Abstract
Diaphyseal prosthetic reconstruction for bone tumors continues to face challenges with failures due to the lack of evidence-based classification systems. This study aimed to develop and validate a novel classification framework to optimize reconstruction strategies through a retrospective analysis of 112 patients undergoing diaphyseal prosthetic reconstruction between 2010–2021. Diaphyseal tumors were classified into five types according to anatomical location and residual medullary cavity length, with each category corresponding to a tailored prosthetic reconstruction strategy. Clinical outcomes demonstrated a mean Musculoskeletal Tumor Society score of 24.3 ± 3.3 with comparable functional outcomes across classification types. The overall complication rate was 16.1% (18/112), primarily involving aseptic loosening (ASL, 8%) and structural failures (3.6%), with a reoperation rate of 6.3% (7/112). Notably, Type I reconstructions using standard stems without plates showed the highest complication rate (21%), while Type IIIa reconstructions exhibited no complications. Competing risk model analysis showed that cumulative mortality rates at 0.5, 1, 3, 5, and 8 years were 6.4%, 17.4%, 54.4%, 59.1%, and 66.5%, respectively, while cumulative complication rates were 2.7%, 6.5%, 12.5%, 19.4%, and 22.5%, respectively. These findings establish diaphyseal prostheses as effective solutions for intercalary defect reconstruction, emphasizing their role in facilitating early weight-bearing and functional recovery. The novel classification system for diaphyseal tumors provides valuable guidance for the design and application of prosthetic reconstructions.
Similar content being viewed by others
Introduction
The incidence of diaphyseal tumors has been progressively increasing, particularly in the context of metastatic bone lesions, which can be attributed to the prolonged survival of cancer patients due to advancements in cancer therapies including targeted treatments. Various techniques have been described for management of diaphysis tumors, including autografts, massive bone allografts, segmental transport techniques, and prosthetic reconstruction1,2,3. Diaphyseal prostheses is a promising option, in part as a result of the ease of use compared with other options and the difficulty of obtaining allografts in some centers in addition to the reported risks of infection, non-union, pseudarthrosis, and fractures4,5.
Diaphyseal prostheses have undergone significant evolution over the past 50 years. A model for segmental bone defects was developed by researchers at the University of Illinois, USA, in 1973 during their investigation into bone integration with metallic joint prostheses, involving the implantation of titanium fiber grafts into femoral shaft defects in baboons to examine bone-prosthesis interactions6. In 1982, Lempberg and Ahlgren7 first reported the clinical application of diaphyseal prostheses in human bone tumor treatment, employing this technique in two cases of femoral tumors and one case of a tibial tumor, with results indicating one revision due to aseptic loosening and no complications in the remaining two cases, thereby demonstrating its potential clinical utility. Subsequent studies have highlighted benefits such as immediate pain relief, early mobilization, and preservation of joint function and growth plates8,9,10,11, with Benevenia et al.12 reporting full weight-bearing within three months after femoral prosthetic reconstruction. Initially used primarily for mid-shaft reconstructions, diaphyseal prostheses have evolved to incorporate extracortical plates, enabling the use of shorter stems and making them applicable for tumors located closer to the metaphyseal region13. Recent advancements in three-dimensional (3D) printing technology have further refined prosthetic designs, enabling more accurate anatomical matching and the creation of porous interfaces that promote bone ingrowth, thereby expanding the potential applications of diaphyseal prostheses to tumors involving the epiphyseal regions14.
Despite these improvements and the clinical benefits of diaphyseal prostheses, their use is associated with significant mechanical complications, particularly aseptic loosening (ASL) and structural failures15,16. Feltri et al.5 reported a 26% complication rate in a meta-analysis of 202 cases of endoprosthetic reconstruction for large diaphyseal defects, including infections, ASL, and structural failures. Similarly, Albergo17 analyzed 36 cases of prosthetic reconstruction, observing a 36% failure rate at five years, primarily due to aseptic loosening and implant fractures. These elevated failure rates may be partly attributed to the fact that many surgeons rely on personal experience when selecting prosthetic designs and indications for diaphyseal tumors, rather than evidence-based guidelines.
In response to these issues, we conducted a retrospective analysis of prosthetic reconstruction for diaphyseal tumors at two musculoskeletal oncology centers. Based on the data, we developed a novel classification system for diaphyseal tumors and assessed the clinical outcomes of prosthetic reconstructions across different types.
Patients and methods
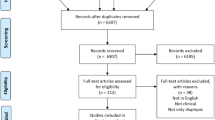
A retrospective analysis was conducted from September 2010 to March 2024, reviewing the medical records of 155 patients diagnosed with diaphyseal tumors who underwent prosthetic reconstruction following tumor resection. According to the inclusion and exclusion criteria, 112 patients were confirmed eligible, included in the study, and completed follow-up. The inclusion criteria were: i) Patients with primary malignant or metastatic diaphyseal tumors; ii) Surgical therapy with tumor resection and reconstruction with diaphyseal prosthesis; iii) Survival expectation of more than 6 months in preoperative evaluation of metastatic bone tumors; iiii) A retrospective observational study. Exclusion criteria were: i) tumor resection with a positive margin or incomplete resection; ii) preoperative poor general status due to cognitive dysfunction, stroke or comorbidities; iii) Major nerve injury during tumor resection; iiii) Lost to follow-up within 3 months.
All patients provided written informed consent for participation in the study. The Institutional Review Board/Ethics Committee of the Department of Bone Oncology of Tianjin Hospital (Tianjin, China) approved the study.
Patient characteristics
A total of 112 patients were included in this study, among which 23.2% (26/112) presented with primary bone tumors, classified as Enneking stage IIA (10 cases), IIB (14 cases), and III (2 cases), with pathological fractures observed in 8 cases, including 15 patients with soft tissue tumors. 76.8% (86/112) had metastatic lesions, with 79 cases exhibiting isolated bone metastases and 7 cases demonstrating widespread skeletal metastases; pathological fractures were identified in 74 of these patients.
The basic patient demographics are summarized in Table 1.
Prosthesis
The diaphyseal prosthesis (Shandong Weigo Company, China) consists of proximal and distal intramedullary stems, which were manufactured using Ti6A14V alloy. The distal and proximal stems are joined with a lap joint connection, secured by two screws, and, if necessary, an extension piece is attached using a Morse taper. For cases where the medullary canal is shortened due to complete tumor excision, shorter stems can be supplemented by extra-cortical plates to improve fixation stability. The prosthesis and plate are designed as modular components. When feasible, the plate is pre-fixed to the prosthesis prior to stem insertion into the cement-filled medullary canal. All screws must be placed before the cement hardens. Cementless stem designs were coated with titanium particle coating at the bone-prosthesis interface, promoting osseointegration and facilitating long-term biological fixation. In certain special cases, such as extreme resections to preserve the growth plate or when precise anatomical matching is required due to complex shapes, 3D-printed prostheses are used.
Preoperative evaluation and planning
Preoperative evaluation for all patients included X-ray, CT, MRI, and ECT imaging, with tumor pathology confirmed through biopsies or examination of resected specimens. Patients with diaphyseal metastases were assessed using the Dutch model scoring system, evaluating projected survival based on the Karnofsky Performance Scale (KPS) score, primary lesion status, and visceral involvement. Tumor resection and reconstruction were performed on patients with a projected median survival exceeding six months. For patients without pathological fractures, diaphyseal prosthesis reconstruction was considered if the Mirels score was greater than 9 for metastases. The osteotomy plane was set based on the most recent imaging results, including X-ray, CT, and MRI. In some cases, an osteotomy guide was designed and fabricated if needed. Each prosthesis was manufactured using computer-aided design and manufacturing technologies after determining the level of bone transection from preoperative plain radiographs and CT/MRI images. Cementation was preferred for elderly patients with limited mobility or comorbidities that compromised bone viability, such as smoking history, cardiovascular disease, diabetes, or prior radiation therapy. Conversely, younger patients without significant comorbidities were better suited for cementless fixation.
Surgical technique
Tumor resection was performed according to the principles defined by Enneking et al.18 with the aim of achieving wide excision without violating the tumor. Osteotomy was performed based on the preoperative plan. After tumor resection, the proximal and distal diaphysis were reamed to accommodate the prosthetic stems, with diameters ≥ 2 mm greater than that of the cemented stem or ≥ 1 mm for non-cemented cases. The prosthesis was trial-fitted to ensure proper seating, and the lap joint was secured with two bolts to restore anatomical alignment. In cemented cases, cement was applied using a standard cement gun, and extra-cortical plates were installed before the cement hardened if needed. For non-cemented cases, a press-fit technique was employed for secure fixation.
Postoperative management
Postoperatively, all patients received intravenous antibiotics for three days. Regardless of whether cemented or cementless fixation was used, patients were encouraged to initiate range of motion exercises for adjacent joints and to begin progressive protective weight-bearing on the second postoperative day. Full weight-bearing was permitted for all patients by three to four weeks after surgery.
Measurement index and classification
The length of the residual medullary cavity refers to the length of the circular intramedullary space remaining at both ends of the resected diaphysis of long bones, which is capable of effectively fixing the prosthetic stem. This measurement excludes the trabecular bone at the metaphyseal ends. For example, in the femur, this corresponds to the segment of the medullary cavity between the lesser trochanter and the femoral condyles. The length of both the residual medullary cavity and the stem was measured from anteroposterior radiographs. A standard stem is defined as having a length greater than 10 cm, whereas a short stem is defined as one with a length of 10 cm or less. Furthermore, information regarding the use of extracortical plates was collected from surgical records and radiographic data. Based on these parameters, the diaphyseal prostheses were classified into distinct categories.
Functional outcome
The functional outcome was evaluated using the Musculoskeletal Tumor Society (MSTS) scoring system, which comprises numerical values from 0 to 5 points assigned for each of the following six categories: pain, level of activity and restriction, emotional acceptance, use of orthopaedic supports, walking ability, and gait. The total score ranges from 0 to 30, with higher scores indicating better functional recovery.
Postoperative complications
Postoperative complications were categorized based on the Henderson classification for limb salvage reconstruction failures, including five types, type 1: soft tissue failures, type 2: ASL for endoprostheses, type 3: structural failure, type 4: infection, type 5: tumor progression.
Survivorship of patients and prostheses
Given that patient death is a competing risk for prosthetic complications, a competing risk model was used to analyze the cumulative incidence of both mortality and complications.
Statistical analysis
Statistical analysis was conducted using R version 4.4.2 (R Foundation for Statistical Computing, Austria). Descriptive statistics, including frequency, percentage, mean, and standard deviation, were calculated. To compare differences, Student’s t-test was used for continuous variables, while Chi-square or Fisher’s exact tests were employed for categorical variables. A multivariate linear regression analysis was conducted to identify independent predictors of the MSTS score. Statistical significance was set at p < 0.05.
Results
General results
All 112 patients were included in the study and followed until death or their last examination, with a mean follow-up period of 24.1 months (range, 0.6–159 months). The mean operative time for all patients was 142.4 ± 41 min and the mean intraoperative blood loss was 658.8 ± 437.3 ml. The mean resection length across all patients was 106.8 ± 33.3 mm. Cementation was performed in 90.2% of cases (n = 101) to enhance initial stability, while the remaining 9.8% (n = 11) underwent non-cemented fixation.
Measurement index and classification
A total of 219 stems were implanted in 112 patients, including 112 standard stems, 107 short stems, and 5 without stems. Additionally, 63.4% of patients (74/112) received extracortical plate fixation. Based on the length of the residual medullary cavities after resection, diaphyseal tumors were classified into distinct categories, and different prosthetic reconstruction strategies were selected accordingly.
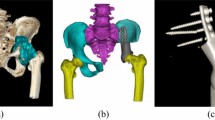
Type I: Both the proximal and distal residual medullary cavities of the lower extremity exceed 10 cm, necessitating a prosthesis with standard stems at both ends, without extracortical plates (Fig. 1).
Type lla: The proximal residual medullary cavity is less than 10 cm and the distal exceeds 10 cm, necessitating a prosthesis with a short stem combined extracortical plate at the proximal end, and a standard stem at the distal end (Figs. 2, 3).
Type llb: The proximal residual medullary cavity exceeds 10 cm and the distal cavity is less than 10 cm, necessitating a prosthesis with a standard stem at the proximal end and a short stem combined extracortical plate at the distal end (Figs. 4, 5).
Type llla: Both the proximal and distal residual medullary cavities are less than 10 cm, necessitating a prosthesis with short stems combined extracortical plates at both ends (Fig. 6).
Type lllb: In humeral reconstructions, regardless of residual medullary cavity length, necessitating a prosthesis with extracortical plates at both ends (Fig. 7).
Type lV: For cases with no residual medullary cavity but preserved epiphysis, necessitating a prosthesis with no stem at one end combined extracortical plates (Fig. 8).
According to the classification system, there were 33.9% (38) in Type I, 25.9% (29) in Type IIa, 11.6% (13) in Type IIb, 4.5% (5) in Type IIIa, 19.6% (22) in Type IIIb, and 4.5% (5) in Type IV. The outcomes based on this classification are summarized in Table 2.
Functional outcome
The mean Musculoskeletal Tumor Society (MSTS) score across all patients was 24.3 ± 3.3 (range, 15–29). The mean MSTS score across Type l, Type lla, Type llb, Type llla, Type lllb, Type lV was 23.2 ± 3.6, 24.9 ± 3.4, 23.9 ± 3.3, 24.8 ± 3.3, 25.3 ± 2.5, 25.6 ± 2.6. Multivariate linear regression showed that none of the factors, such as age, sex, location, resection length, pathological fracture at presentation, or cemented versus cementless prosthesis, were independent risk factors affecting limb function.
Postoperative complications
Among the cohort, 16% of patients (18/112) experienced complications, of which 7 cases underwent reoperation. Complications emerged at a mean of 19.5 months (range, 0.5–44 months), with one patient experiencing two complications.
In Type I diaphyseal tumors, 21% (8/38) of patients experienced complications, including five cases of ASL, one case of structural failure, and two cases of local recurrence. Two patients underwent reoperation due to ASL.
In Type IIa diaphyseal tumors, 10.3% (3/29) of patients experienced complications, including one case of ASL, one case of structural failure, and one case of infection. The patient with infection underwent reoperation for implant removal.
In Type IIb diaphyseal tumors, 15.4% (2/13) of patients experienced complications, including one case of structural failure. One patient experienced both soft tissue failure and local recurrence, ultimately requiring amputation.
In Type IIIa diaphyseal tumors, no complications were reported.
In Type IIIb diaphyseal tumors, 18.2% (4/22) of patients experienced complications, including three cases of ASL and one case of structural failure. One patient required revision due to disengagement of the Morse taper.
In Type IV diaphyseal tumors, 20% (1/5) of patients experienced complications, with the patient requiring amputation due to local recurrence of the tumor.
Survivorship of patients and prostheses
At the last follow-up, 59 patients (52.7%) had succumbed to the disease, with a median survival of 16 months. Among them, 8 of 26 patients (30.8%) with primary bone tumors and 51 of 86 patients (59.3%) with metastatic tumors had died. The mean follow-up duration was significantly longer for patients with primary bone tumors (37.6 months) compared to those with metastatic disease (20.1 months) (P = 0.023). Four patients died within three months postoperatively. Using the competing risk model with death as a competing risk, the cumulative mortality rates at 0.5, 1, 3, 5, and 8 years were 6.39%, 17.4%, 54.4%, 59.12%, and 66.47%, respectively, while the cumulative complication rates were 2.68%, 6.52%, 12.49%, 19.44%, and 22.51%, respectively (Fig. 9).
The competing risk model with 95% confidence with death as a competing risk reveals the cumulative mortality rates at 0.5, 1, 3, 5, and 8 years were 6.39%, 17.4%, 54.4%, 59.12%, and 66.47%, respectively, while the cumulative complication rates were 2.68%, 6.52%, 12.49%, 19.44%, and 22.51%, respectively.
Discussion
Despite numerous reports confirming the superior clinical outcomes of prosthetic treatment for diaphyseal tumors compared to biological reconstructions, which are commonly associated with complications such as infection and non-union, diaphyseal prostheses continue to carry a higher risk of mechanical failure1,3,5. This has contributed to a cautious approach by surgeons when considering prosthetic reconstruction for younger, more active patients. To address this issue, our study proposes a novel classification system for diaphyseal tumors, with the aim of optimizing prosthetic reconstruction strategies and ultimately improving clinical outcomes.
Diaphyseal prosthetic reconstruction has emerged as a reliable approach for the management of diaphyseal tumors. Several subsequent studies have reported favorable outcomes with this method. For instance, Joshua R. et al.19 reported a mean Musculoskeletal Tumor Society (MSTS) score of 70% in 43 patients who underwent humeral diaphyseal prosthesis reconstruction. Zheng et al.20 analyzed data from 49 patients with either primary or metastatic bone tumors, noting mean MSTS scores of 21.2 for femoral reconstructions, 19.6 for humeral, and 17.8 for tibial reconstructions. Johnson et al.4 examined 19 patients with diaphyseal metastases treated with resection and cemented intercalary endoprosthetic reconstruction and observed significant improvements in MSTS scores. In our series, we observed significant improvements in both pain relief and functional outcomes, with a mean MSTS score of 24.3 ± 3.3 points (range 15–29), which aligns closely with the results reported in previous studies. According to our new classification system for diaphyseal tumors, different types of prosthetic reconstructions exhibited similar good functional outcome.
One of the significant advantages of prosthetic reconstruction is the ability to achieve early weight-bearing21, a key benefit over biological reconstruction, which typically involves prolonged postoperative immobilization, significantly delaying the return to full weight-bearing. Clinical results presented by Bus et al.22 in a cohort of 87 patients undergoing intercalary allograft reconstruction showed a median time to full weight-bearing of 9 months. Similarly, studies by Deijkers et al.23 and San Julian Aranguren et al.24 reported mean consolidation times of 17 months for diaphyseal junctions and 13.4 months for metaphyseal junctions, underscoring the extended recovery periods associated with biological methods. Furthermore, systemic chemotherapy and radiotherapy have been shown to contribute to delayed consolidation. The early restoration of function is particularly critical when evaluating reconstructive techniques, especially in patients with limited life expectancy. Aldlyami et al.15 highlighted the importance of early functional recovery for patients with malignant tumors, with a median survival time of 23 months in their study. They suggested that allowing these patients to bear weight sooner would enable them to maintain a relatively normal quality of life during their remaining time. In our series, we encouraged early range-of-motion exercises for adjacent joints and progressive protective weight-bearing starting from the second postoperative day. Most patients in our cohort achieved full weight-bearing within three to four weeks post-surgery.
According to our novel classification system for diaphyseal tumors, different types of prosthetic reconstructions exhibited varying complication rates, with Type I having a complication rate of 21% and Type IIb having a rate of 15.4%, both of which are relatively high. However, due to the small sample sizes for some tumor types, statistical analysis to assess differences between groups was not performed. Published reports indicate that the complication rates associated with diaphyseal prosthetic reconstruction range from 14 to 50%2,3,8,9,10, with common complications including aseptic loosening and structural failure of the prosthesis. Hanna et al.11 reported that implant survival, with failure of the endoprosthesis as the endpoint, was 85% at five years and 68% (95% CI: 42% to 92%) at ten years in 23 patients who underwent limb salvage with endoprosthetic replacement of the femoral diaphysis for primary bone tumors. Compared to prosthetic reconstruction, biological techniques are often associated with higher complication rates, including risks of infection, non-union, pseudarthrosis, and fractures. Brunet et al.25 observed that the cumulative probability of bone union was only 46% at one year in cases involving femoral and tibial intercalary defects. Costantino et al.1 conducted a systematic review of intercalary reconstruction techniques following diaphyseal bone tumor resection, finding the incidence of ASL in diaphyseal prostheses was generally lower than that of non-union in allografts (0–33% vs. 6%–43%). In our cohort, 19 complications were documented in 18 patients, with a mean occurrence time of 19.5 months (range: 0.5–44 months). The overall reintervention rate in our series was 6.25% (7/112), which was relatively low, likely due to the poor overall survival of the cohort, with a median survival of 17 months. Mechanical failures, predominantly aseptic loosening (type II) and structural failure (type III), were the most frequent complications.
The diaphyseal prosthesis is an individually designed implant, and meticulous preoperative planning is essential to guarantee optimal outcomes. This process entails the input of tumor-related imaging data, including CT and MRI scans, into a computer system and the subsequent fusion of these data sets into three-dimensional digital graphics, which facilitates the accurate delineation of the tumor to be excised. The dimensions of the prosthesis and the size of the intramedullary stems, both at the distal and proximal levels, should be accurately delineated to ensure an optimal fit within the bone. It has been suggested that a high rate of ASL of endoprostheses is due to insufficient length of the remaining bone in prosthesis fixation, which reduces the bone-cement and implant-cement contact area, thereby decreasing the strength of the fixation26,27,28. Analyzing the causes of ASL, our data suggests that the anatomical and biomechanical mismatch between the metaphyseal sites and the standard stem configurations contributes to inadequate initial and long-term stability. The epiphysis is characterized by a funnel-shaped structure that lacks the narrow configuration of the diaphysis. Measurements of the femoral marrow cavity revealed a narrow zone between 2 cm below the lesser trochanter and 9 cm above the intercondylar fossa. In the broader region, the marrow cavity diameter ranged from 15 to 35 mm, while in the narrow region, the diameter was 12–15 mm. It is therefore crucial to accurately measure the diameter of the marrow cavity in the wider region via X-ray or CT to prevent the custom-made intramedullary stem from being undersized, which could lead to early prosthesis loosening. To minimise the risk of prosthesis loosening resulting from the substantial metaphyseal metrical cavity and shorter stem, a larger diameter of the intramedullary stem and the incorporation of an extra-cortical plate is employed if necessary. Biomechanical analysis of a novel diaphyseal prosthesis for reconstruction of humeral segmental defects has shown that auxiliary plate fixation enhances anti-tension and anti-torsion stiffness, reducing the risks of ASL and dislocation29. To solve the problem of ASL, extra-cortical plate fixation was used in 71(63.4%) cases of our study, resulting in a significantly lower incidence of ASL (9.8%) compared to other published studies, namely 28.6%16 and 38%30.
Local recurrence was confirmed histologically in four patients in our study, with local disease control rates lower than those in the existing literature13,31,32. The low recurrence rate may be attributed to most patients undergoing en bloc resection33. Preoperative planning for such resections is essential, and employing custom-made osteotomy guides—especially those produced with 3D printing technology—enhances the precision of osteotomy lines during surgery. For cases of metastatic lesions characterized by significant bleeding following fractures, where complete resection is challenging, postoperative radiotherapy coupled with additional adjuvant therapies targeting the primary disease can be effective in reducing recurrence.
In our cohort, four patients died within three months following surgery, highlighting the importance of refining patient selection criteria. Competing risk model analysis showed that cumulative mortality rates at 5 and 8 years were 59.1% and 66.5%, respectively, while cumulative complication rates were 19.4% and 22.5%, respectively. Although approximately 20% of patients developed complications within 5–8 years postoperatively, many prostheses remained functional without requiring revision, with only 8.9% (10/112) of prostheses reaching the endpoint. Hanna et al.11 reported prosthesis survival rates of 85% at five years and 68% at ten years among 23 patients who underwent endoprosthetic replacement of the femoral diaphysis post-resection for primary bone tumors. The relatively higher prosthesis survival observed in our series may be attributed to the application of extra-cortical plates and the fact that many patients succumbed to their disease before revision became necessary. At the last follow-up, 59 out of 112 (52.7%) patients had died of their disease, with a median survival of 16 months.
The present study acknowledges several limitations. Firstly, as a retrospective analysis, it is subject to selection and recall biases, particularly given the rarity of this procedure and the lack of standardized guidelines for managing segmental defects following diaphyseal tumor resection. The high cost of custom prostheses and 3D printing technology in certain regions may limit their widespread application. Additionally, the absence of a true control group, with evaluations limited to patients treated using diaphyseal prostheses, restricts the ability to directly compare outcomes with other reconstruction techniques, introducing potential assessment biases. Although comparisons with other studies employing various reconstruction methods were made, differences in institutional practices and patient demographics—which can significantly impact outcomes—were not controlled. Furthermore, while our cohort included 112 patients, which is larger than previous reports on diaphyseal prosthetic reconstruction, the small sample sizes in certain groups according to the new classification system prevented us from conducting effective statistical analysis.
Conclusion
In conclusion, diaphyseal prostheses represent a viable solution for reconstructing intercalary defects following bone tumor resections, offering advantages in promoting early weight-bearing and improving functional outcomes. The novel classification system for diaphyseal tumors provides valuable guidance for the design and application of prosthetic reconstructions, resulting in promising clinical outcomes.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Errani, C., Tsukamoto, S., Almunhaisen, N., Mavrogenis, A. & Donati, D. Intercalary reconstruction following resection of diaphyseal bone tumors: A systematic review. J. Clin. Orthop. Trauma 19, 1–10. https://doi.org/10.1016/j.jcot.2021.04.033 (2021).
Ahlmann, E. R. & Menendez, L. R. Intercalary endoprosthetic reconstruction for diaphyseal bone tumors. J. Bone Joint Surg. Br.Vol. 88, 1487–1491. https://doi.org/10.1302/0301-620x.88b11.18038 (2006).
Sewell, M. D. et al. Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumors. J. Bone Joint Surg. Br Vol. 93, 1111–1117. https://doi.org/10.1302/0301-620x.93b8.25750 (2011).
Johnson, J. D. et al. What Is the Prosthetic Survival After Resection and Intercalary Endoprosthetic Reconstruction for Diaphyseal Bone Metastases of the Humerus and Femur?. Clin. Orthop. Relat. Res. 481, 2200–2210. https://doi.org/10.1097/corr.0000000000002669 (2023).
Feltri, P. et al. Union, complication, reintervention and failure rates of surgical techniques for large diaphyseal defects: A systematic review and meta-analysis. Sci. Rep. https://doi.org/10.1038/s41598-022-12140-5 (2022).
Galante, J. & Rostoker, W. Fiber metal composites in the fixation of skeletal prosthesis. J. Biomed. Mater. Res. 7, 43–61. https://doi.org/10.1002/jbm.820070305 (1973).
Lempberg, R. & Ahlgren, O. Prosthetic Replacement of Tumor-Destroyed Diaphyseal Bone in the Lower Extremity. Acta Orthop. Scand. 53, 541–545. https://doi.org/10.3109/17453678208992254 (1982).
Damron, T. A. et al. Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin. Orthop. Relat. Res. https://doi.org/10.1097/00003086-199603000-00029 (1996).
Abudu, A., Carter, S. R. & Grimer, R. J. The outcome and functional results of diaphyseal endoprostheses after tumor excision. J. Bone Joint. Surg. Br. 78, 652–657 (1996).
Damron, T. A., Leerapun, T., Hugate, R. R., Shives, T. C. & Sim, F. H. Does the Second-generation Intercalary Humeral Spacer Improve on the First?. Clin. Orthop. Relat. Res. 466, 1309–1317. https://doi.org/10.1007/s11999-008-0246-z (2008).
Hanna, S. A. et al. Femoral diaphyseal endoprosthetic reconstruction after segmental resection of primary bone tumors. J. Bone Joint Surg. Br.Vol. 92, 867–874. https://doi.org/10.1302/0301-620x.92b6.23449 (2010).
Benevenia, J. et al. Outcomes of a Modular Intercalary Endoprosthesis as Treatment for Segmental Defects of the Femur, Tibia, and Humerus. Clin. Orthop. Relat. Res. 474, 539–548. https://doi.org/10.1007/s11999-015-4588-z (2016).
Zhao, J. et al. Intercalary prosthetic reconstruction for pathologic diaphyseal humeral fractures due to metastatic tumors: Outcomes and improvements. J. Shoulder Elbow Surg. 27, 2013–2020. https://doi.org/10.1016/j.jse.2018.03.027 (2018).
Wang, J. et al. Is three-dimensional–printed custom-made ultra-short stem with a porous structure an acceptable reconstructive alternative in peri-knee metaphysis for the tumorous bone defect?. World J. Surg. Oncol. https://doi.org/10.1186/s12957-021-02355-7 (2021).
Aldlyami, E., Abudu, A., Grimer, R. J., Carter, S. R. & Tillman, R. M. Endoprosthetic replacement of diaphyseal bone defects Long-term results. Int. Orthop. 29, 25–29. https://doi.org/10.1007/s00264-004-0614-6 (2005).
Streitbürger, A., Hardes, J., Nottrott, M. & Guder, W. K. Reconstruction survival of segmental megaendoprostheses: a retrospective analysis of 28 patients treated for intercalary bone defects after musculoskeletal tumor resections. Arch. Orthop. Trauma Surg. 142, 41–56. https://doi.org/10.1007/s00402-020-03583-4 (2020).
Albergo, J. I. et al. Failure rates and functional results for intercalary femur reconstructions after tumor resection. Musculoskelet. Surg. 104, 59–65. https://doi.org/10.1007/s12306-019-00595-1 (2019).
Enneking, W. F., Spanier, S. S. & Goodman, M. A. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res, 106–120 (1980).
Labott, J. R. et al. Durability of intercalary endoprosthesis for humeral reconstruction. J. Surg. Oncol. 129, 410–415. https://doi.org/10.1002/jso.27458 (2023).
Zheng, K. et al. Outcome of segmental prosthesis reconstruction for diaphyseal bone tumors: A multi-center retrospective study. BMC Cancer https://doi.org/10.1186/s12885-019-5865-0 (2019).
Liu, W. et al. Three-dimensional-printed intercalary prosthesis for the reconstruction of large bone defect after joint-preserving tumor resection. J. Surg. Oncol. 121, 570–577. https://doi.org/10.1002/jso.25826 (2020).
Bus, M. P. et al. Intercalary allograft reconstructions following resection of primary bone tumors: A nationwide multicenter study. J. Bone Joint Surg. Am. 96, e26. https://doi.org/10.2106/JBJS.M.00655 (2014).
Deijkers, R. L. et al. Epidiaphyseal versus other intercalary allografts for tumors of the lower limb. Clin. Orthop. Relat. Res. 439, 151–160. https://doi.org/10.1097/00003086-200510000-00029 (2005).
Aranguren, M. S., Leyes, M., Mora, G. & Canadell, J. Consolidation of massive bone allografts in limb-preserving operations for bone tumors. Int. Orthop. 19, 377–382. https://doi.org/10.1007/BF00178354 (1995).
Brunet, O. et al. Intercalary defects reconstruction of the femur and tibia after primary malignant bone tumor resection. A series of 13 cases. Orthop. Traumatol. Surg. Res. 97, 512–519. https://doi.org/10.1016/j.otsr.2011.03.021 (2011).
Fuchs, B., Ossendorf, C., Leerapun, T. & Sim, F. H. Intercalary segmental reconstruction after bone tumor resection. Eur. J. Surg. Oncol. (EJSO) 34, 1271–1276. https://doi.org/10.1016/j.ejso.2007.11.010 (2008).
McGrath, A. et al. Custom endoprosthetic reconstruction for malignant bone disease in the humeral diaphysis. Acta Orthop. Belg. 77, 171–179 (2011).
Waanders, D., Janssen, D., Mann, K. A. & Verdonschot, N. The mechanical effects of different levels of cement penetration at the cement-bone interface. J. Biomech. 43, 1167–1175. https://doi.org/10.1016/j.jbiomech.2009.11.033 (2010).
Zhao, L. et al. Biomechanical Analysis of a Novel Intercalary Prosthesis for Humeral Diaphyseal Segmental Defect Reconstruction. Orthop. Surg. 10, 23–31. https://doi.org/10.1111/os.12368 (2018).
Zhao, Z. et al. Intercalary prosthetic replacement is a reliable solution for metastatic humeral shaft fractures: retrospective, observational study of a single center series. World J. Surg. Oncol. https://doi.org/10.1186/s12957-021-02250-1 (2021).
Huang, H. et al. Outcomes of Intercalary Prosthetic Reconstruction for Pathological Diaphyseal Femoral Fractures Secondary to Metastatic Tumors. Orthop. Surg. 9, 221–228. https://doi.org/10.1111/os.12327 (2017).
Pu, F., Zhang, Z., Wang, B., Liu, J. & Shao, Z. En bloc resection and intercalary prosthesis implantation for the treatment of humeral diaphyseal bone metastases. Int. Orthop. 45, 281–288. https://doi.org/10.1007/s00264-020-04845-x (2020).
Shahzad, F. et al. Tandem Reconstruction of the Femoral Diaphysis Using an Intercalary Prosthesis and a Fibular Free Flap. J. Bone Joint Surg. 106, 425–434. https://doi.org/10.2106/jbjs.23.00211 (2024).
Acknowledgements
We appreciate all the participants, local medical workers in the study sites for their collaboration and assistance.
Funding
This work was supported by Weifang Health Commission Research Project (WFWSJK-2022–045), Weifang Science and Technology Development Programme Project (2024YX012) and Weifang Municipal Health Commission Chinese Medicine Research Project (WFZYY2024-1–009).
Author information
Authors and Affiliations
Contributions
L. Mou: Data collection, Patient follow-up, Writing paper. M. Zhang: Editing paper. H. Wang: Editing paper. J. Zhang: Data collection and analysis. D. Tian: Data collection and analysis. Y. Hu: Design of study. D. Lun: Design of study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board/Ethics Committee of Tianjin Hospital in accordance with the Declaration of Helsinki(2024YLJY-020). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Human research participants provided written informed consent for publication of identifying images.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mou, L., Zhang, M., Wang, H. et al. Classification of diaphyseal tumors based on residual medullary cavity length for prosthetic reconstruction. Sci Rep 15, 28118 (2025). https://doi.org/10.1038/s41598-025-12513-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12513-6