Abstract
The effectiveness of microbially induced calcite precipitation (MICP) in reducing the hydraulic conductivity (HC) of soils has been evaluated in several geotechnical engineering applications. However, optimizing MICP parameters to achieve minimal hydraulic conductivity for waste containment liners remains underexplored. This study presents a comprehensive laboratory analysis investigating the influence of bacterial suspension density and compaction energy on compacted lateritic soil, prepared under varying moisture conditions. Bacillus megaterium (B. megaterium) was introduced at different suspension densities, followed by compaction. Results revealed that increased bacterial density and compaction energy significantly reduced hydraulic conductivity due to calcite formation, confirmed through XRD, SEM, XRF, and FTIR analyses. To optimize the hydraulic conductivity, bio-inspired algorithms including particle swarm optimization (PSO), smell agent optimization (SAO), and bacterial foraging optimization (BFO) were deployed. Successive iterations demonstrated that PSO achieved the lowest hydraulic conductivity (k = 2.27 × 10−11 m/s), followed by SAO (k = 2.85 × 10−11 m/s) and BFO (k = 2.66 × 10−9 m/s). These findings highlight the critical role of compaction effort, moisture content, and bacterial density in designing compacted lateritic soil liners for geotechnical applications. Optimization results underscore PSO’s superior performance in achieving design criteria for liners.
Similar content being viewed by others
Introduction
The growing global concern regarding waste management has emphasised the need for effective waste containment systems that minimize environmental risks. In particular, the management of hazardous and non-hazardous waste in landfills requires advanced engineering solutions to prevent leachate migration and soil contamination. Soil stabilization techniques have become increasingly important in this regard, with microbial-induced calcite precipitation (MICP) emerging as a promising approach. This eco-friendly method utilizes naturally occurring microorganisms to precipitate calcium carbonate, thereby enhancing soil strength and reducing hydraulic conductivity1,2,3,4,5. It can be particularly beneficial for weak or loose soils, such as sandy or silty soils, and can help in improve their load-bearing capacity6.
MICP has been used in a variety of applications, including; soil stabilization for construction projects, erosion control7 and the remediation of contaminated soils8,9 restoration of calcareous stone materials10 bioremediation11 reinforcement of concrete12 contaminants immobilization13,14 erosion mitigation and water infiltration control15 lessening of hydraulic conductivity and enhanced soil strength1,16,17 reduction in the saturation as well as liquefaction prospective of soil18 reduce desiccation induced shrinkage in soils19,20,21 improve plasticity of clay soil22,23. MICP compatibility investigations involving lateritic soil relationship with leachate have also been reported by Osinube et al.,24.
Lateritic soils, which have abundance of iron as well as aluminium oxides, possess unique properties that make them suitable for use in waste containment applications. These soils are abundant in tropical regions and have been recognized for their engineering characteristics, such as good load-bearing capacity and resistance to erosion25,26. However, their inherent variability in hydraulic conductivity poses challenges when considered as liners in waste containment structures. Understanding how to optimize these soils through bio-cementation techniques is crucial for improving their performance in real-world applications27 .
Despite the potential benefits of using bio-cemented lateritic soils in waste containment application, significant challenges still exist and need to address. Existing researches done predominantly focused on sandy and coarse-grained soils with limited studies on tropical lateritic soils. Consequently, there is limited understanding of how various factors-such as compaction energy, moisture content, and microbial concentration-interact to affect the hydraulic conductivity of bio-cemented lateritic soils28,29. The current literatures lacks systematic investigations that assess the joint effects of compaction energy and moisture content on the hydraulic properties of bio-cemented lateritic soils. Furthermore, the existing research has not adequately explored the optimization of these parameters using algorithms that can effectively model and predict soil performance under varying conditions. As a result, the application of bio-cementation in waste containment systems remains underexplored and lacks clear practical guidelines. Optimization of MICP processes using advanced techniques such as PSO, SAO and Bacteria Foraging Optimization (BFO) remains underexplored. These optimization methods have demonstrated efficacy in various domains but are yet to be comprehensively employed to enhance MICP efficiency and precision.
The objective of this study is to optimize bio-cemented lateritic soil through a comprehensive laboratory analysis as well as bio-inspired optimization approach. This study is projected to make substantial assistances to the fields of soil bio-stabilization and waste management engineering. The study aims to establish evidence-based protocols for enhancing soil stabilization techniques. The findings could serve as a panacea to engineers and environmental scientists on how to optimize soil properties for effective waste containment solutions, thereby improving the sustainability and safety of landfill operations30. Additionally, the study will contribute to the growing body of knowledge surrounding MICP, by bridging the gap between laboratory research and practical application. The application of optimization algorithms helps in facilitate the translation of experimental results into practical guidelines, enabling the efficient design of bio-cemented soil liners that meet regulatory requirements, and environmental standards31.
Materials and methods
Materials
Soil
Samples of disturbed reddish-brown soil were gathered from a gully erosion site located in Njikoka (6°10′00′′ N and 7°0′0′′ E), Anambra state, Nigeria. The location map of the study area is shown in (Fig. 1). The initial step involved clearing debris of organic materials from the surface within the soil sampling area. This was done to prevent soil contamination with the insitu organic deposits which can affect the soil’s response to MICP and distribution of calcite. Soil samples were obtained at a depth of 1 m to 1.5 m. Ensuring preservation, these samples were carefully stored in waterproof bags and subsequently transported to the Soil Mechanics Research Laboratory at Ahmadu Bello University (ABU). Under natural laboratory conditions, the samples were air-dried, and dried lumps were further broken down into finer particles and sieve through 4.75 mm sieve. It is noteworthy that lateritic soils are not confined to the study location, as residual deposits extend throughout the southern geographical region of Nigeria. Chemical characterization of the samples carried out include X-ray fluorescence (XRF) analysis using the NitonTM XL3t XRF analyzer, X-ray diffraction (XRD), and infrared analysis (IR). The XRF analysis was done at Centre for Minerals Research & Development (CMRD) laboratory at Kaduna Polytechnic, Nigeria. XRD analysis was conducted at National Geo-Science Research Laboratories in Kaduna state, Nigeria. Additionally, IR spectroscopy was carried out in the multipurpose user laboratory ABU Zaria Nigeria.
Constitution of cementation solution and bacterial suspension density
The cementation solution was used to enhance calcite precipitation while at the same time, the presence of calcium chloride in the mixture increases the rate of calcium carbonate precipitated. The weight of individual reagents by composition that make up to 0.5 M concentration are: CO(NH2)2 (30.03 g), NaHCO3 (2.12 g), NH4Cl (10 g), CaCl2 (55.49 g) Nutrient broth (3 g) dissolved in 1 L of water. The preference of 0.5 M of cementing solution applied in this research is the optimal concentration obtained in the earlier part of this study4. Therefore, this section of study is an extension of investigations not covered in the initial report by same authors. The cementing solution was prepared under non-sterile condition as the cementing solution was arranged using tap water and was not subjected to autoclaving in order to mimic the actual occurrence in the field. This approach was used because it will be problematic to conduct autoclaving in large-scale field implementation.
The bacteria used for this study was isolated from the soil through a sequence of biochemical confirmatory test. The bacteria colonies were stored in nutrient agar solution at 4oc. The nutrient agar solution is a composition of agar C14H18011 that solidifies the nutrient agar solution and allow bacteria to attach to the surface and form colonies. Peptone C30H45023N7 rich in substance provides nitrogen, carbon, and other essential nutrients for bacterial growth. In addition, nutrient broth added to the bacterial solution provides appropriate minerals, amino acids in addition to providing vitamins. It also provides a caring environment for inherent soil bacteria culture for bacterial growth. A nutrient broth enrichment solution is considered useful for increasing the culturing of the gram-positive bacteria and consequently, provide a favourable ecosystem for bacterial growth. The nutrient broth was added individually to cementing and enrichment solutions.
Methods
Bacteria inoculation and determination of bacteria cell concentration
The bacteria culture solutions were prepared and placed in a gentle shaker incubator for 24 h at 37 °C to guarantee good aeration and nutrient availability. After incubation, the solutions under observation became turbid. The turbidity expressed the bacteria (B. megaterium) growth in solution (see Fig. 2a). The turbidity was measured using the McFarland turbidity standard for 0.5, 2, 4, 6, and 8 McFarland which are correlated to bacterial suspension densities of 1.5 × 108, 6 × 108, 1.2 × 109, 1.8 × 109, and 2.4 × 109 cells/ml respectively32 .
Treatment, sample preparation and testing
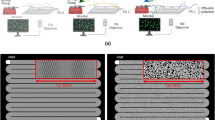
The experimental processes for the various stages involved bacterial isolation, bio-treatment of sample, compaction into mould, bio-cementation inducement by gravity percolation, setting up bio-cemented sample for hydraulic conductivity and calcite content determination as shown in Fig. 2. Prepared soil sample was mixed with one-third pore volume of bacteria solution and then compacted to the required dry density. Bioactivity for urease enzyme was then activated using 0.5 M cementation solution dispensed in three cycles. Two-third 0.5 M cementation solution was dispensed and allowed to diffuse gradually into the sample under gravity in three cycles at interval of 6, 12, and 24 h for 1st, 2nd and 3rd cycles, respectively (see Figs. 2b and c). This account for different degrees of saturation33,34. This method allows bacteria to saturate and circulate efficiently, Also the cementation solutions percolate autonomously by gravity which is cost effective. Cost, time and processes involve in using a specialized pressure or injection equipment for injecting, spraying, or dispensing bacterial suspensions, cementation solutions, or other reagents used in MICP are eliminated. Nonetheless, a key similarity across all methods is that the treated samples ultimately become solidified as a result of calcite precipitation. However, the various methods exist in terms of time required to achieve MICP, with each technique exhibiting its own rate of calcite formation35. The cycle of cementing solution application was considered up to the third cycle because beyond the third cycle from preliminary experimental observation, the level of saturation with cementation solution slowed down due to aggravated precipitate of calcium carbonates formed at the upper top surface of the specimen, thereby clogging the soil pores and fluid pathway and thus inhibit the free flow and immediate saturation of specimen. The amount by volume of bacteria and cementation solutions used by weight of soil were determined using Eqs. (1)−(3).
where omcn = optimum moisture content of natural soil; TPV = total pore volume; Vbsd = volume of bacteria suspension density; Vcs = volume of cementation solution.
The pore volume is defined as the numerical difference between the initial degree of saturation before soaking in water and the final degree of saturation after full saturation of the soil sample in water24. This approach considers the soil’s three-phase composition namely; solid particles, water, and air with the understanding of much of the air is expelled during compaction allowing for a more accurate estimation of the pore volume. Accordingly, the optimum moisture content for natural soil (OMCn) was determined for each level of compactive effort. These values were then used to estimate the required volumes of bacterial suspension and cementation solution for a 3000 g soil sample. Precisely, one-third of the pore volume was allocated for the bacterial suspension and two-thirds for the 0.5 M cementation solution. Table 1 presents the volumes of bacterial suspension and cementation solution used in this study for each of the four compactive efforts.
On completion of the MICP preparation and treatment processes, the specimen were saturated in tap water for 24–48 h, allowed to drain before setting up for hydraulic conductivity test, by falling head method for fine grained soil (see Fig. 2d). Tap water was permeated intermittently until steady head difference was measured with time after 24 h interval and the coefficient, k of hydraulic conductivity was computed, for the various microbial density (cells/ml). Side wall leakage was ignored as recommended by Daniel36. The permeability coefficient is known as saturated hydraulic conductivity k, which was determined as outlined in Daniel36 and Osinubi et al.37. After the termination of permeability test, sample were removed from mould and sliced into five layers for determination of calcite content (see Fig. 2e). The calcite content test was determined at five different layers to measure the spread of calcite with depth. Qualitative microanalysis using scanning electron microscopy (SEM), x-ray diffraction (XRD), x-ray florescence (XRF) and fourier transformed infrared (FTIR) were analysed to validate the correlations of calcite content.

(Source: Etim et al.4) (d) falling head hydraulic conductivity test set up of bio-treated samples and (e) sliced layers of bio-treated lateritic fine grain soil after termination of test for determination of calcite distribution. Keys: 1-Soil, 2-Extracting, isolation and purifying process of bacteria from soil, 3-Isolated bacteria cultured in nutrient agar petri dish, 4-Bacteria inoculated into enrichment solution (bacteria suspension density), 5-Infusion/reinjection of bacteria enrichment solution back into the initial soil, 6-Mixing process after bacteria infusion, 7-Mixed soil-bacteria enrichment solution confined for 12-hours for uniform wetness and bacteria circulation, 8-Sample transferred into mould for compaction, 9-Compacted soil, 10 − 0.5 M Cementation solution, 11-Cementation solution injected in cycles into compacted soil for MICP process, 12- treated zone that shows cementation solution freely percolating/saturating under gravity for continuous bio-cementation, 13-Mould, 14-Filter paper, 15-Circular perforated base plate and 16-Support base of set-up.
(a) Enrichment bacteria suspension density: 1.5 × 108, 6 × 108, 1.2 × 109, 1.8 × 109, and 2.4 × 109 cells/ml equivalent to 0.5, 2, 4, 6 and 8 McFarland standard (MP), respectively (b) introduction of cementation solution (c) overall experimental process.
Calcite content determination
Following the completion of the permeability test, during which a steady flow rate was achieved to establish saturated permeability, the experimental process was terminated. The apparatus was disassembled, and the mould, containing the specimen from the standard proctor test, was removed from water. Subsequently, it was placed on a workbench for a duration of 24–48 h to allow for the complete drainage of moisture. The specimen was meticulously extruded from the mold, and the upper surface was first cleared off. Calcite content for this cleared portion was then evaluated with the understanding that this portion corresponds to the ground surface and/or zero depth of the profile. The remaining block sample was divided into five equal portions; A1, A2, A3, A4, and A5 with each one-fifth layer representing an approximate depth of 20, 40, 60, 80, and 100 mm, respectively. Each section was then spread onto porcelain plates and left to dry under room temperature. After drying, the specimen was crushed into a powder state and sieved through a 75 μm mesh before the determination of calcite content using the acid washing method38. The CaCO3 was liquefied by mixing a 5 g sample with 20 ml of 1 M hydrochloric acid. The consequential mixture was washed using distilled H2O and sieved with filter paper. The residue was then conveyed to an oven for drying at 60 °C for 24 h. The carbonate content (%) was computed by the application of Eq. (4)
where Ccont. = calculated calcite content (%); m(calcium carbonate) = calculated mass of carbonate (CaCO3); mpd = 5 g measured soil prior acid washing; mad = mass of acid washed soil after oven dried (oven dry mass of residue).
Optimization process
Optimization process used in this study was done using MATLAB 2022 software. Three algorithms were used to determine the least hydraulic conductivity value among several outputs. Bacterial Foraging optimization(BFO), Particle Swarm Optimization (PSO) and Smell Agent Optimization(SAO) algorithm programmes were used to define the least hydraulic conductivity, based on the outcome of a combination laboratory measured variables, which include Bacillus megaterium (B. megaterium), void ratio (e), dry density (ρd), water content relative to optimum (WCRO), compactive effort (CE), pH, degree of saturation (Sr), plasticity index (PI), liquid limit (LL) and viscosity (Vs)) that produced the minimum lab hydraulic conductivity value.
The fitness function used in optimising the hydraulic conductivity is given in Eq. (5). The equation was developed using GeneXproTools 5.039,40.
Where: \(\:{x}_{1}\) =B. megaterium suspension density (B.meg), \(\:{x}_{2}\)= void ratio (e), \(\:{x}_{3}\)= dry density (ρd), \(\:{x}_{4}\)= water content relative to optimum (WCRO), \(\:{x}_{5}\)= compactive effort (CE), \(\:{x}_{6}\)= pH, \(\:{x}_{7}\)= degree of saturation (Sr), \(\:{x}_{8}\)= plasticity index (PI), \(\:{x}_{9}\)= liquid limit (LL), \(\:{x}_{10}\)= viscosity (Vs), Y = ln(k)=hydraulic conductivity.
The constraints on the other hand states the margins as well as limits at which the structure is being studied define as follows;
\(\:1.33\text{E}-10\le\:\text{y}\le\:4.05\text{E}-06\) Hydraulic conductivity
\(\:0\le\:{x}_{1}\le\:2.4\text{E}-09\) B.megaterium suspension density
\(\:-1\le\:{x}_{5}\le\:2\) Compactive effort; RBSL=-1, BSL=0, WAS=1, BSH=2
\(\:7.8\le\:{x}_{4}\le\:17.6\) Moulding water content covering dry to wet within the four CE considered
Results and discussion
Material characterization
The summary of the properties of the natural lateritic soil used is given in (Table 2). The soil has a natural moisture content of 11.6%. 35.4% of its particles passed the sieve No. 200 (75 μm opening). The soil is classified as A-4(3), AASHTO41, and SC, ASTM42. Soil samples were compacted with four levels of energies including Reduced British Standard light (RBSL), British Standard light (BSL), West African Standard (WAS) and British Standard heavy (BSH). The gradation distribution of the lateritic soil is shown in (Fig. 3).
Figure 4 shows the XRD results for the lateritic soil, revealing the predominant non-clay minerals (Quartz, SiO2, and Haematite, Fe2O3) alongside the clay mineral (Kaolinite, Al4Si4O10 (OH)8). The spectrum intensity indicates varying concentrations of these minerals. The fundamental component of kaolinite is the structural relative ratio of alumina and silica arranged in repeated sheets and bonded together, as expressed in its chemical formula. The reddish-brown coloration of the soil is attributed to the presence of haematite. Overall, the soil is identified as lateritic, characterized by fine particles with predominantly crystalline constituents, particularly the kaolinitic clay mineral.
Figure 5 shows the FTIR spectra of the natural lateritic soil, a key source of kaolin confirmed by XRD. The results align with XRF and XRD, emphasizing kaolinite dominance. Intensity variations in the spectrum correspond to essential kaolinite functional groups. Notably, bands at 3693.8 and 3652.8 cm−1 indicate in-phase OH stretching of inner-surface hydroxyl groups specific to kaolinite. A 1636.3 cm− 1 band suggests H-O-H bending or water deformation influenced by minor minerals like smectite. The finger print regions showing relative sharp bands of 1032.5, 1036.2, and 913.2 cm− 1 relate to Si-O stretching and OH deformation43. The 779 cm-1 band is likely linked to OH associated with Al3+. Bands from 685.8 to 752.9 cm− 1 pertain to non-clay minerals like quart, while unassigned bands at 2885, 2791, and 2050 cm−1 may be due to trace amounts of minor minerals or structural interference.
Couple effect of bacterial cell concentration and compaction energy on hydraulic conductivity
The hydraulic conductivity (HC) assessment focuses on specimens bio-cemented with a 0.5 M cementation solution concentration, identified as the optimal concentration yielding effective cementation calcite. The results are systematically analyzed to serve as key indicators of Microbially Induced Calcite Precipitation (MICP), compactivity (specifically, compactive effort), and the diverse bacterial cell units. In Fig. 6, the hydraulic conductivity (HC) of bio-cemented lateritic soil is depicted in relation to varying concentrations of B. megaterium cells/ml. The dashed lines represent the criterion limit of hydraulic conductivity for design of waste containment system. Intriguingly, as the amount of bacterial suspension density increased, reaching up to 2.4 × 109 cells/ml, the HC exhibited a consistent decrease across different compactive efforts. For specimens prepared at -2% OMC, the HC values decreased from 4.00 × 10− 6, 4.38 × 10− 7, 2.28 × 10− 7, and 4.84 × 10− 8 m/s at 0 cell/ml (representing the natural specimen) to low values of 5.72 × 10− 9, 8.13 × 10− 9, 3.22 × 10− 9, and 7.21 × 10− 10 m/s when bio-treated with up to 2.4 × 109 cells/ml of B. megaterium suspension density for RBSL, BSL, WAS, and BSH, respectively. This translates to a substantial reduction in HC, with percentages of 99.9%, 98.1%, 98.8%, and 98.3%, underscoring the impactful bio-cementation process. Similar declining trends were also evident for specimens prepared at OMC, + 2, and + 4 OMC (see Figs. 6b-c). The decline in HC is triggered by the hydrolysis of urea, leading to a pH shift that fosters the growth of calcite, partially obstructing soil pores and fabric. The mechanism of calcite formation and bio-cementation is influenced by the size of microorganisms relative to the magnitude of soil pores and throats18,44,45. Furthermore, the observed decrease in HC with increased compaction energy is ascribed to the deformation and destruction of clay peds in the soil fabric, eliminating macro pores44,46. Generally treated soil with a minimal 1.2 × 109 cells/ml compacted with BSL.WAS and BSH met the minimum governing hydraulic conduction value of 1.0 × 10− 7 cm/s for waste containment applications. Further investigation using XRD, SEM and FTIR evaluations was targeted at providing insight that would showed the presence of imprinted calcite which further confirms the MICP effectiveness in deposition of inorganic calcite.
Relationship between moulding water content relative to optimum and hydraulic conductivity
The HC of densified soil is significantly influenced by its MWC47. The relationship between HC and MWC for natural and bio-treated lateritic soil is displayed in (Fig. 7). Experimental results show that HC initially reduced with MWC from − 2% OMC to OMC and afterwards increased with higher MWC (+ 2% and + 4%). It was observed that the lowest values of HC were obtained for bio-treated specimen prepared at OMC for the four compaction energies considered. Similar findings depicting this trend were also reported by Yohanna et al.8. The trend of the results obtained here are like those recorded at 6.0 × 108 cells/ml, 12.0 × 108 cells/ml, 18.0 × 108 cells/ml and 24.0 × 108 cells/ml.
Influence of degree of saturation on hydraulic conductivity of bio-cemented lateritic soil
The relationship derived from the HC of bio-cemented lateritic soil and degree of saturation (DOS) for varying B. megaterium suspension density and compacted using four energy levels is shown in (Fig. 8). The unpredictability in the degree of saturation could probably be due to the uneven or spatial spreading and distribution of the precipitated product (CaCO3) within the soil pores. This may have probably translated to partially filled pore spaces within the soil grains hence permitting fluid passage34,48. The minimum and maximum degrees of saturation that fell within the requisite HC values ≤ 1 × 10− 9 m/s are 82.0% and 99.9% for specimens treated with B. megaterium suspension density up to 24.0 × 108 cells/ml.
Profiles of calcite (CaCO3) with depth/height of specimen with corresponding pH
The calcite content and corresponding pH was assessed to understand its distribution with depth (see Fig. 9). The distribution of calcite content with depth exhibited non-uniform variations, generally decreasing from the top portion to the bottom accordingly. Calcite distribution ranged from 1.1 to 1.7%, 2.8 to 3.3%, 3.2 to 4.2%, 3.5 to 5.4%, 4 to 5.7%, and 5.2 to 5.9% for natural conditions and 1.5, 6, 12, 18, 24 (×108 cells/ml), respectively (see Fig. 9a). This non-uniform distribution of calcite may be linked to the non-continuous access/entrant of the cementation solution to nucleation sites of bacteria cells, un-even percolation of cementation solution due to blockade of micro-pores by calcite formed during the initial biocementation cycle, and fabric/particle size orientation34,49. The non-uniformity and indeed high calcite presence within the upper first layer of one-fifth (1/5th ) layer specimen could probably be as a result of high bacterial aggregation or concentration5,50,51. However, it is possible that the initial bacterial concentration may have likely increased due to or aided by active multiplicity of bacteria caused by breathability of bacteria as observed or caused by adequate exposure and or aeration of topmost layer52. It could be that that oxygen, vital for the metabolic processes of calcite-precipitating bacteria, becomes less available with increasing soil depth, thereby diminishing bacterial activity and subsequently reducing calcite precipitation53. Moreover, the dominance of a clay mineral with a relatively high specific surface area (SSA) at the top surface may contribute to limiting the saturation rate of the cementation solution. This limitation, coupled with variations in pH levels at different depths (see Fig. 9b), affects microbial activity, potentially diminishing the effectiveness of calcite precipitation. The decrease in pH could be attributed to the metabolic process of some unidentified indigenous bacteria in the soil that are not part of the MICP protocol. It is possible that as these bacteria consume nutrients, they release metabolic by-products that may include acidic metabolites or acidic waste products, which contribute to the decrease in pH. The pH except for few cases were in most part alkaline all within the various bacteria concentration and depth that favours the condition for MICP. The decline in pH at greater depths could account for the observed decrease in calcite concentration. The compaction process itself introduces variations in soil structure and density, influencing microbial activity and, consequently, the distribution of calcite. The soil’s pore structure and connectivity further impact the movement of fluid, including enrichment and cementation solutions, as microorganisms influence the transport of calcium ions and microbial cells, thereby shaping calcite distribution. Additionally, substrate availability, exemplified by urease enzyme presence, and the intricate biogeochemical interactions within the soil undergo changes with depth. These factors collectively emphasize the significance of predicting and optimizing microbially induced calcite precipitation (MICP) processes at different depths in fine-grained soils. While this study primarily emanates from laboratory conditions, the challenges encountered underscore potential variations and challenges upon upscaling.
Chemical composition and microanalysis of bio-cemented lateritic soil
The percentage distribution of oxide composition in the natural soil sample, which underwent analysis by XRF after passing over a 0.075 mm sieve, is presented in (Table 3). The ratio of SiO2 to the combined Al2O3 and Fe2O3, denoted as the silica-sesquioxides ratio [(SiO2)/(Al2O3 + Fe2O3)], is calculated to be 1.77. This falls within the recommended or classified range of 1.33–2.0 for lateritic soil, as stipulated by Bell54 ensuring the authenticity of the soil classification. The comparison of chemical composition of natural lateritic soil and bio-treated soil using the XRF analysis shows variation in composition. Each specimen for the respective bio-treated specimen were representative specimen collected from each of the five slice, that make up the depth and mixed before testing. The results confirms formation of binding inorganic precipitate of calcite as seen in increased calcium oxide (CaO) content in contrast to the natural soil having lower percent. Also, other major compound in the soil were represented in the bio-treated specimen. The increase in CaO of bio-treated with bacterial cell specimen is due to the higher formation of calcite triggered by hydrolysis reaction of bacteria urease enzyme.
XRD of Bio-treated specimen
The X-ray diffraction (XRD) analysis of lateritic soil subjected to bio-augmentation with enrichment solution - B. megaterium inoculum of 108 cells/ml was conducted at increasing suspension densities of 1.5, 6, 12, 18, and 24, using an optimal 0.5 M cementation solution. The results confirm the precipitation of calcite, as evidenced by distinct diffraction peaks at various 2theta angles (Fig. 10). Notably, alongside inorganic quartz and hematite minerals, strong diffraction peaks of inorganic calcite, interspersed with intrinsic quartz and hematite, were observed. This observation aligns with findings reported by Oliveira and Neves55 supporting the identification of calcite at specific 2theta values (i.e. 2θ = 27.316, 29.497, and 33.229). It is essential to note that the intensity of diffraction peaks for a mineral is contingent on its relative abundance in the analyzed specimen, as established in studies by Chipera and Bish56 and Sharma et al.,51. The prevalence of calcite, manifested at varying 2theta angles, exhibited an increasing trend corresponding to the rise in bacterial cells/ml but cannot be quantified numerically using XRD. Previous research has shown presence of calcite as prevalent crystal in bio-cementation processes57,58. Precipitation potential of calcite due to influence of biogeochemical interactions in MICP techniques tends to increase with an increase in enzyme content and or bacterial suspension densities45,57.
The natural, untreated lateritic soil image shows the soil’s original fine grain structure, characterized by cohesive to loosely packed particles oriented partly as interfacial dispersed/flocculated structure and cohesive structure. It is obvious that marginal dispersed calcite can be seen clearly as shown in the natural/control lateritic sample. This could probably be due activation of unidentified variants of indigenous bacteria inherit in soil and or principally from the cementation composition source.
The alteration in morphology induced by the formation of inorganic binding calcite as identified in XRD spectroscopy can be conveniently viewed from the image displayed by Phenomworld scan electron microspcope SEM. Photomicrographs from the SEM investigation established numerous fabric features probably associated with the pH level and or bacteria concentration, cementation saturation during bio-treatment biocementation action, interaction/MICP protocol, calcite distribution, pore size orientation and distribution relative to interstitial particle contact and cohesion with calcite and probably degree of saturation by both permeant (tap water) and cementation solution. SEM Image of natural soil specimen show a relatively dispersed grain fabric morphology (Fig. 11a). The bio-cemented specimen (Fig. 11b–f) present a relatively aggregated and thick lumps distributed unevenly59. The general features of both closed and disaggregated structure, suggest possible changes induced by that connectivity and associations between the soil particles translated into an active bonding caused by the products of calcite. The after effect is the aggravated crystal that covers and filled the soil grains but the filling of inter-particle pores and bio-cementing inter-particles grains was more obvious as shown by the cloudy and floppy/ fluffy calcite coverage of photomicrograph of 2.4 × 109 cells/ml sample (Fig. 11f). These activity could be accountable for the low permeability52. This observation corroborate the low hydraulic conductivity value recorded at higher B. megaterium density.
Furthermore, the success of the biotreatment as well as the chemical bonds, mineralogy, and microstructure of lateritic soil was studied again using FTIR. The FTIR analysis was done by means of transparent KBr disks, and the outcomes were evaluated with the aid of an adsorption bands measured at characteristic wave numbers between 700 and 4000 cm − 1. The FTIR spectra of biotreated lateritic soil are shown in (Fig. 12). The band at 3697, 3523, and 3452 cm− 1 point to an octahedral OH stretching vibrations38,60. In the fingerprint region, the sharp bands at 1006.98, indicates the Si-O stretching. The existence of vaterite and calcite correlates to the absorption bands at 745.36. Comparable absorption bands of vaterite (747 and 1085/1084 cm−1) and calcite (708/712 cm−1) were also described in the works of Ferral-Pérez et al.61. Moreover, 1636.3 was gotten based on the H-O-H bending/deformation of water possibly as a results of smectite interference. The deficiency in satisfactory vibrational bands for calcite may be linked to; (1) dissolution of calcite under lengthy permeation and (2) interloping created with other inorganic compounds bearing in mind that the FTIR analysis was not performed on a pure synthesized carbonate.
Optimization approach
The optimizations approach involved the selection of a best possible option or choice from a number of available options using a robust mathematical computer program and approach. The best choice option also known as optimal value is usually a value relative to extreme minimum and maximum in an array of outcomes. A wide range of many engineering specializations have successfully used different optimization techniques40,62,63,64,65,66.
The various output used in measuring the performance of the developed model and its corresponding validation results are contained in (Table 4). Evaluation the performance of BFO, PSO and SAO algorithms involves comparing their outputs using predefined metrics on the fitness/objective function. A comparative evaluation of the results revealed that PSO and SAO optimization approaches recorded good results and met the minimum hydraulic conductivity value of 1E-09 m/s for use as liner material, at all iterations considered, while BFO underperformed and did not meet the minimum requirement of 1E-09 m/s for use as liner material, for all iterations considered. For instance, a comparative study of the three optimizations approaches at 500 alterations each recorded predicted minimum hydraulic conductivity value of 2.7E-09 m/s with 2.4E-09cells/ml B. megaterium, WAS compaction energy, for BFO. A minimum hydraulic conductivity value of 2.27E-011 m/s at 1.2E-09cells/ml B. megaterium, WAS compaction energy, for PSO. In the case of SAO, a minimum hydraulic conductivity value of 4.45E-011 m/s at 2.4E-09cells/ml B. megaterium compacted at WAS compaction energy was recorded. The best recorded result of the least hydraulic conductivity is in the order of PSO > SAO > BFO. Several studies recorded similar findings of impressive results of using PSO in numerous engineering optimization62,63,64,65,66.
The association between predicted HC values against the measured laboratory values is shown in (Figs. 13 and 14). The result showed a resilient association between the predicted and the measured values with documented coefficient of determination R = 0.936. This designate that the independent variables used to generate the regression equation has substential effect on the HC values of soil/B. megaterium mixtures and should thus be prudently considered in the process of design and construction of a landfill system.
The fitness function developed for HC within the basic constraints and self- determining factors is fitted into a BFO, PSO and SAO algorithm programmes which were iterated over a wide number range to obtain minimum hydraulic conductivity. The various hydraulic conductivity values obtained and plotted against iterations number using BFO, PSO and SAO algorithm are shown in Figs. 15, 16 and 17 respectively. The detail rundown and summaries of the optimization results using these algorithms under selected varied iterations number of 10–500, 10–700 and 10 -2000 for BFO, PSO and SAO algorithm, are presented in Tables 5, 6 and 7 respectively.
The basic constraints of compactive effort, moisture content and B. megaterium suspension density should be mainly taken to consideration amongst other factors at both design and construction of bio-treated compacted clay liner for field implementation. These considerations will meet the acceptable designed hydraulic conductivity without compromising other basic engineering parameters, both during the useful life or post active period of the facility or for any associated geotechnical engineering applications.
Conclusion
The following conclusion are derived from this study:
-
(1)
The hydraulic conductivity decreased with increased bacteria suspension density ranging from 1.33 × 10–10 to 4.05 × 10− 8 m/s within 7.8 to 17.6% moulding water content regardless the compactive effort.
-
(2)
SEM and XRD analysis confirmed carbonates precipitation in bio-treated lateritic soil; treated with B. megaterium inoculum density range from 1.5 × 10− 9 cells/ml to 24 × 10− 9 cells/ml. The strengthening of diffraction peaks, particularly at 2θ angles corresponding to calcite, demonstrates a clear correlation between higher bacterial densities and increased calcite formation, reinforcing the effectiveness of bio-mediation in enhancing bio-cementation processes.
-
(3)
The FTIR analysis confirmed the presence of vaterite and calcite in biotreated lateritic soil, highlighting key chemical and mineralogical changes. However, the absence of clear calcite bands suggests possible calcite dissolution or interference from other compounds, indicating complexities in characterizing the treated soil.
-
(4)
Based on the results and data obtained from the statistical analysis and optimization techniques, the three optimization algorithms are ranked in the order of suitability or best performance based on the least hydraulic conductivity (k) which satisfies the regulatory values for liners as follows: PSO ( k: 2.27E-11 m/s) > SAO (k: 2.85E-11 m/s) > BFO (k: 2.66E-09 m/s).
-
(5)
Particle swarm optimization (PSO) and smell agent optimization (SAO) algorithms can be conveniently use to predict the hydraulic conductivity of a typical barrier material made from lateritic soil bio-mediated with Bacillus megaterium species in nutrient media solution of up to 24 × 108 cells/ml.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
DeJong, J. T., Mortensen, B. M., Martinez, B. C. & Santamarina, J. C. Biomediated soil improvement. Ecol. Eng. 36 (2), 197–210 (2010).
Ma, G. et al. Strength and permeability of bentonite-assisted biocemented coarse sand. Can. Geotech. J. 58 (7), 969–981. https://doi.org/10.1139/cgj-2020-0045 (2020).
Jiang, Y. J. et al. Bio-mediated soil improvement: an introspection into processes, materials, characterization and applications. Soil Use Manag. 1–26. https://doi.org/10.1111/sum.12736 (2021).
Etim, R. K., Eberemu, A. O., Ijimdiya, T. S. & Osinubi, K. J. Coupled effect of cementation solution, curing period, moulding water content and compactive effort on strength performance of bio-treated lateritic soil for waste containment application. ASCE J. Hazard. Toxic Radioact. Waste https://doi.org/10.1061/JHTRBP.HZENG-1201 (2023).
Fu, T., Saracho, A. C. & Kenneth, S. Haigh microbially induced carbonate precipitation (MICP) for soil strengthening: A comprehensive review. Biogeotechnics 1100002 https://doi.org/10.1016/j.bgtech.2023.100002 (2023).
Zamani, A., Montoya, B. M. & Gabr, M. Investigating the challenges of in situ delivery of MICP in fine grain sands and silty sands. Can. Geotech. J. 56, 1889–1900 (2019).
Wang, H. et al. Erosion resistance of treated dust soils based on the combined enzymatically induced carbonate precipitation and polyacrylic acid. Bioeng. Translational Technol. 9, 100050. https://doi.org/10.1016/j.bgtech.2023.100050 (2023).
Yohanna., P., Thomas, S. I., Adrian, O. E. & Kolawole, J. O. Diffusion study of municipal solid waste contaminants in compacted lateritic soil treated with bacillus coagulans. Third International Symposium on Coupled Phenomena in Environmental Geotechnics. https://doi.org/10.3208/jgssp.v09.cpeg038, 343–350 (Japanese Geotechnical Society Special Publication, 2021).
Taharia, M. et al. Microbial induced carbonate precipitation for remediation of heavy metals, ions and radioactive elements: A comprehensive exploration of prospective applications in water and soil treatment. Ecotoxicol. Environ. Saf. 271, 115990. https://doi.org/10.1016/j.ecoenv.2024.115990 (2024).
Zhu, T. & Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 4, 4. https://doi.org/10.3389/fbioe.2016.00004 (2016).
Achal, V., Pan, X., Zhang, D. & Fu, Q. Bioremediation of Pb-Contaminated soil based on microbially induced calcite precipitation. J. Microbiol. Biotechnol. 22 (2), 244– (2012).
Ramachandran, S. K., Ramakrishnan, V. & Bang, S. S. Remediation of concrete using micro-organisms. ACI Mater. J. 98 (1), 3–9 (2001).
Fujita, Y., Taylor, J. L., Gresham, T., Delwiche Ivanov, V. & Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol. 7 (2), 139–153 https://doi.org/10.1007/s11157-007-9126-3 (2008).
Jalilvand, N., Akhgar, A., Alikhani, H. A., Rahmani, H. A. & Rejali, F. Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian mine calcareous soils. J. Soil. Sci. Plant. Nutr. 20 (1), 206–219 (2000).
Wang, Z., Zhang, N., Jin, Y., Li, Q. & Xu, J. Application of microbial induced carbonate precipitation (MICP) in sand embankment for scoring/erosion control. Mar. Georesour. Geotechnol. https://doi.org/10.1080/1064119X.2020.1850948 (2020).
Whiffin, V. S., van Paassen, L. A. & Harkes, M. P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J. 24 (5), 417–423 (2007).
Ivanov, V. & Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol., 7(2), 139–153 https://doi.org/10.1007/s11157-007-9126-3 (2008).
Rebata-Landa, V. & Santamarina, J. C. Mechanical limits to microbial activity in deep sediments. Geochem. Geophys. Geosyst. 7, Q11006 (2006).
Osinubi, K. J., Eberemu, A. O., Ijimdiya, T. S., Sani, J. E. & Yakubu, S. E. Volumetric Shrinkage of Compacted Lateritic Soil Treated with Bacillus pumilus. In Proceedings of GeoShanghai 2018 International Conference: Geoenvironment and Geohazard. GSIC 2018 (Farid A., Chen H. eds) https://doi.org/10.1007/978-981-13-0128-5_36 (Springer, 2018).
Etim, R. K., Osinubi, K. J., Eberemu, A. O. & Ijimdiya, T. S. Performance evaluation of compacted lateritic soil bio-cemented with Bacillus megaterium for use as liners and covers in municipal solid waste containment. 351, 176–185 (2024).
Etim, R. K., Osinubi, K. J., Eberemu, A. O. & Ijimdiya, T. S. Suitability of ureolytic microbe in limiting desiccation-induced volumetric shrinkage strain of lateritic soil for waste containment purposes. In Geo-EnvironMeet 2025 (pp. 1–10). https://doi.org/10.1061/9780784485699.001 (2025).
Yohanna, P., Kolawole, J. O., Oshioname, A. E., Thomas, S. I. & Sani, J. E. Effect of treatment compositions on the plasticity of tropical red soil treated with Bacillus Coagulans. J. Civil Eng. Constr. 11 (1), 56–64 (2022).
Osinubi, K. J., Eberemu, A. O., Ijimdiya, T. S. & Etim, R. K. Microbial-Induced calcite precipitation study on the plasticity and compaction characteristics of lateritic soil treated with Bacillus Megaterium in Urea-CaCl2 Culture Medium. ICSID 2020. IOP Conf. Series: Materials Science and Engineering. 1036012031 https://doi.org/10.1088/1757-899X/1036/1/01230331. (2021a).
Osinubi, K. J., Eberemu., A. O., Ijimdiya, T. S. & Yohanna, P. Interaction of landfill leachate with compacted lateritic soil treated with Bacillus coagulans using microbial-induced calcite precipitation approach. J. Hazard. Toxic Radioact. Waste https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000465 (2020).
Mishra, P., Maji, S. & Pradhan, S. Sustainable soil stabilization techniques: A review. J. Environ. Manage. 240, 188–198 (2021).
Afolagboye, L. O., Owoyemi, O. O. & Abdu-Raheem Effect of elevated temperature and treatment duration on selected index and engineering properties of lateritic soil. Discover Civil Eng. 1, 71. https://doi.org/10.1007/s44290-024-00077-9 (2024).
Cao, Y., Zhang, J. & Wang, H. A review on the utilization of biocement in sustainable engineering. Sustainable Mater. Technol. 23, e00136 (2020).
Naseem, A., Bansal, R. & Singh, G. A review on biocementation and its effectiveness in soil stabilization. Constr. Build. Mater. 262, 120334 (2021).
Zhang, Z., Liu, H. & Zhao, Y. Interaction of microbialinduced calcite precipitation and soil structure: implications for soil stabilization. Environ. Earth Sci. 81 (6), 115 (2022).
Xie, C., Wu, H. & Wang, Z. Investigating the effects of microbialinduced calcite precipitation on soil hydraulic properties. Water Res. 212, 118160 (2022).
Santos, P. F., Almeida, J. M. & Canteiro, M. Challenges in soil stabilization: A review. J. Environ. Manage. 240, 188–198 (2019).
Chapin, K. C. & Lauderdalem, T. Reagents, stains, and media: Bacteriology. In Manual of clinical microbiology, 8th ed., (Murray, P. R., Baron, E. J., Jorgensen, J. H., Faller, M. A. P. & Yolken, R. H. eds) (ASM Press, 2003).
Cheng, L., Cord-Ruwisch, R. & Shahin, M. A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 50 (1), 81–90 (2013).
Mujah, D., Shahin, M. A. & Cheng, L. State-of-the-Art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol J. 34 (6), 524–537. https://doi.org/10.1080/01490451.2016.1225866 (2017).
Xiao, Y., Ma, G., Wu, H., Lu, H. & Zaman, M. Rainfall-induced erosion of biocemented graded slopes. Int. J. Geomech. 22 (1). https://doi.org/10.1061/(ASCE)GM.1943-5622.0002239 (2022).
Daniel, D. E. State of the art: laboratory hydraulic conductivity test for saturated soils, Hydraulic Conductivity and Waste Contaminant Transport in Soil. ASTM STP 1142 David. (eds Daniel, E., Stephen, J. & Trautwein) American Society for Testing and Materials, Philadelphia. (1994).
Osinubi., K. J., Yohanna, P., Eberemu., A. O. & Ijimdiya, T. S. A comparative study of soil-water characteristic curves for compacted lateritic soil – bacillus coagulans mixtures. MATEC Web of Conferences 337, 01001 https://doi.org/10.1051/matecconf/202133701001 (2021).
Etim, R. K., Ijimdiya, T. S., Eberemu, A. O. & Osinubi, K. J. Compatibility interaction of landfill leachate with lateritic soil bio-treated with Bacillus megaterium using MICP technique: criterion for barrier material in waste containment. Clean. Mater. https://doi.org/10.1016/j.clema.2022.100110.( (2022).
Ferreira, C. Gene expression programming: A new adaptive algorithm for solving problems. Complex. Syst. 13 (2), 87–129 (2001).
Yohanna, P., Osinubi, K. J., Eberemu, A. O., Ijimdiya, T. S. & Salawudeen, A. T. Evaluation of compacted lateritic soil –bacillus coagulans induced calcite precipitate for landfill application using bacterial foraging optimization algorithm. IOP Conference. Series: Mater. Sci. Eng. https://doi.org/10.1088/1757-899X/1036/1/012029 (2020).
AASHTO. Standard Specification for Transportation, Material and Methods of Sampling and Testing 14th edn (Amsterdam association of state highway and transportation official, 1986).
ASTM. Annual Book of Standards Philadelphia 04.08. Pennsylvania (American Society for Testing and Materials, 1992).
Etim, R. K., Ekpo, D. U., Ebong, U. B. & Usanga, I. N. Influence of periwinkle shell Ash on the strength properties of cement-stabilized lateritic soil. Int. J. Pavement Res. Technol. https://doi.org/10.1007/s42947-021-00072-8 (2021). Chinese Society of Pavement EngineeringSpringer Nature Singapore.
Rowshanbakht, K., Kamehchiyan, M., Sajedi, R. H. & Nikudel, M. R. Effects of injected bacterial suspension volume and relative density on carbonate precipitation resulting from microbial treatment. Ecol. Eng. 89, 49–55. https://doi.org/10.1016/j.ecoleng.2016.01.010 (2016).
Lai, H. J. et al. Factors affecting the effectiveness of biocementation of soil. Biogeotechnics https://doi.org/10.1016/j.bgtech.2024.100087 (2024).
Osinubi, K. J., Yohanna, P., Eberemu, A. O. & Ijimdiya, T. S. Evaluation of hydraulic conductivity of lateritic soil treated with Bacillus coagulans for use in waste containment applications. In: Zhan L, Chen Y, Bouazza A (eds.) Proceedings of the 8th International Congress on Environmental Geotechnics (ICEG 2018). Towards a sustainable geoenvironment. 3, 401–409 https://doi.org/10.1007/978-981-13-2227-3_50 (Springer, 2018).
Moses, G. & Afolayan, J. O. Compacted foundry sand treated with cement kiln dust as hydraulic barrier material. Electron. J. Geotech. Eng. 16, 338–355 (2011).
Ijimdiya, T. S. & Musa, K. A. Hydraulic conductivity behaviour of crude oil contaminated soil bio-remediated using chicken droppings for use in waste containment systems. Nigerian Soc. Eng. Tech. Trans. 51 (2), 19–30 (2017).
Wang, N. et al. Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar. Georesour. Geotechnol. 35, 1135–1146. https://doi.org/10.1080/1064119X.2017.1297877 (2017).
Shahrokhi-Shahraki, R., Zomorodian, S. M. A., Niazi, A. & Okelly, B. C. Improving sand with microbial-induced carbonate precipitation. Proc. Inst. Civil Eng. Ground Improv. 168, 217–230. https://doi.org/10.1680/grim.14.00001 (2015).
Sharma, N., Satyam & Reddy, K. R. Rock-like behavior of biocemented sand treated under non-sterile environment and various treatment conditions. J. Rock Mech. Geotech. Eng. https://doi.org/10.1016/j.jrmge.2020.11.006 (2021).
Chen, Y. et al. Experimental study on permeability and strength characteristics of MICP-treated calcareous sand. Biogeotechnics 1, 100034. https://doi.org/10.1016/j.bgtech.2023.100034 (2023).
Baidya, P., Dahal, B. K., Pandit, A. & Joshi, D. R. Bacteria-induced calcite precipitation for engineering and environmental applications. Adv. Mater. Sci. Eng. https://doi.org/10.1155/2023/2613209 (2023).
Bell, F. G. Engineering geology. (Blackwell, 1993).
Oliveira, P. J. V. & Neves, J. P. G. Effect of organic matter content on enzymatic Bio-cementation process applied to Coarse-Grained soils. J. Mater. Civ. Eng. 31 (7), 04019121. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002774 (2019).
Chipera, S. J. & Bish, D. L. Baseline studies of the clay minerals society source clays: powder X-ray diffraction analysis. Clays Clay Miner. 49, 398–409 (2001).
Li, W., Chen, W. S., Zhou, P. P., Zhu, S. L. & Yu, L. J. Influence of initial calcium ion concentration on the precipitation and crystal morphology of calcium carbonate induced by bacterial carbonic anhydrase. Chem. Eng. J. 218, 65–72 (2013).
Wen, K., Li, Y., Amini, F. & Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 15, 17–27. https://doi.org/10.1007/s11440-019-00899-3 (2020).
Liu, L., Liu, H., Stuedlein, A. & Xiao, Y. Strength, stiffness, and microstructure characteristics of biocemented calcareous sand. Can. Geotech. J. 56 (10), 1502–1513. https://doi.org/10.1139/cgj-2018-0007 (2019).
Madejova, J. & Komadel, P. Baseline studies of the clay minerals society source clays: infrared methods. Clays Clay Miner. 49 (5), 410–432 (2001).
Ferral-Pérez, H. & Galicia-García, M. Bioprecipitation of calcium carbonate by Bacillus subtilis and its potential to self-healing in cement-based materials. J. Appl. Res. Technol. 18 (5) https://doi.org/10.22201/icat.24486736e.2020.18.5.1280 (2020).
Nitin, K. J. & Dipak, A. Comparative analysis of bacterial foraging optimization algorithm with simulated annealing. Int. J. Sci. Res. 3 (3), 10–13 (2014).
Binh Thai et al. A novel hybrid soft computing model using random forest and particle swarm optimization for Estimation of undrained shear strength of soil. Stainability 12, 2218. https://doi.org/10.3390/su12062218 (2020).
Abdullahi, T. S. et al. A particle swarm and smell Agent-Based hybrid algorithm for enhanced optimization. Algorithms 17, 53,1–14. https://doi.org/10.3390/a17020053 (2024).
Farah, A. Z., Mohamad, F. S., Azmira, I., Mohammad, W. A., Hazlie, M. & L. O. & Improved bacterial foraging optimization algorithm with machine Learning–Driven Short–Term electricity load forecasting: A case study in Peninsular Malaysia. Algorithms 17, 510. https://doi.org/10.3390/a17110510 (2024).
Glykeria, K., Ioannis, G. T., Anna, M. G. & Vasileios, C. Parallel smell agent optimization (SAO): collaborative subpopulations for accelerated convergence. Symmetry 17, 592.,1–33. https://doi.org/10.3390/sym17040592 (2025).
Author information
Authors and Affiliations
Contributions
R.K Etim a Ph.D candidate who wrote the draft manuscript and conducted the laboratory experiment.P. Yohanna assisted in the optimization part of the manuscript.K.J Osinubi conceptualization of the research work and main supervisor of the thesis work.A.O. Eberemu a co-supervisor of the thesis work .T.S Ijimdiya a co-supervisor of the thesis work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
The authors confirm that written informed consent was obtained for the publication of the identified image (included in Fig. 2) in this online open-access publication. The participant was made fully aware of the nature of the publication and provided explicit permission for the use of the image, which is a significant part of the experimental process in this study.
Consent for publication
The authors have unanimously agreed to publish their original research work and have sought the consent of Scientific Reports.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Etim, R.K., Yohanna, P., Eberemu, A.O. et al. Assessing the biocementation of lateritic soil using hydraulic conductivity and bioinspired optimization approach. Sci Rep 15, 27356 (2025). https://doi.org/10.1038/s41598-025-12907-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12907-6