Abstract
Coronavirus disease 2019 (COVID-19) patients have a 1.7-fold higher arrhythmia risk with rates of cardiac complications ranging from 2% non-ICU patients to 59% in non-survivors. Atrial fibrillation (AF), the most common arrhythmia, is a frequent complication of acute and long COVID-19. The high expression of ACE2 in the heart suggested that infectious virus may underlie cardiac complications. However, we recently reported in human cardiac tissue from fatal COVID-19 cases perivascular spike protein, elevated pro-inflammatory cytokines, vascular damage, and cardiac remodeling without evidence for direct infection of cardiac cells by SARS-CoV2. Mislocalization of intercalated disc (ID) components, connexin-43 (Cx43) gap junctions and NaV1.5 sodium channels, was also evident in patients’ hearts, recapitulating structural remodeling we previously identified as providing a substrate for atrial arrhythmias following an acute inflammatory insult. Therefore, we hypothesized that the inflammatory response elicited by SARS-CoV2 spike protein is sufficient to provoke atrial arrhythmias. Structural and functional assessments of WT murine hearts were performed five days following a single bolus intravenous injection of the viral spike protein. In vivo ECGs demonstrated increased atrial arrhythmia burden in spike-injected mice vs. control. Immunohistochemistry studies revealed elevated expression of inflammatory markers and evidence of vascular damage in these mice. Additionally, we observed disruption of ID ultrastructure and mislocalization of Cx43 and NaV1.5 in the atria of spike protein-injected mice. Our results suggest that vascular-leak inducing inflammatory insult from viral spike protein, and not direct infection by SARS-CoV2 results in the pathophysiology of cardiac dysfunction in fatal COVID-19.
Similar content being viewed by others
Introduction
Atrial arrhythmias are a frequent complication of acute and long Coronavirus disease 2019 (COVID-19)1 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 patients are nearly twice as likely to develop arrhythmias compared to age- and comorbidity-matched non-infected patients, with incidence increasing with severity of infection2,3. The SARS-CoV-2 receptor, ACE2, is highly expressed in a variety of cell types including endothelial cells and cardiomyocytes in the normal heart4,5 with increased ACE2 expression associated with obesity, diabetes, and heart failure5,6leading to the prevalent assumption that infectious virus likely underlies cardiac complications in COVID-19. However, a recent report by our group using human tissue from fatal COVID-19 cases identified the presence of viral spike protein and absence of SARS-Cov-2 RNA and nucleocapsid protein7 in patients’ hearts8,9,10,11. These results and previous reports that did not identify SARS-CoV-2 virus infecting the hearts of COVID-19 patients7,8,9,12,13,14,−15 suggest that cardiac pathogenesis in COVID-19 may not require direct viral infection of the heart. Thus, in the present study, we sought to demonstrate cardiac impacts of SARS-CoV-2 spike protein on wild-type mice and acknowledge that these effects may be further exacerbated in mice expressing humanized ACE2.
SARS-CoV-2 spike protein components, S1 (ACE2 binding subunit) and S2 subunits, are essential for viral binding and infiltration of host cells and have been implicated in eliciting cytokine storms following viral infection. Indeed, fatal COVID-19 hearts also demonstrated elevated levels of inflammatory markers, TNFα, IL-6, and caspase-3, showing the same perivascular distribution as spike protein. Furthermore, histologic analysis revealed considerable microvascular damage, including enlarged endothelial cells, perivascular edema, and formation of microvascular thrombi, each well documented sequela of severe COVID-19, and findings we reported in our previous study7. Marked lateral migration of both connexin-43 (Cx43) and cardiac sodium channel protein (NaV1.5) were also observed in myocytes from COVID-19 hearts.
The histologic and molecular observations from patient hearts with fatal COVID-19 infection are consistent with an arrhythmia mechanism we previously identified, wherein an acute inflammatory insult – such as elicited by viral spike protein – triggers vascular endothelial dysfunction, thereby initiating cardiomyocyte structural remodeling, particularly of intercalated disc (ID) components including Cx43 gap junctions (GJs) and NaV1.5, thus promoting atrial arrhythmias16. Therefore, we used a previously published mouse model of severe COVID-1917 entailing intravenous injection of the viral spike protein five days prior to structural and functional assessments to test the hypothesis that the inflammatory response elicited by SARS-CoV2 spike protein may be sufficient to elicit the development of a substrate for atrial arrhythmias. Furthermore, we injected the S1 and S2 subunits of the SARS-CoV2 spike protein singly or in combination to delineate ACE2-dependent and –independent impacts.
Methods
All animal procedures were approved by Institutional Animal Care and Use Committee at The Ohio State University and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85 − 23, revised 2011). All methods are reported in accordance with ARRIVE guidelines.
Animals
Wild-type male C57BL/6 mice aged 9 months were used, 6 for each treatment group. Due to the limited availability of the COVID spike protein and the well-documented sex differences in cardiovascular complications— where males are more severely affected18,19 —we focused our study on male mice to ensure a consistent and representative model of disease susceptibility. Mice underwent intravenous administration of the following spike protein components: S1 subunit (10–50 µg), S2 subunit (50 µg), S1 + S2 (50 µg each), or mouse albumin (100 µg). The doses chosen for the mice were based on the reported value of 1.5 ml of blood for a given 20 g mouse. 50 ug spike S1 or S2 (high dose)/1.5 ml is equivalent to the concentrations of spike S1 in the circulating blood of people with severe COVID (often expressed as ug/ml of blood). Additionally, these doses have been previously tested in mice17. All studies were conducted 5 days post injection.
In vivo ECG
Acute ECG recordings (PL3504 PowerLab 4/35, ADInstruments) were obtained from mice anesthetized with isoflurane (1-1.5%) as previously described20. Briefly, after baseline recording (5 min.), animals were injected intraperitoneally with epinephrine (1.5 mg/kg; Sigma) and caffeine (120 mg/kg; Sigma) challenge and ECG recording continued for 20 min (Supplementary Table S1). ECG recordings were analyzed using the LabChart 8 software (ADInstruments). Arrhythmia burden was assessed based on the number and duration of atrial arrhythmic episodes (including atrial flutter and fibrillation but not premature atrial contractions); the presence of a single normal p-wave between episodes was interpreted termination and reinitiation of reentry. At the conclusion of each ECG recording, the heart was excised, leading to euthanasia by exsanguination. Atrial arrhythmias were characterized by the following: irregular p-waves, both in shape and in frequency of occurrence for every QRS complex, distinct from 60 Hz noise. The isolated hearts were prepared in one of the following two ways:
-
i)
Cryopreservation: Three hearts from each treatment group were embedded in optimal cutting temperature compound and frozen using liquid nitrogen for cryosectioning and fluorescent immunolabeling as in previous studies21,22,23,24. These samples were used for light microscopy experiments as described below.
-
ii)
Fixation for Transmission Electron Microscopy (TEM): Three isolated hearts from each treatment group were perfusion fixed with 2% paraformaldehyde + 0.25% glutaraldehyde. Atria were then dissected, cut into 0.5 mm cubes and fixed overnight in 2.5% glutaraldehyde at 4 °C for resin embedding and ultramicrotomy as previously described22,23.
Primary antibodies
The following primary antibodies were used for Western immunoblotting and fluorescence microscopy studies:
-
Connexin43 (Cx43; mouse monoclonal; EMD Millipore Corp. MAB3067).
-
N-cadherin (N-cad; mouse monoclonal; BD Biosciences 610920).
-
Cardiac isoform of the voltage-gated sodium channel (NaV1.5; rabbit polyclonal; custom antibody22.
-
SARS-CoV-2 spike protein.
-
S1 subunit (ProSci 9083).
-
S2 subunit (ProSci 9123).
-
-
Vascular endothelial growth factor A (VEGF-A; mouse monoclonal; Abcam ab1316).
-
Interleukin 6 (IL-6; rabbit polyclonal; Abcam ab6672).
-
Caspase-3 (rabbit polyclonal; Abcam ab4051).
-
Tumor necrosis factor alpha (TNFα; rabbit polyclonal; Proteintech 26405).
Immunohistochemistry
Immunofluorescent labeling of cryosections (5 μm thickness) of fresh-frozen myocardium was performed, as previously described20,22,23,25. Briefly, cryosections were fixed with paraformaldehyde (2%, 5 min at room temperature), permeabilized with Triton X-100 (0.2% in PBS for 15 min at room temperature) and treated with blocking agent (1% BSA, 0.1% triton in PBS for 2 h at room temperature) prior to labeling with primary antibodies (overnight at 4 °C). Samples were then washed in PBS (3 × 5 min in PBS at room temperature) prior to labeling with secondary antibodies.
Samples were then labeled with goat anti-mouse and goat anti-rabbit secondary antibodies conjugated to Alexa 488, Alexa 568 and Alexa 647 were used (1:8000; ThermoFisher Scientific, Grand Island, NY). Samples were then washed in PBS (3 × 5 min in PBS at room temperature) and mounted in ProLong Gold (Invitrogen, Rockford, IL). For simultaneous three-color imaging, immunofluorescent labeling was performed as previously described26. Immuno-labeling of spike protein and inflammatory cytokines were performed as previously described7.
Transmission electron microscopy (TEM)
TEM images of the ID, particularly gap junctions (GJs) and mechanical junctions (MJs), were obtained at 60,000x magnification on a FEI Tecnai G2 Spirit electron microscope. Intermembrane distance was measured at a distance of 50 nm from the edges of GJs and MJs using ImageJ (NIH, http://rsbweb.nih.gov/ij/), as previously described22,23.
Confocal imaging
Confocal imaging was performed using an A1R-HD laser scanning confocal microscope equipped with four solid-state lasers (405 nm, 488 nm, 560 nm, 640 nm, 30 mW each), a 63x/1.4 numerical aperture oil immersion objective, two GaAsP detectors, and two high sensitivity photomultiplier tube detectors (Nikon, Melville, NY). Individual fluorophores were imaged sequentially with the excitation wavelength switching at the end of each frame. Images were collected as z-stacks with fluorophores images sequentially (line-wise) to achieve optimal spectral separation, followed by 3D deconvolution. Images were analyzed using our point pattern analysis-based spatial analysis approach, Spatial Pattern Analysis using Closest Events (SPACE)27,28.
Statistical analysis
Treatments were applied in unblinded fashion for all studies. Distributions of measurements were compared using the two-sample Kolmogorov-Smirnov test, while the Wilcoxon Rank Sum test (for TEM data) and a weighted Student’s T-test (for SPACE outputs) were used for comparisons of central tendency. The Bonferroni correction was applied for multiple comparisons. A p < 0.05 was considered statistically significant. All values are reported as median ± standard error unless otherwise noted.
Results
Intravenous SARS-CoV2 spike protein increases arrhythmia inducibility in mice
To investigate if an acute inflammatory insult from viral spike protein is sufficient to precipitate cardiac arrhythmias, we collected in vivo ECGs from adult wild-type mice 5 days following intravenous injection of viral spike protein or its individual components (S1 or S2). Dosing was determined by levels reported in patients experiencing severe symptoms due to viral infection. Control groups included mice injected with albumin, a globular protein of similar size to spike protein, or a low dose of the S1 subunit mimicking vaccine levels (vS1 [10 µg]). Mice injected with high dose of spike protein showed increased arrhythmia burden (total arrhythmia duration per hour of observation) compared with control groups (Fig. 1; Table 2, Supplementary Fig. 2). Notably, while there was no significant difference in incidence (18 episodes in 6 control vs. 46 episodes in 18 spike-treated mice), high doses of spike protein promoted a more severe arrhythmia phenotype as reflected by episodes lasting more than 30 s (11 episodes in 18 mice vs. 2 episodes in 6 control mice). Further, Supplementary Fig. 1 shows elevated arrhythmia burden in all three subgroups (high dose S1 alone, high dose S2 alone, or both). These data suggest that presence of high levels of spike protein in the heart contributes to the arrhythmia prevalence in COVID-19 patients.
Impact of spike protein on atrial arrhythmia susceptibility. (A) Representative in vivo surface ECG illustrates atrial arrhythmia observed in a spike protein-injected mouse following caffeine + epinephrine challenge. (B) Total atrial arrhythmia burden under caffeine + epinephrine challenge quantified as minutes of arrhythmia per hour of observation (Albumin and vS1 controls n = 6 mice/group, Spike protein n = 18, Spike protein subunits n = 6/group, * p < 0.05 vs. albumin control - Wilcoxon’s rank sum test). (C) Cumulative distribution of atrial arrhythmia duration quantified as seconds of arrhythmia per hour of observation (Albumin control n = 6, Spike-treated (18), † - p < 0.05 vs. albumin control - Kolmogorov-Smirnov test).
Inflammatory markers and spike protein colocalize to vascular endothelium
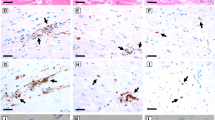
Hearts from patients infected with severe or fatal COVID-19 infection displayed elevated levels of proinflammatory cytokines TNFα and IL-6, and protease caspase-3, localized to vascular endothelial cells7. We, thus, examined hearts from spike protein-injected mice for the presence and distribution of inflammatory markers. Representative images show spike protein subunit S1 was detected in all mice injected with spike protein, and in none of the control groups (Fig. 2A). This is quantified in Table 1. Inflammatory markers were also highly expressed in these hearts, following the same vascular endothelial distribution as spike protein in contrast to control groups which lacked these pro-inflammatory markers (Fig. 2A; Supplementary Fig. 3; Table 1). Further, spike protein-injected mice showed cardiac microvascular damage, including enlarged endothelial cells and perivascular edema with evidence of red blood cell extravasation (Fig. 2B, C).
Histologic observations following spike protein insult. (A) Representative images of mouse heart samples showing presence of spike protein and elevated inflammatory cytokines, IL-6 and VEGF, in spike protein-injected mice compared to albumin control. (B) Representative H&E images demonstrating normal blood vessels in albumin control (black circle) vs. atypical endothelial cells (enlarged endothelial cells, perivascular edema; red arrows) and evidence of red blood cell extravasation, suggesting microvessel rupture, in high dose spike-injected heart (red circles). (C) High magnification view of dashed box in B) show multiple locations of red blood cell extravasation (circles) and perivascular edema (red arrow). All scale bars are 100 μm.
ID structural remodeling following spike protein insult
We previously showed that pathologically elevated vascular leak leads to ultrastructural remodeling within the ID following an acute inflammatory insult16. Therefore, we performed transmission electron microscopy (TEM) to assess ID structure, concentrating on gap junction (GJ) -adjacent perinexal and mechanical junction (MJ) -adjacent nanodomains, in spike protein-injected mice. Representative TEM images show considerable dehiscence, i.e. widening of the extracellular cleft, at these sites in hearts from mice that received the spike protein but not in control groups (Fig. 3A, Supplementary Fig. 4 A). Overall, spike protein levels representative of severe COVID-19 infection significantly increased intermembrane distances compared to albumin and vS1 controls (Fig. 3B; Supplementary Fig. 4B).
Ultrastructural effects of spike protein insult. (A) Representative TEM images of IDs from murine atria. White arrows point to GJs, and black arrows point to MJs. (B) Summary plots of median intermembrane distance measured at 50 nm from GJ (perinexal, solid bars) and MJ (MJ-adjacent, striped bars) edges (> 100 measurements/group/location from n = 3 hearts/group, * p < 0.05 vs. albumin control at perinexal sites, † p < 0.05 vs. albumin control at MJ-adjacent sites).
ID proteins undergo reorganization following spike protein insult
As part of our previously identified arrhythmia mechanism, we identified molecular remodeling within perinexal and MJ-adjacent regions of ID swelling16. We, thus, assessed the effects of spike protein insult on the spatial organization of ID proteins, specifically the cardiac sodium channel (NaV1.5), at these sites.
Low magnification confocal images of ventricular myocardium suggested potential lateralization of Cx43 and NaV1.5 (measured as percent overlap of NaV1.5 with ID marker N-cad) in myocytes of high dose SARS-CoV2 spike protein-treated hearts, but not in albumin-treated controls (Fig. 4; Supplementary Fig. 5). While we observed some instances of Cx43 and NaV1.5 lateralization, the amount of Cx43 and NaV1.5 dissociated from IDs (marked by N-cad) did not reach statistical significance. Representative high magnification views of 3D confocal images of en face IDs show close association of NaV1.5 with GJs and MJs in albumin control hearts (Fig. 5A). In contrast, NaV1.5 evidenced a diffuse, disorganized pattern of localization throughout the ID in hearts treated with high doses of spike proteins (Fig. 5A). Immunosignals for NaV1.5 and Cx43/Ncad were segmented and localized relative to each other, then analyzed using a point process analysis-based spatial statistical approach, Spatial Pattern Analysis using Closest Events (SPACE)28. The observed distributions of nearest-neighbor distances between co-labeled proteins were compared with corresponding distributions predicted under complete spatial randomness, where a leftward shift of the former indicates attraction, and a rightward shift, repulsion. Spatial analysis revealed strong attraction (in excess of levels expected under complete spatial randomness) of NaV1.5 to both GJs and MJs in control hearts, which was significantly reduced in S2 and S1 + S2 –treated mouse hearts (Fig. 5B, C; Supplementary Fig. 6B, C). Interestingly, S1-injected mouse hearts showed no significant change in NaV1.5 organization from albumin control (Supplementary Fig. 6B, C). Together, these data point to inflammation-induced vascular dysfunction following spike protein insult precipitates and perpetuates arrhythmias by ID ultrastructural and protein remodeling.
Confocal survey views of murine atria. Representative 2D confocal images showing some instances of Cx43 lateralization as well as elevated detection of NaV1.5 at the lateral membrane and concurrent loss of NaV1.5 at the ID (white arrows) in hearts from mice injected with high dose spike protein (but not low dose; % Cx43 overlap with N-cad: 53 ± 2% in albumin controls vs. 62 ± 5% in vS1 vs. 53 ± 3% in high dose spike, p = ns vs. control; % NaV1.5 overlap with N-cad: 81 ± 6% in albumin controls vs. 90 ± 3% in vS1 vs. 80 ± 4% in high dose spike, p = ns vs. control), n = 3 hearts/group. All scale bars are 25 μm.
3D Confocal imaging of individual en face IDs. Representative 3D confocal images of en face IDs from murine atria. (A) Albumin control and high dose spike protein (S1 + S2). (B) Cumulative distribution of NaV1.5 distance from Cx43 or Ncad. Dotted lines indicate complete spatial randomness of NaV1.5 relative to Cx43 and Ncad. Solid lines indicate observed NaV1.5 distribution. (C) Summary plot of NaV1.5 attraction to Cx43 or Ncad (n = 3 hearts/group, Albumin control (35 images), S1 + S2 (40), * p < 0.05 vs. albumin, † p < 0.05 vs. complete spatial randomness).
Discussion
Cardiac arrhythmias are a common complication in severe and fatal cases of COVID-19, with increased arrhythmia incidence correlated with severity of viral infection2,3. These patients also have heightened activation of resident macrophages and elevated expression of inflammatory cytokines and show evidence of vascular damage and cardiac remodeling. Identifying the pathophysiologic origins of cardiac arrhythmias in severe and fatal COVID-19 has inspired much debate. A number of studies, using in vitro heart tissue and human induced pluripotent stem cells, have suggested that cardiac damage may result from direct infection of myocytes by SARS-CoV229,30. Yet, others have proposed cardiac arrhythmias as a consequence of the systemic cytokine storm following viral infection9,10,11. We have previously provided strong evidence, using human cardiac tissue from fatal COVID-19 cases, that acute insult from viral spike protein triggers inflammation-induced vascular leak and consequent proarrhythmic disruption of NaV1.5-rich ID nanodomains7. Briefly, histologic and molecular studies in cardiac tissue from 11 fatal COVID-19 cases demonstrated presence of perivascular viral spike protein, elevated expression of pro-inflammatory cytokines, considerable microvascular damage including perivascular edema, and remodeling of key ID components essential for proper impulse propagation. SARS-CoV2 RNA was not detected with an ultrasensitive in situ hybridization method, consistent with other findings that note the lack of viral RNA despite clearly evident cardiac abnormalities in fatal COVID-197,9,12,15. These findings were consistent with results from our previously defined arrhythmia mechanism, whereby inflammation-induced vascular leak, and consequent edema, disrupts ID nanodomains and slows conduction, thereby generating a proarrhythmic substrate16.
Our recently published study in human cardiac tissue suggests insult from viral spike protein alone may be sufficient to precipitate arrhythmias. Therefore, we investigated the functional and structural impacts of spike protein in mice 5 days following initial insult. Mice injected with high levels of SARS-CoV2 spike protein demonstrated increased arrhythmia burden, reflecting arrhythmia prevalence in severe/fatal COVID-19 infection. These mice also mimicked the histologic and molecular findings in human cardiac tissue from fatal COVID-19 cases, including elevated expression of pro-inflammatory cytokines, atypical microvascular endothelial cells, perivascular edema, red blood cell extravasation and extensive ID protein remodeling. As noted above, the absence of evidence for direct infection of the heart by SARS-CoV2 suggests that cardiac pathogenesis in COVID-19 may occur independent of direct viral infection of the heart. We observed significant proarrhythmia following spike protein injection in wild-type mice without humanized ACE2 receptors and observed similar impacts from independent administration of the ACE-binding S1 subunit and/or the S2 subunit of the SARS-CoV2 spike protein. These results, along with the lack of efficacy of ACE2 inhibitors for the treatment of COVID-1915,31, support the notion of proarrhythmic impacts in COVID-19 occurring independent of direct viral infection of the heart and involve ACE2-dependent and independent pathways. Notably, mice injected with vaccine levels of S1 (vS1) exhibited structural and functional phenotypes similar to albumin control mice.
Previous work by our group demonstrated swelling of GJ-adjacent perinexal and MJ-adjacent ID regions and concomitant NaV1.5 reorganization away from these sites, occurring less than 1 h after a pro-inflammatory insult and linked these changes with slowing of action potential propagation in the atria and consequently, elevated AF risk16. Here, we show similar, but more extensive, NaV1.5 remodeling in mouse hearts at a longer timescale, 5 days following spike protein insult. Further, these mice exhibit Cx43 lateralization, a phenomenon that occurs over longer timescales (> 4 h)32,33 than tested in our previous study16. These data suggest a progression of ID protein remodeling following COVID-19 infection; that is, initial insult by spike protein triggers acute NaV1.5 reorganization from ID nanodomains, followed by subacute NaV1.5 and Cx43 lateralization occurring in the continued presence of spike protein and contributing to on-going cardiac abnormalities. Indeed, increased arrhythmia risk persists in long COVID-1934, indicating spike-induced inflammation may underlie arrhythmias in these patients.
Taken together, the results presented here provide strong evidence for a potential role of viral spike protein in not only initiating, but also in perpetuating, arrhythmias in COVID-19 patients. Further, we isolated each spike protein subunit and investigated their impact on cardiac function and structure individually and in combination. While the S1 subunit, containing the receptor binding domain, yielded a significant increase in arrhythmia burden, the S2 subunit, essential for host cell internalization, demonstrated similar detrimental functional and structural cardiac effects. However, it should be noted that our results may underestimate the proarrhythmic impact of the S1, which merits reassessment in mice expressing humanized ACE2 receptors. Histologic examination of S1 and/or S2 -treated mice evidenced increased expression of pro-inflammatory cytokines and compromised microvasculature, linking inflammation-induced vascular leak to the observed arrhythmia burden, consistent with our previous results16. Another possible mechanism driving arrhythmias in COVID-19 is pro-inflammatory cytokine-mediated calcium (Ca2+) mishandling. Interestingly, the viral spike protein S2 subunit has been suggested to mimic Ca2+/calmodulin-dependent protein kinase II (CaMKII) activity35a regulator of cardiac excitation-contraction coupling by modulating Ca2+ dynamics. Notably, increased CaMKII expression has been linked with worse outcomes in long COVID-1936. Together, these data point to viral spike protein mediated inflammation and downstream vascular leak playing a key role in the pathophysiology of cardiac dysfunction in severe and fatal COVID-19. Further, the results presented here highlight an AF patient population in which vascular endothelial barrier protection represents a viable anti-arrhythmic therapy strategy37.
Limitations
SARS-CoV2 S1 recognizes and binds to human ACE2, which is distinct from the murine ACE2. However, the present study utilized wild-type mice and found only moderate proarrhythmic impacts from S1 injection. Future studies in mice expressing humanized ACE2 receptors are needed to fully assess S1-induced proarrhythmia. Nevertheless, we observed significant functional differences in our S1-injected mice compared to control groups, indicating that it can elicit proarrhythmic inflammatory responses even in the absence of human ACE2 receptors. Further, the presence alone of S2, which lacks the ACE2 binding domain, was enough to precipitate arrhythmias, further underscoring the proarrhythmic potential of SARS-CoV2 spike proteins. Additionally, comparison of the proarrhythmic effects between SARS-CoV2 spike protein and those from other envelope viruses would be useful; however, such studies fell beyond the scope of this study. We focus here on mechanisms underlying arrhythmia substrate formation given our previous work on inflammation-induced arrhythmia. However, inflammation can also promote arrhythmia triggers via calcium mishandling. Thus, future work should investigate the mechanisms of trigger formation under inflammatory conditions. Finally, while we recognize the importance of incorporating female mice in these studies, limited availability of spike protein available constrained the scope of our experiments. While our study design is consistent with the male predominance of COVID-induced AF observed in patients, future studies incorporating larger cohorts should investigate sex-specific effects in COVID-19-associated CV risk.
Conclusions
In summary, we provide evidence that the inflammatory response elicited by SARS-CoV2 spike protein components is sufficient to induce a substrate for atrial arrhythmias even in the absence of direct viral infection. Further, we demonstrate that these atrial arrhythmias occur secondary to inflammation-induced vascular leak, and consequent ID nanodomain remodeling, suggesting that severe COVID-19 induces a proarrhythmic mechanism similar to that observed after an acute inflammatory insult. We recently proposed vascular endothelial barrier protection as a mechanistically-derived antiarrhythmic strategy for the prevention of AF in its early stages, especially in inflammation-associated etiologies37. The present study identifies patients with severe COVID-19 as a population at risk for inflammation-induced new onset AF and thus, potential candidates for vascular barrier protection therapy to prevent arrhythmias.
Data availability
All research data pertaining to this study are stored on secure servers at OSU and will be shared freely upon request. Please direct requests for data sharing to R. Veeraraghavan (veeraraghavan.12@osu.edu).
References
Mohammad, M. et al. Cardiac arrhythmias associated with COVID-19 infection: state of the Art review. Expert Rev. Cardiovasc. Ther. 19, 881–889. https://doi.org/10.1080/14779072.2021.1997589 (2021).
Li, X. et al. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID-19: a meta-analysis and systematic review. Crit. Care. 24, 468. https://doi.org/10.1186/s13054-020-03183-z (2020).
Rav-Acha, M. et al. Cardiac arrhythmias amongst hospitalised coronavirus 2019 (COVID-19) patients: prevalence, characterisation, and clinical algorithm to classify arrhythmic risk. Int. J. Clin. Pract. 75, e13788. https://doi.org/10.1111/ijcp.13788 (2021).
Chen, L., Li, X., Chen, M., Feng, Y. & Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 116, 1097–1100. https://doi.org/10.1093/cvr/cvaa078 (2020).
Herman-Edelstein, M. et al. Expression of the SARS-CoV-2 receptorACE2 in human heart is associated with uncontrolled diabetes, obesity, and activation of the Renin angiotensin system. Cardiovasc. Diabetol. 20, 90. https://doi.org/10.1186/s12933-021-01275-w (2021).
Vukusic, K. et al. Overexpression of the SARS-CoV-2 receptor angiotensin converting enzyme 2 in cardiomyocytes of failing hearts. Sci. Rep. 12, 965. https://doi.org/10.1038/s41598-022-04956-y (2022).
Mezache, L. et al. Histologic, viral, and molecular correlates of heart disease in fatal COVID-19. Ann. Diagn. Pathol. 60, 151983. https://doi.org/10.1016/j.anndiagpath.2022.151983 (2022).
Bois, M. C. et al. COVID-19-Associated nonocclusive fibrin microthrombi in the heart. Circulation 143, 230–243. https://doi.org/10.1161/CIRCULATIONAHA.120.050754 (2021).
Delorey, T. M. et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 595, 107–113. https://doi.org/10.1038/s41586-021-03570-8 (2021).
Li, H. et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 395, 1517–1520. https://doi.org/10.1016/S0140-6736(20)30920-X (2020).
Zhang, Y. et al. New Understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 127, 110195. https://doi.org/10.1016/j.biopha.2020.110195 (2020).
Avolio, E. et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. (Lond). 135, 2667–2689. https://doi.org/10.1042/CS20210735 (2021).
Huang, X. et al. Sars-Cov-2 Spike Protein-Induced damage of hiPSC-Derived cardiomyocytes. Adv. Biol. (Weinh). 6, e2101327. https://doi.org/10.1002/adbi.202101327 (2022).
Kato, Y. et al. TRPC3-Nox2 protein complex formation increases the risk of SARS-CoV-2 spike protein-induced cardiomyocyte dysfunction through ACE2 upregulation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24010102 (2022).
Paidi, R. K. et al. ACE-2-interacting domain of SARS-CoV-2 (AIDS) peptide suppresses inflammation to reduce fever and protect lungs and heart in mice: implications for COVID-19 therapy. J. Neuroimmune Pharmacol. 16, 59–70. https://doi.org/10.1007/s11481-020-09979-8 (2021).
Mezache, L. et al. Vascular endothelial growth factor promotes atrial arrhythmias by inducing acute intercalated disk remodeling. Sci. Rep. 10, 20463. https://doi.org/10.1038/s41598-020-77562-5 (2020).
Nuovo, G. J. et al. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the Spike protein. Ann. Diagn. Pathol. 51, 151682. https://doi.org/10.1016/j.anndiagpath.2020.151682 (2021).
Hockham, C. et al. Sex differences in cardiovascular complications and mortality in hospital patients with covid-19: registry based observational study. BMJ Med. 2, e000245. https://doi.org/10.1136/bmjmed-2022-000245 (2023).
Offerhaus, J. A. et al. Sex- and age specific association of new-onset atrial fibrillation with in-hospital mortality in hospitalised COVID-19 patients. Int. J. Cardiol. Heart Vasc. 39, 100970. https://doi.org/10.1016/j.ijcha.2022.100970 (2022).
Koleske, M. et al. Tetrodotoxin-sensitive Navs contribute to early and delayed afterdepolarizations in long QT arrhythmia models. J. Gen. Physiol. https://doi.org/10.1085/jgp.201711909 (2018).
Veeraraghavan, R. & Gourdie, R. G. Stochastic optical reconstruction microscopy-based relative localization analysis (STORM-RLA) for quantitative nanoscale assessment of Spatial protein organization. Mol. Biol. Cell. 27, 3583–3590. https://doi.org/10.1091/mbc.E16-02-0125 (2016).
Veeraraghavan, R. et al. The adhesion function of the sodium channel beta subunit (beta1) contributes to cardiac action potential propagation. Elife https://doi.org/10.7554/eLife.37610 (2018).
Veeraraghavan, R. et al. Sodium channels in the Cx43 gap junction perinexus May constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 467, 2093–2105. https://doi.org/10.1007/s00424-014-1675-z (2015).
Veeraraghavan, R., Lin, J., Keener, J. P., Gourdie, R. & Poelzing, S. Potassium channels in the Cx43 gap junction perinexus modulate ephaptic coupling: an experimental and modeling study. Pflugers Arch. 468, 1651–1661. https://doi.org/10.1007/s00424-016-1861-2 (2016).
Radwański, P. B. et al. Neuronal Na + Channels are integral components of Pro-Arrhythmic Na+/Ca2 + Signaling nanodomain that promotes cardiac arrhythmias during β-Adrenergic stimulation. JACC: Basic. Translational Sci. 1, 251–266. https://doi.org/10.1016/j.jacbts.2016.04.004 (2016).
Struckman, H. L. et al. Unraveling impacts of Chamber-Specific differences in intercalated disc ultrastructure and molecular organization on cardiac conduction. JACC Clin. Electrophysiol. 9, 2425–2443. https://doi.org/10.1016/j.jacep.2023.05.042 (2023).
Bogdanov, V. et al. Distributed synthesis of sarcolemmal and sarcoplasmic reticulum membrane proteins in cardiac myocytes. Basic. Res. Cardiol. 116, 63. https://doi.org/10.1007/s00395-021-00895-3 (2021).
Soltisz, A. M., Craigmile, P. F. & Veeraraghavan, R. Spatial Pattern Analysis using Closest Events (SPACE) – a nearest neighbor point pattern analysis framework for assessing spatial relationships from Image data. bioRxiv https://doi.org/10.1101/2023.05.17.541131 (2023).
Bailey, A. L. et al. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic. Transl Sci. 6, 331–345. https://doi.org/10.1016/j.jacbts.2021.01.002 (2021).
Perez-Bermejo, J. A. et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci. Transl Med. https://doi.org/10.1126/scitranslmed.abf7872 (2021).
Investigators, W. C. f. T. R.-C. Effect of angiotensin-Converting enzyme inhibitor and angiotensin receptor blocker initiation on organ Support–Free days in patients hospitalized with COVID-19: A randomized clinical trial. JAMA 329, 1183–1196. https://doi.org/10.1001/jama.2023.4480 (2023).
Matsushita, T. et al. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ. Res. 85, 1046–1055. https://doi.org/10.1161/01.res.85.11.1046 (1999).
Severs, N. J., Bruce, A. F., Dupont, E. & Rothery, S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc. Res. 80, 9–19. https://doi.org/10.1093/cvr/cvn133 (2008).
Xie, Y., Xu, E., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28, 583–590. https://doi.org/10.1038/s41591-022-01689-3 (2022).
Wenzhong, L. & Hualan, L. COVID-19: the CaMKII-like system of S protein drives membrane fusion and induces syncytial multinucleated giant cells. Immunol. Res. 69, 496–519. https://doi.org/10.1007/s12026-021-09224-1 (2021).
Elseidy, S. A. et al. Cardiovascular complications in the Post-Acute COVID-19 syndrome (PACS). Int. J. Cardiol. Heart Vasc. 40, 101012. https://doi.org/10.1016/j.ijcha.2022.101012 (2022).
Mezache, L., Soltisz, A. M., Johnstone, S. R., Isakson, B. E. & Veeraraghavan, R. Vascular endothelial barrier protection prevents atrial fibrillation by preserving cardiac nanostructure. bioRxiv https://doi.org/10.1101/2023.06.20.545742 (2023).
Funding
This work was supported by grants (R01 HL148736 and R01 HL165751) awarded to RV from the National Institutes of Health and an American Heart Association predoctoral fellowship awarded to LM.
Author information
Authors and Affiliations
Contributions
LM performed the mouse experiments and microscopy image collection in addition to preparing the manuscript and assisting with study design and interpretation of results. AS performed computational image analysis on microscopy data. ET assisted with mouse experiments. GJN and RV conceptualized and designed the study, and assisted with manuscript preparation. RV oversaw the experimental aspects of the study and mentored LM and AS.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mezache, L., Soltisz, A., Tili, E. et al. SARS-CoV-2 spike protein-induced inflammation underlies proarrhythmia in COVID-19. Sci Rep 15, 33991 (2025). https://doi.org/10.1038/s41598-025-12807-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12807-9