Abstract
This study reports the synthesis and characterization of Na2Ca4(PO4)3F phosphor doped with Dy3+, Tb3+, and Eu3+ ions were synthesized using a conventional solid-state method. The phase formation was confirmed by X-ray diffraction (XRD). Functional group analysis through Fourier transforms infrared spectroscopy (FTIR) and morphological evaluation via scanning electron microscope (SEM) and EDS revealed good crystallinity and elemental distribution. The triple-doped phosphor exhibited color-tunable emissions under near-UV excitation, covering the blue, green, yellow, and red regions, resulting in efficient white light output. Photoluminescence (PL) studies indicated effective energy transfer among the dopant ions. Additionally, the phosphor was applied as a down-conversion layer on silicon solar cells using the doctor blade technique. The coated cells showed a 16.33% improvement in efficiency compared to uncoated cells. The combined optical and photovoltaic performance highlights the potential of Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ as a multifunctional material for white light-emitting diodes and solar energy devices.
Similar content being viewed by others
Introduction
The growing global energy demand, driven by population growth and technological advancement, necessitates the development of sustainable, efficient, and eco-friendly energy solutions. Among the various innovations in lighting technologies, white light-emitting diodes (WLEDs) have garnered considerable attention due to their high luminous efficacy, long operational lifespan, low power consumption, and environmental safety. These attributes make WLEDs a promising alternative to conventional fluorescent and incandescent lighting sources1,2,3,4,5. The most common approach to produce white light in WLEDs involves combining a blue-emitting InGaN chip with a yellow-emitting YAG: Ce3+ phosphor. Despite its success, this combination suffers from limitations such as a high correlated color temperature (CCT), low color rendering index (CRI), and undesirable blue light leakage, potentially causing photobiological risks6,7,8. An alternative strategy involves ultraviolet (UV) excitation of RGB (red-green-blue) phosphors to achieve better color balance. However, this method often leads to light reabsorption, spectral mismatch, and complex fabrication processes due to multilayered coatings8,9. To overcome these drawbacks, researchers have focused on single-component phosphors capable of generating white light under near-UV or blue excitation. This approach offers advantages such as simpler device design, better thermal stability, and reduced fabrication costs10,11,12. In this context, rare-earth (RE³⁺)-doped inorganic phosphors are extensively studied for their sharp line emissions, tunable photoluminescence, and excellent thermal and chemical stability.
Among them, Dy³⁺, Tb³⁺, and Eu³⁺ ions are ideal candidates due to their complementary emissions in the blue, green, yellow, and red regions, enabling full-spectrum white light emission when appropriately combined13,14. Phosphate-based host materials such as Na2Ca4(PO4)3F have attracted increasing attention due to their excellent thermal stability, strong absorption in the UV region, high chemical resistance, and ability to accommodate multiple rare-earth dopants13,15,16,17. Furthermore, fluoride incorporation into the phosphate matrix reduces the phonon energy, which suppresses non-radiative losses and enhances luminescence efficiency16.
Considering the limited studies on Na2Ca4(PO4)3F as a host material, the present investigation focuses on the synthesis and characterization of Dy3+, Tb3+, and Eu3+ doped, co-doped, and triple-doped phosphor using a conventional solid-state diffusion method. The primary objective is to explore the luminescence behavior and energy transfer mechanisms among these rare earth ions to achieve tunable and efficient white light emission. Structural, morphological, vibrational, and thermal analyses are carried out to understand the material suitability for lighting applications. Furthermore, to assess its potential in photovoltaic applications, the phosphor was coated on silicon solar cells, and the resulting device performance was evaluated. This dual-functional approach aims to demonstrate the viability of Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ as a promising single-phase material for both next-generation WLEDs and enhanced solar energy conversion.
Even though there has been a lot of progress over the years, but still required highly effective and reasonably priced solar converters18. The world population on the rise, the energy demand has also increased, leading to growing concern about the impact of greenhouse gas emission on climate change19. There is strong motivation among researchers across the globe to explore alternative and sustainable methods of energy production. So here, numerous scientists and researchers published research papers on enhancement of solar efficiency of down-converting inorganic phosphor by dye-sensitized solar cells (DSSCs). Nannan Yao and co-authors et al. presented on the ZnO: Eu3+, Dy3+ down-conversion synthesized by precipitation method and used to prepare the photo anode of dye-sensitized solar cells20. Xiuting Luo et al. explored on Aerosol synthesis and luminescent behavior of CaAl2O4:Eu2+, Nd3+ down-conversion material for enhanced light harvesting of dye sensitized solar cells19. Toshi S. Dhapodkar and other et al. reported on Dy3+, Tb3+, Eu3+ activated/co-activated/triple-activated Mg21Ca4Na4(PO4)18 prepared by melt quenching technique21. Abhijeet R. Kadam et al. studied on Energy transfer mechanism of KAlF4:Dy3+, Eu3+ co-activated down-conversion phosphor sol gel method22. Solar energy is one of the many sources of renewable energy providing an abundant, Eco-friendly and noiseless source of power that has vast potential in meeting the growing global energy consumption demand23,24. The utilization of dye-sensitized solar cells (DSSCs) is an effective method of generating electricity for indoor electronic devices like wireless sensors. DSSCs are known for their affordability and accessibility as well as their capacity to produced lightweight and flexible solar module25,26.
In this work, the series of newly synthesized triple-doped Dy3+/Tb3+/Eu3 + of Na2Ca4(PO4)3 F phosphor with different concentration of dopants was successfully prepared by using traditional solid-state synthesis route. The analysis of properties and possible application of prepared phosphor in WLEDs and photovoltaics is presented below.
Experimental methods
Material preparation
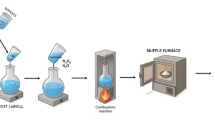
To synthesize powder sample of Na2Ca4(PO4)3F phosphor dopped with Dy3+, Tb3+, Eu3+, a conventional high temperature solid state diffusion method was employed. The scheme of samples preparation is shown on Fig. 1. Table 1. Gives an account of all the precursors used for the preparation of phosphor along with their purity and their sources.
These materials were weighed according to stochiometric proportion and ground together in an agate mortal pestle until a fine mixture was obtained. the resulting homogenous mixture transferred to an alumina crucible and sintered at 800 °C for 24 h in muffle furnace under an air atmosphere. Once the product was obtained, it was furnace cooled to room temperature and ground again to obtain fine powders, which were then subjected to further characterization. Tables 2, 3 and 4 provide the stochiometric amount of the compounds employed in the synthesis of every series presented in this study. The balanced chemical equation for the solid-state synthesis of prepared material;
Na2CO3 + 4CaCO3 + 3NH4H2PO4 + NH4F → Na2Ca4(PO4)3F + 4NH3 + 5CO2 + 5H2O
Instrumentation used
In order to access the phase purity of prepared phosphor Na2Ca4(PO4)3F: Dy3+, Tb3+, Eu3+, powder X-ray diffraction (XRD) pattern was obtain using Cu Kα radiation (λ = 1.5405 Å) and analyzed on a RIGAKU mini flex 600. Fourier transform infrared spectroscopy (FTIR) instrument BRUKER ALPHA II was employed to determine the vibrational characteristics of the sample. Photoluminescence (PL) measurement including excitation and emission spectra as well as lifetime, were conducted using SHIMADZU spectroflurophotometer RF 5301. The morphological properties of the Na2Ca4(PO4)3F: Dy3+, Tb3+, Eu3+ phosphor was analyzed using scanning electron microscope (SEM) EVO18 ZEISS. Additionally, the light and color coordinate parameter were determined by using Commission de I Eclairage (CIE) chromaticity coordinate diagram. The efficiency of the solar cell was evaluated by comparing the I-V characteristics of the coated cell to that of a blank cell with the help of JETSPIN LED SOLAR SIMULATOR.
Results and discussion
Phase and structural identification
The phase purity and structural identification of newly synthesized triple doped Na2Ca4(PO4)3F: xDy3+/Tb3+/Eu3+ (x = 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mol %) phosphor by high temperature solid state method at 8000C were assessed by using XRD. The XRD spectra of obtained sample recorded in the range of 20o – 80o with a step size of 0.02o. The XRD pattern of undoped and doped Na2Ca4(PO4)3F: xDy3+/Tb3+/Eu3+ phosphor with different concentration displayed in Fig. 2. The XRD pattern of the synthesized phosphor shows prominent diffraction peaks at 2θ = 25.8°, 31.7°, 32.9°, 34.7°, 39.6°, 46.5° and 49.3°, which can be assigned to the (0 0 12), (1 1 9), (0 2 7), (2 0 8), (2 1 4), (0 0 21), and (0 3 9) planes respectively. Here, all the positions and diffraction peaks of proposed sample are well treated with standard Inorganic Crystal Structure Database (ICSD) data with reference no. is 00-033-1228. The obtained material is completely crystalline nature in a Rhombohedral system with the space group R-3 m, with no other phases or allotropic phases will observe which indicating a pure crystalline substance was achieved. The intense diffraction peaks in synthesized material as made have a high crystalline character which is good for producing outstanding WLEDs.
Functional group examination
Figure 3. depicted the FTIR spectrum of the phosphor sintered at 800oC by using solid state diffusion method with measurement within the range of (3500 − 500 Cm−1) at room temperature. The spectrum provided valuable insights into the actual formation and identification of functional groups present in the prepared phosphor27,28,29. The FTIR spectrum shows that there are five bands that will be observed at 545 cm−1, 611 cm−1, 1011 cm−1, 1119 cm−1 and 1428 cm−1. The band in the range between 545 cm−1 to 615 cm−1 is due to the ν₃ asymmetric stretching mode of PO43− groups. The shoulder between 1000 cm−1 to 1200 cm−1 is assigned to the symmetric stretching mode v1 (P-O-P) of (PO4)3 groups30. The absorption peak at 1428 cm−1 is assigned to the asymmetric stretching mode (ν3) of residual carbonate (CO32−) groups, which likely originated from incomplete decomposition of carbonate-based precursors such as CaCO3 or Na2CO3 during the high-temperature solid-state synthesis process. Here there is no OH molecule peak in the proposed material was not observed, likely due to the preparation method involving high-temperature solid-state reaction16,17.
Photoluminescence study (PL)
Photoluminescence of single doping in phosphor
Na 2 Ca 4 (PO 4 ) 3 F: Dy 3+ phosphor
Figure 4(a). Displays the PL excitation spectrum of the Na2Ca4(PO4)3F: Dy3+ phosphor with emission wavelength 482 nm. The dominant excitation peak of the sample comes at 351 nm followed by a secondary peak at 365 nm. These peaks correspond to electron transition within Dy3+, specifically 6H15/2-6P7/2 and 6H15/2-6P5/2 respectively31,32,33. Additionally, Dy3+ exhibit characteristic excitation peaks at 325 nm, 388 nm and 428 nm corresponding to the electron transitions 6H15/2 - 4K15/2, 6H15/2− 4M21/2 and 6H15/2−4G11/2. Figure 4(b-d) illustrates the PL emission spectra of Na2Ca4(PO4)3F: xDy3+ phosphor (x = 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mol %) at 351 (Fig. 4.b), 365 (Fig. 4.c) and 388 nm (Fig. 4.d) between the 450–600 nm range. Here, there are two prominent emission peaks observed. One at 482 nm in the blue band and another at 574 nm in the yellow band. These peaks correspond to the electron transition 4F9/2 - 6H15/2 and 4F9/2 - 6H13/2 respectively34,35,36.
(a) Shows PL excitation spectra of synthesized Na2Ca4(PO4)3F: Dy3+ phosphor with different varying concentration located at emission 482 nm. Figure 4. (b-d) The PL emission spectra of synthesized Na2Ca4(PO4)3F: Dy3+ phosphor with different varying concentration located at excitation b) 351 nm, c) 365 nm, and d) 388 nm.
Na 2 Ca 4 (PO 4 ) 3 F: Tb 3+ phosphor
The Tb3+ ion doping in Na2Ca4(PO4)3F fluorophosphate phosphor material has been used in a similar PL study. Figure 5. Displays the excitation and emission spectra at 545 nm (Fig. 5.a) and 378 nm (Fig. 5.b) of the Na2Ca4(PO4)3F: Tb3+ phosphors. The excitation peaks observed in the spectra are attributed to f-d and f-f transition of the Tb3+ ions from the ground state to higher excited state. Specifically, the excitation peaks are observed at wavelength 340, 369 and 378 nm. The pecks at 378 nm originate from lower 7F6 transition to higher state 5G6. Among these excitation peaks, the two most intense peaks occur at 369 nm and 378 nm as shown in Fig. 5.a. When the excitation wavelength is set at 378 nm (Fig. 5.b), the emission spectra of Na2Ca4(PO4)3F: Tb3+ in the range of 400–600 nm are recorded. In the emission spectra, all peaks corresponding to the transition from the 5D state to the 7 F state have the same position except for differences in intensity. The emission pecks at 380, 415, 438 nm correspond to the transition 5D3 - 7F6,5,4, while the peaks at 490, 545, 590 nm correspond to the transitions 5D4 - 7F6,5,4 respectively37,38,39,40. The peak at 545 nm exhibits relatively high intensity, indicating strong emissions in the green region.
(a) The PL excitation spectra of synthesized Na2Ca4(PO4)3F: Tb3+ phosphor with different varying concentration located at emission 545 nm. Figure 5. (b) The PL emission spectra of synthesized Na2Ca4(PO4)3F: Dy3+ phosphor with different varying concentration located at excitation b) 378 nm.
Na 2 Ca 4 (PO 4 ) 3 F: Eu 3+ phosphor
Figure 6. (a) Represents the PL excitation spectrum of Na2Ca4(PO4)3F: Eu3+ with monitoring at 616 nm. The excitation spectrum can be divided into two sections. Firstly, there is a wide range of wavelengths from 200 to 300 nm, forming a broad band. This band is attributed to the charge transfer band (CTB) from O2− to Eu3+41,42,43,44. Secondly, there are distinct excitation peaks ranging from 300 to 600 nm. These sharp peaks are associated with the forbidden intra − 4f transitions of Eu3+. Specifically, the peaks occur at 363 nm (7F0→5D4), 383 nm (7F0→5G3), 395 nm (7F0→5L6), 416 nm (7F0→5D3), and 465 nm (7F0→5D2)45,46. Among these transitions, the 7F0→5L6 transition at 395 nm is the most pronounced that spectra indicating that the Na2Ca4(PO4)3F: Eu3+ compound holds promise as a phosphor for near-ultraviolet (n-UV) based LEDs. The PL emission spectra Na2Ca4(PO4)3F: Eu3+ phosphor located at different excitation wavelengths 246 nm, 395 nm and 466 nm presented in Fig. 6(b-d). The emission spectra exhibit distinct peaks ranging from 570 to 650 nm, which can be attributed to the intra - configurational f-f transition of Eu3+. The PL spectrum primarily comprises two intense and sharp pecks at 595 and 616 nm, corresponding to the characteristics transition of Eu3+ ions due to 5D0 - 7FJ (J = 1,2) respectively47,48,49,50,51,52. The most prominent emission peak at 616 nm corresponds to the 5D0 - 7F2 transition.
(a) The PL excitation spectra of synthesized Na2Ca4(PO4)3F: Tb3+ phosphor with different varying concentration located at emission 616 nm. Figure 6(b-d). The PL emission spectra of synthesized Na2Ca4(PO4)3F: Eu3+ phosphor with different varying concentration located at excitation b) 246 nm, c) 395 nm, and d) 466 nm.
Basically, the concentration quenching mechanism is related with non-radiative energy transfer and this phenomenon completely depends on the critical transfer distance (RC) among Dy3+, Tb3+ and Eu3+ ions. So here, the photoluminescence emission intensity of rare earth ions goes on increasing with respect to the enhancing the concentration up to 2.5 mol % Dy3+, Tb3+ and Eu3+ after that emission intensity is decreasing because of concentration quenching mechanism displayed in Fig. 7(a-c). Blasse formula is used to calculate the Critical transfer distance (RC) as below53,54,55:
Where, V denotes unit cell of volume, Xc represents the highest concentration of Dy3+, Tb3+ and Eu3+ ions and N is the number of available sites for the activator in the unit cell. According to standard ICSD file, the unit cell volume is equal to 1787.64, N = 6 and the value is Xc = 2.5 mol % Dy3+, Tb3+ and Eu3+ ions. putting all these parameter and constant values in above equation then we got the Rc value is 28.344 Å which value is greater than 5. Therefore, we conclude that multipole interactions are responsible for the concentration quenching in non-radiative transition between Na2Ca4(PO4)3F and rare-earth ions.
To identify the specific type of multipolar interaction, Förster–Dexter theory was employed and expressed as;
taking the logarithm of both sides:
Where, I is the emission intensity, X is the activator ion concentration, K and β are constants, and θ is an empirical parameter that characterizes the nature of the interaction including exchange interaction, dipole-dipole (d-d), dipole-quadrupole (d-q) and quadrupole-quadrupole (q-q) interactions for θ = 3, 6, 8 and 10 respectively. This linear fit graph is plot between log (X) Vs log (I/X) yields a straight line with a slope of -θ/3 as shown in Fig. 8. (a-c).
The slope of this fit is −0.7887, −0.9708 and − 0.1.1218. The calculated slope from the experimental data corresponds to θ = 2.366, 2.912 and 3.365, indicating that the exchange interaction is the dominant mechanism responsible for concentration quenching in the Dy3+/Tb3+/Eu3+ doped Na2Ca4(PO4)3F phosphors.
Optical behavior based on co-doping and triple doping
Na 2 Ca 4 (PO 4 ) 3 F: Dy 3+ /Tb 3+ co-activated phosphor
Examining the criteria of spectrum overlapping is crucial to determining the requirements for energy transfer for rare earth ions56 as shown in Fig. 9. There are three types of overlapping for energy transfer mechanism namely as excitation-excitation overlap, emission-emission overlap and excitation-emission overlap11. The PL excitation spectrum of Na2Ca4(PO4)3F: 2.5 mol % of Dy3+, xTb3+ (x = 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mol %) co-doped synthesized phosphor monitored at emission wavelength 545 nm displayed in Fig. 10. (a). From this excitation spectra show, there are three intense peaks that will be observed at 351 nm, 365 nm and 378 nm, respectively. Figure 10. (b-d) Shows the PL emission spectra of Dy3+/Tb3+ co-activated Na2Ca4(PO4)3F with different varying concentration excited by 351 nm, 365 nm and 378 nm in the range from 400 to 600 nm. From this emission spectrum shows several prominent peaks of Dy3+ centered at 490 nm and 576 nm corresponding to the 4F9/2 → 6H15/2, 4F9/2 → 6H13/2 respectively and 545 nm spectra due to 5D4→7F5 transition of Tb3+ ions. It is evident that when the concentration of Dy3+ ions increases, the PL intensity of Tb3+ ions continues to decrease. These results indicate that the emission in these spectra changes from blue/yellow to green. As a result, energy is transferred from Dy3+ to Tb3+.
(a) The PL excitation spectra of synthesized Na2Ca4(PO4)3F: Dy3+/Tb3+ phosphor with different varying concentration located at emission 545 nm. Figure 10. (b-d) The PL emission spectra of synthesized Na2Ca4(PO4)3F: Dy3+/Tb3+ phosphor with different varying concentration located at excitation b) 351 nm, c) 365 nm, d) 378 nm.
Na 2 Ca 4 (PO 4 ) 3 F: Dy 3+ /Eu 3+ co-activated phosphor
The synthesized co-activated phosphor material has been investigated by keeping one lanthanide ion constant with highest intensity and other lanthanide ions is different concentration. So, in this scenario excitation overlapping is possible by Dy3+ excitation and Eu3+ excitation in proposed synthesized material. The PL excitation band of 2.5 mol % of Dy3+, xEu3+ (x = 0.5, 1.0, 1.5,2.0,2.5 and 3.0 mol %) co-doped Na2Ca4(PO4)3F phosphor monitoring at 616 nm depicted in Fig. 11. (a). In this scenario, 395 nm excitation wavelength is most prominent intense peaks as compared to other spectra like 465 nm, 535 nm and 590 nm respectively. So here we plot the emission spectrum at excitation wavelength 395 nm. Figure 11. (b) illustrate the PL emission spectra of Na2Ca4(PO4)3F: 2.5 mol % Dy3+, x mol % Eu3+ (x = 0.5, 1.0, 1.5,2.0,2.5 and 3.0 mol %) material located at excitation wavelength 395 nm. From this emission spectra, there are four prominent peaks of Dy3+ and Eu3+ will be observed here at 482 nm and 576 nm respectively which corresponding to 4F9/2 → 6H15/2 and 4F9/2 → 6H13/2 transitions of Dy3+ while the peak located at 590 and 616 nm attributed to 5D0 – 7F1 and 5D0 –7F2 transition of Eu3+ ions. It can be clearly seen that the photoluminescence intensity of Dy3+ ions goes on diminishing with respect to the enhancing concentration of Eu3+ ions. These findings show that these spectra shift from blue/yellow to orange/red emission. This results in the transfer of energy from Dy3+ to Eu3+.
(a) PL excitation spectra of synthesized Na2Ca4(PO4)3F: Dy3+/Eu3+ phosphor with different varying concentration located at emission 616 nm. Figure 11. (b) PL emission spectra of synthesized Na2Ca4(PO4)3F: Dy3+/Eu3+ phosphor with different varying concentration located at excitation b) 395 nm.
Na 2 Ca 4 (PO 4 ) 3 F: Tb 3+ /Eu 3+ co-activated phosphor
Figure 12. Displayed the PL emission spectra of synthesized Na2Ca4(PO4)3F: 2.5 mol % Tb3+, x mol % Eu3+ (x = 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mol %) phosphor excited by 378 nm. So therefore, under this situation, excitation overlapping between Tb3+ and Eu3+ excitation in the suggested proposed material is achievable. From this spectrum we observe that two intense peaks will observe at 490 and 545 nm corresponding to the 5D4-7F6,5 of Tb3+ rare earth ions and another two peaks will identify in the same emission spectra located at 590 and 612 nm due to 5D0 – 7F1 and 5D0 –7F2 of Eu3+ ions. From Fig. 12, The concentration of Eu3+ ions rises, the emission of Eu3+ increases while the PL emission intensity of Tb3+ decreases. This mechanism shows the complete energy transfer from Tb3+ to Eu3+.
Na 2 Ca 4 (PO 4 ) 3 F: Dy 3+ /Tb 3+ /Eu 3+ triple-doped phosphor
The energy transfer in lanthanide doped co-activated has been analyzed for Dy3+/Tb3+, Dy3+/Eu3+, and Tb3+/Eu3+ co-doped synthesized phosphor as previously discussed in detailed above. The study found that energy was transferred from Dy3+ to Tb3+, Eu3+, and Tb3+ to Eu3+. To achieve perfect white light emission, triple-doping energy transfer was done from Dy3+ to Tb3+ to Eu3+. In this present scenario, the highest concentration of Dy3+ and Tb3+ were held constant, whereas the concentration of Eu3+ changed. Figure 13. (a) displayed the PL excitation spectra of doped with Dy3+/Tb3+/Eu3+ doped Na2Ca4(PO4)3F synthesized material with different concentration under emission wavelength 616 nm. From this PL excitation spectra, there are number intense and prominent excitation peaks will be observed around at 350 nm, 365 nm, 370 nm, 382 nm, 395 nm, 420 nm, 465 nm, 527 nm and 535 nm respectively of Dy3+/Tb3+/Eu3+ rare earth ions. Figure 13. (b-d) shows the PL emission spectra synthesized Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor monitored at different excitation wavelength under 365 nm, 382 nm and 395 nm. From emission plot we observe that there is multiple emission peaks will be showing around at 482 nm (blue spectra) due to 4F9/2→ 6H15/2 transition of Dy3+ ions, 545 nm (green spectra) due to 5D4 →7F5 transition of Tb3+ ions, 575 nm (yellow spectra) due to 4F9/2→6H13/2 transition of Dy3+ ions, Small peaks will be observe at 595 nm (orange spectra) and 616 nm (red spectra) are caused by the 5D0→7F1 and 5D0→7F2 transitions of Eu3+ ions, respectively. Figure 14. PL emission spectra of synthesized Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor of optimum concentration. So, the suggested triple doped synthesized Na2Ca4(PO4)3F: Tb3+/Eu3+ phosphor emits full white light when all these photoluminescence emissions spectra are combined into a single spectrum. Based on the above presented research, the energy transfer mechanism was proposed and it is displayed on Fig. 15. it is explained with the help of works energy level diagram.
(a) PL excitation spectra of synthesized Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor with different varying concentration located at emission 616 nm. Figure 13. (b-d) PL emission spectra of synthesized Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor with different varying concentration located at excitation (a) 365 nm, (b) 382 nm, (c) 395 nm.
Morphological features and energy dispersive x-ray spectroscopy
SEM is a widely employed method for characterizing phosphor material. In this study, both the SEM image and energy dispersive x-ray spectroscopy (EDS) pattern were utilized to examine the morphological properties and compositions of the synthesized sample10. Figures 15 and 18(a-d). Represents the SEM image of Na2Ca4(PO4)3F and Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ sample at room temperature. Clearly, the samples are made up of anomalous microparticles with size varies from 2 to 50 μm. The microparticles are composed of aggregates of small and irregular shaped particles which look like stacking platelet-like particles. Further, the irregular and granular layered structure indicates the crystallographic nature of the powder57,58,59. Figure 17. Displays the energy dispersive X-ray spectroscopy (EDS) spectrum and elemental mapping Na2Ca4(PO4)3F phosphor, which indicates the purpose of confirming the composition of the prepared phosphor. As we can see that, the presence of distinct and intense peaks corresponding to Na, Ca, P, O and F in the spectra validated the successful synthesis of Na2Ca4(PO4)3F prepared phosphor. Figure 19. Show the EDS spectrum and elemental mapping of Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor. From this figure, The EDAX and elemental mapping image of the triple-doped sample reveals a uniform and homogeneous distribution of all constituent elements across the analyzed region. The clear and consistent signals from the dopant elements confirm their successful incorporation into the host matrix. This uniform dispersion supports the formation of a single-phase material and indicates effective synthesis, which is essential for achieving consistent optical and structural properties in the doped phosphor system.
TGA-DTA analysis
Thermogravimetric and Differential thermal analysis (TGA-DTA) instrument used to evaluate the thermal stability60 of obtained Na2Ca4(PO4)3F sample. Figure 20. depict the TGA-DTA plot of proposed Na2Ca4(PO4)3F phosphor measured from room ambient temperature up to 500 °C. The TGA curve exhibited minimum weight loss up to approximately 250 °C, indicating the sample stability within this range. A slight weight loss observed beyond this temperature is likely due to the decomposition of residual impurities or structural changes within the material. The DTA curve showed an endothermic/exothermic peak at 200 °C, suggesting a phase transition or thermal decomposition event. Overall, the data confirm that Na2Ca4(PO4)3F possesses good thermal stability making it suitable for high-temperature applications.
Photo chromaticity analysis
The CIE chromaticity coordinates play a crucial role in evaluating the luminous properties of synthesized material61,62. These color coordinates are determined using the chromaticity coordinate calculation method based on the CIE 1931 system63,64. Specifically, through color calculator software. Figure 21, depict CIE chromaticity diagram from Na2Ca4(PO4)3F: 2.5 mol % Dy3+- 2.5 mol % Tb3+- x mol % Eu3+ (x = 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mol %) phosphors. The measured CIE chromaticity coordinates of Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor obtain with an excitation wavelength of 395 nm are presented in Table 5. Interestingly the CIE values of Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor are very close to the commercially available phosphor Y2O3:Eu3+ (0.61,0.39). Consequently, when excited by UV light, the Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor emits a white color making suitable for color tuning in display devices and solid-state lighting applications. The correlated color temperature (CCT) is used to analyze the quality of emitted light of material which can be calculated by using McCamy empirical equation as below65,66,
where, the value of n denoted as a slope given by (x-xe)/(ye-y), where the epicenter of point xe and ye are 0.332 and 0.186 respectively. The color purity of suggested triple doped phosphor material has been assessed by using given formula67,68,
Where, the X and Y denote CIE co-ordinates, Xd and Yd denotes dominant wavelength of X and Y co-ordinates and Xi, Yi denotes the coordinates of the illuminate point. The calculation of X, Y, Xd, Yd, color purity and CCT for varying concentration of Dy3+/Tb3+/Eu3+ in the synthesized phosphor are shown in Table 5. The obtained CCT values indicate that prepared phosphors could be beneficial for warm light applications since the standard CCT value for such application is below 5000 K. The prepared phosphor Na2Ca4(PO4)3F: 2.5 mol % Dy3+/2.5 mol % Tb3+/0.5 mol % Eu3+ phosphor exhibited the highest CCT value is 4926. The corresponding chromaticity coordinates were found to be x = 0.3433 and y = 0.3097. So therefore, this proposed synthesized material of Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphor which is potential application in the various domain like solid - state lighting and white light emitting diodes applications.
Solar cell efficiency enhancement of synthesized phosphor
The Doctor Blade technique is very important to improve the efficiency of solar cells by allowing for the creation of homogenous and regulated thin coatings of inorganic samples. This technique ensures accurate thickness control, resulting in improved light absorption and lower recombination losses. This method is especially useful for inorganic phosphor material, perovskite solar cells, organic photovoltaics and quantum dot solar cells. Furthermore, this approach is inexpensive, scalable and works with a variety of materials. Initially, the current (I) and voltage (V) properties of blank solar cell were calculated on LED solar simulator maintain at temperature 40OC. The synthesized triple doped Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ phosphors were prepared by using solid state method at 800OC. This synthesized sample crushed finely at mortar pestle for minutes and then crushed fine powder coated on a silicon solar cell by using doctor blade technique. After that the coated silicon cell is dried for 10 h. at an ambient temperature and again, we can calculate I-V characteristics of coated solar cell with the help of JETSPIN solar simulator. Using basic laboratory instruments, like an ammeter and voltmeter, the current and voltage of the solar cell were measured by connecting wires in accordance with the block diagram shown in Fig. 22. The blank and coated solar cells is placed in the LED solar simulator at 40 °C. Variable resistance was used to adjust the output voltage of the solar cell, and current was measured accordingly. The efficiency of solar cell was calculated by using given formula as follows21,
So therefore, we can calculate the solar cell efficiency of the cell and observe that the efficiency of solar cell has increased by 16.33% than the blank solar cell. The current (I) and voltage (V) behavior of the blank and coated solar cell displayed on Fig. 23. The relationship between voltage (v) Vs current density (mA/cm2) of Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ prepared phosphors as shown on Fig. 24.
Solar spectrum
The down converting phosphor doped with Dy3+/Tb3+/Eu3+ of Na2Ca4(PO4)3F triple activated prepared sample function as downshifting materials that can absorb high-energy photons and transmit these lower-energy photons depicted on Fig. 25. Maximum energy is lost due to thermalization when solar energy is transformed into electrical energy. Coating a downshifting layer of material on the silicon solar cell can stop these losses by preventing thermalization losses and acting as a down-conversion material that smoothly transforms high-energy photons into lower-energy for the creation of electron-hole pairs. Therefore, proposed study concluded that Na2Ca4(PO4)3F: Dy3+/Tb3+/Eu3+ triple activated phosphors are the potential candidate for the upcoming generation solar cells.
Conclusion
In summary, Dy3+, Tb3+, and Eu3+ activated Na2Ca4(PO4)3F phosphors were successfully synthesized via a conventional solid-state method. Structural analysis confirmed the formation of a pure rhombohedral phase with good crystallinity. The prepared phosphors exhibited multi-color emissions covering blue, green, yellow, and red regions resulting in tunable white light output suitable for WLEDs applications. Additionally, the application of the phosphor layer on silicon solar cells enhanced the photovoltaic efficiency by 16.33%, demonstrating its potential as a down-conversion material for solar energy conversion.
For future work, optimization of dopant concentrations, particle morphology, and coating techniques is recommended to further enhance the optical performance and solar conversion efficiency. Exploring alternative synthesis routes and integrating these phosphors with advanced solar cell could provide promising pathways for next-generation lighting and photovoltaic applications. Table 6 gives coparative study of recent rare-earth-doped phosphors for WLEDs and solar cell applications (2019–2024).
Data availability
Data availabilityThe datasets generated and/or analysed during the current study are not publicly available due to intellectual property protection but are available from the corresponding authors on reasonable request.
References
Cao, Y. et al. Luminescence properties of Sm3+-doped TiO2 nanoparticles: synthesis, characterization, and mechanism. J. Alloys Compd. 554, 12–20. https://doi.org/10.1016/j.jallcom.2012.11.149 (2013).
Du, P. & Yu, J. S. Facile hydrothermal synthesis of Eu3+-activated NaYF4 nanocrystals and their Judd-Ofelt analysis, photoluminescence and cathodoluminescence properties. Curr. Appl. Phys. 17, 1662–1669. https://doi.org/10.1016/j.cap.2017.09.011 (2017).
Gao, D., Zhang, X., Zheng, H., Gao, W. & He, E. Yb3+/Er3+ codoped β-NaYF4 microrods: synthesis and tuning of multicolor upconversion. J. Alloys Compd. 554, 395–399. https://doi.org/10.1016/j.jallcom.2012.12.010 (2013).
Chen, J. et al. Investigating efficient energy transfer in novel strategy-obtained Gd2O2S:Dy3+, Eu3+ nanofibers endowed with white emitting and magnetic dual-functionality. J. Lumin. 206, 509–517. https://doi.org/10.1016/j.jlumin.2018.10.087 (2019).
Sanjeev Kumar, G., Jain, K., Kumar, B. P., Singh, S. R. & Dhakate A review on polymeric photoluminiscent nanofibers: inorganic, organic and perovskites additives for Solid-State lighting application. Polym. Sci. - Ser. A. 64, 367–392. https://doi.org/10.1134/S0965545X22700213 (2022).
Xie, J. et al. Synthesis and photoluminescence properties of NaBi(WO4)2:Eu3+ red-emitting phosphor for NUV-based WLEDs. J. Lumin. 219, 116841. https://doi.org/10.1016/j.jlumin.2019.116841 (2020).
Gaikwad, A., Parauha, Y. R., Dhoble, S. J. & Dabre, K. V. Development of colour tunable phosphor via rare Earth doping in Eulytite type Ba3Bi2(PO4)4 host material. J. Mater. Sci. Mater. Electron. 34, 1–10. https://doi.org/10.1007/s10854-023-10880-2 (2023).
Chen, Q., Miao, B., Kumar, P. S. & Xu, S. Enhanced luminescence properties and Judd-Ofelt analysis of novel red emitting Sr2LiScB4O10: Eu3+ phosphors for WLED applications. Opt. Mater. (Amst). 116, 111093. https://doi.org/10.1016/j.optmat.2021.111093 (2021).
Jiao, M. et al. The structures and luminescence properties of Sr4Gd3Na3(PO4)6F2:Ce3+,Tb3+ green phosphors with zero-thermal quenching of Tb3+ for WLEDs. Dalt Trans. 49, 667–674. https://doi.org/10.1039/c9dt04320a (2020).
Tikale, R. V., Kadam, A. R. & Dhoble, S. J. Optical properties and crystal structure analysis of Sr3AlO4F:Sm3+, Eu3+ phosphors: an approach towards color tunability. J. Mater. Sci. Mater. Electron. 34 https://doi.org/10.1007/s10854-023-11356-z (2023).
Tikale, R. V., Kadam, A. R., Mathur, A. & Dhoble, S. J. Combustion assisted optical and structural investigation of Dy3+/Eu3+ co-doped KMg3Si3AlO10F2 phosphor by energy transfer mechanism. Inorg. Chem. Commun. 161, 112146. https://doi.org/10.1016/j.inoche.2024.112146 (2024).
Seeta Rama Raju, G., Pavitra, E. & Yu, J. S. Pechini synthesis of lanthanide (Eu3+/Tb3+or Dy3+) ions activated BaGd2O4 nanostructured phosphors: an approach for tunable emissions. Phys. Chem. Chem. Phys. 16, 18124–18140. https://doi.org/10.1039/c4cp02266a (2014).
Reddy, L. A review of the efficiency of white light (or Other) emissions in singly and Co-Doped Dy3+ ions in different host (Phosphate, silicate, Aluminate) materials. J. Fluoresc. 33, 2181–2192. https://doi.org/10.1007/s10895-023-03250-y (2023).
Haque, M. M., Kudrat-E-Zahan, M., Jahan, N. A. & Lee, H. I. Eu3+-activated potential red-emitting phosphor for solid-state lighting. Optik (Stuttg). 133, 1–8. https://doi.org/10.1016/j.ijleo.2016.12.068 (2017).
Bawanthade, B., Mistry, A., Bawanthade, V., Mathur, A. & Dhoble, S. J. Luminescence characterization of rare Earth (RE = Tb, dy, Eu) ions activated Ca8NaGd(PO4)6F2 halophosphor synthesis by modified pechini method. J. Mater. Sci. Mater. Electron. 35, 1–20. https://doi.org/10.1007/s10854-024-12525-4 (2024).
Verma, B., Baghel, R. N., Bisen, D. P., Brahme, N. & Jena, V. Judd-Ofelt analysis and luminescent characterization of Eu3+ activated Li2Zr(PO4)2 phosphor, opt. Mater. (Amst). 118, 111196. https://doi.org/10.1016/j.optmat.2021.111196 (2021).
Yadav, R. S., Monika, E., Rai, L. P., Purohit, S. B. & Rai Realizing enhanced downconversion photoluminescence and high color purity in Dy3+ doped MgTiO3 phosphor in presence of li++ ion. J. Lumin. 217, 116810. https://doi.org/10.1016/j.jlumin.2019.116810 (2020).
Kaur, S., Kaur, H., Rao, A. S. & Prakash, G. V. A review on photoluminescence phosphors for biomedical, temperature sensing, photovoltaic cell, anti-counterfeiting and white LED applications. Phys. B Condens. Matter. 690, 416224. https://doi.org/10.1016/j.physb.2024.416224 (2024).
Luo, X., Ahn, J. Y. & Kim, S. H. Aerosol synthesis and luminescent properties of CaAl2O4:Eu2+, Nd3+ down-conversion phosphor particles for enhanced light harvesting of dye-sensitized solar cells. Sol Energy. 178, 173–180. https://doi.org/10.1016/j.solener.2018.12.029 (2019).
Yao, N. et al. Efficiency enhancement in dye-sensitized solar cells with down conversion material zno: Eu3+, Dy3+. J. Power Sources. 267, 405–410. https://doi.org/10.1016/j.jpowsour.2014.05.135 (2014).
Dhapodkar, T. S., Kadam, A. R., Brahme, N. & Dhoble, S. J. Efficient white light-emitting Mg21Ca4Na4(PO4)18: Dy3+, Tb3+, Eu3+ triple-doped glasses: a multipurpose glasses for wleds, solar cell efficiency enhancement, and smart windows applications. Mater. Today Chem. 24. https://doi.org/10.1016/j.mtchem.2022.100938 (2022).
Kadam, A. R. & Dhoble, S. J. Energy transfer mechanism of KAlF4_Dy3+, Eu3+ co-activated down-conversion phosphor as spectral converters_ an approach towards improving photovoltaic efficiency by downshifting layer. J. Alloys Compd. 884, 161138. https://doi.org/10.1016/j.jallcom.2021.161138 (2021).
Aslam, A. et al. Dye-sensitized solar cells (DSSCs) as a potential photovoltaic technology for the self-powered internet of things (IoTs) applications. Sol Energy. 207, 874–892. https://doi.org/10.1016/j.solener.2020.07.029 (2020).
Rahmatian, M., Sayyaadi, H. & Ameri, M. Indoor photovoltaics: A numerical model of dye-sensitized solar cells based on indoor illumination for the internet of things applications, energy convers. Manag X. 22, 100606. https://doi.org/10.1016/j.ecmx.2024.100606 (2024).
Devadiga, D., Selvakumar, M., Shetty, P., Santosh, M. S. & Mini-Review Dye-Sensitized Solar Cell for Indoor Applications: A J. Electron. Mater. 50 3187–3206. https://doi.org/10.1007/s11664-021-08854-3. (2021).
Saeed, M. A., Yoo, K., Kang, H. C., Shim, J. W. & Lee, J. J. Recent developments in dye-sensitized photovoltaic cells under ambient illumination. Dye Pigment. 194, 109626. https://doi.org/10.1016/j.dyepig.2021.109626 (2021).
Mahajan, R. et al. X-ray photoemission and spectral investigations of Dy3+ activated magnesium pyrophosphate phosphors. J. Alloys Compd. 777, 562–571. https://doi.org/10.1016/j.jallcom.2018.10.355 (2019).
Taha, M. A., Youness, R. A., El-Bassyouni, G. T. & Azooz, M. A. FTIR spectral characterization, mechanical and electrical properties of P2O5-Li2O-CuO Glass-Ceramics. Silicon 13, 3075–3084. https://doi.org/10.1007/s12633-020-00661-5 (2021).
Kumar, S., Kumar, K., Singh, M., Jain, D. & Dhakate, S. R. Fabrication of novel red light emitting pva/zno: Eu3+ electrospun composite nanofibers. Results Mater. 26, 100682. https://doi.org/10.1016/j.rinma.2025.100682 (2025).
Ibrahim, S. & Marzouk, M. A. El komy, structural characteristics and electrical conductivity of Vanadium-doped lithium ultraphosphate glasses. Silicon 9, 403–410. https://doi.org/10.1007/s12633-016-9405-0 (2017).
Tejas, A. et al. Yellowish light emitting Dy3+ doped single phase – BaNb2O6 phosphors for solid state lighting applications. J. Lumin. 275, 120741. https://doi.org/10.1016/j.jlumin.2024.120741 (2024).
Muralimanohar, P. et al. Preparation and luminescence properties of Dy3+ doped BaAlBO3F2 glass ceramic phosphor for solid state white leds. Optik (Stuttg). 225, 165807. https://doi.org/10.1016/j.ijleo.2020.165807 (2021).
Tikale, R. V., Kadam, A. R. & Dhoble, S. J. Synthesis and optical properties of LiZr2(PO4)3: Eu3+, Dy3+ phosphor for display devices application. Chem. Phys. Impact. 8, 100525. https://doi.org/10.1016/j.chphi.2024.100525 (2024).
Chhillar, P. & Doon, P. B. Facile synthesis and photophysical properties of combustion derived Dy3+doped Ca9La(PO4)7 nanophosphors for advanced solid-state lighting applications. Inorg. Chem. Commun. 159, 111844. https://doi.org/10.1016/j.inoche.2023.111844 (2024).
Chandrappa, V. et al. A critical review and future prospects of Dy3+-doped glasses for white light emission applications. Optik (Stuttg). 266, 169583. https://doi.org/10.1016/j.ijleo.2022.169583 (2022).
Kumar, G. et al. Tunable photoluminescence properties of Dy3+ doped LLZO phosphors for WLED and dosimetry applications. Ceram. Int. 48, 1402–1407. https://doi.org/10.1016/j.ceramint.2021.09.226 (2022).
Wang, X. et al. Structure, properties and application of Tb3+ doped CaMO4 (M: W, Mo) phosphors prepared by solid-state double exchange method. Phys. Lett. Sect. Gen. Solid State Phys. 511, 129553. https://doi.org/10.1016/j.physleta.2024.129553 (2024).
Devi, S. et al. Achieving bright green emission in Tb3+ doped ternary yttrate-based nanocrystalline powder for solid-state illumination applications. Mater. Sci. Eng. B. 289, 116273. https://doi.org/10.1016/j.mseb.2023.116273 (2023).
Kumar, P. et al. Preparation, structural and photometric properties of single-phased Gd3Al5O12:Tb3+ green-emitting phosphors for solid state lighting purpose. Mater. Sci. Eng. B. 288, 116189. https://doi.org/10.1016/j.mseb.2022.116189 (2023).
Gupta, I. et al. Phase recognition and spectroscopic characteristics of single-phase Tb3+ doped Gd4Al2O9 nanophosphors for NUV energized advanced photonic appliances. J. Lumin. 252, 119327. https://doi.org/10.1016/j.jlumin.2022.119327 (2022).
Priya, M., Dhavamurthy, M., Suresh, A. A. & Mohapatra, M. M. Luminescence and spectroscopic studies on Eu3+-doped Borate and boro-phosphate glasses for solid state optical devices. Opt. Mater. (Amst). 142, 114007. https://doi.org/10.1016/j.optmat.2023.114007 (2023).
Tiwari, K. et al. Study of morphological, elemental, optical and excitation wavelength dependent red photoluminescence in Eu3+ doped Li2SrSiO4 for solid state lighting. Mater. Sci. Semicond. Process. 171, 107997. https://doi.org/10.1016/j.mssp.2023.107997 (2024).
Tikale, R. V., Kadam, A. R., Halwar, D. K. & Dhoble, S. J. Luminescence investigation of red emitting CaAlSiO4F: Eu3+ doped phosphor for white leds based on oxyfluoride matrix. Mater. Lett. X. 21, 100224. https://doi.org/10.1016/j.mlblux.2023.100224 (2023).
Gangwar, A. K. et al. Highly Luminescent Dual Mode Polymeric Nanofiber-Based Flexible Mat for White Security Paper and Encrypted Nanotaggant Applications, Chem. - Eur. J. 24 9477–9484. https://doi.org/10.1002/chem.201800715. (2018).
Kumar, V. et al. Energy transfer mechanism in Eu3+ doped Tin oxide nanophosphors for red solid state lighting. J. Lumin. 250, 119085. https://doi.org/10.1016/j.jlumin.2022.119085 (2022).
Girisha, H. R. et al. Amalgamation of composite flux as luminescent armor in Eu3+ doped BaLa2ZnO5 phosphor for enhanced luminescence, combating counterfeiting, improving thermal sensing and advanced forensic investigations. Inorg. Chem. Commun. 169, 113109. https://doi.org/10.1016/j.inoche.2024.113109 (2024).
Krishnapriya, T. et al. Investigation of the structural and photoluminescence properties of Eu3+ doped Na6CaP2O9 phosphors for solid state lighting. Mater. Res. Bull. 139, 111259. https://doi.org/10.1016/j.materresbull.2021.111259 (2021).
Monisha, M. et al. Judd-Ofelt analysis and luminescence characteristics of Eu3+ doped nepheline (NaAlSiO4)-based glass ceramics for solid-state lighting applications. J. Non Cryst. Solids. 599, 121971. https://doi.org/10.1016/j.jnoncrysol.2022.121971 (2023).
Princy, A., Rathina Mala, V., Albert, K. J. & Masilla Moses Kennedy, S. Synthesis, optical properties and Judd-Ofelt analysis of Eu3+ doped Ca2KMg2V3O12. Inorg. Chem. Commun. 158, 111574. https://doi.org/10.1016/j.inoche.2023.111574 (2023).
Kameshwaran, R. et al. Synthesis, structure and luminescence properties of bifunctional KCaF3 phosphor influenced by incorporating Eu3+ ions for solid state lighting and TL dosimetry applications. Appl. Radiat. Isot. 191, 110520. https://doi.org/10.1016/j.apradiso.2022.110520 (2023).
Kumar, S. et al. Stress-Induced Structural Phase Transition in Polystyrene/NaYF4: Eu3+Photoluminescent Electrospun Nanofibers, J. Nanomater. (2022). (2022) https://doi.org/10.1155/2022/2173629
Kumar, S. et al. A novel fabrication of electrospun polyacrylonitrile/NaYF4:Eu+3light emitting nanofibers. RSC Adv. 10, 24855–24861. https://doi.org/10.1039/d0ra03984e (2020).
Rajendran, M., Samal, S. K. & Vaidyanathan, S. A novel self-activated (bluish-green) and Eu3+ doped (red) phosphors for warm white leds. J. Alloys Compd. 815, 152631. https://doi.org/10.1016/j.jallcom.2019.152631 (2020).
Yashodha, S. R., Dhananjaya, N., Manohara, S. R. & Yogananda, H. S. Investigation of photoluminescence and dielectric properties of europium-doped LaOCl nanophosphor and its Judd–Ofelt analysis. J. Mater. Sci. Mater. Electron. 32, 11511–11523. https://doi.org/10.1007/s10854-021-05726-8 (2021).
Tikale, R. V., Kadam, A. R. & Mathur, A. Dhoble, Sm3+ activated Ba3LaNa(PO4)3F fluorophosphate phosphor: synthesis, characterization and their photoluminescence investigation for warm WLEDs. Chem. Phys. Impact. 9, 100741. https://doi.org/10.1016/j.chphi.2024.100741 (2024).
Kadam, A. R., Mishra, G. C., Deshmukh, A. D. & Dhoble, S. J. Enhancement of blue emission in Ce3+, Eu2+ activated BaSiF6 downconversion phosphor by energy transfer mechanism: A photochromic phosphor. J. Lumin. 229, 1–9. https://doi.org/10.1016/j.jlumin.2020.117676 (2021).
Letswalo, M. L. A., Reddy, L., Balakrishna, A., Swart, H. C. & Ntwaeaborwa, O. M. Influence of SO42– anionic group substitution on the enhanced photoluminescence behaviour of red emitting CaMoO4:Eu3+ phosphor. J. Alloys Compd. 854 https://doi.org/10.1016/j.jallcom.2020.157022 (2021).
Kumari, S., Rao, A. S. & Sinha, R. K. Investigations on photoluminescence and energy transfer studies of Sm3+ and Eu3+ ions doped Sr9Y2W4O24 red emitting phosphors with high color purity for w-LEDs. J. Mol. Struct. 1295, 136507. https://doi.org/10.1016/j.molstruc.2023.136507 (2024).
Upadhyay, K., Thomas, S., Tamrakar, R. K., Kalarikkal, N. & Butoliya, S. Comparative study of structural and optical behavior of Er3+ doped Y2SiO5 phosphor prepared by different methods. Mater. Sci. Eng. B. 275, 115511. https://doi.org/10.1016/j.mseb.2021.115511 (2022).
Khursheed, S., Sheergojri, G. A. & Sharma, J. Phosphor polymer nanocomposite: SrAl2O4:Eu2+, Dy3+ embedded PMMA for solid-state applications, Mater. Today Proc. 21 2096–2104. (2020). https://doi.org/10.1016/j.matpr.2020.01.329
Guan, H. & Li, Y. Na(Y1.5Na0.5)F6:RE3+(Dy3+,Tb3+,Eu3+,Tm3+,Ho3+):Controllable morphology, multicolor light and thermal properties. J. Alloys Compd. 859, 157833. https://doi.org/10.1016/j.jallcom.2020.157833 (2021).
Pavitra, E. et al. Desired warm white light emission from a highly photostable and single-component Gd2TiO5:Dy3+/Eu3 + nanophosphors for indoor illuminations. J. Alloys Compd. 875, 160019. https://doi.org/10.1016/j.jallcom.2021.160019 (2021).
Kumar, S., Jain, G., Singh, B. P. & Dhakate, S. R. Tunable Photoluminescence of Polyvinyl Alcohol Electrospun Nanofibers by Doping of NaYF4: Eu + 3 Nanophosphor, J. Nanomater. (2020). (2020) https://doi.org/10.1155/2020/1023589
Kumar, S. et al. A facile fabrication of poly(methyl methacrylate)/ α -NaYF 4:eu 3 + tunable electrospun photoluminescent nanofibers. Appl. Nanosci. 10, 3857–3864. https://doi.org/10.1007/s13204-020-01499-4 (2020).
Kumari, C., Manam, J. & Sharma, S. K. Strong red emission in double perovskite Sr3LiSbO6: Eu3+ phosphor with high color purity for solid-state lighting applications. Mater. Sci. Semicond. Process. 158, 107385. https://doi.org/10.1016/j.mssp.2023.107385 (2023).
Gopinath, A., Jose, J. R., Unnikrishnan, A. M. S. N. V., Joseph, C. & Biju, P. R. Photoluminescence investigation of novel red emitting NaLa(1–x)MgTeO6: XSm3+ phosphor with high color purity for n-UV based warm WLEDS and anti-counterfeiting applications. Mater. Res. Bull. 177, 112846. https://doi.org/10.1016/j.materresbull.2024.112846 (2024).
Wang, J. et al. Spectroscopic investigation of the novel orange-red phosphor Ca3La2W2O12:Sm3+ with the high color purity for w-LED applications. J. Lumin. 241, 118459. https://doi.org/10.1016/j.jlumin.2021.118459 (2022).
Yang, Y. et al. Synthesis and luminescence properties of double perovskite red-emitting NaSrLaTeO6:Sm3+ phosphors with high thermal stability and color purity for w-LEDs. J. Lumin. 252, 119262. https://doi.org/10.1016/j.jlumin.2022.119262 (2022).
Khode, P. P., Kadam, A. R., Nikolay, N. G. & Dhoble, S. J. Structural investigation and color tunability in Dy3+, Sm3+co-doped La2O3 phosphors for WLED applications. J. Mol. Struct. 1345, 143030. https://doi.org/10.1016/j.molstruc.2025.143030 (2025).
Acknowledgements
One of the authors RVT is thankful to the Mahatma Jyotiba Phule research fellowship-2022 (MJRF-2022) for financial assistance and R.T.M. Nagpur University, Nagpur for constant encouragement.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
Rahul V. Tikale: Conceptualization, Methodology, Investigation, Writing - original draft. Abhijeet R. Kadam: Methodology, Investigation, Writing - review & editing, Marta Michalska-Domańska: Methodology, Investigation, Writing - review & editing and S. J. Dhoble: Supervision, Writing - review &editing, Resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tikale, R.V., Kadam, A.R., Michalska-Domańska, M. et al. Triple-doped Dy3+/Tb3+/Eu3+ activated Na2Ca4(PO4)3F halo-phosphors for next-generation WLEDs and solar cell efficiency enhancement. Sci Rep 15, 27748 (2025). https://doi.org/10.1038/s41598-025-12538-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12538-x