition of patients with AIG after eradication therapy differed significantly from that of patients with chronic gastritis, with decreased Shannon, Pielou’s evenness, and Simpson indices. The gastric microbiota of patients with AIG after eradication therapy was characterized by an increased abundance of the genus Streptococcus and a reduced abundance of the genus Prevotella compared with those in patients with chronic gastritis.
Materials and methods
Participants
Patients with AIG and control patients with atrophic gastritis who visited Kyorin University Hospital for endoscopy between November 28, 2018, and July 30, 2021, were recruited for this study. AIG was diagnosed endoscopically and serologically5. Briefly, antigastric parietal cell or anti-intrinsic factor antibodies or both were detected in the serum. Control individuals were patients diagnosed with H. pylori infection at Kyorin University Hospital, who were treated with first- or second-eradication regimen(s) and were currently H. pylori-negative (Fig. 1).
In the first regimen, clarithromycin (CLR) 400 mg bid and amoxicillin (AMX) 750 mg bid with PPI or vonoprazan (P-CAB) were administered for 7 d. Patients whose H. pylori infection was not eradicated by the first-line eradication regimen received a second-line regimen, which included a 7-d administration of PPI or P-CAB, metronidazole (MNZ) 250 mg bid, and AMX 750 mg bid. In cases not eradicated by the first- and second-line eradication regimens, patients were treated with a third-line regimen, which included administering a high dose of AMX and P-CAB for 14 d or sitafloxacin, AMX, and P-CAB for 7 d.
13C-UBT was used to determine successful eradication. Control patients with atrophic gastritis were previously infected with H. pylori, had received eradication therapy for chronic gastritis, and were not infected with H. pylori at the time of the study. This study was conducted in accordance with the Declaration of Helsinki and ethics review procedures concerning research involving humans, with approval from the Ethics Committee of Kyorin University, Tokyo (624-04).
Biopsy specimens and DNA extraction
Gastric biopsy tissues from the gastric corpus (A) and antrum (B) were collected from patients at the time of gastric endoscopy for microbiota analysis. The fresh specimens were placed in DNA/RNA Shield (Zymo Research, Irvine, CA, USA) and stored at 4 °C until use. Tissue samples were centrifuged at 10, 000 × g for 10 min, and the supernatant was discarded. Total DNA was extracted using the QIAamp Power Fecal Pro DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To improve the PCR amplification efficiency, the host DNA was removed from the extracted DNA that had not been sufficiently amplified. The extracted DNA was removed using the NEBNext® Microbiome DNA Enrichment Kit (New England Biolabs, Ipswich, MA, USA), following the manufacturer’s instructions.
16S rRNA gene amplicon sequencing analysis
Total DNA (< 12.5 ng) extracted from gastric biopsy tissues was amplified using universal primers targeting the 16S rRNA V3-V4 region; forward primer (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′), reverse primer (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′), including a tag region. The first PCR reaction was performed by incubating at 95 °C for 3 min, followed by 30–35 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and 72 °C for 5 min using the Platinum II Hot-Start PCR Master Mix (Invitrogen, Thermo Fisher Scientific, San Diego, CA, USA). The generated amplicon was attached to dual indices and Illumina sequencing adapters using the Nextera XT index kit (Illumina, Inc., San Diego, CA, USA), according to the manufacturer’s instructions. Amplicon sequencing was performed using the Illumina MiSeq (Illumina).
The obtained sequence data were aligned against the human reference genome (GRCh38) using the Kraken2 software33 to remove host sequences, and the remaining sequences were analyzed using the quantitative insights into microbial ecology 2 (QIIME2 October 2019) pipeline34. The nonhost paired-end sequence reads were quality-filtered, denoised, merged, and chimeras were removed using the DADA2 plugin. Taxonomy was assigned to the amplicon sequence variants (ASVs) obtained from DADA235 using the feature-classifier-classify sklearn plugin in the SILVA database v138.136. The alpha diversity of each sample microbiota was determined using Chao-1, Pielou’s evenness, Shannon, and Simpson indices.
Statistical analysis
The significance of differences between the AIG and control groups was determined using the Wilcoxon rank-sum test. Intergroup comparisons of beta diversity were performed using PERMANOVA. Microbiome Multivariable Associations with Linear Models (MaAslin2)37 was also performed to identify biomarkers between AIG and control individuals.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Strickland, R. G. & Mackay, I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am. J. Dig. Dis. 18, 426–440 (1973).
Notsu, T. et al. Prevalence of autoimmune gastritis in individuals undergoing medical checkups in Japan. Intern. Med. 58, 1817–1823 (2019).
Terao, S. et al. Multicenter study of autoimmune gastritis in Japan: clinical and endoscopic characteristics. Dig. Endosc. 32, 364–372 (2020).
Lenti, M. V. et al. Autoimmune gastritis. Nat. Rev. Dis. Primers 6, 56 (2020).
Kamada, T., Maruyama, Y., Monobe, Y. & Haruma, K. Endoscopic features and clinical importance of autoimmune gastritis. Dig. Endosc. 34, 700–713 (2022).
Toyokawa, T. et al. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: a long-term prospective cohort study. J. Gastroenterol. Hepatol. 25, 544–547 (2010).
Haruma, K. et al. Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus-evaluation of 24-h pH monitoring. Aliment. Pharmacol. Ther. 13, 155–162 (1999).
Wang, L. et al. Helicobacter pylori and autoimmune diseases: Involving multiple systems. Front. Immunol. 13, 833424 (2022).
Amedei, A. et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+ –adenosine triphosphatase in human gastric autoimmunity. J. Exp. Med. 198, 1147–1156 (2003).
Zhang, X. & Pan, Z. Influence of microbiota on immunity and immunotherapy for gastric and esophageal cancers. Gastroenterol. Rep. 8, 206–214 (2020).
Kaźmierczak-Siedlecka, K., Daca, A., Roviello, G., Catalano, M. & Połom, K. Interdisciplinary insights into the link between gut microbiome and gastric carcinogenesis—what is currently known?. Gastric Cancer 25, 1–10 (2022).
Wang, L. et al. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 28, 261–266 (2016).
Ndegwa, N. et al. Gastric microbiota in a low-Helicobacter pylori prevalence general population and their associations with gastric lesions. Clin. Transl. Gastroenterol. 11, e00191 (2020).
Han, H. S. et al. Correlations of the gastric and duodenal microbiota with histological, endoscopic, and symptomatic gastritis. J. Clin. Med. 8, 312 (2019).
Cornejo-Pareja, I. et al. H. pylori eradication treatment alters gut microbiota and Glp-1 secretion in humans. J. Clin. Med. 8, 451 (2019).
Chen, C. C. et al. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 13, 1909459 (2021).
Guo, Y. et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut 69, 1598–1607 (2020).
Parsons, B. N. et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 13, e1006653 (2017).
Shin, C. M. et al. Changes in gastric corpus microbiota with age and after Helicobacter pylori eradication: a long-term follow-up study. Front. Microbiol. 11, 621879 (2020).
Tsuboi, M. et al. Distinct features of autoimmune gastritis in patients with open-type chronic gastritis in Japan. Biomedicines 8, 419 (2020).
Sung, J. J. Y. et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 69, 1572–1580 (2020).
Hsiao, W. W. L. et al. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 13, 345 (2012).
Paroni-Sterbini, F. et al. Effects of proton pump inhibitors on the gastric mucosa-associated microbiota in dyspeptic patients. Appl. Environ. Microbiol. 82, 6633–6644 (2016).
Cheung, K. S. et al. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 67, 28–35 (2018).
Ferreira, R. M. et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67, 226–236 (2018).
Coker, O. O. et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 67, 1024–1032 (2018).
Liu, C. et al. Meta-analysis of mucosal microbiota reveals universal microbial signatures and dysbiosis in gastric carcinogenesis. Oncogene 41, 3599–3610 (2022).
Fu, K. et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell 187, 882-896.e17 (2024).
Arai, J. et al. Association between vonoprazan and the risk of gastric cancer after Helicobacter pylori eradication. Clin. Gastroenterol. Hepatol. 22, 1217-1225.e16 (2024).
Rugge, M. et al. Autoimmune gastritis: long-term natural history in naïve Helicobacter pylori-negative patients. Gut 72, 30–38 (2023).
Waldum, H. L. Conclusion that autoimmune gastritis does not predispose to gastric cancer is unproven. Gut 73, 379 (2024).
Yu, Y. F. et al. Research status and hotspots of autoimmune gastritis: A bibliometric analysis. World J. Gastroenterol. 29, 5781–5799 (2023).
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
SILVA documentation. https://www.arb-silva.de/documentation/release-1381/ (2024).
Mallick, H. et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 17, e1009442 (2021).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI: 20K08365 and 24K11176), AMED (24fk0108678h0302, 24ym0126140j0001) and the Yakult Bio-Science Foundation. We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
K.T. and T.O. conceived the study, designed the experiments, and prepared the manuscript; K. T., S.M., and Y. I. collected clinical specimens; F.H. and H.Y. performed the experiments; K.O. and M.T. provided analysis tools; J. M. analyzed the data; and S.K. and T.H. supervised the manuscript preparation. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Dr Tokunaga’s work has been funded by the Yakult Bio-Science Foundation. Dr Shigeru Kamiya receives remuneration as a medical advisor for Miyarisan Pharmaceutical Co., Ltd. Dr Kentaro Oka and Motomichi Takahashi were employees of Miyarisan Pharmaceutical Co. No other author has a competing interest.
Informed consent
Informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tokunaga, K., Miyoshi, S., Hojo, F. et al. Comparative study of gastric microbiota between patients with autoimmune gastritis and those with atrophic gastritis. Sci Rep 15, 27658 (2025). https://doi.org/10.1038/s41598-025-12211-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12211-3