Abstract
Non-invasive preoperative assessment of HER2 status is critical for identifying candidates for targeted therapy and personalizing treatment strategies in endometrial cancer (EC). This study aims to assess the preoperative value of multiparametric magnetic resonance imaging (MRI)-based radiomics in predicting HER2 status and prognosis of EC patients. We included 492 patients with EC divided into training (n = 215), internal validation (n = 92), and external validation cohorts 1 (n = 64) and 2 (n = 121). Models were constructed using six machine learning algorithms based on radiomics features derived from multiparametric MRI, including T2-weighted, diffusion-weighted, and contrast-enhanced T1-weighted sequences. A fusion model integrating key clinical predictors with the radiomics score (Rad-score) was created. Its predictive performance was evaluated through receiver operating characteristic (ROC) analysis, and its prognostic significance was assessed through survival analysis. HER2 (+) status was associated with poor differentiation and myometrial invasion in patients with EC. A support vector machine (SVM)-based model comprised of multiparametric MRI-based radiomics features demonstrated excellent performance in predicting HER2 status, with a mean area under the ROC curve (AUC) of 0.814 in the validation cohorts. A fusion model combining the SVM-based Rad-score with clinical factors significantly improved prediction accuracy, achieving AUCs of 0.914 in the training cohort, and 0.809–0.865 in the validation cohorts. Kaplan–Meier analysis revealed that patients with EC with predicted HER2 (+) status had worse progression-free survival than those with predicted HER2 (−) status. The fusion model based on multiparametric MRI-based radiomics features can potentially aid in the accurate preoperative prediction of HER2 status and prognosis of patients with EC, providing essential insights for clinical decision-making.
Similar content being viewed by others
Introduction
Endometrial cancer (EC) ranks among the most prevalent gynecological cancers, and its incidence is increasing in younger populations1,2. While surgical treatment offers significant survival benefits, 10%–20% of patients with EC still experience poor prognoses, reflected in a 5-year survival rate of less than 20%3. The recent development of targeted therapies has opened new avenues for treating EC. Numerous research efforts have suggested that therapies aimed specifically at human epidermal growth factor receptor-2 (HER2)-positive EC could enhance survival rates among affected patients4,5. As a receptor tyrosine kinase, HER2 is crucial in the processes of tumor development, invasion, and metastasis, primarily because of its amplification and overexpression at the genetic level6. Consequently, it is essential to predict HER2 status accurately before surgery to inform tailored treatment approaches and enhance patient results.
Immunohistochemical (IHC) analysis of biopsy samples remains the gold standard for assessing HER2 status preoperatively7. Nonetheless, this approach has various drawbacks, such as its invasiveness, long procedure time, and significant costs. Moreover, due to limited biopsy size, IHC analysis may fail to comprehensively capture tumor heterogeneity8. Although polymerase chain reaction can accurately detect HER2 gene amplification, it is not routinely used in clinical practice due to its technical complexity, higher cost, and limited ability to reflect intratumoral protein expression heterogeneity. Magnetic resonance imaging (MRI) is frequently employed in diagnosing, staging, and monitoring subsequent treatment of EC9,10,11. One study has indicated that quantitative MRI techniques, such as amide proton transfer and intravoxel incoherent motion, may serve as valuable imaging biomarkers for HER2 status evaluation in EC patients12. However, it was constrained by small sample sizes and single-slice image analysis, which may have led to the loss of crucial tumor-related information. These functional imaging sequences are also not routinely included in standard clinical MRI protocols, potentially increasing scanning time and limiting their widespread clinical adoption. Therefore, developing a non-invasive and practical tool for assessing HER2 status preoperatively remains urgent.
Radiomics involves the extraction of intricate quantitative features from medical imaging modalities, including MRI, which go beyond human visual perception capabilities13. This advanced analysis focuses on the relationships between these extracted imaging features and various clinical, pathological, and genetic features. Therefore, radiomics provides valuable insights into tumor heterogeneity and elucidates their biological behavior14. It has been applied to non-invasively evaluate tumor grading15, gene expression16, and lymph node (LN) status17 in EC. Recently, MRI-based radiomics has shown promising predictive capabilities regarding HER2 status across multiple malignancies, including breast, gastric, and bladder cancers18,19,20. Despite these encouraging findings, its effectiveness for predicting HER2 status in EC remains ambiguous and thus warrants further investigation.
Therefore, we sought to evaluate the viability of utilizing of employing multiparametric MRI-based radiomics to predict HER2 status in EC. We develop a prediction model capable of risk stratification to improve prediction accuracy and investigate its predictive ability for the progression-free survival (PFS).
Materials and methods
The Ethics Review Committee of the Fifth Affiliated Hospital of Wenzhou Medical University approved this study (ethics approval number 2025-136), waiving the requirement for informed consent due to its retroactive character. All procedures adhered to the ethical norms established by the Helsinki Declaration.
Patient selection
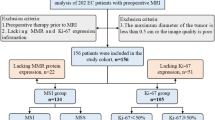
This study enrolled 492 patients with EC from three different medical centers. Figure 1 depicts the enrollment process. Patients were selected based on the subsequent criteria: (1) histopathologically confirmed EC diagnosis following surgical resection, (2) clear determination of HER2 status in tumor specimens through IHC, and (3) preoperative MRI examination conducted within two weeks before surgery. Patients were excluded if they met any of the following criteria: (1) maximum tumor diameter of < 1.0 cm, (2) incomplete clinical or pathological data, and (3) prior radiotherapy or curettage before the MRI examination.
The 307 patients treated at the Fifth Affiliated Hospital of Wenzhou Medical University (Center 1) from January 2016 to May 2023 were randomly assigned to training (n = 215) and internal validation (n = 92) cohorts at a 7:3 ratio. External validation cohort 1 comprised 64 patients treated at the Third Affiliated Hospital of Wenzhou Medical University (Center 2) from January 2020 to May 2023. External validation cohort 2 comprised 121 patients treated at the Sixth Affiliated Hospital of Wenzhou Medical University (Center 3) from January 2018 to May 2023. Each patient’s HER2 positive (HER2 (+)) or negative (HER2 (−)) status was determined based on their postoperative pathological examination results. The detailed evaluation process is explained in Appendix E1.
MRI examination and radiological assessment
All patients underwent pelvic contrast-enhanced MRI scans at three centers. The imaging protocols employed included T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and contrast-enhanced T1-weighted imaging (CE-T1WI) with fat saturation. Detailed information about the MRI scanners and parameters used for acquisition is provided in Table S1.
Two radiologists, possessing 9 and 20 years of experience in MRI interpretation, respectively, independently evaluated the images while remaining unaware of both the clinical and pathological information. The radiological features encompassed tumor size, tumor margin, tumor composition, myometrial invasion depth, LN status, and abnormal ascites, as detailed in Appendix E2.
Follow up
All patients underwent regular monitoring every six months postoperative to assess disease progression. Disease progression was characterized by either local recurrence in the pelvis or the emergence of new metastases in the abdomen or other distant locations, which comprised nodal, peritoneal, or visceral metastases. PFS was defined as the interval from the surgery date to the first instance of disease recurrence, death due to any cause, or the latest follow-up in March 2025.
Tumor segmentation, feature extraction and selection
Figure 2 illustrates the study workflow. Tumor segmentation and imaging preprocessing were conducted before feature extraction, as described in Appendix E3. Initially, 1688 radiomics features were obtained from each whole-tumor volume of interest (VOI) of each MRI sequence for each patient. Appendix E4 contains further information regarding radiomics feature extraction. Next, all extracted radiomics features were standardized using the z-score method. Features were selected in the training cohort, utilizing the T2WI, DWI, and CE-T1WI subsets both individually and in combination. The three-step procedure used for feature selection—reproducibility analysis via interclass correlation coefficients (ICCs), Spearman’s correlation analysis, and least absolute shrinkage and selection operator (LASSO) analysis—is described in Appendix E5. The subsets of radiomics features with the highest predictive value were utilized to construct the radiomics model.
Radiomics model building
Six machine learning (ML) algorithms, including k-nearest neighbor (KNN), support vector machine (SVM), logistic regression (LR), random forest (RF), naïve Bayes (NB), and extreme gradient boosting (XGBoost), were employed to construct radiomics models that could predict the HER2 status of EC based on the chosen features. The radiomics model with the highest average area under the receiver operating characteristic (ROC) curve (AUC) across the three validation cohorts was deemed the optimal radiomics model and was utilized to calculate the radiomics score (Radscore).
Clinical and fusion nomogram development and validation
In the training cohort, univariate and multivariate LR analyses were conducted to identify clinical predictors of HER2 status in EC, which were utilized to develop the clinical model. A fusion model was established alongside the respective nomogram for predicting HER2 status by integrating the Radscore and clinical predictors through multivariate LR analysis. ROC analysis was employed to assess the predictive capabilities of these models. Metrics such as sensitivity, specificity, and accuracy were computed and visualized in a confusion matrix. The calibration curves were generated using 1000 bootstrap resamples to evaluate the alignment between predicted risks and actual outcomes. It was also conducted decision curve analysis (DCA) to ascertain the clinical relevance.
Statistical analysis
Statistical analyses were performed using R (v 4.1.0) and Python (v 3.9.6). Student’s t-test or Mann–Whitney U-test was used to compare continuous variables across groups, while Chi-square or Fisher’s exact test was used for categorical variables. Subgroup analyses accounting for age, menopausal status, and tumor size were conducted to assess the model’s generalizability. DeLong’s test was employed to compare the AUCs. The cut-off for the risk score was determined by the fusion model within the training cohort through the Youden index. This process enabled the classification of patients into predicted HER2 (−) and HER2 (+) categories. The prognostic significance of the fusion model regarding PFS was evaluated through Kaplan–Meier analysis and log-rank tests across different cohorts. To pinpoint risk factors influencing PFS in EC patients, both uni- and multi-variate Cox regression analyses were performed. A two-tailed P-value of less than 0.05 was deemed statistically significant.
Results
Baseline characteristics of the enrolled patients
This study enrolled 492 patients with EC from three centers. Tables 1 and S2 provide a summary of the clinical and radiological characteristics of all patients. Notable differences in both differentiation and myometrial invasion were found between the HER2 (−) and HER2 (+) groups across all four cohorts (P < 0.05). HER2 (+) was associated with poor differentiation (odds ratio [OR]: 3.036, 95% confidence interval [CI]: 1.508–6.111) and myometrial invasion (OR: 3.621, 95% CI 1.895–6.915), which were subsequently employed to develop the clinical model (Table S3).
Radiomics model construction
After conducting the reproducibility analysis, we identified stable radiomics features with ICCs > 0.75. Specifically, 1,411 features were retained from T2WI, 1,428 from DWI, and 1,472 from CE-T1WI (Fig. S1). Subsequent correlation analysis resulted in 42, 47, and 52 radiomics features being retained in these three sequences, respectively. During the final selection step utilizing LASSO analysis, the T2WI, DWI, and CE-T1WI models included 7, 5, and 6 radiomics features, respectively. Ultimately, 10 radiomics features were preserved from multiparametric MRI, comprising 3 from T2WI, 3 from DWI, and 4 from CE-T1WI (Fig. S2). The optimal radiomics feature subsets and their corresponding coefficients are listed in Table S4.
Based on the optimal feature subset from multiparametric MRI, six ML algorithms were employed to construct radiomics models. Hyperparameter optimization for each ML algorithm was conducted using grid search with five-fold cross-validation implemented in Python to identify the best-performing parameter combinations. The grid search was applied to systematically evaluate combinations of key hyperparameters (e.g., C, gamma, max_depth, n_estimators, etc.) within predefined ranges. The optimal hyperparameters for the SVM algorithm were C = 0.6, kernel = ‘rbf’, and gamma = 0.1. The other ML algorithm parameters are detailed in Appendix E6.
The predictive capabilities of the various ML models according to ROC analyses are shown in Table 2 and Fig. S3. The SVM model surpassed the other ML models (Table S5), attaining the highest average AUC of 0.814 and an average accuracy of 79.58% across the validation cohorts (Table 2). In comparison to SVM models that utilized single MRI sequences, the SVM model utilizing multiparametric MRI demonstrated superior AUC values (Fig. S4). Therefore, the SVM-based model using multiparametric MRI was identified as the best radiomics model, and utilized to calculate the Radscore.
Fusion nomogram development and validation
By incorporating differentiation, myometrial invasion, and the SVM-based Radscore, a fusion model was created. The fusion model showed favorable and robust performance, with an AUC of 0.914 in the training cohort, 0.846 in the internal validation cohort, and 0.809 and 0.865 in the external validation cohorts 1 and 2, respectively (Table 3 and Fig. 3A–D), which were superior to the clinical model in all cohorts (all P < 0.05; Table S6). The confusion matrices of HER2 status classification by different models are shown in Fig. S5. Subgroup analyses based on age, menopausal status, and tumor size further validated the robustness of the fusion model (Table S7 and Fig. S6). The calibration curves showed good consistency between the fusion model-predicted risks and the actual outcomes (Fig. 3E–H). DCA indicated that the fusion model yielded more net benefit compared with the clinical and radiomics models in all cohorts (Fig. 3I–L). Ultimately, the fusion model was effectively visualized as an easy-to-use nomogram, and two instances of the nomogram application are depicted in Fig. 4.
Two instances in which the nomogram and multiparametric MRI images are used to assess the individual risk of HER2 status in patients with EC. Multiparametric MRI includes axial T2WI, DWI, and CE-T1WI. (A) Patient 1 exemplifies a case where HER2 (+) was correctly predicted. (B) Patient 2 exemplifies a case where HER2 (−) was correctly predicted. The pathological images underwent IHC analysis at 200 × magnification.
Model risk stratification and prognostic value assessment
Figures 5A–C and S7A show the risk score distributions generated by the fusion model for each patient across all cohorts. Next, we investigated the prognostic value of the fusion model across the different cohorts. The PFS of patients with EC used in the survival analyses are listed in Table S8. Individual patients’ progression status and PFS time are shown in Figs. 5D–F and S7B. The patients classified as predicted HER2 (+) demonstrated significantly worse PFS than those classified as predicted HER2 (−) in the internal validation (hazard ratio [HR] = 2.838, 95% CI 1.100–7.324), external validation 1 (HR = 3.195, 95% CI 1.042–9.800), and external validation 2 (HR = 2.913, 95% CI 1.380–6.147) cohorts (Fig. 5G–I). This is consistent with the PFS results of patients with actual HER2 status, that is, the PFS of patients with actual HER2 (+) was also significantly worse than that of patients with actual HER2 (−) (internal validation: HR = 3.026, 95% CI 1.239–7.394; external validation 1: HR = 3.967, 95% CI 1.287–12.235; external validation 2: HR = 3.083, 95% CI 1.462–6.500). A comparable trend was observed in the training cohort (Fig. S7C). The forest plots indicated a significant association between the risk score and PFS across various subgroups in the validation cohorts, including age, menopause status, and tumor size (Fig. 5J). Cox regression analyses also showed that the predicted HER2 status was independently correlated with PFS (HR: 2.166, 95% CI 1.032–4.546, P = 0.041; Fig. 5K and Table S9).
Associations between the fusion model-predicted risk score and PFS. (A–C) Plots show the fusion model risk score and the actual HER2 status of each patient in the validation cohorts. The dotted line represents the optimal cut-off of 0.353 for the risk score. (D–F) The correlation between progression status and survival time for each patient. (G–I) Kaplan–Meier survival curves of PFS according to predicted HER2 status and actual HER2 status in the validation cohorts. (J) Forest plot of Cox regression analysis of the fusion model-predicted risk scores in different subgroups of the validation cohorts. The center of the error bar represents the HR. (K) Forest plot of independent risk factors for PFS.
Discussion
EC is typically characterized by heightened tumor invasiveness and a less favorable prognosis. HER2 is a pivotal predictive biomarker for identifying candidates suitable for targeted therapies. Therefore, precise preoperative assessment of HER2 status holds considerable clinical importance. This study illustrated that radiomics features obtained from multiparametric MRI can successfully predict HER2 status in EC patients. The fusion model, which combined clinical features with SVM-based Radscore, demonstrated exceptional performance. In the training cohort, it predicted the HER2 status of patients with EC with an AUC of 0.914, and in the three validation cohorts, it had AUCs of 0.809–0.865. It is worth noting that only a HER2 prediction model was trained in this study; the risk probability output by this model facilitates the stratification of EC patients into predicted HER2 (+) and HER2 (−) groups. In addition, we verified that EC patients with predicted HER2 (+) had worse PFS than those with predicted HER2 (−).
Radiomics enables the extraction of high-dimensional quantitative features from medical images, facilitating the characterization of intratumoral heterogeneity in morphology, texture, and function14. This heterogeneity reflects variations in cellularity, necrosis, vascularity, and underlying molecular alterations. HER2 status has been shown to influence cell proliferation, angiogenesis, and immune infiltration in breast cancer, reshaping the tumor microenvironment21. These biological changes can be indirectly captured through radiomics features quantifying structural and textural complexity. In this study, radiomics analysis of tumors with different HER2 status revealed distinct feature patterns, suggesting a link between HER2-driven processes and imaging heterogeneity. Feature selection enhanced model interpretability, with wavelet-based features (7/10) being most predictive. These features, which decompose imaging data into multiple spatial frequency components, are sensitive to subtle texture and intensity variations, reflecting increased tissue complexity associated with HER2 positivity. HER2 overexpression promotes cellular proliferation, increased cell density, and disrupted glandular architecture, all contributing to imaging heterogeneity22. Additionally, HER2-driven angiogenesis leads to irregular microvascular patterns, detectable by wavelet-transformed features. Thus, wavelet-based features may serve as proxies for architectural disorganization and vascular abnormalities characteristic of HER2-positive tumors. The feature most strongly correlated with HER2 status was zone entropy from the GLSZM. This feature quantifies the irregularity of gray-level patterns; higher values reflect more heterogeneous textures, potentially indicating structural instability driven by HER2-related changes in the tumor microenvironment.
MRI has emerged as a crucial element in radiomics analysis due to its outstanding resolution for soft tissues, lack of ionizing radiation, and ability to perform multiparametric imaging. Earlier MRI-based radiomics studies mainly focused on EC diagnosis and prognosis23,24,25,26. Their results indicate that multiparametric MRI could offer a broader understanding of tumor heterogeneity. Thus, our study identified multiparametric MRI-based radiomics features to predict HER2 status in EC. Additionally, six ML algorithms were implemented to develop radiomics models that were structured around multiparametric MRI-based radiomics features. Among these ML-based models, the SVM-based radiomics model proved the most effective in predicting HER2 status.
The SVM algorithm is particularly adept at identifying subtle patterns in intricate datasets, primarily because of its ability to minimize classification errors on unseen data, regardless of any prior assumptions regarding the data’s probability distribution27. An important benefit of SVM is its capacity to manage minor nonlinear fluctuations28. Mainenti et al.29 reported that their SVM-based radiomics model offered significant benefits in risk stratification for EC. Similarly, Song et al.23 employed algorithms such as SVM, RF, and LR to identify the microsatellite instability status of EC patients, reporting that the SVM-based model exhibited the best diagnostic results. However, the optimal classification algorithm may depend on the clinical application. Meng et al.30 reported that their RF classifier outperformed other classifiers in predicting EC with a high tumor mutation burden. Moreover, Mao et al.25 showed that a NB model performed exceptionally well in distinguishing type I and II EC preoperatively. Therefore, further research is warranted to identify the most suitable algorithms for specific clinical applications.
Multivariate LR analysis identified low tumor differentiation and deep myometrial invasion as independent risk factors for HER2 (+) status, consistent with prior studies12,31,32. The potential mechanism is that HER2 expression typically activates cellular signaling pathways, promoting cancer cell proliferation, invasion, and migration, thereby exacerbating the tumor’s biological aggressiveness. Nonetheless, the clinical model constructed utilizing clinical predictors demonstrated only moderate efficacy (AUC: 0.635–0.716). This moderate efficacy stems from clinical features only representing visual anatomical data, which do not sufficiently reflect intratumoral heterogeneity. Consequently, we combined clinical attributes with the Radscore to develop a fusion model. The clinical model’s predictive power and classification accuracy were significantly enhanced by the inclusion of the SVM-based Radscore. Notably, subgroup analyses also validated the excellent robustness of the fusion model. These results further emphasize the shortcomings of clinical features while illustrating the significant benefits of multiparametric MRI-based radiomics features in predicting HER2 status in EC.
Our study’s proposed nomogram can serve as an effective tool for classifying patients with EC into two distinct categories: predicted HER2 (−) and HER2 (+). Among the patients with EC, those with predicted HER2 (+) had notably worse PFS than those with predicted HER2 (−). The HER2 status predicted by the fusion nomogram was also recognized as a distinct risk factor for PFS in EC, suggesting that our prediction nomogram for HER2 status could effectively guide the recurrence stratification of patients with EC. For those at high risk, the nomogram could assist in formulating more timely individual treatment strategies, such as targeted therapy. They may also require more regular follow-ups to enhance their prognosis. Consequently, this nomogram may be an effective resource for directing healthcare professionals in managing EC.
There were various restrictions on our study. Firstly, it was retrospective, which carries inherent biases and involves a limited sample size. Larger datasets obtained through multicenter prospective studies are needed to confirm the validity of our predictive model. Secondly, it only included patients with EC whose tumors had a maximum diameter > 1 cm to improve the delineation of tumor boundaries and ensure an adequate pixel size for radiomics analysis. Future investigations should include patients with EC with tumors with smaller diameters to enhance the model’s applicability. Lastly, the manual segmentation process is not only intricate but also requires considerable time; therefore, future studies would benefit from an automated, dependable, and reproducible segmentation technique.
Conclusions
In conclusion, our study comprehensively investigated using multiparametric MRI-based radiomics features to predict HER2 status in EC patients preoperatively. Our proposed fusion nomogram integrating clinical features and the Radscore could be a useful tool to non-invasively determine HER2 status and assess prognosis in patients with EC, offering valuable guidance for personalized treatment regimens and intensive follow-up strategies.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Makker, V. et al. Endometrial cancer. Nat. Rev. Dis. Primers 7(1), 88 (2021).
Sun, D., Cao, M., Li, H., He, S. & Chen, W. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin J. Cancer Res. 32(2), 129–139 (2020).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74(1), 12–49 (2024).
Banet, N. et al. HER-2 amplification in uterine serous carcinoma and serous endometrial intraepithelial carcinoma. Am. J. Surg. Pathol. 45(5), 708–715 (2021).
Fader, A. N. et al. Randomized phase ii trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 36(20), 2044–2051 (2018).
Tymon-Rosario, J. et al. Trastuzumab tolerability in the treatment of advanced (stage III–IV) or recurrent uterine serous carcinomas that overexpress HER2/neu. Gynecol. Oncol. 163(1), 93–99 (2021).
Sekmek, S. et al. Prognostic significance of HER2 positivity in endometrium cancer. Oncol. Res. Treat. 48(3), 75–81 (2025).
Visser, N. C. M. et al. Accuracy of endometrial sampling in endometrial carcinoma: A systematic review and meta-analysis. Obstet. Gynecol. 130(4), 803–813 (2017).
Soneji, N. D., Bharwani, N., Ferri, A., Stewart, V. & Rockall, A. Pre-operative MRI staging of endometrial cancer in a multicentre cancer network: Can we match single centre study results?. Eur. Radiol. 28(11), 4725–4734 (2018).
Bakir, V. L. et al. Role of diffusion-weighted MRI in the differential diagnosis of endometrioid and non-endometrioid cancer of the uterus. Acta Radiol. 58(6), 758–767 (2017).
Ahmed, M. et al. Can MRI help assess aggressiveness of endometrial cancer?. Clin. Radiol. 73(9), 833.e811-833.e818 (2018).
Li, X. et al. Multimodal MRI for estimating Her-2 gene expression in endometrial cancer. Bioengineering 10(12), 1399 (2023).
Mayerhoefer, M. E. et al. Introduction to Radiomics. J. Nucl. Med. 61(4), 488–495 (2020).
Lambin, P. et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14(12), 749–762 (2017).
Yamada, I. et al. Endometrial carcinoma: Texture analysis of apparent diffusion coefficient maps and its correlation with histopathologic findings and prognosis. Radiol. Imaging Cancer 1(2), e190054 (2019).
Wang, X. et al. Additional value of PET/CT-based radiomics to metabolic parameters in diagnosing lynch syndrome and predicting PD1 expression in endometrial carcinoma. Front. Oncol. 11, 595430 (2021).
Yan, B. C. et al. Radiologists with MRI-based radiomics aids to predict the pelvic lymph node metastasis in endometrial cancer: A multicenter study. Eur. Radiol. 31(1), 411–422 (2021).
Chen, Y. et al. Multiparametric MRI radiomics with machine learning for differentiating HER2-zero, -low, and -positive breast cancer: model development, testing, and interpretability analysis. AJR Am. J. Roentgenol. 224(1), e2431717 (2025).
Shen, L., Li, Y., Huang, H., Lu, Z. & Chen, B. HER2 in gastric cancer: a comprehensive analysis combining meta-analysis and DCE-MRI radiomics. Cancer Control 31, 10732748241293700 (2024).
Yu, R. et al. MRI-based machine learning radiomics for preoperative assessment of human epidermal growth factor receptor 2 status in urothelial bladder carcinoma. J. Magn. Reson. Imaging 60(6), 2694–2704 (2024).
Buchynska, L. G., Brieieva, O. V. & Iurchenko, N. P. Assessment of HER-2/neu, c-MYC and CCNE1 gene copy number variations and protein expression in endometrial carcinomas. Exp. Oncol. 41(2), 138–143 (2019).
Xu, K., Lu, S., Huang, B., Wu, W. & Liu, Q. Stage-by-stage wavelet optimization refinement diffusion model for sparse-view CT reconstruction. IEEE Trans. Med. Imaging 43(10), 3412–3424 (2024).
Song, X. L. et al. Multisequence magnetic resonance imaging-based radiomics models for the prediction of microsatellite instability in endometrial cancer. Radiol. Med. 128(2), 242–251 (2023).
Li, Q. et al. Radiogenomics for predicting microsatellite instability status and PD-L1 expression with machine learning in endometrial cancers: A multicenter study. Heliyon 9(12), e23166 (2023).
Cao, Y. et al. Multiparameter MRI-based radiomics analysis for preoperative prediction of type II endometrial cancer. Heliyon 10(12), e32940 (2024).
Lefebvre, T. L. et al. Development and validation of multiparametric MRI-based radiomics models for preoperative risk stratification of endometrial cancer. Radiology 305(2), 375–386 (2022).
Huang, S. et al. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genom. Proteom. 15(1), 41–51 (2018).
Noble, W. S. What is a support vector machine?. Nat. Biotechnol. 24(12), 1565–1567 (2006).
Mainenti, P. P. et al. MRI radiomics: A machine learning approach for the risk stratification of endometrial cancer patients. Eur. J. Radiol. 149, 110226 (2022).
Meng, X., Yang, D., Jin, H., et al. MRI-based radiomics model for predicting endometrial cancer with high tumor mutation burden. Abdom Radiol (NY) 2024.
An, H. J. et al. Significance of HER2 and VEGFR2 in early-stage endometrial cancer. In Vivo 36(2), 723–730 (2022).
Cui, Z. J., Zhou, X. H. & Zhang, H. Y. DNA methylation module network-based prognosis and molecular typing of cancer. Genes 10(8), 571 (2019).
Funding
This study is supported by Zhejiang Public Welfare Research Program (LGF20H220002, LGF19H180010), and Zhejiang Provincial Healthcare Program (2024KY562).
Author information
Authors and Affiliations
Contributions
Guihan Lin: Writing-original draft, Visualization, Project administration, Methodology, Investigation, Formal analysis, Data curation. Weiyue Chen: Writing-review & editing, Project administration, Data curation, Formal analysis. Yongjun Chen, Changsheng Shi, Jingjing Cao, Weibo Mao, Chuchu Zhao, Hongyou Zhou, Yumin Hu, Shuiwei Xia: Project administration. Weibin Yang and Min Xu: Validation, Resources. Minjiang Chen and Jiansong Ji: Supervision, Resources, Conceptualization. Chenying Lu: Validation, Supervision, Conceptualization, Methodology, Investigation. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, G., Chen, W., Chen, Y. et al. Preoperative prediction of the HER2 status and prognosis of patients with endometrial cancer using multiparametric MRI-based radiomics: a multicenter study. Sci Rep 15, 35590 (2025). https://doi.org/10.1038/s41598-025-12894-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12894-8