Abstract
This study aims to explore the brain network connectivity patterns in elderly individuals with cognitive impairment at varying levels of inhibitory function and to identify key brain connectivity features that influence inhibitory function. The study analyzed data from 120 elderly individuals with cognitive impairment, including the Montreal Cognitive Assessment ( MoCA ), muscle strength, Stroop task performance, and 5-minute resting-state EEG signals. Pearson correlation and analysis of variance were used to identify significant targets. BrainNet Viewer was utilized to create visualizations of EEG-based brain networks to identify connectivity features. (1) Stroop task accuracy under congruent/incongruent conditions showed a significant positive correlation with MoCA scores (r = 0.599, p < 0.01; r = 0.474, p < 0.01), while reaction times under these conditions exhibited a significant negative correlation with MoCA scores (r=-0.475, p < 0.01; r=-0.354, p < 0.01). (2) Significant differences were observed among the four groups of elderly individuals with cognitive impairment in grip strength, 30-second sit-to-stand, and SPPB performance (P < 0.05). In EEG metrics, significant differences were identified among the four groups in Fp1θ, Fp1α1, Fp1α2, Fp2α1, Fp2α2, F3α1, F4α1, F4α2, C3α1, C4α1, C4α2, P3α1, P4α1, P4α2, O1α1, O2α1, O2α2, F7α1, F8α1, F8α2, T4α1, T4α2, T5α1, T6α1, T6α2 (P < 0.05). (3) Higher accuracy in inhibitory function was mainly associated with stronger and denser connectivity in the prefrontal and parietal regions, whereas faster reaction times were linked to the central and occipital regions. The observed balance in connectivity between the left and right hemispheres was associated with differences in inhibitory function and task execution efficiency in elderly individuals with cognitive impairment. Training in physical function and muscle strength may enhance EEG activity in individuals with lower levels of inhibitory function, thereby improving their cognitive abilities.
Similar content being viewed by others
Introduction
The aging population is a pressing global issue, with the incidence of neurodegenerative diseases among the elderly increasing as they age. Statistics indicate that in China, around 6.0% of individuals aged 60 and above suffer from dementia, 3.9% from Alzheimer’s disease, 1.6% from vascular dementia, and 0.5% from other types of dementia. The overall prevalence of mild cognitive impairment stands at approximately 15.5%, which translates to 38.77 million patients in China1. Cognitive impairment, which is closely associated with aging, has become a significant public health concern at various governmental levels. It refers to the varying degrees of cognitive function damage caused by multiple factors, and its incidence rises with age, reaching up to 33.59%2. Emerging research has highlighted the crucial role of physical health in influencing cognitive function. Regular physical activity and muscle strength have been linked to better cognitive performance, particularly in tasks involving inhibitory control and executive functions. Studies suggest that maintaining physical health can support cognitive function by improving cerebral blood flow and enhancing the release of neurotransmitters such as dopamine, which are vital for cognitive processes3. Therefore, improving physical fitness may help delay the onset of cognitive decline, offering an additional strategy for healthy aging.As cognitive decline progresses with age, the extreme outcome of continuous deterioration—dementia—has emerged as the fourth leading cause of health problems among the elderly, following cardiovascular, cerebrovascular diseases, and tumors4.
Inhibitory function is considered one of the cognitive functions most severely affected by aging5. Hasher et al. proposed the Inhibitory Deficit Theory, suggesting that the decline in inhibitory function is a major contributor to cognitive decline in the elderly. A decline in inhibitory function allows more irrelevant information to enter working memory, affecting the processing of current information. Inhibitory function development involves increased brain activation that directly affects behavioral responses6. The neural structures involved include the dorsolateral prefrontal cortex, frontal eye fields (FEF), anterior cingulate cortex (ACC), and basal ganglia (BG), forming the fronto-striatal circuitry, along with the cerebellum. Changes in these neural structures also impact cognitive function. In this study, the Stroop task, which essentially reflects the conflict effect, was used to measure inhibitory function7. Recent theories have suggested that the decline in inhibitory function may be compensated by the brain’s ability to recruit additional neural circuits. The CRUNCH hypothesis8 proposes that as cognitive functions decline, the brain compensates by engaging more brain regions, particularly those involved in executive function, to maintain performance. This compensation is often more pronounced in individuals who maintain better physical health, as their neural networks remain more efficient and flexible, enabling them to better adapt to cognitive demands. Physical interventions such as exercise or physical training may enhance prefrontal connectivity and improve overall cognitive performance by boosting neural integration in the brain’s executive control regions9.When faced with stimuli where color and word meaning are inconsistent, reaction times are prolonged, and error rates increase, indicating that more cognitive resources are required to process conflicting stimuli10.
Currently, the diagnosis of cognitive impairment and dementia primarily involves psychological assessments, blood tests, cerebrospinal fluid tests, neurological examinations, and magnetic resonance imaging. Due to its non-invasive nature, low cost, portability, and high temporal resolution, electroencephalography (EEG) has become an effective screening tool. The EEG signals of elderly individuals with declining inhibitory function exhibit certain specificities. Studies have shown that lower frequency bands (such as delta and theta) in the EEG of the elderly are closely related to the decline in inhibitory function11. This decline may lead to increased cortical instability, resulting in more delta and theta bands during wakefulness12. Other studies have found that resting-state delta and alpha1 power values are related to inhibitory function tasks, with an increase in delta bands corresponding to a decrease in task accuracy, while an increase in alpha1 bands corresponds to shorter task reaction times13,14.
With the rapid development of brain science, research on brain network connectivity provides profound insights into the structure, function, and pathological mechanisms of the brain15. Functional brain networks have become powerful tools for studying brain connectivity and have been widely applied in neurodegenerative disease research16. These networks typically describe dynamic interaction patterns between brain regions based on time series data from techniques such as EEG and functional magnetic resonance imaging (fMRI). Due to its high temporal resolution and lower cost, EEG is widely used in neurological disease research17. Studies have shown that the functional connectivity strength of Alzheimer’s disease patients is significantly reduced, especially in low-frequency bands like theta and alpha18. Weakened functional connectivity between the frontal lobe and other brain regions in these patients may affect their executive function and cognitive control abilities. Inhibitory function relies on the highly connected prefrontal cortex regions, particularly the activity in theta and alpha bands, which are closely related to inhibitory control. Changes in these bands may reveal the neural mechanisms underlying cognitive decline19.
Based on existing research, the decline in inhibitory function is closely related to abnormal brain network connectivity, especially the functional connectivity impairment in the prefrontal cortex, which is considered an important mechanism of cognitive impairment. The brain network connectivity patterns may differ among individuals with varying levels of inhibitory function. While some studies have explored the relationship between brain network connectivity and inhibitory function, the specific differences in brain connectivity at different levels of inhibitory function remain unclear20. This study aims to further investigate the brain network connectivity patterns in individuals with different levels of inhibitory function, focusing on the differences in accuracy and reaction times across different brain regions. The goal is to identify brain network connectivity characteristics that influence inhibitory function, providing evidence and clinical value for healthy aging.
Participants and methods
Participants
This study complies with the latest version of the Helsinki Declaration’s ethical requirements and has been reviewed by the Ethics Committee of Shanghai University of Sport (ethics registration number 102772020RT059). Utilizing health lectures and recruitment posters in four elderly service centers in Shanghai, a convenience sampling method was used to recruit 247 elderly individuals aged 70 and above. Eligibility was preliminarily assessed based on inclusion and exclusion criteria. Participants were informed of the study’s purpose and content, and they signed informed consent forms, indicating their voluntary participation. Recruitment and screening took place from March 2023 to July 2023. Using the Montreal Cognitive Assessment, researchers screened the elderly participants and identified 120 individuals with cognitive impairment, with a detection rate of 48.58%. These participants constituted the final sample used in this study. The inclusion criterion of age 70 and above was chosen to reflect the increased risk of cognitive decline and inhibitory dysfunction observed in this age group.
Inclusion criteria: ① Elderly individuals aged 70 and above; ② Right-handed; ③ In good physical condition; ④ No severe cardiovascular or cerebrovascular diseases; ⑤ Normal vision and hearing; ⑥ Normal mental state, capable of verbal communication, and able to cooperate with the investigation; ⑦ Willing to sign the informed consent form.Exclusion criteria: ① Severe cardiovascular diseases or major organic diseases; ② Severe muscular diseases preventing prolonged standing or standing at all; ③ Presence of exercise contraindications; ④ Poor vision or hearing, preventing completion of tests; ⑤ Long-term or recent use of medications that could potentially affect the study results, specifically psychotropic drugs, drugs affecting physical activity levels, or cholinesterase inhibitors. This exclusion criterion was applied to minimize any confounding effects of these medications on EEG and cognitive measurements. The recruitment process is shown in Fig. 1.
Testing procedure
The testing sessions for this study were conducted daily between 13:30 and 16:30. Subjects were required to visit the laboratory on two occasions. During the first visit, the testing procedures were explained, subjects signed the informed consent form, and completed the basic information questionnaire as well as the Montreal Cognitive Assessment (MoCA). Subsequently, their height, weight, and muscle strength were assessed. The second visit involved EEG data collection and Stroop task. The testing procedure is shown in Fig. 2. Subjects were advised to refrain from strenuous exercise and avoid consuming caffeine or alcohol for 24 h prior to the test.
Testing tools
Basic information questionnaire
The basic information questionnaire collected data on name, age, gender, height, weight, marital status, previous occupation, education level, smoking status, drinking status, dietary habits, hearing condition, vision condition, sleep quality, presence of exercise contraindications, medical history, and medication use.
Montreal cognitive assessment (MoCA)
The MoCA scale is widely used for assessing cognitive function in the elderly. It covers eight domains: visuospatial/executive function, naming, memory, attention, language fluency, abstract thinking, delayed recall, and orientation. The total score is 30, with higher scores indicating better cognitive function. A score of 26 or higher is considered normal, 18–26 indicates mild cognitive impairment, 10–17 indicates moderate impairment, and below 10 indicates severe impairment. To account for the effect of education level, individuals with ≤ 12 years of education receive an additional point, but the total score does not exceed 30. The test-retest reliability of the MoCA scale used in this study is 0.85721.
Height, weight, and muscle strength measurement
Height was measured using a stadiometer. The subject stood barefoot with their back against the stadiometer column, torso straight, head upright, and eyes looking forward. The measurement was recorded in centimeters, accurate to one decimal place. Weight was measured using a scale. The subject stood barefoot naturally in the center of the scale, maintaining body stability. The measurement was recorded in kilograms, accurate to one decimal place. Both height and weight were measured twice, with the average values used. Body Mass Index (BMI) was calculated by dividing weight (kg) by the square of height (m), using the formula BMI = weight (kg)/height² (m).
Stroop task
The Stroop task paradigm was used to test inhibitory function. The task was divided into two blocks: each block began with a practice mode followed by a formal experiment module. The practice mode consisted of 20 trials. Feedback on correctness and reaction time was provided after each trial. The formal experiment began once the accuracy of the 20 practice trials reached 75%; otherwise, the practice module continued, but no more than three times. After the practice mode, subjects rested for 30 s before starting the formal module. The stimuli consisted of Chinese characters “红” (red), “黄” (yellow), “蓝” (blue), and “绿” (green), displayed in red, yellow, blue, and green colors. There were 16 types of stimuli: 4 with congruent color words and 12 with incongruent color words, all randomized. After a fixation point “+”, a colored word appeared in the center of the screen. The formal experiment had one block. In the congruent condition (block 1), subjects judged the color of the word. If the color was red, they pressed “D”; yellow, “F”; blue, “J”; green, “K”. There were 24 trials, presented randomly. In the incongruent condition, subjects also judged the color of the word, pressing the same keys accordingly. Each stimulus was presented for 1500ms, with a total of 48 trials.
EEG signal collection and processing
The testing environment was a soundproof, ventilated room with appropriate lighting, temperature, and humidity, free from electromagnetic interference. Subjects washed and dried their hair before the test and sat comfortably. They were instructed to close their eyes, relax, remain quiet and awake, and avoid blinking, clenching, swallowing, or moving. EEG data was recorded using an NCERP-190,012 EEG machine from Shanghai Nuocheng Electrical Co., Ltd. Sixteen electrodes were placed according to the international 10/20 system: Fp1 (left frontal), Fp2 (right frontal), F3 (left frontal), F4 (right frontal), C3 (left central), C4 (right central), P3 (left parietal), P4 (right parietal), O1 (left occipital), O2 (right occipital), F7 (left frontal), F8 (right frontal), T3 (left temporal), T4 (right temporal), T5 (left posterior temporal), and T6 (right posterior temporal), with the ground electrode on Fz and reference electrodes on both mastoids (A1 and A2). The device, equipped with a preamplifier, had a sampling rate of 500 Hz, high-pass filter of 0.3 Hz, low-pass filter of 30 Hz, and electrode-skin impedance below 5 kΩ. EEG waves were divided into six frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha1 (8–10.5 Hz), alpha2 (10.5–13 Hz), beta1 (13–20 Hz), and beta2 (20–30 Hz). Five minutes of resting-state EEG data were collected, and the first 30 s and any obvious artifacts were discarded. Data were subjected to fast Fourier transform to obtain power values.
Brain functional connectivity
Brain connectivity shows significant potential in assessing brain function in patients with various neurological disorders and tracking cognitive and emotional brain states in healthy participants. Widely distributed complex brain networks generate diverse topological signal processing and communication patterns under different conditions. BrainNet Viewer is a MATLAB-based brain network visualization toolbox22,23. It uses a graphical user interface (GUI) for easy data import and processing, including brain surface, node, and edge data, to generate 3D neuroimaging visualizations. The graphics can be exported as commonly used image files or presentation videos, allowing for multi-level observation and analysis of brain connectivity data. Heatmaps use adjacency matrices to represent quantitative brain connectivity, effectively displaying overall relationships between available EEG channel pairs. By observing brain activity patterns in a resting state, functional connectivity mainly analyzes connectivity strength and density. Using cut-off thresholds from 0.1 to 0.9, brain network visualization maps were constructed and analyzed individually for connectivity strength and density. “No connection” denotes missing functional connectivity; connections are analyzed by “color” representing functional connectivity and “thickness” representing the proportion of different connection strengths at the same cut-off threshold. (Fig. 3)
Statistical analysis
All data processing and chart creation in this study were performed using MATLAB 2020a and toolboxes such as EEGLAB and BrainNet Viewer. Model computation and validation were conducted using SPSS 29.0 and R 4.2.1. For approximately normally distributed metric data, the mean ± standard deviation (x ± SD) was used for description, and analysis of variance (ANOVA) was used for group comparisons. For skewed distribution metric data, the median (interquartile range) [M (P25, P75)] was used for description, and the Mann-Whitney U non-parametric test was used for group comparisons. Categorical data were described using n (%), and group comparisons were performed using the χ2 test. Pearson correlation analysis was used to explore the relationships between various indicators. To reduce the risk of Type I error caused by multiple testing, Bonferroni correction was applied to the correlation analyses between Stroop test measurements and MoCA scores (adjusted significance threshold: p < 0.0125).Prior to statistical analysis, EEG data were standardized. All statistical inferences were conducted using two-tailed tests, with significance levels set at P < 0.05, P < 0.01, and P < 0.001 indicating statistical significance.
Stroop grouping justification
In this study, the grouping of participants based on inhibitory function was conducted using Stroop test performance metrics, specifically the average accuracy and reaction time under both congruent and incongruent conditions. Given the continuous nature of the Stroop measurements, participants were divided into four distinct groups using the median values of average accuracy and reaction time as cut-off thresholds. This approach allows for the categorization of individuals into: (1) High Accuracy & Fast Reaction, (2) High Accuracy & Slow Reaction, (3) Low Accuracy & Fast Reaction, and (4) Low Accuracy & Slow Reaction.
Results
Differences in basic information among elderly with different levels of Stroop test measurements
A total of 107 elderly participants (Table 1) were divided into four groups based on their Stroop test measurements (reaction time and accuracy): high-accuracy/fast group (n = 40, high accuracy and fast reaction time), high-accuracy/slow group (n = 19, high accuracy but slow reaction time), low-accuracy/fast group (n = 14, low accuracy but fast reaction time), and low-accuracy/slow group (n = 34, low accuracy and slow reaction time). The table reveals the differences in various basic information variables among the groups, highlighting the relationship between Stroop test measurements levels and individual characteristics.
Significant differences were observed in age among the different Stroop test measurements groups (F = 5.971, P < 0.001), with the low-accuracy/slow group being the oldest, suggesting that increased age may be associated with poorer Stroop test measurements. Gender differences were also statistically significant (P = 0.01), with a notable variation in the proportion of males across the groups. Education level was significantly associated with Stroop test measurements (P = 0.004), with individuals having higher Stroop test measurements generally possessing higher education levels, indicating that lower education levels may be linked to reduced Stroop test measurements.
Overall, the differences in Stroop test measurements showed significant associations with several demographic variables, particularly age, gender, and education level. These findings contribute to understanding the variations in Stroop test measurements across different backgrounds.
Correlation between Stroop test measurements and MoCA scores
To further investigate the relationship between Stroop test performance and cognitive function (as measured by MoCA scores), correlation analyses were conducted. Considering that age, gender, and educational level may confound the association between Stroop test outcomes and cognitive performance, we included these three variables as covariates. Partial correlation analysis was performed to control for the potential effects of these confounding factors and to better reflect the independent association between Stroop performance and MoCA scores.The relationship between Stroop test measurements and MoCA scores was examined using Pearson correlation coefficients (Table 2). There was a significant correlation between Stroop test measurements and MoCA scores. Specifically, consistent accuracy was significantly positively correlated with MoCA scores (r = 0.506, p < 0.001), while consistent reaction time was significantly negatively correlated with MoCA scores (r = −0.332, p < 0.001). Similarly, inconsistent accuracy was positively correlated with MoCA scores (r = 0.385, p < 0.001). However, the correlation between inconsistent reaction time and MoCA scores did not reach statistical significance (r = −0.184, p > 0.05).
These results suggest that higher accuracy and shorter reaction times are associated with better cognitive performance. This indicates that Stroop test measurements is an essential component of MoCA scores, and improvements in Stroop test measurements can positively impact cognitive abilities.
Differences in physical function, upper and lower limb strength, and EEG indicators among elderly with cognitive impairment and different levels of Stroop test measurements
To further explore the differences in physical function, upper and lower limb strength, and EEG indicators among individuals with varying levels of Stroop test measurements, cognitively impaired elderly participants were divided into four groups: HF (high accuracy, fast reaction time), HS (high accuracy, slow reaction time), LF (low accuracy, fast reaction time), and LS (low accuracy, slow reaction time). The results revealed significant differences among the four groups in grip strength, 30-second chair stand test, and SPPB (Short Physical Performance Battery) scores (Table 3). The HF group outperformed the LS group significantly in grip strength, the 30-second chair stand test, and SPPB.
Regarding EEG indicators (Table 4), there were significant differences among the four groups in Fp1θ, Fp1α1, Fp1α2, Fp2α1, Fp2α2, F3α1, F4α1, F4α2, C3α1, C4α1, C4α2, P3α1, P4α1, P4α2, O1α1, O2α1, O2α2, F7α1, F8α1, F8α2, T4α1, T4α2, T5α1, T6α1, and T6α2. These findings indicate that individuals with high levels of Stroop test measurements (HF and HS groups) exhibit better physical function and muscle strength, as well as higher θ and α frequency components in their EEG activity. Conversely, those with lower levels of Stroop test measurements (LF and LS groups) performed worse in these areas, particularly the LS group, which demonstrated significantly lower physical function and muscle strength, along with reduced θ and α frequency components in their EEG activity.
These results suggest that physical function and muscle strength training may help improve EEG activity in individuals with lower levels of Stroop test measurements, thereby potentially enhancing their MoCA scores.
Differences in brain connectivity among cognitively impaired elderly with different levels of Stroop test measurements
Differences in brain connectivity in the alpha 1 frequency band at a correlation coefficient of 0.5
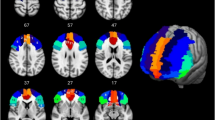
This study examined brain connectivity maps of the four groups of elderly participants at correlation coefficients of 0.5, 0.7, and 0.9. By comparing brain connectivity under different correlation coefficients and frequency bands (alpha1 and alpha2), we analyzed the differences among the high-accuracy/fast group, high-accuracy/slow group, low-accuracy/fast group, and low-accuracy/slow group. The results (Fig. 4) revealed significant differences in brain connectivity in the alpha1 frequency band at a 0.5 correlation coefficient among the high-accuracy/fast, high-accuracy/slow, and low-accuracy/slow groups. Notably, the low-accuracy/fast group had no data at this correlation coefficient.
The findings showed that the high-accuracy/fast group had superior connection strength (thicker lines) and connection density (more lines) compared to the high-accuracy/slow and low-accuracy/slow groups. Specifically, the high-accuracy/fast group demonstrated strong alpha1 connectivity across multiple brain regions, with weaker connections between the right temporal lobe and other brain regions, and no connections on the left side. The high-accuracy/slow group exhibited slightly weaker connection strength and density in the prefrontal region compared to other brain regions, with significantly lower connection density in the left hemisphere compared to the right. The low-accuracy/slow group had lower connection strength and density in both hemispheres compared to the high-accuracy/fast group. Compared to the high-accuracy/slow group, the low-accuracy/slow group had weaker connection strength and density in the right hemisphere but higher connection strength and density in the left hemisphere.
These results suggest that the accuracy of Stroop test measurements is primarily associated with the connection strength and density in the prefrontal and parietal regions of the brain, while the speed of reaction time is related to the central and occipital regions.
Differences in brain connectivity in the alpha 2 frequency band at a correlation coefficient of 0.5
The results (Fig. 5) indicated significant differences in brain connectivity in the alpha2 frequency band at a correlation coefficient of 0.5 between the high-accuracy/fast group and the low-accuracy/slow group. The high-accuracy/fast group exhibited stronger and denser connections compared to the low-accuracy/slow group. Specifically, the high-accuracy/fast group showed notable connectivity in the central, parietal, and occipital regions, with dense and robust connections in these areas. In contrast, the low-accuracy/slow group displayed weaker brain connectivity, particularly in the prefrontal and occipital regions, where the connection strength was substantially lower than that of the high-accuracy/fast group. The low-accuracy/slow group’s brain connectivity was primarily concentrated in a few specific regions, overall exhibiting weaker connection strength.
These findings suggest that elderly individuals with high accuracy and fast reaction times in the alpha2 frequency band have significantly stronger brain connectivity compared to those with low accuracy and slow reaction times, especially in the connections between the prefrontal cortex and other brain regions. This underscores the prefrontal cortex as a critical control region for Stroop test measurements.
Differences in brain connectivity in the alpha 1 frequency band at a correlation coefficient of 0.7
The results(Fig. 6) indicated significant differences in brain connectivity in the alpha1 frequency band at a correlation coefficient of 0.7 among the four groups. The high-accuracy/fast group exhibited significantly greater connection strength and density compared to the high-accuracy/slow and low-accuracy/slow groups, particularly in the connections between the prefrontal cortex and other brain regions. However, the low-accuracy/fast group showed even higher connection strength and density in certain areas, such as the right temporal lobe, compared to the high-accuracy/fast group.
The high-accuracy/slow group displayed weaker connectivity in the prefrontal cortex but stronger connections in the central, parietal, and occipital regions. Additionally, their left hemisphere connectivity was noticeably lower compared to the right hemisphere. The low-accuracy/fast group demonstrated overall strong connection strength and density, especially in the connections between the right temporal lobe and other regions. Although the low-accuracy/slow group had slightly higher connection strength in the prefrontal cortex compared to the high-accuracy/slow group, its overall connection strength and density remained inferior to both the high-accuracy/fast and low-accuracy/fast groups.
These findings suggest that the strength of prefrontal cortex connections is highly correlated with levels of Stroop test measurements, particularly in the high-accuracy/fast group, where robust prefrontal connections likely play a crucial role in efficient task execution. While the low-accuracy/fast group exhibits good overall performance, especially in the right temporal lobe, its advantage in Stroop test measurements may rely more on the collaborative efforts of various brain regions rather than solely on prefrontal cortex function.
The high-accuracy/slow group exhibited an uneven distribution of connectivity between the left and right hemispheres, with weaker connections on the left and stronger connections on the right, which might affect the balance and efficiency of task execution. Despite the low-accuracy/slow group having slightly stronger prefrontal connections compared to the high-accuracy/slow group, their overall deficiency in connection strength and density likely contributes to poorer performance in Stroop test measurements tasks.
These results underscore the importance of the strength of prefrontal cortex connections and the balance of connectivity between the left and right hemispheres for optimal Stroop test measurements and task execution efficiency.
Differences in brain connectivity in the alpha 2 frequency band at a correlation coefficient of 0.7
The results (Fig. 7) indicated notable differences in brain connectivity in the alpha2 frequency band at a correlation coefficient of 0.7 among the three groups. The high-accuracy/fast group exhibited stronger and denser connections compared to the low-accuracy/slow group, especially in the prefrontal cortex, central, and parietal regions. However, the low-accuracy/fast group showed even higher connection strength and density in certain areas, such as the right temporal lobe, demonstrating its strong regional connectivity advantage.
The low-accuracy/slow group displayed the weakest overall connection strength and density, particularly in the prefrontal and occipital regions, indicating poorer coordination among brain regions during task execution. These findings suggest that while comparing the high-accuracy/fast and low-accuracy/fast groups, the impact of reaction time on brain connectivity is relatively minor. Instead, overall Stroop test measurements performance is likely influenced by the connectivity between the prefrontal cortex and other brain regions.
Differences in brain connectivity in the alpha 1 frequency band at a correlation coefficient of 0.9
The results (Fig. 8) indicated a notable decrease in brain connectivity strength and density across all four groups. At a correlation coefficient of 0.9 in the alpha1 frequency band, the high-accuracy/fast group exhibited significantly higher connection strength and density compared to the high-accuracy/slow and low-accuracy/slow groups, yet lower than the low-accuracy/fast group. Except for the low-accuracy/fast group, the other three groups displayed partial deficits in connection strength and density in the prefrontal cortex.
The low-accuracy/fast group showed smaller connection strength between the prefrontal cortex and other regions, but it had the highest overall connection strength and density among the four groups. These findings suggest that as the correlation coefficient increased from 0.3 to 0.7 and then to 0.9, the differences in connectivity among the groups became more pronounced. Particularly, at the 0.7 threshold, the low-accuracy/fast group began to surpass the high-accuracy/fast group in connection strength and density, a trend that became even more evident at a correlation coefficient of 0.9.
The low-accuracy/fast group demonstrated the strongest connection density and extensive connection strength in non-prefrontal regions, indicating that individuals in this group might rely on the collaborative efforts of multiple brain regions during task execution, rather than solely depending on the prefrontal cortex.
Differences in brain connectivity in the alpha 2 frequency band at a correlation coefficient of 0.9
The results (Fig. 9) demonstrated a significant decrease in brain connectivity strength and density within the alpha2 frequency band at a correlation coefficient of 0.9 for the high-accuracy/fast group. In contrast, the high-accuracy/slow group showed the strongest connectivity at this coefficient. Except for the high-accuracy/slow group, the other three groups exhibited partial deficits in connectivity between the prefrontal cortex and other regions.
For the high-accuracy/fast group, connectivity was primarily concentrated in the central, parietal, and occipital regions, though the strength was relatively weak. The high-accuracy/slow group maintained connections between the prefrontal cortex and other regions, albeit with slightly weaker strength, and had stronger connectivity in the central, parietal, and occipital regions as well.
The connectivity patterns of the low-accuracy/fast and low-accuracy/slow groups were similar to those of the high-accuracy/fast group, with the low-accuracy/fast group showing higher connection strength compared to both the high-accuracy/fast and low-accuracy/slow groups.
Discussion
The results of this study indicate that cognitive impairment in elderly individuals is characterized by an age-related decline in inhibitory functions. There are significant differences in brain connectivity strength and density among individuals with varying levels of inhibitory function. Additionally, higher muscle strength is associated with better cognitive and inhibitory function performance, which aligns with previous findings24,25,26. West27 suggested that the decline in inhibitory functions is a direct result of frontal cortex integrity being compromised, rendering it unable to prevent irrelevant information from entering the brain. This leads to a diminished ability in elderly individuals to inhibit irrelevant responses. Hasher and colleagues proposed the inhibitory deficit hypothesis28, which posits that the decline in cognitive functions in the elderly is largely due to an inability to effectively suppress irrelevant information29,30,31.Hausman et al.32 further demonstrated that cingulo-opercular and frontoparietal network connectivity are critical for executive functioning, reinforcing the idea that compromised connectivity in these networks could exacerbate inhibitory deficits in the elderly.
Our study found that there is a degree of plasticity in the aging process of inhibitory functions in the elderly33. There is a close relationship between muscle strength and inhibitory function, possibly because individuals with higher muscle strength have better cerebral blood flow and oxygen supply, leading to higher cortical activity34. Moreover, muscle strength is linked to the release of neurotransmitters such as dopamine and norepinephrine, which can influence brain neural activity patterns, increase the number of central neurons in specific brain regions, and alter EEG indicators related to inhibitory function in those areas35,36. Friedman and Robbins3 emphasized that prefrontal cortex activity is modulated by neurotransmitters such as dopamine, which are enhanced through physical fitness. This provides a plausible mechanistic pathway linking muscle strength with improved inhibitory function.
The study identified significant differences in brain connectivity strength and density among individuals with different levels of inhibitory function, particularly in the prefrontal cortex. Individuals with high levels of inhibitory function (such as those in the high-accuracy/fast group) exhibited higher connectivity strength and density between the prefrontal cortex and other brain regions in the alpha1 and alpha2 frequency bands, especially at correlation coefficients of 0.7 and 0.9. This connectivity advantage was more pronounced in these individuals37,38. Conversely, individuals in the low-accuracy/slow group displayed lower prefrontal connectivity strength and density, indicating reduced collaborative ability of the prefrontal cortex during cognitive tasks. Additionally, the low-accuracy/fast group showed increased connectivity strength and density in non-prefrontal regions compared to the high-accuracy/fast group, suggesting that they might compensate for prefrontal deficiencies through the collaborative efforts of other brain regions37. The functional significance of these differences can be explained in terms of neural efficiency and network integration. Stronger connectivity at the 0.7 and 0.9 thresholds may suggest more efficient processing within specific brain networks, particularly those involved in executive functions such as inhibitory control and working memory. In contrast, the lower threshold (0.5) may highlight a broader but less efficient network, possibly indicating the involvement of compensatory mechanisms or less focused cognitive resources. These findings align with the neural plasticity hypothesis, which suggests that the brain can reconfigure its network to compensate for cognitive decline, as seen in the low-accuracy/fast group39.
Recent evidence has highlighted the crucial role of theta-band oscillations (4–8 Hz) in organizing prefrontal connectivity, particularly during tasks requiring inhibitory control and conflict adaptation. For example, a recent study by Martínez-Molina et al.40 demonstrated that theta activity in the lateral prefrontal cortex not only correlates with, but also causally contributes to, conflict expectation and adaptive cognitive control. In their TMS-EEG experiments, theta stimulation over the superior frontal gyrus (SFG) increased the behavioral effect of conflict expectation and enhanced theta oscillatory power in lateral frontal regions. Moreover, their cross-frequency coupling analysis revealed that theta oscillations modulate alpha/beta amplitudes, possibly facilitating top-down regulation from prefrontal to motor and premotor regions. These findings align with our observations that elderly individuals with stronger cognitive performance exhibited more efficient prefrontal connectivity. However, by primarily focusing on alpha-band differences in our study, we may have overlooked important theta-related dynamics that also support cognitive control mechanisms. Future research should explore the combined roles of theta and alpha oscillations, including theta-alpha or theta-gamma coupling, to better understand the neural mechanisms underlying inhibitory function decline in aging populations.
The emergence of different connectivity patterns at varying thresholds could reflect the functional specialization of brain regions. For example, higher thresholds might represent the synchronization of functionally related areas, while lower thresholds could reflect a more distributed network with less selective neural interactions. These differences in connectivity strength might therefore provide insights into how the brain adapts to cognitive demands and how it utilizes different brain networks to support task performance.This observation aligns with the compensation-related utilization of neural circuits hypothesis (CRUNCH) proposed by Kang et al.8, suggesting that the low-accuracy/fast group compensates for prefrontal deficiencies by recruiting alternative neural strategies.These findings imply that efficient collaboration between the prefrontal cortex and other brain regions is crucial for superior inhibitory function performance41,42.
Existing research has shown that alpha frequency band activity is closely related to inhibitory control, and that alpha oscillations in the prefrontal cortex may regulate information processing during inhibitory tasks. High-functioning individuals enhance task execution efficiency through increased prefrontal connectivity, whereas low-functioning individuals might rely on other brain regions due to insufficient prefrontal connectivity. Doval et al.43found that alterations in alpha band connectivity might reflect compensatory mechanisms rather than mere deficits, supporting the idea that these oscillations facilitate adaptive neural strategies in response to cognitive decline.The integrative function of the prefrontal cortex is vital for performing complex cognitive tasks, as it helps allocate attention resources more effectively through collaboration with other brain regions during inhibition processes44,45.
This study also revealed that cognitive-impaired elderly individuals with varying levels of inhibitory function exhibit significant differences in brain connectivity. Although previous research has demonstrated a close relationship between inhibitory function and brain connectivity, particularly in the prefrontal cortex3,17,46, this study further identified changes in brain connectivity across different frequency bands and under varying correlation thresholds (e.g., r = 0.5, 0.7, 0.9), offering a more nuanced understanding of network characteristics across Stroop performance levels. At the alpha1 frequency band and a correlation coefficient of 0.7, the high-accuracy/fast group showed significantly higher connectivity strength and density between the prefrontal cortex and other brain regions compared to other groups. The low-accuracy/fast group exhibited stronger connectivity in non-prefrontal regions, which may reflect the need for collaborative efforts across multiple brain regions for efficient task execution, not solely relying on the prefrontal cortex8. Neuroimaging studies suggest that the strength of prefrontal connectivity and the balance between hemispheric connections play crucial roles in inhibitory tasks. However, elderly individuals with different levels of inhibitory function show varied activation patterns, possibly due to functional specialization among different brain regions32.Based on these findings, several intervention strategies could be considered to improve brain connectivity and cognitive function. One promising approach is cognitive training aimed at enhancing executive function, particularly inhibitory control and working memory. Such training could target the prefrontal cortex, which is essential for high-level cognitive control. Programs designed to improve task-switching, cognitive flexibility, and inhibitory control could promote more efficient brain network integration, particularly between the prefrontal cortex and other brain regions. Previous research has shown that cognitive training can lead to structural and functional changes in the brain, improving the connectivity between prefrontal areas and enhancing cognitive performance.
Despite controlling for numerous influencing factors and including a large sample size, some limitations must be acknowledged. The cross-sectional design of this study prevents causal conclusions, and current findings can only infer hypotheses regarding the relationship between brain connectivity and inhibitory function. Future research should utilize longitudinal studies to validate these hypotheses and explore how brain connectivity evolves over time. Longitudinal studies are essential to establish causal relationships and observe changes in brain connectivity over time. Additionally, this study did not include a cognitively healthy control group, which limits the ability to directly compare impaired and non-impaired populations. Future studies should consider including such groups to enhance interpretability and generalizability of the findings. Research should also explore how interventions like physical exercise and cognitive training can modify brain connectivity, providing insights into strategies for promoting healthy cognitive aging.
Conclusion
The findings suggest that better inhibitory function is associated with higher cognitive performance among cognitively impaired elderly individuals, highlighting the potential relevance of inhibitory control in cognitive health. Physical function and strength training may help improve EEG activity in individuals with low inhibitory function, thereby enhancing their cognitive functions. The accuracy of inhibitory functions is primarily related to the connectivity strength and density between the prefrontal and parietal regions, while reaction time is related to regions such as the central and occipital areas. The strength of prefrontal connectivity and the balance between hemispheric connections were found to be associated with inhibitory function and task execution efficiency, highlighting their potential relevance in understanding cognitive impairment.
Data availability
The findings of this study will be supported by data provided upon request by the study sponsor, Shanghai Sport University. In accordance with specific standards, conditions, and exceptions, relevant de-identified personal data of participants may also be provided by Shanghai Sport University. Additional information may be obtained by contacting Shanghai Sport University. Enquiries may also be directed to the corresponding author.
References
Jia, L. et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in china: A cross-sectional study. Lancet Public. Health. 5 (12), e661–e71 (2020).
He, S. et al. Spatial distribution and influencing factors of cognitive impairment in Chinese population aged 45 years and above. J. South. Med. Univ. 43 (04), 611–619 (2023).
Friedman, N. P. & Robbins, T. W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47 (1), 72–89 (2022).
Jin, L. et al. Research progress in mild cognitive impairment. Adv. Mod. Biomed. 17(11), 2170–2173 (2017).
Zhiguang, J. Characteristics and Mechanism of Cognitively Demanding Exercise in Improving Inhibitory Function in Elderly People (Shanghai Institute of Physical Education, 2018).
Houdt, C. A. V. et al. Executive Function Training in Very Preterm Children: A Randomized Controlled Trial. 5 (Steinkopff-Verlag, 2021).
Stroop, J. R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 18 (6), 643 (1935).
Kang, W., Wang, J. & Malvaso, A. Inhibitory control in aging: the compensation-related utilization of neural circuits hypothesis. Front. Aging Neurosci. 13, 771885 (2022).
Bamidis, P. D. et al. A review of physical and cognitive interventions in aging. Neurosci. Biobehavioral Reviews. 44, 206–220 (2014).
Peskar, M. et al. Stroop in motion: neurodynamic modulation underlying interference control while sitting, standing, and walking. Biol. Psychol. 178, 108543 (2023).
Schmiedt-Fehr, C. & Basar-Eroglu, C. Event-related delta and theta brain oscillations reflect age-related changes in both a general and a specific neuronal inhibitory mechanism. Clin. Neurophysiol. 122 (6), 1156–1167 (2011).
Nitish, T. & Shanbao, T. Quantitative EEG analysis methods and applications.Artech. (2009).
Hasher, L. & Zacks, R. T. Working Memory, Comprehension, and Aging: A Review and a New View. In Psychology of Learning and Motivation (ed. Bower, G. H.) 193–225 (Academic Press, 1988).
Karamacoska, D. et al. Intrinsic EEG and task-related changes in EEG affect go/nogo task performance. Int. J. Psychophysiol. 125, 17–28 (2018).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10 (3), 186–198 (2009).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52 (3), 1059–1069 (2010).
Stam, C. J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 15 (10), 683–695 (2014).
Stam, C. J. et al. Small-world networks and functional connectivity in alzheimer’s disease. Cereb. Cortex. 17 (1), 92–99 (2007).
Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29 (2-3), 169–195 (1999).
Jobson, D. D. et al. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 3 (3), fcab125 (2021).
Lixiu, Z. & Xueqin, L. Study on the reliability and validity of the Chinese version of the Montreal cognitive assessment scale. Nurs. Res. 31: 2906–2907 (2007).
Xia, M., Wang, J. & He, Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 8 (7), e68910 (2013).
Song, X. et al. Neural mechanism for dynamic distractor processing during video target detection: insights from time-varying networks in the cerebral cortex. Brain Res. 1765, 147502 (2021).
Menant, J. C. et al. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people: time to abandon the term sarcopenia? Osteoporos. Int. 28 (1), 59–70 (2017).
Sui, S. X. et al. Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci. Rep. 10 (1), 10367 (2020).
Zhidong, C., Wanting, J. & Xing, W. Effects of elastic band resistance exercise on working memory of elderly people: Evidence from fNIRS. J. Shanghai Univ. Sport 48(03), 65–74 (2024).
West, R. L. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 120 (2), 272–292 (1996).
Hasher, L. Inhibitory deficit hypothesis. The encyclopedia of adulthood and aging. 1–5 (2015).
Hasher, L., Lustig, C. & Zacks, R. T. Inhibitory mechanisms and the control of attention. Variation Working Memory. 19, 227–249 (2007).
Zavagnin, M. & Riboldi, F. Training Di Potenziamento Della memoria Di lavoro e Delle funzioni esecutive Nella popolazione anziana: Una Rassegna. Giornale Italiano Di Psicologia 39(4), 745–770 (2012).
May, C. P. & Hasher, L. Synchrony effects in inhibitory control over thought and action. J. Exp. Psychol. Hum. Percept. Perform. 24 (2), 363 (1998).
Hausman, H. K. et al. Cingulo-opercular and frontoparietal control network connectivity and executive functioning in older adults. Geroscience 1–20 (2022).
Liqin, Y. et al. Effects of multi-modal exercise on cognitive function and neuroplasticity in the elderly with mild cognitive impairment. Sports Sci. 42 (01), 78–87 (2022).
Tsai, C-L. et al. Executive function and endocrinological responses to acute resistance exercise. Front. Behav. Neurosci. 8, 262 (2014).
Wu, C-H. et al. Effects of acute aerobic and resistance exercise on executive function: an ERP study. J. Sci. Med. Sport. 22 (12), 1367–1372 (2019).
Yarrow, J. F. et al. Training augments resistance exercise induced elevation of Circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 479 (2), 161–165 (2010).
Hwang, K. et al. Cortical neurodynamics of inhibitory control. J. Neurosci. 34 (29), 9551–9561 (2014).
Kupferschmidt, D. A. et al. Prefrontal interneurons: populations, pathways, and plasticity supporting typical and disordered cognition in rodent models. J. Neurosci. 42 (45), 8468–8476 (2022).
Ji, L. et al. A new measure for neural compensation is positively correlated with working memory and gait speed. Front. Aging Neurosci. 10, 71 (2018).
Martínez-Molina, M. P. et al. Lateral prefrontal theta oscillations causally drive a computational mechanism underlying conflict expectation and adaptation. Nat. Commun. 15 (1), 9858 (2024).
Zaizhi, W., Jianhua, Z. & Xiaochuan, P. Quantitative analysis of functional connectivity between prefrontal cortex and striatum. J. East. China Univ. Sci. Technol. (Natural Sci. Edition) 45(2), 316–327 (2019) (in Chinese).
Kang, W. et al. Inhibitory control development: a network neuroscience perspective. Front. Psychol. 13, 651547 (2022).
Levin, F. et al. Longitudinal trajectories of cognitive reserve in hypometabolic subtypes of alzheimer’s disease. Neurobiol. Aging. 135, 26–38 (2024).
Rae, C. L. et al. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J. Neurosci. 35 (2), 786–794 (2015).
Kim, C. et al. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J. Neurosci. 31 (13), 4771–4779 (2011).
Sundermann, B. & Pfleiderer, B. Functional connectivity profile of the human inferior frontal junction: involvement in a cognitive control network. BMC Neurosci. 13, 1–13 (2012).
Acknowledgements
We thank Shanghai Sport University for their support and sponsorship of this study. We thank Shanghai Nuocheng Limited Company for providing equipment support for this study.
Funding
The work was supported by National key research and development program (NO.2020YFC2007205), Shanghai Key Lab of Human Performance (Shanghai University of Sport) (NO. 11DZ2261100) and National Social Science Foundation of China (NO.22BTY076).
Author information
Authors and Affiliations
Contributions
The authors have developed the ideas and model described in this article in joint discussions. XX, SJ, XW and XW: conception, design, and revision of the article, these authors contributed equally to this work. XX has written a first draft of the manuscript that has then been revised (amended, commented, expanded) by ZW. and SJ. in two extensive rounds of revision. SJ, SL, JW and QL: data acquisition and analysis. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was obtained from the Ethics Committee of Shanghai Institute of Physical Education (Approval No. 102772020RT059), and all participants provided informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xin, X., Wang, Z., Jia, S. et al. Brain functional connectivity characteristics at various levels of inhibitory function in elderly individuals with cognitive impairment. Sci Rep 15, 27828 (2025). https://doi.org/10.1038/s41598-025-12334-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12334-7