Abstract
The stress hyperglycemia ratio (SHR) represents an emerging biomarker linked to poor clinical outcomes. However, its association with fatal outcomes in patients experiencing respiratory failure (RF) remains poorly understood. This research was designed to evaluate the utility of SHR in predicting both in-hospital mortality and intensive care unit (ICU) mortality among RF patients. This retrospective cohort analysis utilized data from the MIMIC-IV version 3.0 database. Patients diagnosed with RF in the ICU were divided into four groups according to the SHR index quartiles (group1, group2, group3, and group4), and the outcomes were in-hospital and ICU mortality. Survival outcomes among different groups were analyzed through Kaplan-Meier curves. Based on the results of the schoenfeld residual test, choose the Cox model or the model with the time interaction term to report the association between SHR and the outcome. Furthermore, restricted cubic splines analyses were conducted to explore potential non-linear relationships of SHR with both in-hospital and ICU mortality. This study enrolled 2,250 participants, demonstrating in-hospital mortality and ICU mortality rates of 23.91% and 14.31%, respectively. Kaplan-Meier analysis revealed that the group4 exhibited the lowest survival rates (P < 0.001). Through multivariate Cox regression, when comparing the group1 to the group4, three analytical models consistently showed increased in-hospital and ICU mortality in group4. The time interaction model revealed significant increases in hospital mortality risk across SHR quartiles compared to group1. Specifically, in model1, group2 showed a 62%-69% higher risk (HR = 1.69, 95% CI: 1.17–2.44), group3 exhibited a 358%-370% higher risk (HR = 4.58, 95% CI: 1.83–11.5) ,while group4 demonstrated exponential risk escalation. All quartile groups exhibited a daily risk attenuation of approximately 57% (time interaction term HR = 0.43, all p < 0.001). The associations remained consistent after adjusting the variables in models 2 and 3. In contrast, no significant risk association was observed between SHR and ICU mortality in the time interaction model. Besides, a U-shaped relationship was observed between SHR and both in-hospital mortality and ICU mortality. The study revealed that elevated SHR levels in ICU-admitted RF patients were significantly associated with increased risks of in-hospital mortality. Clinicians should closely monitor patients with high admission SHR values, especially patients in the highest SHR quantile (Q4 group) during the early admission period, underscoring the need for prioritized clinical intervention in this high-risk population.
Similar content being viewed by others
Background
Respiratory failure (RF) ranks as a leading disease in critical care and emergency department settings1,2. It can be precipitated by diverse pathological conditions, the resultant hypoxia or carbon dioxide retention are prone to precipitate damage to crucial organs including the heart, cerebrum, and kidneys. In recent years, the diagnosis of respiratory failure has been gradually increasing, and approximately 30% intensive care unit (ICU) admissions are patients with severe RF3. Despite apparent improvements in the respiratory support and quality of care over time, severe RF leading to ICU admission is associated with high mortality4,5,6,7,8.
Emerging evidence from multiple investigations has demonstrated a consistent association between hyperglycemia and poorly clinical outcomes across diverse pathological conditions, particularly in cardiovascular disorders, renal impairment, cerebrovascular events, and systemic infections9,10,11,12,13.The reliability of admission glucose as a stress hyperglycemia marker was questioned cause it does not reflect the overall hyperglycemia condition or evaluate chronic glucose levels. In response to these clinical needs, researchers have developed a novel biomarker, the stress hyperglycemia ratio (SHR), to more accurately assess glycemic status in critically ill patients14. Research by Yan et al. indicate that elevated SHR levels are linked to a higher risk of adverse events in those people suffering from sepsis11. A review of 32 studies demonstrated that patients with acute myocardial infarction and higher SHR were significantly more likely to encounter major adverse events and mortality than those with lower SHR15. Lin et al. have shown that SHR independently relates to the risk of pulmonary infection among patients with ST-segment elevation myocardial infarction16. According to Pan et al. stress hyperglycemia is linked to higher mortality rates in patients with ischemic stroke17. However, the association between SHR and mortality risk in RF patients remains insufficiently explored.
This investigation sought to evaluate the prognostic value of SHR in predicting clinical outcomes among ICU-admitted RF patients, utilizing data from the MIMIC-IV database. The findings may provide valuable insights for optimizing risk stratification and improving therapeutic decision-making in critical care settings.
Method
Data source
This retrospective study analyzes data from the publicly accessible Medical Information Mart for Intensive Care IV (MIMIC-IV, version 3.0) database. The dataset comprises clinical records spanning from 2008 to 2019, collected at Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts, USA. The use of this database was authorized by the Institutional Review Committee at Massachusetts Institute of Technology and BIDMC. Furthermore, the research team completed an online training program focused on safeguarding human research participants, offered by the National Institutes of Health, and successfully passed the Collaborative Institutional Training Initiative certification exams (author certification number: 13980804). The database repository has undergone complete anonymization, with all identifiable information removed. Ethical approval and participant consent were not required as the study exclusively utilized publicly available datasets.
Data extract and study design
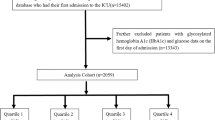
Our research encompassed 2,250 patients who were in the ICU according to the MIMIC-IV version 3.0 database. The variables we extracted include demographic characteristics, vital signs, disease severity scores, length of stay, outcomes, comorbidities and laboratory parameters. Variables were removed from the analysis if they had missing values above 20%. The included criteria were: (1) first admitted to hospital; (2) first admitted to ICU; (3) Patients diagnosed with RF between 18 and 100 years old. The excluded criteria were: (1) without glucose on admission (2) without glycated hemoglobin [HbA1c]) on admission. Ultimately, 2,250 patients were classified into four groups based on the SHR index quartiles (shown in Fig. 1).
Calculation of SHR and outcome
The formula [admission glucose (mg/dl)/[28.7×HbA1c (%) − 46.7)] was used to calculate SHR, using admission glucose and HbA1c data obtained from MIMIC IV18. The outcomes of this study were in-hospital mortality and ICU mortality within patient admission.
Statistical analysis
Participants were stratified according to SHR index quartiles for baseline characterization. Numerical data are expressed as mean ± SD, while qualitative measures are reported in frequencies and proportions. Statistical comparisons were performed using one-way ANOVA for numerical parameters and Pearson’s chi-square test for categorical measures. Mortality events were quantified throughout the observation period.
The differences in mortality among the four groups were explored using Kaplan-Meier (K-M) survival analyses. Restricted cubic spline (RCS) analyses using four knots were employed to examine the link between SHR and mortality. The proportional hazards assumption was formally assessed using Schoenfeld residual tests. Based on the results of the schoenfeld residual test, choose the Cox model or the model with the time interaction term to report the association between SHR and the clinical outcome. To account for potential confounders, three adjusted regression models were constructed. Model 1 was implemented without adjusting for covariates. Model 2 was adjusted for age, sex, and race. Model 3 was adjusted for age, gender, race, hypertension, diabetes mellitus type2 (DM2), heart failure (HF), stroke, myocardial infarction (MI), chronic kidney disease (CKD), acute renal failure (ARF), hepatitis, pneumonia, cirrhosis, hyperlipidemia (HLP), chronic obstructive pulmonary disease (COPD), sepsis, fluid balance status, and use of insulin, prednisone, midazolam, meropenem, vancomycin, piperacillin-tazobactam, propofo, and morphine. In cases where the relationship was nonlinear, we determined the cut-off value and identified the inflection point. We applied a two-piece wise Cox proportional hazards model on either side of the inflection point to explore the link between SHR and mortality.
In addition, we further performed subgroup analyses by using gender, age, complications including hypertension, DM2, HF, MI, ARF, CKD, COPD, HLP, and stroke, use of continuous renal replacement therapy (CRRT) and ventilator to verify the robustness of the results. R studio (version R4.2.3) and Empower Stats (version 4.1) were used for statistical analysis in this study, with a two-sided P value < 0.05 indicating statistical significance.
Results
Baseline characteristics
Our study involved 2,250 patients with RF, who were sorted into four groups by SHR index quartiles (group1, group2, group3, and group4), with group1 being the reference group. The average age of the patients included in the study was 66.47 years old, with 57.96% of them being male. The average day of stay in the hospital and ICU was 18.54 days and 7.77 days respectively. The range of SHR in the group1, group2, group3, and group4 was 0.12–0.94, 0.95–1.18, 1.19–1.50, and 1.51–15.04, respectively. There were no statistically differences in gender, marital status, race, length of stay in hospital, length of stay in ICU, Glasgow Coma Scale score (GCS score), Charlson Comorbidity Index score (CCI score), use of ventilation, or complications of HF, malignant tumor (MT), CKD and COPD among the different SHR groups. Notably, significant differences were observed between the different quartiles regarding SHR index, including sequential organ failure assessment score(SOFA score), acute physiology score III (APS III score), simplified acute physiology score II (SAPS II score) and oxford acute severity of illness score (OASIS score), mortality in-hospital, mortality in ICU, complications of hypertension, DM2, MI and ARF, use of medications (insulin, prednisone, midazolam, and vancomycin), also includes some laboratory parameters. When compared with the patients in the other 3 groups, those with higher SHR levels had higher lactate, total bilirubin, urine protein, troponint, activated partial thromboplastin time, alanine aminotransferase and worse disease severity score including SOFA score, APS III score, SAPS II score and OASIS score; while the proportion of patients use of ventilation and CRRT, with complication of MI, cirrhosis, ARF, and COPD increased significantly(shown in Table 1).
Kaplan-Meier (K-M) survival analysis
K-M survival analyses showed a significant difference in the incidence of in-hospital mortality and ICU mortality between the four groups. The details of the K-M survival analyses are presented in Fig. 2a and b, the highest in-hospital mortality and ICU mortality being in the group4 (P < 0.001). Moreover, K-M analyses indicated that patients with high SHR were linked to worse survival (p < 0.001), while no notable differences in survival rates were found among the other three groups (group1, group2, and group3).
Cox proportional-hazards regression model and time interaction term model
After adjusting for all confounding factors, Cox proportional-hazards regression model revealed (Tables 2 and 3): When SHR was treated as a continuous variable, each unit increase was associated with a 22% higher risk of in-hospital mortality (HR = 1.22,95% CI: 1.12–1.33, P < 0.001) and a 16% increased risk of ICU mortality (HR = 1.16,95% CI: 1.06–1.28, P = 0.002). When SHR was categorized into quartiles, compared to the group1, patients in the Q4 group exhibited significantly elevated risks of both in-hospital mortality (HR = 1.92, 95% CI: 1.44–2.55, P < 0.001) and ICU mortality (HR = 1.86, 95% CI: 1.29–2.69, P = 0.001).
P-values of SHR and global schoenfeld residual tests being less than 0.05, as show in the supplementary Fig. 1. Similar trends were observed in the time interaction model (Tables 2 and 3). The results revealed significant increases in hospital mortality risk across SHR quartiles compared to group1 (reference group). Specifically, in model1, group2 showed a 62%−69% higher risk (HR = 1.69, 95% CI: 1.17–2.44), group3 exhibited a 358%−370% higher risk (HR = 4.58, 95% CI: 1.83–11.5),while group4 demonstrated exponential risk escalation. All quartile groups exhibited a daily risk attenuation of approximately 57% (time interaction term HR = 0.43, all p < 0.001). The associations remained consistent after adjusting the variables in models 2 and 3. Notably, while the group4 quartile demonstrated extreme risk elevation (p ≤ 0.001), the interpretation requires caution due to the wide confidence interval. In contrast, no significant risk association was observed between SHR and ICU mortality in the time interaction model.
Non-linear relationships between the SHR and mortality
The non-linear link between SHR and mortality in RF patients was evaluated using Cox proportional hazards regression models with RCS. The RCS analysis showed a U-shaped correlation between SHR and both in-hospital and ICU mortality, even when other confounding factors were considered. (P values for non-linearity < 0.05; shown in Fig. 3a and b). The cut-off value of SHR corresponding to the lowest risk of in-hospital mortality according to multivariate-adjusted RCS analyses was 1.052 for the population.We further divide SHR into high SHR groups and low SHR groups according to the inflection point. K-M survival analysis showed that the in-hospital mortality and ICU mortality in the high SHR group were higher than those in the low SHR group (shown in Fig. 4a and b).
Subgroup analysis
To ensure the robustness of the results, we performed a subgroup analysis to examine the associations between SHR and in-hospital mortality and ICU mortality, considering variables like gender, age, CRRT, ventilation, complications of hypertension, DM2, HF, MI, ARF, CKD, COPD, HLP, and stroke (shown in Fig. 5). The forest plot showed significant interaction between SHR and in-hospital mortality in subgroups of patients ages < 60 years, use the ventilation and CRRT, those with complications of DM2, HF, MI and HLP. The HRs of those patients use the CRRT and ventilation, combined with DM2, HF, and HLP, were 1.53, 1.47, 1.51, 1.69, and 1.37 respectively. However, almost no significant interaction between SHR and ICU mortality in each subgroup of patients were observed. This evidences that SHR maybe is an independent prognostic factor in ICU mortality.
Discussion
To the best of our awareness, this is the initial study exploring the relationship between SHR and mortality for RF patients. Findings from our research suggest that elevated SHR levels in RF patients are associated with higher in-hospital mortality, demonstrating clear time-dependent effects. Especially, patients in the highest SHR quantile (Q4 group) exhibited exceptionally high mortality risk during the early admission period. Besides, there was a U-shaped association between SHR and in-hospital mortality rates and ICU mortality rates. Significantly, it may provide a straightforward and efficient biomarker for assessing prognosis in RF patients.
Stress hyperglycemia highlights the acute increase in blood glucose levels due to stress reaction or serious illness. For the past few years, SHR as a novel effective indicator has gradually attracted people’s attention. Previous reports have confirmed that SHR is a powerful predictor of elevated mortality and morbidity risk in individuals with various health conditions. For example, Lei et al. reported SHR correlates with both all-cause and cardiovascular mortality in patients, with a U-shaped association for all-cause mortality and an L-shaped association for cardiovascular mortality19. Mohammed et al. found that elevated SHR was independently linked to a greater risk of composite events of all-cause and cardiovascular mortality than lower SHR, serving as a valuable tool for predicting adverse risks in HFpEF patients20. In brief, SHR is associated with risk factors for several diseases and might also be a valuable clinical prognostic indicator. Our study partially aligns with the studies discussed above, analysis of 2,250 RF patients within the American MIMIC-IV cohorts revealed that individuals in the high SHR levels exhibited significantly elevated risks for both in-hospital mortality and ICU mortality. Furthermore, the study identified a non-linear, U-shaped correlation between SHR levels and patient mortality among critically ill cases.
While the precise mechanisms behind this observation require further investigation, we hypothesize that elevated mortality risks in RF patients with high SHR levels may stem from a combination of impaired pulmonary function and the detrimental impacts of stress-related hyperglycemia. Patients with respiratory failure usually accompanied by inflammatory reactions, such as severe pneumonia, acute respiratory distress syndrome and systemic inflammatory response syndrome21,22. On the other hand, stress-related hyperglycemia is a common occurrence in critically ill individuals, it can trigger insulin resistance, systemic inflammatory responses, compromised immune function, and significant disruptions in glucose metabolism regulation. In other words, SHR may somewhat have a certain relationship with the inflammatory response.
First of all, patients with hyperglycemia could be increase the oxidative stress and stimulate the release of stress-related counterregulatory hormones, including catecholamines, cortisol, glucagon, and growth hormone, alongside proinflammatory mediators such as TNF-α and IL-6. Studies have demonstrated that insulin therapy aimed at normalizing blood glucose levels in patients significantly decreases inflammatory markers, including adhesion molecules, hepatic iNOS expression, and circulating NO metabolites23,24,25. In addition, research indicates that elevated glucose concentrations trigger the activation of reactive oxygen species, proinflammatory cytokines, chemokines, and signaling pathways such as NF-κB, PKC, and p38 MAPK within immune cells23,26. In other words, elevated glucose levels may impair T lymphocyte activity, immunoglobulin production, and complement function, the compromising the immune system and heightening infection susceptibility. Ultimately, ICU patients with elevated SHR often require more invasive interventions, including mechanical ventilation, CRRT, and central venous catheterization, which may elevate the risk of pulmonary infections27. As mentioned above, the stress hyperglycemia may inhibit the immune system and increase the risk of infection; and the inflammatory response is aggravated due to the promotion of the release of various inflammatory mediators. It is reasonable to assume that the combination of severe pulmonary dysfunction and systemic stress response—reflected by higher SHR levels—contributes to the increased mortality rate in patients with respiratory failure. However, further investigation is necessary to elucidate the precise mechanisms linking stress-induced hyperglycemia and clinical outcomes in RF patients.
Previous literature has reported that both hyperglycemia and hypoglycemia can increase mortality in ICU patients, highlighting the critical importance of maintaining glucose homeostasis in critically ill patients28,29,30,31. Our research also showed that a lower SHR level is associated with elevated mortality in patients with RF, may potentially mediated through disturbances in energy metabolism, neuroendocrine dysregulation, and systemic inflammatory responses. First, lower SHR levels may indicate hypoglycemia, which leads to insufficient glucose supply to brain tissue, potentially causing impaired consciousness and respiratory center depression, thereby further worsening ventilation function. Second, under severe stress conditions, insufficient insulin secretion or resistance to counter-regulatory hormones (such as cortisol, glucagon, and epinephrine) results in impaired gluconeogenesis, making it difficult to effectively correct hypoglycemia. Meanwhile, hypoglycemia can trigger sympathetic nervous system excitation, increasing oxygen consumption and aggravating respiratory muscle fatigue. Finally, conditions like sepsis and ARDS that cause stress-induced hypoglycemia are often accompanied by excessive inflammatory responses. Cytokines such as TNF-α and IL-6 inhibit insulin signaling pathways while promoting mitochondrial dysfunction. Hypoglycemia itself can also enhance oxidative stress, further damaging pulmonary vascular endothelial cells and alveolar epithelial cells.In summary, low SHR levels in respiratory failure patients not only reflect severe metabolic disorders but can also independently exacerbate multiple organ dysfunction, ultimately leading to increased mortality.
Our study demonstrated a significant association between SHR levels and RF patient in hospital mortality, with this relationship exhibiting distinct time-dependent characteristics that were particularly pronounced during the early admission period. This temporal pattern may be attributed to the underlying pathophysiological mechanism: abnormal SHR levels upon admission (whether elevated or reduced) directly reflect more severe glucose metabolism dysregulation under acute stress conditions. Such metabolic disturbance typically indicates more profound systemic pathophysiological alterations and, consequently, predicts poorer clinical outcomes.
Numerous biomarkers have evaluated the prognosis of patients with RF32,33,34. These methods face challenges such as restricted availability, significant expenses, and technical limitations, making rapid implementation difficult. In contrast, the SHR, derived from readily available admission glucose and HbA1c measurements through standard lab tests, offers a more practical and accessible alternative for clinical use. However, it is imperative to emphasized that additional prospective studies are necessary to investigate the relationship between SHR and clinical outcomes in RF patients. Validation of SHR as a prognostic indicator requires evaluation across varied patient populations and healthcare settings.
Limitation
Our research suggests that the SHR index could serve as a valuable clinical tool for evaluating critically ill RF patients. However, this study has several limitations. First, the absence of data on potential confounders, including the etiology and severity of respiratory failure, may influence the results. Second, the inability to pinpoint the exact timing of RF onset and the specific causes of death could reduce the clinical applicability of our findings. Third, while the exclusion of variables with over 20% missing data aligns with common practice in the field, we recognize that this approach may introduce bias if the omitted variables are clinically or biologically relevant. Furthermore, as a single-center retrospective analysis, the conclusions require validation through additional multicenter cohort studies to strengthen the reliability of the results.
Conclusion
The findings of our research highlight that among RF patients admitted to the ICU, elevated SHR levels were significantly associated with increased risks of in hospital mortality, demonstrating clear time-dependent effects. Especially, patients in the highest SHR quantile (group 4) exhibited exceptionally high mortality risk during the early admission period, underscoring the need for prioritized clinical intervention in this high-risk population. Notably, a nonlinear, U-shaped correlation was demonstrated between SHR index and both outcomes among this patient population. However, additional controlled clinical trials are required to validate the clinical implications of SHR in managing severe RF patients.
Data availability
The data that support the findings of current study are available in the MIMIC repository at [https://physionet.org/content/mimiciv/3.0/]. The data examined in this study is available upon reasonable request from the corresponding author.
Abbreviations
- ICU:
-
intensive care unit
- SD:
-
standard deviation
- F:
-
ANOVA
- χ²:
-
Chi-square test
- LOS:
-
length of stay
- HR:
-
heart rate
- WBC:
-
white blood cell
- RBC:
-
red blood cell
- RDW:
-
red blood cell distribution width
- PH:
-
potential of hydrogen
- PO2:
-
Partial pressure of oxygen
- PaCO2:
-
Partial pressure of carbon dioxide
- Spo2:
-
Peripheral Oxygen Saturation
- PT:
-
Prothrombin Time
- APTT:
-
Activated Partial Thromboplastin Time
- INR:
-
International Normalized Ratio
- CK:
-
Creatine Kinase
- CK-MB:
-
Creatine Kinase-MB
- LDH:
-
Lactate dehydrogenase
- ALT:
-
glutamic pyruvic transaminase
- AST:
-
glutamic oxaloacetic transaminase
- ABPs:
-
Artery Systolic Blood Pressure
- ABPd:
-
Artery Diastolic Blood Pressure
- ABPm:
-
Artery Mean Blood Pressure
- DM2:
-
Diabetes mellitus type2
- HF:
-
heart failure
- MT:
-
malignant tumor
- MI:
-
myocardial infarct
- CKD:
-
chronic kidney disease
- ARF:
-
acute renal failure
- HLP:
-
hyperlipidemia
- COPD:
-
chronic obstructive pulmonary disease
- SOFA:
-
sequential organ failure assessment
- OASIS:
-
Oxford acute severity of illness score
- APS III:
-
acute physiology score III
- SAPS II:
-
simplified acute physiology score II
- GCS:
-
Glasgow Coma Scale
- CCI:
-
Charlson Comorbidity Index
- CRRT:
-
continuous renal replacement therapy
- F:
-
female
- M:
-
male
References
Vincent, J. L. et al. The epidemiology of acute respiratory failure in critically ill patients(*). Chest 121, 1602–1609 (2002).
Chen, L. & Rackley, C. R. Diagnosis and epidemiology of acute respiratory failure. Crit. Care Clin. 40, 221–233 (2024).
Kempker, J. A. et al. The epidemiology of respiratory failure in the united States 2002–2017: a serial cross-sectional study. Crit. Care Explor. 2, e128 (2020).
Ippolito, M., Galvano, A. N. & Cortegiani, A. Long-term outcomes in critically ill patients with acute respiratory failure. Curr. Opin. Crit. Care. 30, 510–522 (2024).
Bellani, G. et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. Jama-J Am. Med. Assoc. 315, 788–800 (2016).
Grasselli, G. et al. Esicm guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 49, 727–759 (2023).
Urner, M. et al. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Resp. Med. 8, 905–913 (2020).
Chi, Y., Zhao, Z., Frerichs, I., Long, Y. & He, H. Prevalence and prognosis of respiratory Pendelluft phenomenon in mechanically ventilated Icu patients with acute respiratory failure: a retrospective cohort study. Ann. Intensive Care. 12, 22 (2022).
Yang, J. et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. 45, 947–956 (2022).
Zhang, C. et al. Relationship between stress hyperglycemia ratio and allcause mortality in critically ill patients: results from the mimic-iv database. Front. Endocrinol. 14, 1111026 (2023).
Yan, F. et al. Association between the stress hyperglycemia ratio and 28-day all-cause mortality in critically ill patients with sepsis: a retrospective cohort study and predictive model establishment based on machine learning. Cardiovasc. Diabetol. 23, 163 (2024).
Cheng, S., Shen, H., Han, Y., Han, S. & Lu, Y. Association between stress hyperglycemia ratio index and all-cause mortality in critically ill patients with atrial fibrillation: a retrospective study using the mimic-iv database. Cardiovasc. Diabetol. 23, 363 (2024).
Ge, T., Hu, J. & Zhou, Y. The association between stress hyperglycemia ratio with mortality in critically ill patients with acute heart failure. Front. Cardiovasc. Med. 11, 1463861 (2024).
Nathan, D. M. et al. Translating the a1c assay into estimated average glucose values. Diabetes Care. 31, 1473–1478 (2008).
Esdaile, H. et al. The association between the stress hyperglycaemia ratio and mortality in cardiovascular disease: a meta-analysis and systematic review. Cardiovasc. Diabetol. 23, 412 (2024).
Lin, Z. et al. Positive association between stress hyperglycemia ratio and pulmonary infection in patients with st-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 22, 76 (2023).
Pan, H., Xiong, Y., Huang, Y., Zhao, J. & Wan, H. Association between stress hyperglycemia ratio with short-term and long-term mortality in critically ill patients with ischemic stroke. Acta Diabetol. 61, 859–868 (2024).
Roberts, G. W. et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J. Clin. Endocrinol. Metab. 100, 4490–4497 (2015).
Ding, L. et al. The prognostic value of the stress hyperglycemia ratio for all-cause and cardiovascular mortality in patients with diabetes or prediabetes: insights from Nhanes 2005–2018. Cardiovasc. Diabetol. 23, 84 (2024).
Mohammed, A. Q. et al. Stress hyperglycemia ratio as a prognostic indicator for long-term adverse outcomes in heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 23, 67 (2024).
Choudhary, T. et al. Derivation and validation of generalized sepsis-induced acute respiratory failure phenotypes among critically ill patients: a retrospective study. Crit Care. 28, 321 (2024).
Azoulay, E., Mokart, D., Kouatchet, A., Demoule, A. & Lemiale, V. Acute respiratory failure in immunocompromised adults. Lancet Resp. Med. 7, 173–186 (2019).
Dandona, P., Mohanty, P., Chaudhuri, A., Garg, R. & Aljada, A. Insulin infusion in acute illness. J. Clin. Invest. 115, 2069–2072 (2005).
Van den Berghe, G., Vanhorebeek, I., Langouche, L. & Gunst, J. Our scientific journey through the ups and downs of blood glucose control in the Icu. Am. J. Respir Crit. Care Med. 209, 497–506 (2024).
Rybka, J. [Glycaemia control in critically ill patients is justified and effective]. Vnitr Lek. 56, 977–987 (2010).
Shomali, N. et al. Harmful effects of high amounts of glucose on the immune system: an updated review. Biotechnol. Appl. Biochem. 68, 404–410 (2021).
Mellado-Artigas, R. et al. Effect of immediate initiation of invasive ventilation on mortality in acute hypoxemic respiratory failure: a target trial emulation. Crit. Care. 28, 157 (2024).
Guo, J. Y. et al. The paradox of the glycemic gap: does relative hypoglycemia exist in critically ill patients? Clin. Nutr. 40, 4654–4661 (2021).
Bellaver, P. et al. Association of multiple glycemic parameters at intensive care unit admission with mortality and clinical outcomes in critically ill patients. Sci. Rep. 9, 18498 (2019).
Kataja, A. et al. The association of admission blood glucose level with the clinical picture and prognosis in cardiogenic shock - results from the cardshock study. Int. J. Cardiol. 226, 48–52 (2017).
Krinsley, J. S., Meyfroidt, G., van den Berghe, G., Egi, M. & Bellomo, R. The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr. Opin. Clin. Nutr. Metab. Care. 15, 151–160 (2012).
Delerme, S. & Ray, P. Acute respiratory failure in the elderly: diagnosis and prognosis. Age Ageing. 37, 251–257 (2008).
Cervantes-Alvarez, E. et al. Galectin-3 as a potential prognostic biomarker of severe covid-19 in SARS-cov-2 infected patients. Sci. Rep. 12, 1856 (2022).
Asif, S. et al. Plasma endostatin correlates with hypoxia and mortality in covid-19-associated acute respiratory failure. Biomark. Med. 15, 1509–1517 (2021).
Acknowledgements
We express our gratitude to all individuals who contributed to this project.
Author information
Authors and Affiliations
Contributions
Author contributions: HHB, DN, BLL, YZ, and YW designed the study. HHB extracted clinical data from the MIMIC-IV database. HHB, DN, and BLL performed the statistical analysis of the data. HHB authored the first draft. DN provides the references. BLL, YZ and YW examined and revised the paper. All authors contributed to manuscript revision, read, and endorsed the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki. The review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center approved the use of the MIMIC-IV database. Because the data were publicly available, the study was exempt from the requirements of an ethics approval statement and informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bai, H., Niu, D., Li, B. et al. Elevated stress hyperglycemia ratio associated with higher hospital mortality in patients with respiratory failure. Sci Rep 15, 27972 (2025). https://doi.org/10.1038/s41598-025-12853-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12853-3