Abstract
The survival of coral reefs depends on the rejuvenation of coral populations with the potential to adapt and survive a changing climate. Assisted sexual reproduction has become an important tool in reef management. One bottleneck is the efficient and manageable induction of coral larval settlement. Here we report cycloprodigiosin (CYPRO) as a multispecies cue which induces complete larval settlement at rates between 40 and 93% in four brooding and five broadcast spawning species. All nine tested species showed similar uptake and conversion of CYPRO (photolytic degradation), which together initiate and complete the transition to successful settlement. Due to its chemical stability and low water solubility, the component can be easily stored, transported and applied on clean substrates, which might increase the chances of survival of the settled recruits. Thus, this broad settlement cue has the chance to advance reef restoration projects.
Similar content being viewed by others
Introduction
Coral reefs are exceptional ecosystems that face a multitude of anthropogenic stressors such as overfishing, pollution, but foremost climate change and the associated increase in heat events1. Extreme heat over a prolonged period of time disrupts the essential symbiosis between coral hosts and their associated symbiotic algae2. The interrupted symbiosis can lead to mass bleaching events as globally witnessed in 2023-24 already for the fourth time3. Mass bleaching often results in large coral community die-offs and, associated with it, long-term ecosystem shifts4,5. To counteract this environmental crisis, methods have been developed to restore damaged reefs on a small scale. However, most of these methods rely on asexual reproduction through coral fragmentation and growth of these clones, that have the same characteristics as their parental colony. Juvenile corals obtained from sexual reproduction, in contrast, possess both a large genetic diversity as well as an inherent phenotypical plasticity, resulting in a higher adaptive potential6. Therefore, coral restoration procedures in recent years have extended from cloning via fragmentation towards the production of sexually reproduced coral generations7,8,9,10. The latest research showed that these sexually reproduced corals might be more tolerant to global warming11 and also retain the genetic diversity of natural coral populations12.
Scleractinian corals reproduce sexually in two distinct modes: brooding and broadcast spawning. Both reproductive strategies result in swimming coral larvae that need to detect a suitable place to settle. After attachment to a solid substrate, coral larvae metamorphose into a sessile coral recruit and cement their foundations by calcification towards a juvenile coral colony. This initial step of settlement on a specific substrate is driving the survival of young corals. The choice of settlement location is influenced by many environmental factors10 such as light spectra and intensity13,14reef sound15substrate colors16,17substrate microtopography18but is primarily triggered by biochemical cues that are produced by reef organisms such as crustose coralline algae (CCA19,20,21,22), and their associated microbiome23,24. Two methods are commonly used to promote the settlement of coral larvae during restoration measures: (1) establishment of CCA with their natural bacterial community on settlement substrates25 and (2) mature natural biofilms that cover substrates8,9,26. However, both methods have their limitations. With CCA, settlement rates can vary greatly depending on the coral and CCA species27,28,29. Also, the bacterial community of CCA is highly dynamic and shows sensitivity to environmental shifts such as warming30 or acidification of the ocean31. Once CCA are transferred from the reef into an aquarium facility, their microbial community may change32 and their ability to induce settlement of coral larvae may diminish over time. Some CCA species utilize competitive strategies such as overgrowth and sloughing, which can significantly reduce post-settlement survival rates of coral recruits25,33. By comparison, long incubated biofilms on suitable settlement tiles are independent of CCA but the established microbial communities (e.g., bacteria, microalgae, protists) strongly depend on the prevailing bacterial community of the seawater, the culturing time and the selected culturing conditions (i.e., temperature, light regime and nutrient composition)34. The established biofilm may also include harmful organisms, which could have a significant negative impact on post settlement survival35,36,37. Thus, settlement triggered by barely controllable biological systems is unpredictable and can vary greatly in its efficiency among batches.
In the attempt to decouple biological variability (i.e., CCA or microbial biofilms) in the fragile settlement reaction of coral larvae, isolated chemical agents are becoming increasingly recognized. In 2011, the compound tetrabromopyrrole (TBP) was isolated and identified from the CCA-associated bacterial strain Pseudoalteromonas sp. and found to induce settlement in multiple coral species38,39,40,41. The cnidarian neuropeptide Hym-248, member of the Glycine-Leucine-Tryptophan-amide family neuropeptides (GLW-amides), was found to be a highly species-specific settlement cue. This compound induces high settlement rates in Acroporids, but very low or no settlement in species of the genera Dipsastrea, Favia, Lobophyllia, Pachyseris, Porites, Platygyra and Montipora36,42,43,44. Other neurotransmitters such as Epinephrine and Dopamine also showed inductive capacity in coral larvae, with success rates of 35–40%, and these compounds were solely tested on the brooding species Leptastrea purpurea45. Another example of a chemical settlement inducer is the bromotyrosine derivative 11-deoxyfistularin-3, which was isolated from 15 kg of CCA rubble. Application of this compound as a singular settlement agent to larvae of Pseudosiderastrea tayamai only resulted in moderate settlement success rates of 28%, but settlement was enhanced up to almost 90% in combination with the carotenoid fucoxanthinol and/or fucoxanthin46. Most recently, the red pigment cycloprodigiosin (CYPRO), identified and isolated from the CCA-associated bacterial strain Pseudoalteromonas rubra24,47induced settlement in larvae of L. purpurea at an efficiency close to 90% of settlement success. A follow-up study, also using L. purpurea as test species, showed that chemically induced settlement with CYPRO followed the same development pathway compared to CCA induced settlement and that both strategies led to successful transformation of settlers into young adult colonies (observed > 1 year of age48).
In this study, we tested the settlement cue CYPRO on nine widespread coral species including four brooding species (i.e., Leptastrea purpurea, L. transversa and Pocillopora acuta and Favia fragum) and five broadcast spawning species (i.e., Acropora humilis, A. hemprichii, A. kenti, A. millepora and A. microclados). Successful settlement across all species corroborate its potential as a multispecies settlement inducer with possible applications in reef restoration.
Results
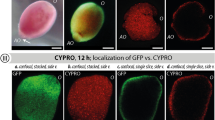
During this study, settlement experiments with nine different coral species (four brooding and five broadcast spawning species) were conducted. In all tested species, the absorption of the red pigment was clearly visible (cf. Figure 1) and ultimately resulted in larval settlement with mean success rates that varied between 40 and 93%, depending on the species and the concentration of CYPRO tested (Figs. 2 and 3). Similarly, metamorphosis in the water column was observed in all species in a concentration dependent manner (cf. Supplemental Figs. 2–10). At the CYPRO concentrations showing the highest settlement success rates, mean water column metamorphosis rates ranged from 7 to 42% and mean mortality rates ranged from 0 to 27%, depending on the species. For each of the nine species (i.e., Figs. 2 and 3), it was possible to identify at least one CYPRO concentration that induced significant settlement (* = p > 0.05; ** = p > 0.005 and *** = p > 0.001) compared to the negative control (NEG) of the same timepoint.
CYPRO uptake and settlement process. (A) Conceptual model of settlement with the pigment CYPRO. (A-I) swimming coral larvae accumulate the red pigment CYPRO from the bottom of the well in darkness; (A-II) swimming larvae appear red due to the time-dependent accumulation of CYPRO within their ectodermal layer during the dark period, under light exposure the pigment CYPRO steadily degrades, the strong red color fades over time and with that releases a constant, µM level of H2O2 50. Ultimately, the larvae attach to the bottom of the well and proceeds with their metamorphosis into a sessile recruit; (A-III) in the following altering 12 h dark and 12 light phases the pigment CYPRO fades completely, and the larvae further develop into a sessile coral recruits. (B) During the initial dark phase of the experiments (first 12 h) the swimming coral larvae of all tested species visibly changed their color and appeared red (cf. CYPRO row). For comparison the pictures at the Control row show the normal coloration of the larvae (without CYPRO).
Mean coral larval settlement of brooding species in response to CYPRO. Settlement was monitored after one and two days. Note that CYPRO has a very low water solubility and thus the concentration of the pigment is specified in µg cm− 2. Pictures in the upper right corner of each graph show a corresponding coral recruit after 2 days. Error bars represent standard error (SE). Differences within each time point between the negative control and the different CYPRO treatments as well as the positive control were evaluated per experiment using non-parametric Kruskal-Wallis tests, followed by pairwise Dunn tests (α = 0.05). Asterisk indicate the level of significance difference between NEG control and CYPRO treatment or POS control within each timepoint, with n.s. = not significant; * = p > 0.05; ** = p > 0.005 and *** = p > 0.001. Leptastrea transversa and purpurea: 3 replicates with 5 larvae each, Pocillopora acuta: 3 replicates with 3 larvae each and Favia fragum: 6 replicates with 5 larvae each.
Mean coral larval settlement of broadcast spawning species in response to CYPRO. Settlement was monitored after one and two days. Note that CYPRO has a very low water solubility and thus the concentration of the pigment is specified in µg cm− 2. Pictures in the upper right corner of each graph show a corresponding coral recruit after 48 h (day 2). Error bars represent standard error (SE). Differences within each time point between the negative control and the different CYPRO treatments as well as the positive control were evaluated per experiment using non-parametric Kruskal-Wallis tests, followed by pairwise Dunn tests (α = 0.05). Asterisk indicate the level of significance difference between NEG control and CYPRO treatment or POS control within each timepoint, with n.s. = not significant; * = p > 0.05; ** = p > 0.005 and *** = p > 0.001. Acropora humilis, kenti and microclados: 3 replicates with 5 larvae each, Acropora hemprichii: 3 replicates with 4 larvae each (in one control well were 5 instead of 4 larvae), Acropora millepora: 3 replicates for the concentrations 0.05; 0.08; 0.31 and 0.42 µg cm− 2 and 6 replicates for NEG; POS; 0.1 and 0.21 µg cm− 2.
In the brooding species, mean settlement success in the species-specific optimal concentrations varied from 55 to 93% after two days of CYPRO exposure (Fig. 2), while mean metamorphosis in the water column occurred between 7 and 23% (Supplemental Figs. 2–5). Leptastrea transversa and L. purpurea both had their settlement optimum at a concentration of 0.29 µg cm− 2 with mean settlement success at 93 (± 6.7 SE) and 66% (± 6.7 SE), respectively. For L. purpurea, 7% of the settled larvae died between the first and the second day. Pocillopora acuta larvae settled with 55% (± 29.4 SE) mean success at their optimal CYPRO concentration of 0.31 µg cm− 2. Favia fragum larvae settled most effectively at a concentration of 0.52 µg cm− 2 with a mean success rate of 63% (± 10.9 SE) after two days (Fig. 2) and 73% (±12.3 SE) after 3 days (see Supplemental Fig. 5).
In the spawning species, mean settlement success among the (species-specific) optimal concentrations varied from 40 to 73% after two days of CYPRO exposure (see Fig. 3), while metamorphosis in the water column occurred between 13 and 42% (Supplemental Figs. 6–10). Acropora humilis larvae settled most successfully at the highest tested CYPRO concentration of 0.21 µg cm− 2, with 40% (± 11.5 SE) mean success rate. A. kenti larvae settled best at a CYPRO concentration of 0.26 µg cm− 2 with a mean success rate of 60% (± 11.5 SE). A. hemprichii larvae settled best when exposed to 0.21 µg cm− 2 with 50% mean (± 14.4 SE) success, which was significantly higher compared to NEG, although a small fraction of NEG larvae settled (1 out of 13). This single larva was the only one settling of all 158 NEG control larvae throughout all species. A. millepora settled best when treated with 0.31 µg cm− 2 with 73% (± 6.7 SE) mean success. Interestingly, larvae of this species did not settle at all in the applied POS control treatment. A. microclados settled best when exposed to 0.37 µg cm− 2 with 60% (± 11.5 SE) mean success. A detailed description of each data set including all observed larval states such as swimming, attached, metamorphosis in the water column, settlement, deformation and dissolved are provided in the Supplementary section (Supplemental Tables 2 and Supplemental Figs. 2–10).
For the tested coral species, settlement was induced at species-specific optimal CYPRO concentrations (see Fig. 4). For the brooding species, this optimal concentration varied from 0.29 µg cm− 2 for both Leptastrea species to up to 0.52 µg cm− 2 for F. fragum larvae. The five spawning Acropora species settled most successfully in response to 0.21–0.37 µg cm− 2 of CYPRO. For A. humilis and A. hemprichii the optimal concentration might not have been found, since 0.21 µg cm− 2 was the highest tested and most successful settlement concentration.
Species-specific settlement-inducing CYPRO concentration ranges separated in brooding and spawning species. Note that for both Leptastrea species, smaller well sizes (3.5 cm2) were used compared to all other species (9.6 cm2 cf. Table S1). Triangles display the mean settlement in percentage; transparent ribbons display the standard error. Lines in the graph were “loess” smoothed (span = 0.55) using the ggplot2 package.
Discussion
Coral larval settlement is a crucial process in the life cycle of corals. Yet, it is still not fully understood which biochemical cues are involved and how exactly these cues trigger the settlement reaction of swimming coral larvae. To date, TBP40 and CYPRO47 are the only identified bacterially produced, multispecies settlement cues for coral larvae. Both compounds were originally isolated from microbial biofilms, or more precisely from the marine bacterial genus Pseudoalteromonas. However, recent studies suggest that other bacterial families besides Pseudoalteromonadaceae, not associated with the production of TBP and/or CYPRO, also lead to high settlement rates24,27,32. This observation suggests that TBP and CYPRO are likely two of potentially many other settlement cues occurring in natural coral reefs that may function either individually or in combination to induce successful settlement. Nevertheless, the investigation and comparison of purified chemical cues such as TBP and CYPRO offers the opportunity to better understand the settlement induction on a deeper mechanistical level. Both compounds were observed to induce to some extent metamorphosis in the water column (without prior attachment), if applied in a non-optimal concentration range (cf. Supplemental Figs. 2–1038,40,41). Furthermore, both compounds are associated to the production of reactive oxygen species such as H2O2 49,50 which, if solely applied, induces metamorphosis in the water column50,51. Petersen and colleagues50 hypothesized that the role of H2O2 released during CYPRO degradation is one of the main drivers of this chemically induced settlement mechanism. Using light and confocal microscopy, the latter authors observed that larvae of the brooding species L. purpurea were able to absorb CYPRO into their outer ectodermal layer, clearly changing their appearance from a translucent-brownish color to a red coral larva after a few hours of CYPRO exposure. This study extends the use of CYPRO as settlement cue to four brooding and four broadcast spawning coral species and found that all eight additionally tested species rapidly took up CYPRO, which likewise resulted in a clearly visible red coloration (cf. Figure 1B). However, this “blushing” of the larvae only occurred in complete darkness and when exposed to rather high CYPRO concentrations. The addition of CYPRO under direct exposure to light always led to a rapid and visible decrease of the pigment at the bottom of the well, and the larvae retained its translucent-brownish color. Thus, this study supports the generality of the conceptual model of a CYPRO-induced coral settlement mechanism (time-dependent accumulation of CYPRO within the larvae during darkness followed by photodegradation of CYPRO during daylight and with that release of µM H2O2 levels, cf. Figure 1A) proposed by Petersen and colleagues50. Given the high settlement rates of CYPRO in all nine brooding and broadcast spawning coral species, we propose CYPRO as a general settlement cue for scleractinian corals, although future research should identify species-specific optimal concentrations of CYPRO to maximize settlement success and possible applications in coral restoration.
Factors such as larval size and their swimming behavior might influence the uptake rate of the chemical cue by the searching larvae. For example, larger larvae with a greater surface area need to accumulate more of the lipophilic cue. In addition, larvae that search horizontally (i.e., L. purpurea) appear to collect more of the pigment within a given time interval than larvae that mainly move vertically in the water column, such as observed in the five tested Acropora species. Thus, the offered CYPRO concentrations may have been too low to trigger maximum settlement rates for some tested species. For example, A. hemprichii and A. humilis achieved highest settlement rates with the highest tested CYPRO concentration of 0.21
µg cm− 2 (cf. Figure 3). The species’ optimal concentration might be higher than 0.21 µg cm− 2, although this could not be tested due to a lack of larval supply. P. acuta, on the other hand, settled with highest success rates at the lowest applied CYPRO concentration of 0.31 µg cm− 2 and might settle with higher success when exposed to lower CYPRO concentrations (< 0.31 µg cm− 2, cf. Figure 2). If too high CYPRO concentrations were applied, it resulted in the mortality of the larvae (cf. Supplemental Figs. 2–10). Other factors influencing success in settlement may be caused by differences in uniform illumination of the experimental set ups or in the thickness and surface distribution of the applied CYPRO film at the bottom of the well. For example, if CYPRO is applied in a 10-fold higher concentrated MeOH solution (e.g. 1 mg mL− 1), the distribution of the dried pigment is less homogenous, and the searching larvae may take up either too much or too little of CYPRO depending on the area that the individual larva has swept. All these aspects may lead to variations in settlement rates among individual larvae and coral species and should be considered to optimize settlement success. In some experiments, only low amounts of larvae were available, resulting in a low number of replicates, especially in the case of P. acuta, which was tested using three replicates per treatment with three larvae each, but nevertheless showed significant results compared to the NEG control. Even though the experimental design could be improved, this study clearly shows that CYPRO is a multispecies settlement cue and thus holds a great potential for broad usage.
In coral restoration based on larval recruitment, more attention has recently been directed towards chemically induced settlement7,10 as it appears to be more reliable, better available and easier to manage than biological cues (e.g., CCA, biofilms). This approach currently utilizes purified chemical settlement inducers such as TBP or Hym-248, that are both commercially available (TBP - Aaron Chemicals LLC, USA) and (Hym-248 - NovoPro, USA). However, aside from their varying efficiency and the species specificity of Hym-248 36,38,39,40,41,42,43,44, both chemical cues are highly water soluble, making their use in large aquarium systems or open ocean reef environments extremely challenging. Furthermore, the chemical properties of TBP such as its instability in storage and transport to remote areas39 as well as the elevated toxicity for phytoplankton52 may be problematic for large-scale reef restoration programs. CYPRO (available at Sirius Fine Chemicals SiChem GmbH, Germany), is suitable for long term storage (> 2 years at -20 °C) and prolonged transport at room temperatures if stored in complete darkness. Furthermore, CYPRO strong lipophilicity allows its application on substrates such as settlement tiles, regardless of the water volume. Its use on non-conditioned, biofilm-free tiles might have positive effects on coral post-settlement survival. Specifically, it could provide more time for corals to reach a size-escape threshold before tiles are colonized by strong benthic competitors, such as macroalgae and bryozoans.
In conclusion, we showed that CYPRO is functioning as a multispecies settlement cue for a wide range of brooding and broadcast spawning scleractinian coral species. Since an increased effectiveness of restoration efforts in the early life-stages holds the potential to significantly improve the restoration outcome, CYPRO, is an interesting settlement cue for large-scale coral reef restoration. Future studies should use CYPRO as a tool for reef restoration, focusing on settlement efficiency and post-settlement survival rates.
Materials and methods
Larval acquisition
Coral larvae of the brooding species Leptastrea purpurea and Leptastrea transversa were collected in the facility of the Marine Institute in Guam (USA) and larvae of the species Pocillopora acuta were collected in the aquarium facility of the Environmental Chemistry Group of the University of Oldenburg in Wilhelmshaven (GER). For these three species, a similar collection procedure was used53. In brief, parental colonies were placed in a potassium chloride (KCl) bath (5–7.5 g L− 1) for a period of 5 to 10 min after which they released fully developed and competent swimming larvae. Larvae were immediately collected using a plastic Pasteur pipette and washed at least two times by transferring them into a bath of filtered (using a 0.2 μm nylon filter) natural sea water for both Leptastrea species or artificial sea water (FASW, Tropic Marin® Pro-Reef Salt, Wartenberg, Germany) for Pocillopora acuta. Brooded larvae were stored for a maximum of 3 weeks in an incubator (Lovibond) at a steady temperature of 26 °C and a 12 h dark/light rhythm. The FASW media was exchanged weekly. Favia fragum larvae were collected at the aquarium facility of the University of Wageningen (NL) following the collection procedure described in54. In brief, adult colonies were placed individually in 1 L beakers during the night. The beaker had a constant inflow of 27 °C FASW that continuously caused buoyant larvae to overflow over the handle into a collection container. Larvae were transferred into a storage container filled with 500 mL of 0.5 μm FASW.
Spawned larvae were obtained within the artificial spawning system of the Environmental Chemistry Group of the University of Oldenburg in Wilhelmshaven (GER), based on the technology of the Coral Spawning Lab Ltd (GB). The underlying principle of these systems is to induce bundle generation and synchronized spawning in parental coral colonies by imitating the lunar cycle as well as seasonal variations in the natural day length and water temperature55. As soon as the setting (i.e., appearance of sperm-egg bundles) of the parental colonies was visible, a tube of acrylic glass was placed around the colonies to capture ascending sperm-egg bundles. After bundle collection and their disintegration, sperm and eggs were separated with a 100 μm mesh. Sperm fractions of all spawned colonies were combined and used to fertilize the eggs of all suitable colonies to increase potential genetic diversity. Fertilized eggs further developed into swimming coral larvae in circular mesh-bottom containers floating in a larger holding tank of a recirculating aquaculture system with a steady but slow water movement. The layers of fat accumulating on the water surface from remaining sperm and dissolving eggs were removed periodically using pieces of plastic wrap to ensure sufficient water quality. Depending on the coral species, larvae gained competence to settle within 5 to 7 days after fertilization. For more specific information see Table S1.
CYPRO production
CYPRO was acquired using the cultivation and extraction protocol as described by47 but is also commercially available at Sirius Fine Chemicals SiChem GmbH. In brief, Marine Broth (MB, Carl Roth GmbH) agar plates were inoculated with a Pseudoalteromonas rubra cryo-conserved stock that was stored at -80 °C. After 48 h, an individual colony was picked and transferred into MB liquid media. After another 24 h, the yellow-transparent liquid media changed into a milky-cloudy state. In that state, the liquid media was used to inoculate MB agar plates, which were incubated at room temperature and in darkness for another 24 h. The grown bacterial lawn was scraped off and extracted in methanol (MeOH) first by homogenization using an immersion blender (Ultra-Turrax T25, Janke & Kunkel IKA Labortechnik) at 12,000 rpm for 30 s followed by a 10 s ultrasonic bath (Bandelin Sonorex). The extract was then filtered through cellulose filter paper (grade 3 hw, sartorius) and dried with a speed-vac system (Christ, Gefriertrocknungsanlagen GmbH). The dried crude extract was re-dissolved in MeOH and further purified using a combined approach of liquid-liquid partitioning (LLP), a C18-based solid-phase extraction (SPE, SUPELCO) and high-performance liquid chromatography with a semi-preparative C18-column and for peak-base separation a diode array detector (HPLC-DAD, Agilent Technologies). The quality of the separation process was tested subsequently, using ultrahigh resolution mass spectrometry47. All production steps were performed in low-light environments to prevent a light-induced breakdown of CYPRO. Purified CYPRO was stable for at least 2 years when stored in the dark either dry or dissolved in MeOH at -20 °C. The stored CYPRO amount was quantified by HPLC-MS and additionally analyzed for degradation products.
Settlement experiments
To obtain high settlement rates with each coral larval species, CYPRO was tested at a range of concentrations (i.e. 0.05–0.86 µg cm− 2). Settlement experiments were conducted in 6-well (9.6 cm2) or 12-well (3.5 cm2) cell culture plates using a similar protocol as described in48 (cf. Figure 5). The purified settlement cue CYPRO was dissolved in MeOH to a concentration of 0.1 mg mL− 1 and the tested quantities were pipetted on the bottom of the well plate. The organic solvent MeOH was evaporated at room temperature for approximately 30 min in the absence of light to prevent degradation of the pigment CYPRO. Subsequently, a thin CYPRO film remained on the bottom of the well, however not homogenously distributed over the entire well surface. As negative control (NEG), only MeOH without CYPRO was pipetted onto the bottom of the well, using the corresponding maximum volume used in the assays with CYPRO. A positive control (POS) was used in experiments in which sufficient larvae were available (see Table S1). For that, an approximately 1 cm2 piece of live CCA either cultured in the aquarium facilities in Wilhelmshaven or freshly collected CCA pieces of the species Hydrolithon reinboldii from the Luminao Reef in Guam (USA) was used (cf. Table S1). Finally, the wells were filled either with 5 mL (12-well plate) or 10 mL (6-well plate) of FASW and 3 or 5 larvae were added to each well (cf. Table S1). Since CYPRO has an extremely high lipophilicity it hardly dissolves in the FASW and remains on the bottom of the well plate. All laboratory experiments were incubated constantly at 26 °C and began with a 12 h dark phase followed by 12 h of light exposure (65 µmol m− 2 s− 1; see Supplemental Fig. 1 for detailed light spectra) alternating until the end of the experiment. Experiments with both Leptastrea species were conducted in the outdoor facilities of the University of Guam Marine Laboratory at an average temperature of 28 °C and started with 8 h darkness followed by the natural light rhythm and composition. Settlement was determined as a change from the free-swimming, pear-shaped larval form to a disc-shaped recruit, that was attached solidly to the well, with septal mesenteries radiating from the mouth as defined by Heyward and Negri56. The experiments were monitored after approx. 24 h and 48 h. Larvae were tested for adhesion after two days with a gentle flow of water induced by a pipette. For detailed specifications of each experiment see Table S1.
Stepwise instructions to the conducted settlement experiments. (1) Addition of CYPRO dissolved in MeOH, (2) evaporation of MeOH with a remaining CYPRO film on the bottom, (3) addition of FASW and (4) addition of larvae. For specific information about each experiment see Figure S1. CYPRO has an extremely high lipophilicity and therefore does not dissolve in the FASW (step 3). Since it remains on the bottom of the plate, CYPRO concentrations are provided in µg cm− 2.
Statistical analysis
In each experiment at least three replicates (wells) per control and treatment with at least three larvae per well were tested for settlement (for detailed information about each experiment see Table S1). Differences in settlement percentage between the negative control and the different CYPRO treatments as well as the positive control were evaluated per treatment and within timepoint (day 1, day 2) using non-parametric Kruskal-Wallis tests, followed by pairwise Dunn tests (α = 0.05) without corrections for multiple comparisons. R (version 4.3.2) with the RStudio IDE (version 2023 9.1.494) was used for data analysis. The packages dunn.test 1.3.5 57 and tidyverse 2.0.0 58 were used for statistical testing, data manipulation and plotting.
Data availability
All data generated or analyzed during this study are included in this published article or in the Supplemental Materials.
References
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017). https://doi.org/10.1038/nature21707
Boilard, A. et al. Defining coral bleaching as a microbial dysbiosis within the coral holobiont. Microorganisms 8, 1682 (2020). https://doi.org/10.3390/microorganisms8111682
ICRI & NOAA. NOAA and ICRI Confirm Fourth Global Coral Bleaching Event. (2024). https://icriforum.org/4gbe/
Reverter, M., Helber, S. B., Rohde, S., de Goeij, J. M. & Schupp, P. J. Coral reef benthic community changes in the anthropocene: biogeographic heterogeneity, overlooked configurations, and methodology. Glob Change Biol. 1–16. (2021). https://doi.org/10.1111/gcb.16034
Reverter, M., Helber, S. B., Rohde, S., Goeij, J. M. D. & Schupp, P. J. Drivers of Coral Reef Benthic Changes and Implications on Ecosystem Functioning and Services. in Oceanography and Marine Biology 215–247 (2024). https://doi.org/10.1201/9781003477518-5
Torda, G. et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Change. 7, 627–636 (2017). https://doi.org/10.1038/nclimate3374
Banaszak, A. T. et al. Applying coral breeding to reef restoration: best practices, knowledge gaps, and priority actions in a rapidly-evolving field. Restor. Ecol. 31, e13913 (2023). https://doi.org/10.1111/rec.13913
Chamberland, V. F. et al. New seeding approach reduces costs and time to outplant sexually propagated corals for reef restoration. Sci. Rep. 7, 18076 (2017). https://doi.org/10.1038/s41598-017-17555-z
Dela Cruz, D. W. & Harrison, P. L. Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Sci. Rep. 7, 13985 (2017). https://doi.org/10.1038/s41598-017-14546-y
Randall, C. et al. Sexual production of corals for reef restoration in the anthropocene. Mar. Ecol. Prog Ser. 635, 203–232 (2020). https://doi.org/10.3354/meps13206
Miller, M. W. et al. Assisted sexual coral recruits show high thermal tolerance to the 2023 Caribbean mass bleaching event. PLOS ONE. 19, e0309719 (2024). https://doi.org/10.1371/journal.pone.0309719
Baums, I. B. et al. Considerations for maximizing the adaptive potential of restored coral populations in the Western Atlantic. Ecol. Appl. 29, e01978 (2019). https://doi.org/10.1002/eap.1978
Mundy, C. N. & Babcock, R. C. Role of light intensity and spectral quality in coral settlement: implications for depth-dependent settlement? J. Exp. Mar. Biol. Ecol. 223, 235–255 (1998). https://doi.org/10.1016/S0022-0981(97)00167-6
Strader, M. E., Davies, S. W. & Matz, M. V. Differential responses of coral larvae to the colour of ambient light guide them to suitable settlement microhabitat. R Soc. Open. Sci. 2, 150358 (2015). https://doi.org/10.1098/rsos.150358
Vermeij, M. J. A., Marhaver, K. L., Huijbers, C. M., Nagelkerken, I. & Simpson, S. D. Coral larvae move toward reef sounds. PLoS ONE. 5, e10660 (2010). https://doi.org/10.1371/journal.pone.0010660
Foster, T. & Gilmour, J. Seeing red: coral larvae are attracted to healthy–looking reefs. Mar. Ecol. Prog Ser. 559, 65–71 (2016). https://doi.org/10.3354/meps11902
Mason, B., Beard, M. & Miller, M. W. Coral larvae settle at a higher frequency on red surfaces. Coral Reefs. 30, 667–676 (2011). https://doi.org/10.1007/s00338-011-0739-1
Whalan, S., Abdul Wahab, M. A., Sprungala, S., Poole, A. J. & De Nys, R. Larval settlement: the role of surface topography for sessile coral reef invertebrates. PLOS ONE. 10, e0117675 (2015). https://doi.org/10.1371/journal.pone.0117675
Morse, D. E., Hooker, N., Morse, A. N. C. & Jensen, R. A. Control of larval metamorphosis and recruitment in sympatric agariciid corals. J. Exp. Mar. Biol. Ecol. 116, 193–217 (1988). https://doi.org/10.1016/0022-0981(88)90027-5
Kitamura, M., Schupp, P. J., Nakano, Y. & Uemura, D. Luminaolide, a novel metamorphosis-enhancing macrodiolide for scleractinian coral larvae from crustose coralline algae. Tetrahedron Lett. 50, 6606–6609 (2009). https://doi.org/10.1016/j.tetlet.2009.09.065
Price, N. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163, 747–758 (2010). https://doi.org/10.1007/s00442-010-1578-4
Ritson-Williams, R., Arnold, S. N., Paul, V. J. & Steneck, R. S. Larval settlement preferences of Acropora palmata and Montastraea faveolata in response to diverse red algae. Coral Reefs. 33, 59–66 (2014). https://doi.org/10.1007/s00338-013-1113-2
Negri, A., Webster, N., Hill, R. & Heyward, A. Metamorphosis of broadcast spawning corals in response to bacteria isolated from crustose algae. Mar. Ecol. Prog Ser. 223, 121–131 (2001). https://doi.org/10.3354/meps223121
Petersen, L. E. et al. Mono- and multispecies biofilms from a crustose coralline Alga induce settlement in the scleractinian coral Leptastrea purpurea. Coral Reefs. 40, 381–394 (2021). https://doi.org/10.1007/s00338-021-02062-5
Page, C. A., Giuliano, C. & Randall, C. J. Benthic communities influence coral seeding success at fine Spatial scales. Restor. Ecol. 32, e14212 (2024). https://doi.org/10.1111/rec.14212
Dela Cruz, D. W. & Harrison, P. L. Enhancing coral recruitment through assisted mass settlement of cultured coral larvae. PLOS ONE. 15, e0242847 (2020). https://doi.org/10.1371/journal.pone.0242847
Jorissen, H. et al. Coral larval settlement preferences linked to crustose coralline algae with distinct chemical and microbial signatures. Sci. Rep. 11, 14610 (2021). https://doi.org/10.1038/s41598-021-94096-6
Wahab, M. A. A. et al. Hierarchical settlement behaviours of coral larvae to common coralline algae. Sci. Rep. 13, 5795 (2023). https://doi.org/10.1038/s41598-023-32676-4
Whitman, T. N., Negri, A. P., Bourne, D. G. & Randall, C. J. Settlement of larvae from four families of corals in response to a crustose coralline Alga and its biochemical morphogens. Sci. Rep. 10, 16397 (2020). https://doi.org/10.1038/s41598-020-73103-2
Webster, N. S., Soo, R., Cobb, R. & Negri, A. P. Elevated seawater temperature causes a microbial shift on crustose coralline algae with implications for the recruitment of coral larvae. ISME J. 5, 759–770 (2011). https://doi.org/10.1038/ismej.2010.152
Webster, N. S., Uthicke, S., Botté, E. S., Flores, F. & Negri, A. P. Ocean acidification reduces induction of coral settlement by crustose coralline algae. Glob Change Biol. 19, 303–315 (2013). https://doi.org/10.1111/gcb.12008
Turnlund, A. C. et al. Linking differences in microbial network structure with changes in coral larval settlement. ISME Commun. 3, 114 (2023). https://doi.org/10.1038/s43705-023-00320-x
Harrington, L., Fabricius, K., De’ath, G. & Negri, A. Recognition and selection of settlement substrata determine Post-Settlement survival in corals. Ecology 85, 3428–3437 (2004). https://doi.org/10.1890/04-0298
O’Brien, P. A. et al. Light and dark biofilm adaptation impacts larval settlement in diverse coral species. Environ. Microbiome. 20, 11 (2025). https://doi.org/10.1186/s40793-025-00670-0
Box, S. & Mumby, P. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog Ser. 342, 139–149 (2007). https://doi.org/10.3354/meps342139
Erwin, P. M. & Szmant, A. M. Settlement induction of Acropora palmata planulae by a GLW-amide neuropeptide. Coral Reefs. 29, 929–939 (2010). https://doi.org/10.1007/s00338-010-0634-1
Fong, J. et al. Allelopathic effects of macroalgae on Pocillopora acuta coral larvae. Mar. Environ. Res. 151, 104745 (2019). https://doi.org/10.1016/j.marenvres.2019.06.007
Sneed, J. M., Sharp, K. H., Ritchie, K. B. & Paul, V. J. The chemical cue tetrabromopyrrole from a biofilm bacterium induces settlement of multiple Caribbean corals. Proc. R Soc. B Biol. Sci. 281, 20133086 (2014). https://doi.org/10.1098/rspb.2013.3086
Sneed, J. M. et al. Coral settlement induction by tetrabromopyrrole is widespread among Caribbean corals and compound specific. Front. Mar. Sci. 10, 1298518 (2024). https://doi.org/10.3389/fmars.2023.1298518
Tebben, J. et al. Induction of larval metamorphosis of the coral Acropora millepora by tetrabromopyrrole isolated from a Pseudoalteromonas bacterium. PLoS ONE. 6, e19082 (2011). https://doi.org/10.1371/journal.pone.0019082
Tebben, J. et al. Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 5, 10803 (2015). https://doi.org/10.1038/srep10803
Hirose, M., Yamamoto, H. & Nonaka, M. Metamorphosis and acquisition of symbiotic algae in planula larvae and primary polyps of Acropora spp. Coral Reefs. 27, 247–254 (2008). https://doi.org/10.1007/s00338-007-0330-y
Iwao, K., Fujisawa, T. & Hatta, M. A cnidarian neuropeptide of the GLWamide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs. 21, 127–129 (2002). https://doi.org/10.1007/s00338-002-0219-8
Randall, C. J. et al. Larval precompetency and settlement behaviour in 25 Indo-Pacific coral species. Commun. Biol. 7, 142 (2024). https://doi.org/10.1038/s42003-024-05824-3
Moeller, M., Nietzer, S. & Schupp, P. J. Neuroactive compounds induce larval settlement in the scleractinian coral Leptastrea purpurea. Sci. Rep. 9, 2291 (2019). https://doi.org/10.1038/s41598-019-38794-2
Kitamura, M., Koyama, T., Nakano, Y. & Uemura, D. Characterization of a natural inducer of coral larval metamorphosis. J. Exp. Mar. Biol. Ecol. 340, 96–102 (2007). https://doi.org/10.1016/j.jembe.2006.08.012
Petersen, L. E., Kellermann, M. Y., Nietzer, S. & Schupp, P. J. Photosensitivity of the bacterial pigment Cycloprodigiosin enables settlement in coral Larvae—Light as an understudied environmental factor. Front. Mar. Sci. 8, 749070 (2021). https://doi.org/10.3389/fmars.2021.749070
Fiegel, L. J. et al. Detailed visualization of settlement and early development in Leptastrea purpurea reveals distinct bio-optical features. Front. Mar. Sci. 10, 984656 (2023). https://doi.org/10.3389/fmars.2023.984656
Whalen, K. E. et al. The chemical cue tetrabromopyrrole induces rapid cellular stress and mortality in phytoplankton. Sci. Rep. 8, 15498 (2018). https://doi.org/10.1038/s41598-018-33945-3
Petersen, L. E. et al. Photodegradation of a bacterial pigment and resulting hydrogen peroxide release enable coral settlement. Sci. Rep. 13, 3562 (2023). https://doi.org/10.1038/s41598-023-30470-w
Ross, C., Fogarty, N. D., Ritson-Williams, R. & Paul, V. J. Interspecific variation in coral settlement and fertilization success in response to hydrogen peroxide exposure. Biol. Bull. 233, 206–218 (2017). https://doi.org/10.1086/696215
Whalen, K. E. et al. Bacterial alkylquinolone signaling contributes to structuring microbial communities in the ocean. Microbiome 7, 93 (2019). https://doi.org/10.1186/s40168-019-0711-9
Nietzer, S., Moeller, M., Fiegel L. & Schupp, P. Coral larvae on demand: a novel method for an instant acquisition of healthy brooded larvae. Research Square (Preprint). https://doi.org/10.21203/rs.3.rs-4616138/v1
Geertsma, R. C., Wijgerde, T., Latijnhouwers, K. R. W. & Chamberland, V. F. Onset of zooplanktivory and optimal water flow rates for prey capture in newly settled polyps of ten Caribbean coral species. Coral Reefs. 41, 1651–1664 (2022). https://doi.org/10.1007/s00338-022-02310-2
Craggs, J. et al. Inducing broadcast coral spawning ex situ: closed system mesocosm design and husbandry protocol. Ecol. Evol. 7, 11066–11078 (2017). https://doi.org/10.1002/ece3.3538
Heyward, A. J. & Negri, A. P. Natural inducers for coral larval metamorphosis. Coral Reefs. 18, 273–279 (1999). https://doi.org/10.1007/s003380050193
Dinno, A. dunn.test: Dunn’s test of multiple comparisons using rank sums. (2017).
Wickham, H. et al. Welcome to the tidyverse. J. Open. Source Softw. 4, 1686 (2019). https://doi.org/10.21105/joss.01686
Acknowledgements
We thank Petra Schwarz and Birgit Brinkmann for assistance in the lab and the aquarium facility. Also, we are grateful to Laurie Raymundo and the staff of the Marine Lab of the University of Guam for providing facilities and materials to conduct the Leptastrea experiments. We would also like to thank the German Research Foundation (DFG) for funding the instrument grant INST 184/147-1FUGG for the high-resolution mass spectrometer Waters Synapt G2-Si.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
L.J.F, P.J.S and M.Y.K designed the research. L.J.F and M.Y.K carried out the microbial and chemical lab work to produce the settlement cue. L.J.F and M.Y.K performed the experiments. L.J.F and D.B analyzed the data. S.N. maintained the aquarium facilities. S.N, R.C.G and R.O provided the coral larvae. L.J.F, D.B. and M.Y.K drafted the manuscript. All authors contributed to revisions thereafter and gave final approval for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fiegel, L.J., Nietzer, S., Brefeld, D. et al. Cycloprodigiosin: A multispecies settlement cue for scleractinian coral larvae. Sci Rep 15, 27075 (2025). https://doi.org/10.1038/s41598-025-12409-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12409-5