Abstract
Bariatric surgery is an effective treatment for moderate-to-severe obesity, however, reliable vascular access during the perioperative period remains a challenge in this population. This study compared the safety and efficacy of midline catheters (MCs) and long peripheral catheters (LPCs) in patients who underwent bariatric surgery. This single-blind, randomised controlled trial was conducted at a tertiary acute hospital between September 2023 and January 2024. A totol of 224 patients were assigned to receive either MC or LPC. The primary outcome was catheter failure; secondary outcomes included insertion attempts, time to insert the device, indwelling time, complications, requirements for additional vascular access devices, and patient satisfaction. The incidence of catheter failure was significantly lower in the MCs group (10.71%) than in the LPCs group (20.54%; odds ratio [OR] 0.46; 95% confidence interval [CI], 0.22–0.99; P = 0.043). Additionally, MCs had longer median indwelling times (7 d vs. 5 d; P < 0.001), fewer complications (13.39% vs. 27.68%; OR 0.40; 95% CI, 0.20–0.80; P = 0.008), and required fewer additional devices (4.46% vs. 16.07%; OR 0.24; 95% CI, 0.09–0.68; P = 0.004). These findings suggest that MCs are a superior choice for vascular access devices in patients undergoing bariatric surgery.
Trial registration: Trial registered at ClinicalTrials.gov (NCT06031545 11/09/2023).
Similar content being viewed by others
Introduction
Obesity is a significant global health challenge, with its prevalence rising steadily worldwide, leading to substantial reductions in life expectancy and overall quality of life in affected individuals1,2,3,4. Bariatric surgery has emerged as a key intervention for achieving sustained improvement or complete remission of obesity-related metabolic diseases5. The success of bariatric surgery is closely linked to effective perioperative management, where intravenous (IV) access plays a crucial role in fluid administration, medication delivery, and blood sampling throughout the surgical process6.
Peripheral IV catheters (PIVCs) are the standard devices used for IV therapy (IVT), with approximately 85% of hospitalised patients requiring IVT, and up to 70% receiving PIVCs7. However, obtaining peripheral IV access in patients with obesity poses unique anatomical challenges owing to thick subcutaneous fat and deeper veins, complicating venous puncture and increasing the likelihood of complications and multiple attempts to puncture the vein8. Additionally, comorbidities common in patients with obesity, such as diabetes, further complicate peripheral venipuncture, making reliable PIVC access more difficult9.
To address these challenges, central vascular access devices (CVADs) are often used, offering longer indwelling times but incurring higher costs and an increased risk of catheter-related complications, such as bloodstream infections10,11. Given these limitations, there is a need to explore alternative vascular access options for patients with obesity, such as long peripheral catheters (LPCs) and midline catheters (MCs). Both are classified as PIVCs and have shown lower catheter failure rates and longer dwell times compared to standard short PIVCs12,13,14.
LPCs, ranging from 6 to 15 cm, and MCs, typically measuring longer than 15 cm, provide distinct advantages in terms of insertion techniques and cost-effectiveness6. LPCs can be inserted using direct or accelerated Seldinger techniques, whereas MCs are inserted using a modified Seldinger technique. Some studies suggest similar costs for both types15, while others indicate that LPCs are more economical than MCs16. Both LPCs and MCs are increasingly used in patients with difficult venous access, such as in paediatric patients and patients with coronavirus disease 201917,18.
Despite their growing use in patients with difficult venous access and medium-term therapeutic plans19,20,21, the efficacy of LPCs and MCs remains unclear in patients undergoing bariatric surgery due to specific vascular challenges and the typical 6- to 7-day hospitalisation. In most Chinese metabolic and bariatric surgery centres, patients are routinely kept hospitalised until postoperative day 7, reflecting national reimbursement policies and local perioperative care pathways, therefore, reliable venous access throughout this period is essential. Moreover, interventional studies on catheter use in patients with obesity undergoing bariatric surgery are limited, and existing clinical comparative studies of LPCs and MCs are primarily observational16,22. This gap underscores the need for high-quality evidence from randomised controlled trials (RCTs) to compare the safety and efficacy of LPCs and MCs in this context.
This study represents the first RCT to investigate the use of LPCs and MCs in patients undergoing bariatric surgery. The primary objective was to compare the safety and efficacy of LPCs and MCs in this patient population. We hypothesised that MCs would demonstrate a lower catheter failure rate before the treatment completion. The secondary objectives included reducing the need for additional vascular devices and improving patient satisfaction.
Methods
Study design
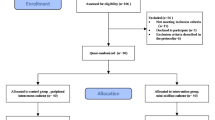
This single-center randomised controlled trial was conducted at Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China. The study included two groups: (1) the control group, which received LPCs, and (2) the experimental group, which received MCs. Ethics approval was obtained from the hospital’s Institutional Review Board (approval no. 2023 Research No. 0456), and informed consent was obtained from all patients. The trial adhered to the principles of the Declaration of Helsinki and was registered at ClinicalTrials.gov (NCT06031545 registered on 11/09/2023). Data reporting complied with the guidelines for reporting of RCTs (the Consolidated Standards of Reporting Trials statement). All methods were performed in accordance with the relevant guidelines and regulations. The study enrolled inpatients who underwent bariatric surgery between September 2023 and January 2024. All enrolled patients underwent laparoscopic sleeve gastrectomy, without conversion to open surgery or additional procedures.
Participants
Patients who met the clinical criteria were eligible to participate, and written informed consent was obtained from each participant or their legally authorised representatives. The inclusion criteria were as follows: (1) age between 18 and 65 years and (2) scheduled for bariatric surgery. The exclusion criteria were as follows: (1) concurrent treatment for other diseases during hospitalisation and (2) requirement for CVADs. Patients who were voluntarily discharged during catheter retention or who could not comply with follow-up visits were excluded from the study. All patients received structured preoperative education from a multidisciplinary team (surgeons, nurses, and dietitians). The educational sessions covered nutritional guidelines, postoperative recovery expectations, and breathing exercises.
Sample size
The primary outcome was the difference in the catheter failure rate. Sample size calculations were based on data from prior observational studies and RCTs15,16,22,23. A clinically significant reduction in catheter failure was anticipated, from 25% for LPCs to 10% for MCs. With a significance level (α) of 0.05 and a power of 80% (β = 0.2), the required sample size was 200 patients (100 in each group). To account for a possible 10% attrition rate, 224 participants were included.
Randomisation
Of the 278 eligible patients, 224 consented to participate. Random numbers were generated using SPSS 26.0 by an individual not involved in the study, assigning participants to either the experimental or control group in a 1:1 ratio. An independent researcher distributed the randomised sequences in sealed, opaque envelopes. The envelopes were opened sequentially upon patient enrollment. Owing to the nature of the procedure, blinding of the interventionists, participants, and outcome evaluators was not feasible; however, the statisticians were blinded to the group assignments.
Interventions
Patients were randomised to receive:
-
1.
LPC group: 4Fr, 10 cm catheter (Shandong Ande Healthcare Apparatus Co. Ltd, Shandong, China).
-
2.
MC group: 4Fr, 25 cm catheter (Foshan Special Medical Co. Ltd, Guangdong, China).
Catheters were inserted by experienced nurses in a dedicated procedure room using ultrasound guidance.
Device insertion and maintenance
LPC insertion (control group)
LPCs were inserted using the direct Seldinger technique, guided by ultrasound to identify the optimal vein based on its diameter and depth. After preparing the site with a 2% alcohol-based chlorhexidine solution, a sterile cover and gel were applied to the ultrasound probe. Nurses injected 0.4–0.6 mL of 2% lidocaine subcutaneously before venipuncture while maintaining real-time ultrasound guidance. The catheter was advanced into the vein and inserted to a depth of 10cm, followed by flushing with 0.9% sodium chloride and secured with a transparent dressing.
MC insertion (experimental group)
MCs were inserted using the modified Seldinger technique, following the same pre-catheterisation assessment and sterile precautions as in the control group. Nurses injected 0.4–0.6 mL of 2% lidocaine subcutaneously before venipuncture. A micro-introducer needle was inserted at a 30°–45° angle, and a floppy-tipped guidewire was threaded into the vein. A peel-away dilator/introducer was advanced over the guidewire, and the catheter was inserted to a depth of 23 cm. After aspirating blood and flushing with saline, the introducer sheath was removed, and the catheter was secured as in the control group.
Catheter retention and maintenance
Catheter function was assessed and maintained according to the Infusion Therapy Standards of Practice.⁶ Patency, fixation, potential dislodgement, and the puncture sites were evaluated regularly. The catheter was flushed with 5 mL of 0.9% sodium chloride before and after each infusion and at least once every 24 h between infusions.
Postoperative management
Limited Enhanced Recovery After Surgery (ERAS) measures were implemented, including early postoperative ambulation and multimodal analgesia. At our center, as in most Chinese metabolic and bariatric surgery units, patients are routinely hospitalised until postoperative day 7, rather than discharged earlier. This approach reflects the national reimbursement policies, availability of dedicated postoperative care resources, and traditional perioperative pathways in China. However, standardised ERAS pathways advocating early discharge were not followed, as our protocol emphasised extended hospitalisation for metabolic and cardiopulmonary monitoring.
Discharge readiness was assessed using the following predefined criteria: (1) stable haemoglobin, electrolyte, and glucose levels; (2) afebrile status, normotension, and oxygen saturation ≥ 95% on room air; (3) confirmation of anatomical integrity via upper gastrointestinal contrast study; (4) tolerance of liquid diet without nausea/vomiting; and (5) removal of all surgical drains.
Outcome measurements
The primary outcome was catheter failure, defined as unexpected or premature removal before the completion of therapy, due to any catheter-related complications, including occlusion, infiltration, extravasation, dislodgement, phlebitis, thrombosis, and infection. Catheter-related infections included both local infections and bloodstream infections (based on the Centres for Disease Control and Prevention National Healthcare Safety Network criteria)24. The secondary outcomes are detailed in Supplement Table 1.
Data collection
Data were collected using a customised form, including baseline patient demographics (e.g., age, sex, and weight) and device characteristics (e.g., allocation, insertion site, insertion method, number of insertion attempts, and placement time). Preoperative data collection also included baseline vascular comorbidities, specifically a history of coronary artery disease, deep vein thrombosis/pulmonary embolism, and chronic kidney disease stage ≥ 3. Research nurses recorded patient data at enrollment, monitored the devices daily, and documented any complications. Complications were assessed daily until catheter removal. Suspected infections underwent immediate diagnostic evaluation (e.g., blood cultures and site swabs). At device insertion and removal, data on removal reasons and patient satisfaction were also collected.
Quality control
Quality standards were maintained throughout the study, with all catheters and needleless connectors from the same brand and using consistent securement methods. Participants were strictly screened according to the criteria, and only experienced nurses (with over three years of relevant experience and more than 500 catheter placements) performed the insertions. The upper arm was selected as the puncture site for both catheters. All data were stored in a secure electronic database, and any discrepancies were verified and corrected.
Statistical analysis
Descriptive data were presented as mean (standard deviation [SD]) or median ( interquartile range [IQR]) for continuous variables and as frequency (percentage) for categorical variables. For the primary outcome, logistic regression was used, and the results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). Poisson regression was applied for count outcomes, with incidence rates reported per 1,000 h of indwelling time. Kaplan-Meier curves were used for time-to-failure analysis, and the log-rank test was used to assess curve equality. Linear regression was used for continuous outcomes, with mean differences reported with 95% CIs. Analyses followed the intention-to-treat principle, and an α-level < 0.05 was considered statistically significant. All analyses were performed using Stata, version 13.1.
Results
Participant characteristics and attrition
Participants were recruited between September 2023 and January 2024, with the final data cut-off on 28 January 2024. Of the 278 patients screened, 40 were excluded for not meeting the inclusion criteria, and 14 declined to participate (Fig. 1). A total of 224 patients were randomly assigned to the LPC (n=112, 50%) and MC (n=112, 50%) groups. All patients received their allocated catheters. However, three patients in the MC group and two in the LPC group did not meet the surgical indications, two in the MC group and one in the LPC group withdrew from surgery, and one patient in the LPC group received alternative treatment. All patients were included in the intention-to-treat (ITT) analyses.
The baseline characteristics and catheter insertion details were comparable between the two groups (Table 1). Importantly, none of the 224 patients had a history of coronary artery disease, deep vein thrombosis/pulmonary embolism, or chronic kidney disease stage ≥ 3. The mean age in the MC group was 31.46 years (SD 7.31), similar to that of the LPC group (31.56 years, SD 7.31). Female patients predominated in both groups, with 80 and 94 females in the MC group and LPC groups, respectively. The mean body mass index was slightly higher in the MC group (39.36 kg/m², SD 5.27) compared to the LPC group (38.27 kg/m², SD 5.33). Other baseline parameters, including comorbidities, haematological profiles, and hospital stay duration, showed no significant intergroup differences. No intraoperative or postoperative surgical complications (e.g., anastomotic leaks, haemorrhage, or wound infections) occurred in either group. All patients met the discharge criteria within the planned hospitalisation period.
Primary outcome
The primary outcome, device failure, was lower in the MC group than that in the LPC group. The device failure rate was 10.71% in the MC group compared to 20.54% in the LPC group, yielding an OR of 0.46 (95% CI, 0.22–0.99; P = 0.043) (Table 2). This corresponded to 16.22 device failures per 1,000 catheter-days in the MC group compared to 40.00 per 1,000 catheter-days in the LPC group.
Secondary outcomes
Insertion success rate and placement time
There was no significant difference in the number of insertion attempts between the groups, with 92.86% of MCs successfully inserted on the first-attempt compared with 88.39% of LPCs (relative risk, 1.71; 95% CI, 0.68–4.29; P = 0.252) (Table 2). However, the time required for device insertion was significantly longer for MCs, with a median of 17 min (IQR, 21-27.5) compared to 5 min (IQR, 4–6) for LPCs (median difference [MedD], 15 min; 95% CI, 14 to 17; P < 0.001).
Catheter indwelling time and complication incidence
MCs had a longer indwelling time, with a median of 7 d (IQR, 6–7) compared with 5 d (IQR, 4–7) for LPCs (median difference, 1 d; 95% CI, 1–2; P < 0.001) (Table 2). Additionally, MCs were associated with fewer device complications during the indwelling period, with a composite complication rate of 13.39% compared with 27.68% for LPCs (OR, 0.40; 95% CI, 0.20–0.80; P = 0.008), corresponding to 20.27 complications per 1,000 catheter-days in the MC group versus 53.91 in the LPC group.
Infiltration or extravasation occurred significantly less frequently in the MC group (5.36%) than in the LPC group (15.18%), with an OR of 0.32 (95% CI, 0.12–0.84; P = 0.015) (Table 2). Other complications, such as occlusion, thrombosis, phlebitis, and dislodgement, did not differ significantly between the groups. Notably, no infections were reported in either of the groups. No catheter-related complications prolonged hospitalisation.
Patients in the MC group required fewer additional devices to complete treatment (4.46%) than those in the LPC group (16.07%), with an OR of 0.24 (95% CI, 0.09–0.68; P = 0.004).
Patient satisfaction
Patient-reported satisfaction at the time of catheter insertion did not significantly differ between the MC and LPC groups. However, satisfaction at the time of device removal was significantly higher in the MC group (mean satisfaction score of 8.24) than in the LPC group (mean satisfaction score of 7.63; mean difference, 0.61; 95% CI, 0.29–0.92; P < 0.001) (Table 2).
Device survival analysis
Kaplan-Meier survival analysis (Fig. 2) demonstrated a significant difference in device survival between the groups, with MCs showing a slower rate of device failure compared with LPCs. The first device failures were observed within two days of placement in both groups, with MCs exhibiting a delayed onset of failure compared with LPCs.
Discussion
Bariatric surgery is widely recognised as an effective intervention for obesity and its associated metabolic disorders. However, perioperative management remains complex and challenging due to altered body fluid compartments and increased cardiac output in patients with obesity25. These physiological changes present unique challenges in selecting appropriate vascular access during bariatric surgery9. In patients with obesity, ultrasound-guided LPCs and MCs placement achieves a first-attempt success rate of 85–90% versus 50–60% for PIVCs without ultrasound, thus minimising repeated needle sticks in drowsy, oedematous postoperative patients10. LPCs and MCs are usually left in place for 1–4 weeks6, whereas PIVCs often require replacement every 48–72 h due to mechanical failure or phlebitis19, reducing nursing workload and patient anxiety. LPCs and MCs facilitate frequent postoperative blood sampling and fluid management without the need for repeated venipunctures. To our knowledge, this is the first RCT to compare the clinical efficacy and safety of LPCs and MCs in this patient population. Our findings demonstrate a significant reduction in catheter failure rates with MCs compared to LPCs, along with a longer duration of use, fewer catheter-related complications, a reduced need for additional vascular access devices, and higher patient-reported satisfaction.
Both groups achieved high success rates for first-puncture insertions (92.86% for MCs and 88.39% for LPCs), surpassing previous studies14,26. This high success rate is likely attributable to the use of ultrasound guidance and the expertise of the specialised nursing staff. No significant complications occurred during insertion in either group, and there was no statistically significant relationship between the number of venous punctures. However, the insertion time for MCs was significantly longer than for LPCs, reflecting the procedural differences between the accelerated and modified Seldinger techniques: the accelerated Seldinger approach used for LPCs streamlines access by integrating needle insertion, guidewire placement, and dilator advancement into a single step, whereas the modified Seldinger technique required for MCs necessitates sequential needle withdrawal, guidewire introduction, dilator passage, and catheter advancement under full sterile conditions, establishing a maximum sterile barrier similar to peripherally inserted central catheter procedures, and therefore prolongs the overall procedure6. In emergencies, LPCs may offer a faster option for patients with difficult venous access, but further research is needed to confirm this16,26.
Catheter-related complications, a major cause of catheter failure17, were significantly less frequent in the MC group (13.39%) than in the LPC group (27.68%). This reduction aligns with previous studies, highlighting the safety profile of MCs16,23,27. The differences in complication rates between LPCs and MCs may be attributed to the catheter length and tip location. LPCs, being shorter, often position the tip at the distal axillary vein, where blood flow is slower, while MCs, with a length of 25 cm, typically reach the axillary or subclavian veins, where faster blood flow aids in drug dilution and reduces coagulation risk28. This likely contributes to a lower incidence of complications, such as phlebitis and catheter blockage, in the MC group. These findings are consistent with previous research, which showed higher complication rates when the catheter tip is located in the distal axillary vein versus the axillary or subclavian veins28,29.
Infiltration, the most common catheter-related complication in adults15, was also observed more frequently in the LPC group (15.18%) than in the MC group (5.36%, P = 0.015), suggesting that longer catheter lengths may reduce the risk of inadvertent infusion into surrounding tissues. Thrombosis, a potentially serious complication, poses particular risks for patients with obesity due to impaired venous return and an increased risk of deep vein thrombosis30. In this study, thrombosis referred to symptomatic cases, and the overall risk was low in both groups.
Previous studies have shown a low incidence of catheter-related bloodstream infections (CRBSIs) in PIVCs, typically less than 0.5 per 1,000 catheter-days21. Although patients with obesity are generally more susceptible to CRBSIs during hospitalisation31, we observed no CRBSIs in either group by the end of data collection. This result aligns with findings from a systematic review reporting similarly low CRBSI rates (0.3 to 0.4 per 1,000 catheter-days) for long peripheral and MCs in adults32. The favourable outcome may be attributed to the rigorous aseptic techniques and systematic training provided to the nursing staff responsible for catheter placement.
The catheter failure rate was significantly lower in the MC group than in the LPC group (10.71% vs. 20.54%, P = 0.043), consistent with previous studies demonstrating the superior performance of MCs in maintaining vascular access, particularly in patients with difficult venous access16,28,29. Furthermore, the MC group required fewer additional devices to complete treatment (4.46% vs. 16.07%, P = 0.004), indicating that the use of MCs may reduce the need for multiple catheter replacements and help preserve vascular access for future treatments. Kaplan-Meier survival analysis also revealed a longer median dwell time for MCs, suggesting enhanced stability and a lower risk of complications. Although the dwell times for both MCs and LPCs were statistically significant, they were shorter than those reported in previous studies12,18,19, likely due to the study’s focus on patients undergoing bariatric surgery, with hospital stays typically limited to one week. In this study, patients who underwent bariatric surgery remained hospitalised for a median of 7 d (IQR 6–7), with catheters retained throughout the hospitalisation period. This aligns with standard protocols in Chinese tertiary hospitals, where bariatric surgery is classified as a Level IV procedure and excluded from day surgery programmes due to the inherent risks in patients with obesity (e.g., cardiopulmonary comorbidities and metabolic instability). Although many Western ERAS protocols target discharge by postoperative day 2–3, most high-volume Chinese bariatric centres continue to discharge patients on postoperative day 7. This practice ensures uninterrupted postoperative monitoring and aligns with the national insurance and rehabilitation structures. Extended hospitalisation and catheter retention prioritise patient safety by ensuring uninterrupted therapy (e.g., anticoagulants and analgesics) and reducing the need for repeated venous access. While this contrasts with ERAS protocols advocating for discharge by postoperative day 1 and prompt catheter removal, our findings highlight the need to evaluate catheter performance in ERAS-driven settings to align with global trends toward shorter hospital stays.
Limitations
This study had some limitations. First, it was a single-centre study with a relatively small sample size. Second, all participants were of Asian descent, as the study was conducted in China, potentially restricting the applicability of the results to other demographic populations. Additionally, the prolonged hospitalisation observed in this study may not be representative of settings that implement ERAS protocols, where discharge typically occurs within 1–2 postoperative days. Third, the cost-effectiveness of the catheters was not assessed, which could influence both clinical decision-making and patient satisfaction. Future research should address these limitations by conducting multicentre RCTs with larger and more diverse populations, incorporating cost-effectiveness analyses as a key outcome, and evaluating catheter performance in outpatient bariatric cohorts to align with global health care trends.
Conclusion
This randomised controlled trial demonstrated that MCs are superior to LPCs regarding catheter failure rates and overall clinical outcomes, making them the preferred choice for patients with obesity undergoing bariatric surgery. While the results are promising, the study’s limitations, including its single-centre design and specific patient population, may limit the generalisability of the findings. Further research, particularly multicentre trials with diverse patient populations, is required to validate these results. Additionally, cost-effectiveness studies comparing these devices are essential to balance catheter efficacy with economic considerations in various clinical settings.
Data availability
The data that support the findings of this study are available on request from the corresponding author, Yiyu Zhuang (zhuangyy@zju.edu.cn). The data are not publicly available due to restrictions e.g. their containing information that could compromise the privacy of research participants.
References
Blüher, M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15 (5), 288–298. https://doi.org/10.1038/s41574-019-0176-8 (2019).
Zheng, Y. et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. Jama-J Am. Med. Assoc. 318 (3), 255–269 (2017).
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. New. Engl. J. Med. 377 (1), 13–27. https://doi.org/10.1056/NEJMoa1614362 (2017).
Schauer, P. R. et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-Year outcomes. New. Engl. J. Med. 376 (7), 641–651. https://doi.org/10.1056/NEJMoa1600869 (2017).
Eisenberg, D. et al. 2022 American society for metabolic and bariatric surgery (ASMBS) and international federation for the surgery of obesity and metabolic disorders (IFSO): indications for metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 18 (12), 1345–1356. https://doi.org/10.1016/j.soard.2022.08.013 (2022).
Nickel, B. et al. Infusion therapy standards of practice, 9th edition. J Infus Murs. 47(1S Suppl 1), S1-S285. https://doi.org/10.1097/NAN.0000000000000532 (2024).
Qin, K. R., Nataraja, R. M. & Pacilli, M. Long peripheral catheters: is it time to address the confusion? J. Vasc Access. 20 (5), 457–460. https://doi.org/10.1177/1129729818819730 (2019).
Juvin, P., Blarel, A., Bruno, F. & Desmonts, J. M. Is peripheral line placement more difficult in obese than in lean patients? Anesth. Analg. 96 (4), 1218. https://doi.org/10.1213/01.ANE.0000050570.85195.29 (2003).
Houston, P. A. Obtaining vascular access in the obese patient population. J. Infus Murs. 36 (1), 52–56. https://doi.org/10.1097/NAN.0b013e31827989d8 (2013).
Egan, G., Healy, D., O’Neill, H., Clarke-Moloney, M. & Walsh, S. R. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg. Med. J. 30 (7), 521–526 (2012).
Shokoohi, H. et al. Ultrasound-guided peripheral intravenous access program is associated with a marked reduction in central venous catheter use in noncritically ill emergency department patients. Ann. Emerg. Med. 61 (2), 198–203. https://doi.org/10.1016/j.annemergmed.2012.09.016 (2013).
Qin, K. R. et al. Standard versus long peripheral catheters for multiday IV therapy: A randomized controlled trial. Pediatrics 147 (2). https://doi.org/10.1542/peds.2020-000877 (2021).
Marsh, N. et al. Safety and efficacy of midline catheters versus peripheral intravenous catheters: A pilot randomized controlled trial. Int. J. Nurs. Pract. 29 (2), e13110. https://doi.org/10.1111/ijn.13110 (2023).
Kleidon, T. M. et al. Midline compared with peripheral intravenous catheters for therapy of 4 days or longer in pediatric patients: A randomized clinical trial. Jama Pediatr. 177 (11), 1132–1140. https://doi.org/10.1001/jamapediatrics.2023.3526 (2023).
Fabiani, A., Aversana, N., Santoro, M. & Sanson, G. Complications associated to midline- and long peripheral catheters in adults. Systematic review of literature and proposal for a standardized model for data collection. Thromb. Res. 236, 117–126. https://doi.org/10.1016/j.thromres.2024.02.022 (2024).
Fabiani, A., Eletto, V., Dreas, L., Beltrame, D. & Sanson, G. Midline or long peripheral catheters in difficult venous access conditions? A comparative study in patients with acute cardiovascular diseases. Am. J. Infect. Control. 48 (10), 1158–1165. https://doi.org/10.1016/j.ajic.2019.12.025 (2020).
Gilardi, E. et al. Mini-midline in difficult intravenous access patients in emergency department: A prospective analysis. J. Vasc Access. 21 (4), 449–455. https://doi.org/10.1177/1129729819883129 (2020).
Gilardi, E. et al. Long peripheral cannula in COVID-19 patients: 769 catheter days experience from a semi-intensive respiratory COVID unit. J. Vasc Access. 11297298221115002. https://doi.org/10.1177/11297298221115002 (2022).
Chopra, V. et al. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann. Intern. Med. 163 (6), S1–S40. https://doi.org/10.7326/M15-0744 (2015).
Qin, K. R., Pittiruti, M., Nataraja, R. M. & Pacilli, M. Long peripheral catheters and midline catheters: insights from a survey of vascular access specialists. J. Vasc Access. 22 (6), 905–910. https://doi.org/10.1177/1129729820966226 (2021).
Pittiruti, M. et al. European recommendations on the proper indication and use of peripheral venous access devices (the ERPIUP consensus): A WoCoVA project. J. Vasc Access. 24 (1), 165–182. https://doi.org/10.1177/11297298211023274 (2023).
Fabiani, A., Santoro, M. & Sanson, G. The catheter-to-vein ratio at the tip level, not the catheter type, as a risk factor for a catheter failure. A retrospective comparative study of polyurethane midline and long peripheral catheters. Heart Lung. 60, 39–44. https://doi.org/10.1016/j.hrtlng.2023.02.027 (2023).
Bahl, A., Diloreto, E., Jankowski, D., Hijazi, M. & Chen, N. W. Comparison of 2 midline catheter devices with differing antithrombogenic mechanisms for catheter-Related thrombosis: A randomized clinical trial. Jama Netw. Open. 4 (10), e2127836. https://doi.org/10.1001/jamanetworkopen.2021.27836 (2021).
Horan, T. C., Andrus, M. & Dudeck, M. A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 36 (5), 309–332. https://doi.org/10.1016/j.ajic.2008.03.002 (2008).
Alpert, M. A. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am. J. Med. Sci. 321 (4), 225–236. https://doi.org/10.1097/00000441-200104000-00003 (2001).
Pavelkova, K. et al. Comparison of 12-cm versus 6-cm long peripheral catheters in patients with difficult intravenous access (DIVA). J. Vasc Access. 23 (1), 94–97. https://doi.org/10.1177/1129729820983151 (2022).
Jeon, M. H., Kim, C. S., Han, K. D. & Kim, M. J. Efficacy and safety of midline catheters with integrated wire accelerated Seldinger technique. Vasc Specialist Int. 38, 2. https://doi.org/10.5758/vsi.210062 (2022).
Zhao, L. et al. Midline catheter tip position and catheter-related complications in antimicrobial therapy: A multi-center randomized controlled trial. Int. J. Nurs. Stud. 141, 104476. https://doi.org/10.1016/j.ijnurstu.2023.104476 (2023).
Qin, K. R. et al. Long peripheral catheters for intravenous access in adults and children: A systematic review of the literature. J. Vasc Access. 22 (5), 767–777. https://doi.org/10.1177/1129729820927272 (2021).
Willenberg, T. et al. Impact of obesity on venous hemodynamics of the lower limbs. J. Vasc Surg. 52 (3), 664–668. https://doi.org/10.1016/j.jvs.2010.04.023 (2010).
Wang, Y., Xiang, Q., Wu, J., Xiao, N. & Chen, J. Obesity and the risk of catheter-related bloodstream infection: a systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 11 (1), 141. https://doi.org/10.1186/s13756-022-01166-z (2022).
Tripathi, S., Kumar, S. & Kaushik, S. The practice and complications of midline catheters: A systematic review. Crit. Care Med. 49 (2), e140–e150. https://doi.org/10.1097/CCM.0000000000004764 (2021).
Funding
This study has no financial support, including external funding.
Author information
Authors and Affiliations
Contributions
Linfang Zhao: Writing – original draft, Investigation, Data curation, Formal analysis. Xiaohui Yang: Writing – original draft, Investigation, Data curation, Formal analysis. Chang Liu: Methodology, Investigation. Weihua Yu: Conceptualization, Methodology, Supervision. Xiuzhu Cao: Supervision, Writing – review & editing. Xiangyun Li: Investigation, Supervision, Data curation.Jie Wang: Investigation, Supervision. Yiyu Zhuang: Conceptualization, Methodology, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethics approval was obtained from the Sir Run Run Shao Hospital’s Institutional Review Board (Approval No. 2023 Research No. 0456), and informed consent was secured from all patients.
Consent for publication
All participants signed the informed consent form and agreed to publish.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, L., Yang, X., Liu, C. et al. Comparative safety and efficacy of midline catheters versus long peripheral catheters in patients undergoing bariatric surgery: a randomised controlled trial. Sci Rep 15, 30534 (2025). https://doi.org/10.1038/s41598-025-12551-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12551-0