Abstract
Among chemical warfare agents (CWAs), mustard gas (HD) is a lethal blister agent that causes prolonged chemical burns and skin blistering, often without immediate symptoms. HD has been used as a chemical weapon due to its potent toxicity, which becomes more pronounced as temperatures rise after its release in colder conditions. As temperature increases, HD also undergoes more active desorption, making its detection increasingly difficult. Therefore, temperature-sensitive detection methods are critical for effectively identifying HD for practical applications in this field. In this study, we investigated the detection of 2-chloroethyl ethyl sulfide (2-CEES), a simulant for HD, using a surface acoustic wave (SAW) sensor coated with a polyphosphonamide-based polymer (PPD-F2). Experiments were conducted across a temperature range of − 20 °C to 50 °C. The results demonstrated that the sensor’s frequency shift (∆f) increased as the temperature decreased, with a particularly notable rise in ∆f observed at temperatures below 10 °C.
Similar content being viewed by others
Introduction
Chemical Warfare Agents (CWAs) are chemical compounds that disperse rapidly at low concentrations and can cause fatal harm to humans1,2. Therefore, In April 1994, the Organization for the Prohibition of Chemical Weapons (OPCW) completely banned the development, production, acquisition, stockpiling, possession, transfer, and use of CWAs through the Chemical Weapons Convention (CWC)3. CWAs are classified into nerve, blister, blood, and choking agents, as shown in Table S11,4,5,6.
Among CWAs, mustard gas (HD) is a blister agent that causes continuous chemical burns and large blisters on the skin, eyes, and lungs of exposed individuals5,7. Moreover, it can irreversibly alkylate deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and proteins, influencing cell division and leading to tissue necrosis, oxidation, carcinogenesis, and even death6,8,9. HD was first employed by the German army during World War I in 1917 and was utilized in the Iran-Iraq War from 1983 to 19888,10.
Recently, technology for detecting such CWAs has advanced. However, the need for rapid onsite detection remains unmet because of issues such as bulky size, high cost of detection equipment, complex operational procedures, and the need for specialized training. However, among chemical detectors, Surface Acoustic Wave (SAW) sensor offers advantages such as small size, high sensitivity, rapid response, reversibility, affordability, light weight design, and very low operating power, making them highly portable and suitable for various applications2,5,6,7,11,12,−13.
The experiments were conducted using 2-Chloroethyl ethyl sulfide (2-CEES), considering the toxicity and hazards of HD2,9,13,14. As shown in Figure S1, the torsion angles of Cl-C-S-S and C-C-S-C in 2-CEES were 177.38° and 83.17°, respectively, showing values very close to HD (179.9° and 82.2°), as indicated15. This demonstrates that 2-CEES exhibits chemical properties similar to those of HD16. Therefore, 2-CEES is a less toxic simulant than HD. Although 2-CEES is a simulant of HD, it is a highly toxic substance and is referred to as half-HD. Considering its inherent toxicity, it is likely to be utilized as a substitute for HD in low-temperature environments where HD cannot be used.
HD has been employed based on the principle that it is dispersed at temperatures lower than its melting point, and its toxicity becomes apparent when the environmental temperature increases above this melting point. Additionally, infield environments, when the temperature increases, HD vaporizes, increasing its toxicity and decreasing its persistence. This makes the detection of HD challenging and leads to significant casualties. HD exhibits a pattern of flowing from higher to lower altitudes owing to its greater density compared to air, progressively exacerbating additional harm and environmental contamination in the affected areas9,16,17,18.
In such chemical and biological warfare scenarios, technology for detecting CWAs is critically important. By conducting HD detection experiments based on environmental temperatures, it is possible to analyze the sensor response according to the ambient temperature under field conditions. This allows the development of proactive technology to counteract the threat of HD2,6. Previous studies on 2-CEES detection have primarily focused on high-temperature environments, and those utilizing SAW sensors have shown limited sensitivity and responsiveness. Yin Long et al.17 employed a SAW sensor coated with a strongly hydrogen-bond acidic (HBA) polymer, functionalized polymer (PLF), for the detection of 2-CEES, demonstrating rapid adsorption and equilibrium behavior at vapor concentrations of 1, 2, and 5 mg/m3. Raj et al.19 utilized oxide thin films (ZnO, TeO2, SnO2, and TiO2) as sensing materials in a SAW-based electronic nose for the detection of chemical warfare agent simulants. The experiments demonstrated that each oxide film exhibited selective sensitivity and rapid response characteristics to specific gases, with TiO2, and SnO2 showing particularly high sensing performance. However, research on 2-CEES detection at low temperatures remains scarce, underscoring the need for further investigation in this area20,21,22,23,24. Therefore, there is a need for technology that can detect 2-CEES based on changing environmental temperatures.
In this study, the polyphosphonamide-based polymer (PPD-F2) material was synthesized to use in the SAW sensor for detection of 2-CEES. The characterization methods such as Fourier-Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD), and X-ray Photoelectron Spectroscopy (XPS) were used to study the chemical structure and bonds between them. Their performance in detection of 2-CEES was analyzed by frequency shift (∆f), selectivity, linearity, response/recovery times, repeatability, effect of temperature, and relative humidity on the sensing response. Thus, we proposed the adsorption theory based on sensing mechanism between the 2-CEES and PPD-F2.
Experimental methods and materials
Reagents and instruments
2-Chloroethyl ethyl sulfide (2-CEES, 98%), Dimethyl methyl phosphonate (DMMP, 98%), Malathion (98%), Cyanogen chloride (CK, 98%), sulfur dioxide (SO2, 98%), and Ethylene oxide (EO, 98%) were obtained from Kin-Tek (504 Laurel St., La Marque, TX 77568, USA) in two types: Liquid Filled High-rate (LFHA, for all gases except for low concentration) and High-Rate Tube (HRT, for low-concentration applications). The gases were of certified grade and used without further purification. N, N-Dimethylformamide (DMF, ≥ 99%), used as a solvent for receptor synthesis, was purchased from Sigma-Aldrich and used without additional purification.
The frequency characteristics of the SAW sensors were measured using a network analyzer (Keysight E5063A). FT-IR spectroscopy of synthesized material was conducted on a Vertex 80 v FT-IR Spectrometer (Bruker, Billerica, MA, USA). XRD of PPD-F2 was performed using an X’pert PRO MRD diffractometer (Philips/PANalytical, Malvern, UK). XPS (K-Alpha, Thermo Fisher Scientific, Waltham, MA, USA) was used to analyze the elemental composition of the composite materials. Gas concentration and flow rate were controlled using a Flex Stream gas dilution system (Kin-Tek), and environmental temperature and humidity were regulated using a TH-JG-460 environmental chamber, with temperature maintained between − 20 °C and 50 °C and relative humidity controlled at 30% RH, 50% RH, and 70% RH.
Material characterization methods
With a scan range of 4000 –800 cm− 1, Vertex 80 v FT-IR Spectrometer was used for the FT-IR examinations. The 400∼4000 nm Mid-IR Infrared energy is transmitted through the substance to obtain the substance’s unique absorption spectrum and analyze the substance’s structure and special functional groups (possible for solid and liquid). By using XRD studies, which were performed using a X’pert PRO MRD diffractometer (Max power: 3 kW, Cu source, Scan range: 3° ~ 145°, Minimum step size: 0.0001°, Goniometer radius: 300 mm), it was possible to determine the crystallographic and phase composition of the synthesized materials. X-ray photoelectron spectroscopy (XPS) was used to further examine the compositions of the samples (K-Alpha, Thermo Fisher Scientific). Its specifications are : X-ray source: monochromated Al Ka, spot size : 30 μm to 400 μm, ion source energy: 100 eV to 3 keV.
SAW sensor design
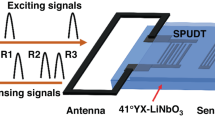
As shown in Figure S2, the SAW sensor used in the experiment consisted of a substrate, delay line, reflector, and Inter Digital Transducer (IDT) operated based on the piezoelectric effect. ST-cut quartz with a + 42° tilt angle relative to the z-axis was employed as the piezoelectric material because of its low coefficient of thermal expansion and high-temperature stability25,26,27. The delay line was set to an optimal length of 1 mm with minimal loss to coat the receptor. The IDT of the SAW sensor transmitted a constant resonant frequency towards the delay line. The IDT generates surface acoustic waves, with the delay line enabling signal processing, the reflector directing the waves back towards the IDT, while the coating area interacts with the target analyte, affecting wave propagation and enabling sensing functionality. The IDT wavelength of the SAW sensor used in this system has a resonant frequency of 250 MHz and a wavelength of 12.632 μm, as shown in Table S228,29. The resonant frequency was determined by the wavelength of the IDT, and the target gas was detected based on changes in the resonance frequency that occurred as the signal passed through the delay line.
As shown in Figure S3, the delay line was coated with the sensing material to actively induce adsorption reactions with the target gas, resulting in frequency changes corresponding to the mass variation caused by adsorption. The mechanism for detecting the target gas using the shifted frequency value can be explained by the mass-loading effect (Δm), as shown in Eq. 1.30,31
.
where k1 and k2 are of piezoelectric constants of substrate, f0 is the resonant frequency, and A is the film area for sensing.
Synthesis of PPD-F2
A PPD-F2 was synthesized and used as a sensing material. The synthesis process of PPD-F2 is shown in Fig. 1; it is modular, and various diamines can be introduced. Here, 2, 2-bis[4-(4-aminophenoxy) phenyl] hexafluoro propane (518 mg, 1.0 mMol) was mixed for 1 h at 60 °C. Next, triethylamine (TEA, 0.28 mL, 2.0 mMol) was introduced, and subsequently, phenyl phosphoric dichloride (195 mg, 1.0 mMol) was added dropwise to the solution. Afterward, the mixture was heated to 82 °C and refluxed overnight under a nitrogen atmosphere. Once the reaction was complete, the precipitated formed were filtered, washed with water several times, and dried under vacuum, resulting in a white powder-like product (Yield: 618.5 mg, 96%)32.
Deposition of sensing materials onto the SAW sensor
The process of coating the sensing material onto the delay line of the SAW sensor is shown in Figure S4. First, the inner space of the vial was cleaned using an air gun. Furthermore, the receptor was manufactured by mixing the powdered sensing material and solvent dimethylformamide (DMF) in a 1:1 ratio. The two substances were mixed using a Voltex mixer. The prepared receptor was coated onto the delay line of the SAW sensor using a drop-casting method. The amount of receptor used at this stage is 0.5 µL, which is the optimized quantity that minimizes loss while ensuring excellent reactivity. The receptor coated SAW sensor underwent 12 h drying process in a vacuum chamber at room temperature, forming a thin film. Through this process, a sensor capable of detecting the target gas was developed.
Schematic diagram—test setup
The target gas was generated and controlled using the Kin-Tek ‘Flex Stream base’ model with a permeation tube and blown into the chamber housing the SAW sensor via the gas out pathway. Gas detection experiment was performed using the designed system shown in Figure S5. The permeation tube functions as a flow control mechanism that dispenses small amounts of vapor through a polymeric membrane (Thin Film Encapsulation, TFE or Fluorinated Ethylene Propylene Teflon®, FEP). The emission rate is determined by the membrane’s permeability, the vapor pressure of the compound, and the operating temperature.
For an efficient blending system, the carrier flow must remain continuous and steady to ensure the stability of component concentrations and rapid system response. In the absence of carrier flow, the permeation chamber and surrounding elements are at high risk of contamination; thus, maintaining a continuous flow is essential. Switching the carrier flow to a vent can help maintain a “zero gas” state, allowing online verification of the system’s cleanliness33.
Characterization methods
In Figure S6, a constant flow of nitrogen (100 sccm) was supplied to the permeation tube containing 2-CEES to generate an appropriate concentration of 2-CEES. Mixing nitrogen with the 2-CEES produced in this way makes it possible to create 2-CEES with a concentration of 5 mg/m3. In this process, solenoid valves and Mass Flow Controllers (MFCs) were used to control the flow direction and flow rate. The SAW sensor was exposed with only nitrogen flow rate before exposure to the target gas, allowing for a stabilization period. This minimizes noise with respect to the flow rate of the 2-CEES and the cleaning of foreign substances. SAW sensors exhibit sensitivity to increasing temperature, resulting in a linear decrease in the resonant frequency. Therefore, stabilization was performed for 1 h at each temperature. The environmental temperature was controlled within a range of – 20 °C to 50 °C using a temperature chamber.
Results and discussions
Characterization
FT-IR data
Figure 2a presents the FT-IR spectra of the PPD-F2. A broad peak observed around 3342 cm− 1 corresponds to N–H stretching vibrations, suggesting the presence of amine groups, potentially involved in the formation of hydrogen bonds34. Peaks at 3053 cm− 1 and 2852 cm− 1 are attributed to = C–H stretching of aromatic rings and aliphatic C–H stretching, respectively.
Strong absorption bands around 1612 cm− 1 and 1502 cm− 1 are assigned to aromatic C = C stretching vibrations, indicating the presence of conjugated or aromatic structures within the polymer backbone. Additionally, peaks in the region of 1247 cm− 1 to 1049 cm− 1 (at 1247 cm− 1, 1203 cm− 1, 1172 cm− 1, and 1049 cm− 1) are attributed stretching vibration of P = O group35,36. Out-of-plane C–H bending vibrations observed at 831 cm− 1 and 700 cm− 1 are indicative of substituted benzene rings32,37.
XRD data
Figure 2b presents the XRD pattern of the PPD-F2. The XRD pattern of the polymer exhibits a broad halo centered around 2θ = 20°, indicative of an overall amorphous structure. However, several distinct diffraction peaks are observed at 2θ values of 13.06°, 14.86°, 15.72°, 17.22°, 18.62°, and 21.58°, which correspond to the (020), (201), (111), (211), (100), and (230) crystallographic planes, respectively38. The presence of these sharp reflections suggests the existence of short-range ordering or partially crystalline domains embedded within the predominantly disordered polymer matrix. These features imply that the polymer has a semi-crystalline nature, potentially arising from the periodic packing of polymer chains or specific intermolecular interactions39. Notably, no additional diffraction peaks are observed beyond 2θ = 50°, indicating the absence of long-range crystalline order at higher scattering angles.
XPS data
XPS was conducted to analyze the surface chemical composition of the synthesized sample. The high-resolution P2p spectrum (Fig. 3a) shows a dominant peak at 144.08 eV. The N1s spectrum (Fig. 3b) presents a peak centered at 410.08 eV, while the C1s spectrum (Fig. 3c) exhibits a strong peak at 298.08 eV. In the O1s region (Fig. 3d), a peak is observed at 545.08 eV. The F1s spectrum displays a peak at 698.08 eV (Fig. 3e).
Quantitative analysis based on atomic percentages revealed that carbon (68.75%) and fluorine (16.3%) were the most abundant elements, followed by oxygen (8.41%), nitrogen (4.77%), and phosphorus (1.77%).
These results indicate that the XPS analysis reveals the highest intensities for carbon and fluorine, which is consistent with their high content in the PPD-F2 sample40,41. The XPS survey spectrum (Fig. 3f) confirms the presence of carbon (C), nitrogen (N), oxygen (O), fluorine (F), and phosphorus (P) in the material.
Selectivity test
In the selectivity test, an experiment was performed to evaluate whether PPD-F2 could selectively detect a specific gas by exhibiting a strong response. PPD-F2 was exposed to 2-CEES, dimethyl methyl phosphonate (DMMP), malathion, cyanogen chloride (CK), sulfur dioxide (SO2), and ethylene oxide (EO) at a concentration of 10 mg/m3. The results revealed frequency shifts of 832.08 Hz for 2-CEES, 290.99 Hz for DMMP, 49.43 Hz for malathion, 47.63 Hz for CK, 41.82 Hz for SO2, and 41.85 Hz for EO, as illustrated in Fig. 4. 2-CEES exhibited approximately 2.85 times higher reactivity compared to DMMP, the second most reactive substance, confirming that PPD-F2 shows a significantly higher response specifically to 2-CEES.
To understand this selectivity, it is important to consider the mechanism by which PPD-F2 interacts with target gases. The target gases are exposed to a SAW sensor coated with PPD-F2, where they interact with the fluorine (F) functional group of the sensing material via their respective functional groups—hydrogen (2-CEES, DMMP, Malathion, EO), nitrogen (CK), and oxygen (SO2). The bond strength between the functional group of the gas and the fluorine atom of PPD-F2 plays a crucial role in the selectivity of the sensor.
PPD-F2 forms a particularly strong bond with 2-CEES compared to other gases due to the high bond dissociation energy and the strong electronegativity of sulfur (S) in 2-CEES. The hydrogen-containing gases (2-CEES, DMMP, Malathion, EO) exhibit a bond dissociation energy of 569.4 kJ/mol with fluorine, while the nitrogen-containing gas CK has a bond dissociation energy of 280.5 kJ/mol, and the oxygen-containing gas SO2 has a bond dissociation energy of 191.7 kJ/mol42. This difference in bond dissociation energy explains why 2-CEES forms a stronger and more stable interaction with PPD-F2, leading to significantly larger frequency shifts.
Furthermore, the bond strength can be compared based on the electronegativity of the central atoms in the functional groups. The central atom of 2-CEES is sulfur (S) with an electronegativity of 2.58, while DMMP and Malathion have phosphorus (P) at 2.19, and EO has carbon (C) at 2.55. Due to the higher electronegativity of sulfur, PPD-F2 forms a stronger bond with 2-CEES compared to other gases, resulting in the highest frequency shifts. Therefore, the selectivity of PPD-F2 for 2-CEES is attributed to both the bond dissociation energy and the electronegativity of the functional group’s central atom. Hence, these materials can be used as potential candidates for 2-CEES sensing43.
2-CEES sensing performances under different temperature ranges
Figure 5a-h show the real-time frequency response and repeatability evaluation of PPD-F2-coated SAW sensors exposed to 2-CEES vapor at a concentration of 5 mg/m3 under temperatures ranging from – 20 °C to 50 °C. The observed characteristics can be explained by the interaction between the carboxyl groups of the PPD-F2 sensing material and the carbonyl functional groups of the target gas. The repeatability test results showed that the average ∆f was 723.12 Hz at – 20 °C, 500.19 Hz at – 10 °C, 310.19 Hz at 0 °C, 220.33 Hz at 10 °C, 205.36 Hz at 20 °C, 162.21 Hz at 30 °C, 131.35 Hz at 40 °C, and 91.21 Hz at 50 °C. Since there were no significant variations between the frequency shift values and their averages at each temperature, the sensors demonstrated excellent repeatability. Figure 6a-h show the response time and recovery time of SAW sensors coated with PPD-F2 for detecting 2-CEES vapor at a concentration of 5 mg/m3 under environmental conditions ranging from – 20 °C to 50 °C. The reaction times required to reach 90% of the final equilibrium value upon exposure to 2-CEES at various temperatures are summarized as follows. The shortest response time was approximately 69 s at 40 °C, followed by 71 s at 50 °C. The shortest recovery time was 82 s at 50 °C, with 113 s at 30 °C. These results indicate that as the environmental temperature increases, both the adsorption and desorption reactions become more active, leading to longer response and recovery times44.
To ensure that the frequency shift observed in the above experiments is solely due to the interaction between 2-CEES and the sensing layer, and not a result of the intrinsic thermal sensitivity of the SAW sensor itself, control measurements were conducted using a bare (un-coated) SAW device under identical temperature conditions. As shown in Fig. 7, the resonance frequency of the bare sensor displays a slight drift depending on the ambient temperature but remains stable over time after reaching thermal equilibrium. In Fig. 7-a shows the absolute resonance frequency across the temperature range of – 20 °C to 50 °C, and 7-b presents the ∆f relative to the initial frequency at each temperature. These results indicate that the frequency drift due to temperature alone was minimal compared to the shifts observed with 2-CEES exposure.
Consequently, baseline correction was applied where appropriate, confirming that the frequency responses described in Fig. 7-a and -b were primarily driven by the chemical interaction between PPD-F2 and 2-CEES rather than the thermal sensitivity of the sensor substrate.
Temperature-dependent behavior of a bare SAW sensor without any sensing layer. (a) Resonance frequency variation as a function of temperature from – 20 °C to 50 °C. (b) Frequency shift (Δf) over time relative to the initial value at each temperature, demonstrating thermal stability after equilibration.
Effect of relative humidity
Figure 8a shows the response of the SAW sensor coated with PPD-F2 under varying humidity conditions of 30% RH, 50% RH, and 70% RH when exposed to 25 mg/m³ of 2-CEES. The sensor exhibited frequency shifts of 2218.13 Hz at 30% RH, 2553.95 Hz at 50% RH, and 2640.88 Hz at 70% RH, indicating enhanced responsiveness with increasing humidity. This trend is attributed to the adsorption of water molecules on the sensing layer, which increases the surface mass and consequently reduces the velocity of the surface acoustic wave. In our sensor system, this results in a greater ∆f, which directly reflects improved sensitivity. Therefore, the increased humidity facilitates mass loading and enhances the detection performance of the PPD-F2-coated SAW sensor under humid conditions45,46.
Limit of detection (LOD) test
Figure 8b illustrates the investigation of linearity to determine the LOD of the target gas. PPD-F2-coated SAW sensors were exposed to various concentration of 2-CEES under controlled environmental conditions at a temperature of – 20 °C. The concentrations of 2-CEES were set to 1/2, 2/5, 3/10, 1/5, and 1/10 of the previously tested concentrations, corresponding to 2.5, 2.0, 1.5, 1.0, and 0.5 mg/m3, respectively. The mean and standard deviation (SD) values for each concentration were calculated from three repeated experiments, and the results are as follows: for 2.5 mg/m3, the mean is 60 with SD of 3; for 2.0 mg/m3, the mean is 94 with SD of 2; for 1.5 mg/m3, the mean is 137 with SD of 3; for 1.0 mg/m3, the mean is 165 with SD of 11; and for 0.5 mg/m3, the mean is 197 with SD of 10. Error bars were added to the data points to represent the variability of the measurements. The experimental data were used to derive a linearity curve, with coefficient of determination (R2) of 0.99289. High R2 indicates excellent linearity between the gas concentration and the sensor’s frequency response, demonstrating the reliability and accuracy of the PPD-F2-coated SAW sensor in detecting 2-CEES.
Based on this linear relationship, the LOD is estimated as the lowest gas concentration at which the sensor can reliably distinguish a signal from baseline noise. Based on this linear relationship, the LOD is estimated as the lowest gas concentration at which the sensor can reliably distinguish a signal from baseline noise. To determine the theoretical LOD, the standard deviation σblank of the frequency shift at the lowest tested concentration (0.5 mg/m3) was used, which was 2.7517 Hz. The slope of the calibration curve was 74.0123, derived from the linear regression of the experimental data.
The LOD is then calculated using the standard formula:
.
Although this theoretically derived LOD indicates the minimum detectable concentration under ideal conditions, our practical experiments showed that the sensor begins to produce a clearly distinguishable signal from 0.5 mg/m3. Therefore 0.5 mg/m3 is reported as the practical LOD, reflecting the actual detection capability of the sensor47.
These results highlight the sensor’s high sensitivity and its potential for detecting low concentrations of 2-CEES, confirming its suitability for applications requiring precise gas detection48.
Stability test
Figure 8c presents the frequency shift data collected over 7 days. The mean and SD values for each day were calculated from three repeated experiments. For day 1, the mean is 428 with an SD of 18; for day 4, the mean is 336 with an SD of 19; and for day 7, the mean is 294 with an SD of 33. All sensors showed a ~ 30% reduction in signal over 7 days. The results show a gradual decrease in frequency shift over time, suggesting a decline in sensor sensitivity possibly due to chemical degradation on the sensing surface49. A linear regression analysis was performed, and the fitted line shows a negative slope. Notably, the regression yielded a high R² value is 0.953, which indicates a strong linear relationship. This high coefficient of determination suggests that the performance degradation follows a predictable trend, which is beneficial for long-term applications where calibration or signal correction may be necessary to maintain reliable sensor performance50.
Measured results of SAW sensor (a) Effect of relative humidity on the sensing performances in detection of 2-CEES, (b) Linearity test for detection of 2-CEES at low concentration ranging from 0.5 to 2.5 mg/m3 at the temperature of – 20 °C, and (c) Long term stability results for detection of 2-CEES for seven days at three-day interval.
Freezing point test
To investigate the underlying cause of the highest reactivity observed at – 20 °C, an experimental analysis was conducted to explore the possibility that 2-CEES has a freezing-point higher than – 20 °C, among various factors. The experiment was conducted in separate vials containing 2-CEES and water, followed by a gradual reduction in temperature using an environmental chamber. The temperature was decreased from 50 to – 20 °C at a rate of 2 °C per minute51,52.
In Fig. 9, the results of the freezing point experiments for 2-CEES and water are shown. As the environmental temperature decreased, the temperatures of both the 2-CEES and water also decreased. In the case of water, the temperature dropped after super-cooling and the phase change process. Super-cooling is a phenomenon in which a liquid remains unfrozen despite being below its freezing point. To initiate the phase change of water into ice, a process known as nucleation is necessary, during which water molecules arrange themselves in a specific configuration. If this nucleation is insufficient, super-cooling occurs. Various factors contribute to this phenomenon, such as a lack of nucleation sites, a rapid cooling rate, and the presence of impurities.
During the phase-change process, as water molecules come closer to each other and form an increased number of hydrogen bonds, the interconnection between water molecules strengthens, ultimately forming a solid ice structure. Through this process, we defined a temperature of 0 °C as the freezing point of water, where a phase change occurs53. However, in the case of 2-CEES, there is no observable phase change region or super-cooling range similar to that of water. This indicates that the freezing point of 2-CEES is lower than – 20 °C. Therefore, the experiment confirmed that the increase in responsiveness was not due to an increase in reactivity as 2-CEES solidified.
Adsorption theory
The following investigates the relationship between temperature and adsorption rate as the observed cause for the highest reactivity at – 20 °C. The adsorption reaction refers to the process in which atoms, molecules, or ions in the gaseous, liquid, or dissolved state adhere to the surface of a solid or liquid and involves simultaneous and rapid adsorption and desorption reactions. The adsorption process can be categorized into physical and chemical, depending on the type of bonding involved. During physical adsorption, physical binding occurs through Van der Waals forces, releasing a small amount of heat. In chemical adsorption, chemical bonds (ionic and covalent bonds) are formed between the molecules and the adsorbent, resulting in the release of a higher heat of adsorption compared to physical adsorption. The adsorption reactions are spontaneous processes with a negative Gibbs free energy value (∆G = ∆H – T × ∆S < 0, where ∆H < T × ∆S), where H is the enthalpy, T is the absolute temperature, and S is the entropy during the process. Furthermore, the adsorption reaction is exothermic, resulting in a negative enthalpy change in value (∆H < 0). When gas molecules are adsorbed onto the adsorbent, the degree of molecular freedom decreases, leading to a decrease in entropy (∆S < 0). As the adsorption capacity increases, ∆H approaches zero, and when ∆G becomes zero with the change in adsorption capacity, the adsorption and desorption reactions reach the same reaction rate and establish equilibrium54.
Le Chatelier’s principle (equilibrium law) states that when conditions such as concentration, pressure, and temperature change, the equilibrium state shifts to counteract the changes in these conditions. According to the above principle, with increasing temperature, the equilibrium of the reaction shifts towards desorption rather than adsorption, leading to a decrease in the adsorption amount. Therefore, according to Le Chatelier’s principle, as the temperature decreases, the reaction equilibrium shifts towards adsorption, increasing the adsorption amount55,56.
In addition to the thermodynamic considerations, the adsorption behavior can also be interpreted from a kinetic perspective. The pseudo-second-order kinetic model is often employed to describe chemisorption processes, where the adsorption rate is proportional to the square of the difference between the equilibrium adsorption capacity (qe) and the amount adsorbed (qt) at time t57,58. This relationship is expressed as:
where y2 is the rate constant, A is Arrhenius pre-exponential factor, Ea is activation energy, R is gas constant, and T is absolute temperature. The rate constant itself is temperature-dependent and follows the Arrhenius equation:
This implies that although higher temperatures may accelerate the adsorption rate due to increased molecular energy, they may simultaneously reduce the overall adsorption capacity due to the exothermic nature of the reaction. Thus, the observed decrease in sensor response at elevated temperatures may be attributed to this kinetic-thermodynamic trade-off relation.
Conclusions
In this study, we experimentally investigated the effect of environmental temperature conditions on the frequency shift characteristics to detect 2-CEES. The results showed that there was a decrease in the reactivity as the temperature increased; conversely, there was an increase in the reactivity as the temperature decreased. A significant frequency shift difference began to occur at temperatures below 10 °C. This indicates that the temperature conditions significantly impact the frequency shifts, emphasizing the importance of temperature experiments for analysis. Two types of analyses were conducted to determine the underlying causes of these phenomena. First, we considered the influence of the phenomenon in which the freezing point of 2-CEES was higher than the experimental temperature, leading to an increase in responsiveness owing to cooling effects. To observe the phase transition range occurring during the cooling process, a cooling test was conducted with a temperature change rate of 2 °C per min, using water as a control group. The experimental results showed that, while the phase transition process of water was observed, no phase transition process was observed in 2-CEES. Therefore, the increase in responsiveness can be attributed to temperature changes rather than an increase due to cooling effects. Second, we considered the influence of temperature changes during the adsorption/desorption reactions. According to Le Chatelier’s principle, as the temperature decreases, the reaction equilibrium shifts towards adsorption, increasing the adsorption capacity. Conversely, as the temperature increased, the equilibrium shifted towards the desorption reaction, decreasing the adsorption capacity. By utilizing this, detection technology can be developed for target gases at low concentrations. Future studies should be directed towards optimizing this technology to enhance its capabilities.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. The raw datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Davidson, C. E. et al. Detection of chemical warfare agents by colorimetric sensor arrays. ACS Sens. 5, 1102–1109 (2020).
Pan, Y. et al. Effects of temperature and humidity on the performance of a PECH polymer coated SAW sensor. RSC Adv. 10, 18099–18106 (2020).
Guidotti, M. & Trifirò, F. Chemical risk and chemical warfare agents: science and technology against humankind. Toxicol. Environ. Chem. 98, 1018–1025 (2016).
Joo, B. S., Huh, J. S. & Lee, D. D. Fabrication of polymer SAW sensor array to classify chemical warfare agents. Sens. Actuators B Chem. 121, 47–53 (2007).
Kim, J., Park, H., Kim, J., Seo, B. I. & Kim, J. H. SAW chemical array device coated with polymeric sensing materials for the detection of nerve agents. Sensors-Basel 20, 7028 (2020).
Sferopoulos, R. A Review of Chemical Warfare Agent (CWA) detector technologies and commercial-off-the-shelf items. Defense Science and Technology Organisation Technical Report (2009).
Saladi, R., Smith, E. & Persaud, A. Mustard: a potential agent of chemical warfare and terrorism. Clin. Exp. Dermatol. 31, 1–5 (2006).
Chauhan, S. et al. Chemical warfare agents. Environ. Toxicol. Pharmacol. 26, 113–122 (2008).
Fan, Y. et al. A highly selective gas sensor based on the WO3/WS2 Van der Waals heterojunction for the 2-chloroethyl ethyl sulfide (2-CEES) sensing application. J. Mater. Chem. C. 9, 17496–17503 (2021).
Wattana, M. & Bey, T. Mustard gas or sulfur mustard: an old chemical agent as a new terrorist threat. Prehosp. Disaster Med. 24, 19–29 (2009).
Pan, Y. et al. Development of a SAW Poly (epichlorohydrin) gas sensor for detection of harmful chemicals. Anal. Methods. 14, 1611–1622 (2022).
Bartelt-Hunt, S. L., Knappe, D. R. & Barlaz, M. A. A review of chemical warfare agent simulants for the study of environmental behavior. Crit. Rev. Environ. Sci. Technol. 38, 112–136 (2008).
Gros-Désormeaux, F., Caffin, F., Igert, A., Guatto, N. & Piérard, C. Is CEES a good analog of sulfur mustard? Macroscopic aspect, histology, and molecular biology comparisons between sulfur mustard and CEES-induced skin lesions. Toxicol. Lett. 361, 21–28 (2022).
Talmage, S. S., Watson, A. P., Hauschild, V., Munro, N. B. & King, J. Chemical warfare agent degradation and decontamination. Curr. Org. Chem. 11, 285–298 (2007).
Pan, Y. et al. A novel surface acoustic wave sensor array based on wireless communication network. Sensors-Basel 18, 2977 (2018).
Grabka, M., Witkiewicz, Z., Jasek, K. & Piwowarski, K. Acoustic wave sensors for detection of blister chemical warfare agents and their simulants. Sensors-Basel 22, 5607 (2022).
Long, Y. et al. The different sensitive behaviors of a hydrogen-bond acidic polymer-coated SAW sensor for chemical warfare agents and their simulants. Sensors-Basel 15, 18302–18314 (2015).
Stout, S. C., Larsen, S. C. & Grassian, V. H. Adsorption, desorption and thermal oxidation of 2-CEES on nanocrystalline zeolites. Microporous Mesoporous Mater. 100, 77–86 (2007).
Raj, V. B., Singh, H., Nimal, A., Sharma, M. & Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E-nose for the detection of chemical warfare agents. Sens. Actuators B Chem. 178, 636–647 (2013).
Lee, J. H. et al. Real-time selective detection of 2-chloroethyl Ethyl sulfide (2-CEES) using an Al-doped ZnO quantum Dot sensor coupled with a packed column for gas chromatography. Sens. Actuators B Chem. 284, 444–450 (2019).
Li, B. et al. In situ construction of hierarchical Fe2O3 nanotube arrays for real-time detection and degradation of 2-CEES gas. Sens. Actuators B Chem. 383, 133590 (2023).
Yang, L. et al. Highly sensitive and selective MEMS gas sensor based on WO3/Al2O3/Graphite for 2-Chloroethyl Ethyl sulfide (2-CEES) detection. Chemosensors 12, 5 (2024).
Yang, J. et al. High-performance metal-oxide gas sensors based on hierarchical core–shell ZnFe2O4 microspheres for detecting 2-chloroethyl ethyl sulfide. Anal. Methods. 15, 3084–3091 (2023).
Wang, Y. et al. Sensitive materials used in surface acoustic wave gas sensors for detecting Sulfur-Containing compounds. Polymers 16, 457 (2024).
Chiashi, S. et al. Growth of Horizontally Aligned Single-Walled Carbon Nanotubes on the Singular R-Plane (10–11) of Quartz. J. Phys. Chem. C. 116, 6805–6808 (2012).
Kim, E., Kim, J., Ha, S., Song, C. & Kim, J. H. Improved performance of surface acoustic wave sensors by plasma treatments for chemical warfare agents monitoring. J. Nanosci. Nanotechnol. 20, 7145–7150 (2020).
Oh, J. G., Choi, B. & Lee, S. Y. SAW based passive sensor with passive signal conditioning using MEMS A/D converter. Sens. Actuators A Phys. 141, 631–639 (2008).
Bae, B. G., Jang, H. C., Choi, H. S., Lee, Y. J. & Kim, J. H. High performance and reusable SAW sensor coated with Thiourea-Decorated POSS with different functional groups for DMMP detection. Coatings 13, 348 (2023).
Kim, J. et al. Four-Channel Monitoring System with Surface Acoustic Wave Sensors for Detection of Chemical Warfare Agents. J. Nanosci. Nanotechnol. 20, 7151–7157 (2020).
Ramakrishnan, N., Nemade, H. B. & Palathinkal, R. P. Resonant frequency characteristics of a SAW device attached to resonating micropillars. Sensors-Basel 12, 3789–3797 (2012).
Ricco, A., Martin, S. & Zipperian, T. Surface acoustic wave gas sensor based on film conductivity changes. Sens. Actuators. 8, 319–333 (1985).
Zhao, W. et al. Synthesis, structure–property and flame retardancy relationships of polyphosphonamide and its application on epoxy resins. RSC Adv. 7, 49863–49874 (2017).
Murphy, L. R., Meek, T. L., Allred, A. L. & Allen, L. C. Evaluation and Test of Pauling’s Electronegativity Scale. J. Phys. Chem. A. 104, 5867–5871 (2000).
Yang, Z. et al. Isolated Cu-N5 sites engineered polypyrrole-reduced graphene oxide hybrids for enhancing room-temperature DMMP sensing. Sens. Actuators B Chem. 385, 133671 (2023).
Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd Ed. (John Wiley and Sons, 2004).
Qi, P. et al. Flame retardant and anti-dripping surface treatment through a co-deposition of polydopamine/polyphosphonamide for fabric and foam materials. Compos. Part. B: Eng. 247, 110262 (2022).
Lama, S. et al. Synthesis and characterization of MnO2@cellulose and Polypyrrole-Decorated MnO2@cellulose for the detection of chemical warfare agent simulant. Materials 15, 7313 (2022).
Swanson, H. E. Standard X-ray Diffraction Powder Patterns. NBS Circular 539, Vol. 25 US Department of Commerce, National Bureau of Standards (1953).
Ramesh, S. et al. Nanostructurally fabrication of nickel oxide-interfaced carbon nanotubes for supercapacitors and exploration of electrochemical correlation via computer vision techniques and artificial intelligence. J. Energy Storage. 82, 110429 (2024).
Lama, S., Choi, H. S., Ramesh, S., Lee, Y. J. & Kim, J. H. Synthesis and characterization of nitrogen-doped-MWCNT@Cobalt oxide for nerve agent simulant detection. Sci. Rep. 14, 11605 (2024).
Ramesh, S. et al. Fabrication of nickel-copper sulfide nanoparticles decorated on metal-organic framework composite for supercapacitor application by hydrothermal process. J. Alloys Compd. 977, 173375 (2024).
Allred, A. L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 17, 215–221 (1961).
Haghighi, E. & Zeinali, S. Nanoporous MIL-101 (Cr) as a sensing layer coated on a quartz crystal microbalance (QCM) nanosensor to detect volatile organic compounds (VOCs). RSC Adv. 9, 24460–24470 (2019).
Lama, S. et al. Nano-sheet-like morphology of nitrogen-doped graphene-oxide-grafted manganese oxide and polypyrrole composite for chemical warfare agent simulant detection. Nanomaterials 12, 2965 (2022).
Wu, T. T., Chen, Y. Y. & Chou, T. H. A high sensitivity nanomaterial based SAW humidity sensor. J. Phys. D. 41, 085101 (2008).
Angelopoulos, M., Ray, A., Macdiarmid, A. G. & Epstein, A. J. Polyaniline: processability from aqueous solutions and effect of water vapor on conductivity. Synth. Met. 21, 21–30 (1987).
International Conference on Harmonisation. ICH Harmonised Tripartite Guideline: Validation of analytical procedures: text and methodology. Q2(R1) (2005).
Taleuzzaman, M. Limit of blank (LOB), limit of detection (LOD), and limit of quantification (LOQ). Org. Med. Chem. Int. J. 7, 127–131 (2018).
Rabus, D. et al. Degradation of Sub-Micrometer Sensitive Polymer Layers of Acoustic Sensors Exposed to Chlorpyrifos Water-Solution. Sensors-Basel 22, 1203 (2022).
Kajmakovic, A., Diwold, K., Römer, K. Pestana, J. & Kajtazovic, N. Degradation detection in a redundant sensor architecture. Sensors-Basel 22, 4649 (2022).
Angell, C. A. Supercooled water. In Water and Aqueous Solutions at Subzero Temperatures(ed. Franks, F.) 1–81 (Springer US, 1982).
Bigg, E. K. The supercooling of water. Proc. Phys. Soc. B 66, 688 (1953).
Dąbrowski, A. Adsorption—from theory to practice. Adv. Colloid Interface Sci. 93, 135–224 (2001).
Ihde, J. Le Châtelier and chemical equilibrium. J. Chem. Educ. 66, 238 (1989).
Fahim, F., Mainuddin, M., Mittal, U., Kumar, J. & Nimal, A. Novel SAW CWA detector using temperature programmed desorption. IEEE Sens. J. 21, 5914–5922 (2020).
McKinley, J. Permeation tubes: A simple path to very complex gas mixtures. Gases Instrumentation Jan/Feb 22–26 (2008).
Bullen, J. C., Saleesongsom, S., Gallagher, K. & Weiss, D. J. A revised pseudo-second-order kinetic model for adsorption, sensitive to changes in adsorbate and adsorbent concentrations. Langmuir 37, 3189–3201 (2021).
Revellame, E. D., Fortela, D. L., Sharp, W., Hernandez, R. & Zappi, M. E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 1, 100032 (2020).
Acknowledgements
This work was supported by Korea Institute of Energy Technology Evaluation and Planning(KETEP) grant funded by the Korea government(MOTIE) (20222020800130, Development and demonstration of hybrid power system using ORC(Organic Rankine Cycle) and TED(Thermoelectric Generator) for low and medium temperature industrial waste heat recovery) and also supported by space funding from Korea government KASA (Korea AeroSpace Administration) under Grant (NRF-2022M1A3C2076483, Space HR&D Center).
Author information
Authors and Affiliations
Contributions
Conceptualization and Approaches: [Joo-Hyung Kim]; Methodology: [Young-Jun Lee]; Formal analysis and investigation: [Hee-Chan Jang]; Writing - original draft preparation: [Hee-Chan Jang]; Writing - review and editing: [Sanjeeb lama, Young-Jun Lee, Joo-Hyung Kim]; Funding acquisition: [Joo-Hyung Kim]; Supervision: [Joo-Hyung Kim]; Data curation: [Sanjeeb lama]; Material synthesis: [Jaeyoung Heo, Changsik Song]; Material design: [Changsik Song]; Visualization: [Young-Jun Lee, Jun-Soo Lee]. All authors have read and agreed to the published version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jang, HC., Lama, S., Heo, J. et al. An experimental study on the detection mechanism of 2-CEES using SAW sensors under various temperature conditions. Sci Rep 15, 28329 (2025). https://doi.org/10.1038/s41598-025-12329-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12329-4