Abstract
Zymoseptoria tritici, the causal agent of Septoria tritici blotch (STB) in bread wheat (Triticum aestivum), leads to significant global yield losses. Resistance breeding is vital for managing STB, but there is limited information on Z. tritici infection behaviour in Ethiopia. This study examined the virulence variability of Z. tritici isolates from Ethiopia’s Central Highlands and evaluated the effectiveness of known wheat STB-resistance genes. Eight wheat lines were tested against six Z. tritici isolates, showing significant differences (p < 0.0001) in necrotic leaf area (%NLA) and pycnidia coverage (%PC) among the tested Z. tritici isolates, wheat lines and their interactions. Wheat genotype TE9111 exhibited specific resistance to 50% of the isolates, while Taichung 29 showed no resistance. Isolate ZSE158 was the most aggressive, causing 61.4% PC and 54% NLA. The Ethiopian isolates displayed broad virulence against resistance genes, including Stb2 – Stb7. TE9111, carrying Stb11, showed resistance to 50% of isolates, making it a valuable source for resistance breeding against STB. This study identified highly virulent pathogen isolates useful for wheat germplasm screening for STB resistance and also key resistance source materials for use in wheat resistance breeding in Ethiopia.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum) is a crucial staple food crop in Ethiopia, contributing significantly to food and nutritional security. The country’s diverse agro-ecologies allow for wheat cultivation across various regions, making it an essential crop for both smallholder farmers and commercial production systems. In Ethiopia, wheat ranks fourth after teff (Eragrostis tef), maize (Zea mays) and sorghum (Sorghum bicolor) in area coverage, and third after maize and teff in total production1. It is cultivated by more than 5 million householders on about 2.3 million ha for various uses, such as food, feed and income generation. Both durum and bread wheat are cultivated in Ethiopia, the latter accounting for nearly 80% of the country’s total wheat production2,3. During the last 15 years, wheat harvest area, production and productivity in Ethiopia have increased from 1.4 M ha, 2.31 million metric tons and 1.62 t/ ha in 2003, to 2.3 M ha, 7 million metric tons and 3.04 ton/ha in 2022, respectively4. However, this national average wheat productivity (3.04 t/ha) is far lower than the global average of 3.69 t/ha5, resulting in a production limit to meeting the growing demand for food by an ever-increasing population6. Wheat production in Ethiopia can be curtailed by several factors, such as limited access to advanced agricultural production technologies and low agricultural inputs (improved varieties and fertilizer), biotic stresses such as disease, pests and weeds, and abiotic stresses including drought, soil acidity and salinity7.
Among the biotic factors, diseases caused by fungal pathogens represent the major constraints affecting wheat production and productivity globally. In Ethiopia, wheat cultivation is persistently affected by over 30 fungal diseases8, among which stripe rust (yellow rust) caused by Puccinia striiformis f. sp. tritici, stem rust caused by Puccinia graminis f. sp. tritici, and Septoria tritici blotch (STB) caused by Zymoseptoria tritici are the major infections8,9,10. Rusts can result in grain yield losses of 60–100%11. Up to 82% losses in wheat yield have been reported to be the result of STB, and currently the prevalence of STB has increased considerably in the major wheat-growing areas of Ethiopia12,13. STB affects grain yield by causing reduced tillering, poor seed set, poor grain fill or shriveled kernels, and the death of leaves, spikes or the entire plant14,15 .
The development and use of resistant varieties is the most economical, durable and environmentally safe approach to controlling crop diseases16. The gene pool utilized in wheat breeding efforts has expanded as a result of extensive research into additional sources of STB resistance17. Similar to many other plant diseases, wheat has two types of STB resistance: qualitative and quantitative. Quantitative resistance is regulated by polygenic characteristics and provides insufficient resistance to Z. tritici. In contrast, qualitative resistance confers complete or near complete resistance to particular isolates and follows a gene-for-gene model. So far, 22 resistance genes in Z. tritici have been reported and mapped on the wheat genome17,18,19. However, the expression patterns and effects of these genes on STB resistance vary between the seedling and adult plant stages20. For example, Stb16 is expressed and effective at both seedling and adult stages, while Stb17 is only expressed during the adult stage21. Stb18 is an isolate-specific resistance gene, displaying varying resistance to Z. tritici depending on the isolate, in both seedling and adult stages21. Stb6 is the only qualitative gene for STB resistance22, and its corresponding avirulence gene, AvrStb6, in Z. tritici23 has been shown to follow a gene-for-gene relationship. However, because of its reproductive biology (sexual life cycle), Z. tritici changes its genome rapidly, favoring adaptions to host resistance genes (R genes)24. In line with this25, suggest that the narrow genetic basis of modern wheat cultivars and the rapidly changing fungal genomes have together resulted in the frequent breakdown of host resistance.
Disease can happen when the balance between a host and a pathogen is altered. Thus, host defense can never be considered independent of a pathogen’s virulence factor. In other words, effective resistance breeding relies on a clear understanding of the disease-causing Pathotype and its genetic structure. The term ‘Pathotype’ describes populations of a fungus species that are recognized as having identical morphology but distinct infection behaviors, which may be distinguished by the way the populations respond to a series of test host cultivars called ‘differentials’ 26,27 used wheat differential lines with known Stb genes to assess virulence variability in Mycosphaerella graminicola and Z. tritici populations. Screening of seedlings based on the gene-for-gene concept provides the opportunity to determine the effectiveness of resistance against a broad range of isolates21.
Despite the greater incidence and severity of STB disease on wheat, there is limited information on the infection behavior of Z. tritici populations in Ethiopia. Therefore, the purpose of this study was to evaluate the effectiveness of Stb resistance genes and assess the pathotype diversity of Zymoseptoria tritici isolates from Ethiopia through artificial inoculation under greenhouse conditions.
Results
Integrative analysis of variance
Variance analysis is used to assess the significance of isolate-by-genotype interactions within the plant–pathogen relationship and identify differential outcomes. Six Z. tritici isolates were chosen from a pool of 200, by comparing the isolates identified in a phylogenetic tree following internal transcribed spacer (ITS) region sequencing in accordance with the location of their collection. The infection behavior of the six Z. tritici isolates was assessed on eight wheat differential genotypes with known Stb genes (Table 1). Plants with a score of 0–2 were categorized as resistant, while those with a score of 3–5 were considered susceptible. The data on disease severity revealed highly significant differences (p < 0.0001) (Table 1) in the percentage of necrotic leaf area (%NLA) and pycnidia coverage (%PC) between the isolates, wheat differential lines, and their two-way interactions (Table 2). The symptoms of each genotype was compared with Taichung 29 used as susceptible control. The variation based on wheat genotypes was the largest. Based on an ANOVA, the isolates’ main effect was also significant (Table 1), suggesting that Ethiopian Z. tritici isolates have significant variability in their virulence, providing the second highest source of variation. Similarly, %NLA and %PC values were significantly different (p < 0.0001) among the wheat differential lines, indicating that the genotypes differed greatly in their responses to the Z. tritici isolates. Furthermore, the extremely significant differences in the isolate-by-genotype interactions indicated the existence of specificity among the wheat differential lines to the pathogen inoculum, and also the presence of considerable differences among the pathogen inoculum. This suggested a distinct interaction between the genotypes of the isolates and hosts.
Pathogenicity, aggressiveness and virulence of Z. tritici isolates
Aggressiveness is a pathogen’s relative capacity to infect a host, and is a quantitative aspect of pathogenicity, whereas virulence is a distinguishable isolate-specific relationship28. Differential interactions always relate to virulence (which is linked to the presence or absence of R genes), whereas ‘aggressiveness’ describes a quantitative component of pathogenicity that is, by definition, non-specific relative to host genotypes. The classic susceptible wheat cultivar, Taichung 29, demonstrated high virulence for all isolates. Examination of the isolates’ aggressiveness showed that the wheat genotypes varied significantly in their response to them (manifest as mean disease severity). The Z. tritici isolates tested displayed broad-spectrum virulence across the wheat genotypes. Isolate ZSE158 was the most aggressive, with the highest mean PC of 61.4% and NLA of 54% (Fig. 1.). With a mean PC of 56% and NLA of 50%, ZSET206 was the second most aggressive. In contrast, isolate ZSET218 was the least aggressive, with a mean PC and NLA of 46% and 39.8%, respectively (Figs. 1, 2 and 3).
Resistance spectra for wheat septoria differential lines against Ethiopian Z. tritici isolates
The ANOVA results showed that the wheat differential lines, isolates, and their two-way interactions, varied significantly in terms of disease severity (%PC and %NLA) (Table 2). Among 48 isolate-by-wheat genotype interactions, four (8.3%) and five (10.4%) showed mean PC and NLA values lower than the LSD values at p < 0.01 and p < 0.05 levels, respectively, and thus they could be considered resistant to one or more of the tested Z. tritici isolates (Table 3). Among the wheat genotypes used in this study, TE9111, which carries Stb11, was resistant to three (50%) Z. tritici isolates (Table 3; Fig. 4). Likewise, the genotypes CS Synthetic and Tadinia showed were resistant to two and one isolates, respectively (Table 3).
Five of the tested differential lines, Estanzuela Federal, Israel 493, Shafir (Stb6), Taichung 29 and Veranopolis, showed 100% susceptibility to the tested Z. tritici isolates (Table 3). None of the tested wheat genotypes showed resistance to all the Z. tritici isolates used. Only TE9111 and CS Synthetic (6x) showed significant specific resistance to one or more of the tested Z. tritici isolates, suggesting that they possessed one or more Stb genes effective against a limited number of Z. tritici isolates (Table 3). Among the tested wheat differential lines, TE9111 and Taichung 29 were found to be the most resistant and susceptible, respectively (Table 3; Fig. 5 and Table 4).
Cluster analysis of wheat genotypes
Hierarchical cluster analysis based on mean disease severity (NLA and PC) grouped the eight differential lines (wheat genotypes) into six clusters (Fig. 6). Cluster I contained nine (18.75%) of the pairwise isolate-by-genotype interactions; the genotypes were categorized as moderately resistant (MR) and the isolates as moderately avirulent (MAv). Cluster II comprised 13 (27.08%) of the interactions, which were considered to have moderately susceptible (MS) genotypes and moderately virulent (MV) isolates. Cluster III contained 11 (22.9%) interactions, which were categorized as susceptible (S) genotypes and virulent (V) isolates. Cluster IV contains six (12.5%) genotype-by-isolate combinations that comprise very susceptible (VS) genotypes and highly virulent (HV) isolates. Cluster V contained six (12.5%) combinations categorized as resistance (R) genotypes and avirulent (Av) isolates. Cluster VI consisted of three (6.25%) combinations that were categorized as highly resistance (HR) genotypes and highly avirulent (HAv) isolates.
Efficacy of Stb-resistant genes against Ethiopian Z. tritici isolates
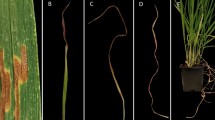
Table 3 presents a summary of the virulence level of the six Z. tritici isolates on the eight differential lines with known major Stb genes. All six isolates showed variations in their virulence against the Stb genes. Accordingly, TE9111, which possessed Stb11, was highly resistant (HR) to the isolates ZSET168 and ZSET218, moderately resistant (MR) to ZSET033, moderately susceptible (MS) to ZSET121, and susceptible (S) to ZSET206. Genotype CS Synthetic (6x), which possessed Stb5 + Stb6, showed resistance (R) to the isolate ZSET206, and moderate resistance (MR) to ZSET218, but was susceptible (S) to all other Z. tritici isolates. The differential lines Israel 493 and Tadinia were found to be moderately susceptible to ZSET121 and ZSET218, respectively. The other wheat differential lines, such as Veranopolis (Stb2 + Stb6), Shafir (Stb6), Estanzuela Federal (Stb7) and the susceptible control Taichung 29 (Stb2 + Stb6, Stb6, Stb7), were found to be totally susceptible to the Ethiopian Z. tritici isolates. Among the Stb genes, Stb11 in TE9111 was the most effective, conferring resistance to four Z. tritici isolates (Table 3). Differential lines with known Stb genes that scored mean disease severity values higher than the critical LSD values were considered as susceptible and designated S, while those with mean disease severity values (NLA and PC) lower than the LSD values at α = 1% were considered resistant and denoted as R (Table 4). Figure 7 illustrates the resistance response of the eight wheat Septoria differential lines with known Stb genes against the Z. tritici isolate ZSET158. The resistant Israel 493 and TE9111 lines developed no/minimum symptoms of STB infection compared with susceptible lines. This was due to that TE9111 differential was the first resistant variety and Israel was the second resistant variety according to the result of the investigation.
Discussions
This study evaluated the efficacy of known Stb resistance genes and assessed the Pathotype diversity of Zymoseptoria tritici isolates collected from Ethiopia. The findings highlight significant variability in both the effectiveness of Stb genes and the virulent patterns of Z. tritici populations, emphasizing the dynamic nature of host-pathogen interactions in wheat production.
The virulence patterns of six Z. tritici isolates (Fig. 7) obtained from Ethiopia’s major wheat-growing areas were examined on eight differential lines of bread wheat (Table 7) that possessed different Stb genes. Using %PC and %NLA as disease parameters16,29, the results provide relevant information for wheat breeders regarding the virulence spectrum and aggressiveness of Ethiopian populations of Z. tritici isolates, as well as the response of previously reported Stb genes. In a study conducted by30 in Ethiopia, several Z. tritici isolates were found to be virulent on the differential lines such as Veranopolis, Tadinia, Km 7 and Kavkaz-K4500, out of the seven tested. Similarly31 reported that six isolates out of eight tested isolates were virulent on the genotypes tested. This finding is similar to the current results. Collectively, the isolates from both the present study and the earlier investigation demonstrate that Ethiopian Z. tritici populations are capable of overcoming resistance in differential lines tested which was previously considered one of the most resistant differential lines.
The combined analysis of variance for both parameters (%PC and %NLA) revealed extremely significant differences (p < 0.0001) in the interaction effects, indicating that the isolates varied considerably in their virulence patterns across the wheat genotypes (Table 1). The particular interactions between isolates and genotypes32 verify the presence of genotype specificity and virulence diversity in Ethiopian Z. tritici isolates (Table 2). Among the 48 isolate-by-genotype interactions, four (8.3%) and five (10.4%) had mean %PC and %NLA values lower than the LSD values at p < 0.01 and p < 0.05 significance levels, respectively (Table 4), indicating that these interactions could be focus points for sources of resistance.
These results are consistent with31, who reported highly significant differences between wheat genotypes, Z. tritici and their interactions, confirming that Ethiopian Z. tritici populations display broad spectrum virulence. The findings of the current study confirm that the isolates possess broad-spectrum virulence, making them valuable tools for use in wheat resistance breeding programs, particularly for screening germplasm against diverse Pathotype which is similar result with previous study by30 in the same country. Likewise33, identified highly significant levels of virulence diversity among Iranian Z. tritici isolates, ranging from 40 to 90%, and34 have demonstrated the existence of substantial virulence differences among Z. tritici isolates in Iran.
Among the tested wheat genotypes, TE9111 exhibited specific resistance to three isolates (50%) (Fig. 4.), indicating that it is a promising source of resistance against STB for use in future wheat breeding programs3536. have reported that the same genotype has wide resistance to M. graminicola. Likewise, CS/ Synthetic (6X) and Tadinia showed specific resistance to two (33.3%) and one Z. tritici isolates, respectively, indicating that they could also be used as sources of resistance, and thus increase the genetic basis of wheat resistance to STB in Ethiopia. The majority of the wheat differential lines studied with known Stb genes were highly susceptible to Ethiopian Z. tritici populations. Stb2–Stb7 were ineffective against the Ethiopian Z. tritici Pathotype, and the differential lines Israel 493, Veranopolis, Tadinia, Shafir and Estanzuela Federal offered no protection against one or a few Z. tritici isolates. These findings are in line with previous reports by28,37, who identified these differential lines as susceptible to most Z. tritici isolates in Iran. TE9111, which carries the major gene Stb11, exhibited a 50% isolate-specific resistance, and thus represents a key resistance gene for Ethiopian Z. tritici isolates. This is in accordance with a study by20, who demonstrated that Quantitative trait loci (QTL) associated with STB resistance as identified by Genome wide association studies (GWAS) is mapped on chromosome 1B. Another study has mapped Stb11 on the short arm of chromosome 1B in the TE9111 genotype38.
The analyses revealed high levels of diversity in the aggressiveness of the Z. tritici isolates tested (Fig. 1). ZSE158 was the most aggressive, with a mean PC of 61.4% and NLA of 54%. These results are comparable with the findings of31, who reported the highest mean PC and NLA (58%), for an isolate collected from the Bale Administrative zone in Ethiopia. The small differences observed between these two studies could be because of isolation and/or differential line variations. The Oromia region’s Arsi-Bale area is known as a ‘wheat belt’ because it produces the most wheat in Ethiopia. On susceptible wheat varieties planted in hot-spot areas in Ethiopia, STB has caused yield losses of up to 82%39. Additionally, recent research has revealed that STB causes yield losses of up to 41% and 48%, respectively, at agricultural research centers in Holeta40 and Areka41 in Ethiopia. Conversely, isolate ZSET218 appears to be the least aggressive, indicating that it may have an avirulence gene that is recognized by a common resistance gene found in the majority of the tested wheat genotypes. A study conducted by30 in Ethiopia reported that most Z. tritici isolates were avirulent, which contrasts with the findings of the current study, where the majority of Ethiopian isolates were virulent on both the tested genotypes and differential lines. This observation is, however, consistent with the findings of a previous report by31. The discrepancy may be explained by the genetic composition of the isolates either the earlier isolates produced multiple avirulence effectors recognized by resistance genes in the differential lines, or they carried a specific avirulence gene that was targeted by a resistance gene commonly present across several of the differential lines34.
Pathogen evolution is a major challenge in the management of plant diseases, especially in systems relying heavily on genetic resistance. Zymoseptoria tritici, the causal agent of Septoria tritici blotch (STB), is known for its high evolutionary potential. This is largely due to its mixed reproductive system (both sexual and asexual reproduction), high gene flow, large population sizes, and ability to rapidly adapt under selection pressure. When resistant wheat varieties carrying Stb genes are deployed over large areas, they exert strong selective pressure on the pathogen population. As a result, virulent strains capable of overcoming these resistance genes can emerge and become dominant, a phenomenon known as resistance breakdown. Over time, the effectiveness of resistance genes like Stb6, Stb11, or others may diminish as matching virulent Pathotype evolve. Studies such by42,43 have documented such breakdowns and emphasized the role of genetic diversity and recombination in Z. tritici. This underlines the importance of using resistance genes in combination (pyramiding), integrating them with quantitative resistance, and rotating cultivars to reduce the selection pressure on any single resistance gene.
To mitigate resistance breakdown, breeding programs must stay ahead of pathogen evolution by monitoring virulent shifts in pathogen populations, avoiding monocultures of single-resistance cultivars and promoting gene deployment strategies across different agro-ecological zones. Ultimately, a deeper understanding of pathogen evolution will inform more resilient breeding strategies and sustainable disease control measures.
Conclusions
Wheat cultivation in Ethiopia is constrained considerably by several fungal diseases, including STB, which is caused by the hemibiotrophic fungus Z. tritici. Resistance breeding is the most efficient, cost-effective and environmentally friendly approach to managing STB, but developing sustainable management through a breeding program requires a clear understanding of the pathogen’s infection behavior and thorough screening of the available germplasm for potential resistance sources44. This research offers valuable data for Ethiopian breeding programs with the aim of initiating resistance against the devastating wheat disease STB.
This study’s findings offer important information regarding the resistance patterns of wheat differential lines against Z. tritici isolates recovered from the major wheat-growing areas of Ethiopia. This study has also profiled the virulence pattern of isolates. Ethiopian Z. tritici isolates exhibit broad pathogenicity against previously reported Stb genes, including Stb2–Stb7, suggesting that the Z. tritici populations may have successfully evolved to overcome these resistance genes through pathogenic host adaptation. Among the tested wheat differential lines, TE9111, which carries Stb11, showed resistance to 50% of the tested Z. tritici isolates, suggesting that it could be a good candidate source for wheat resistance breeding against STB. Overall, the broad virulence pattern of Ethiopian Z. tritici isolates indicates the need to look for possible sources of resistance and deploy them in susceptible but high yielding wheat genotypes. The insights provided into the infection behavior of Z. tritici isolates recovered from major wheat-growing areas of Ethiopia can inform development programs for STB-resistant wheat cultivars and efficient management plans within agricultural environments. Greater efforts should be directed toward identifying and characterizing resistance gene resources through both conventional and molecular approaches to effectively manage Septoria tritici blotch (STB) in Ethiopia. Additionally, more attention should be given to understanding the evolutionary potential of the pathogen, particularly its capacity to overcome genetic resistance over time.
Generally, this study highlights the complexity of host-pathogen interactions in Z. tritici and underscores the importance of strategic breeding and disease management to combat Septoria tritici blotch (STB) in Ethiopia. The observed variability in Stb gene efficacy and the high Pathotype diversity of the pathogen emphasize the need for a diversified approach to resistance breeding. Future research should focus on identifying novel resistance sources, monitoring pathogen evolution, and optimizing breeding strategies to ensure sustainable wheat production in the region.
Materials and methods
Determining the pathogenicity and virulence spectrum of Z. tritici isolates
Virulence analyses of the pathogen Zymoseptoria tritici were performed at the Swedish University of Agricultural Sciences (SLU, Alnarp, Sweden). The pathogen was shipped for use in a stock culture maintained in 25% glycerol. The cultures were plated in Petri dishes on potato dextrose agar (PDA) and incubated at 24 °C for 8 days. Single-spore-derived colonies were multiplied in liquid medium for 2 weeks for use as inoculum. Two hundred Z. tritici isolates which were used in previous study45 were molecularly identified by sequencing an internal transcribed spacer (ITS) rDNA region of 760 bp (Table 5), and a phylogenetic tree was generated. Based on the generated tree, six isolates (Table 6; Fig. 2) were selected for the virulence variability study. The infection behavior of the fungal isolates was tested on eight wheat differential lines with known Stb genes (obtained from SLU; Table 7) through artificial inoculation at the seedling stage under greenhouse conditions [with temperatures of 22 °C/21°C (day/night), a 12-hour photoperiod and relative humidity of 25–80%] at Biotron (Department of Plant Breeding, SLU, Alnarp, Sweden). Five seeds of each of the differential lines were planted in plastic pots (12 cm in diameter and 15 cm in depth) arranged in a factorial randomized complete block design (RCBD) and with four replicates. The soil type used in the study was potting soil clay and silica with a nutritional composition (g/m3) of nitrogen 182, phosphorus, 91, potassium, 194, magnesium, 274, sulfur, 99, calcium, 2186, iron, 8.6, manganese, 3.2), copper, 2.0, zinc, 1.0, boron, 0.4 and molybdenum, 2.6, and a pH of 5.5–6.5 (produced by SW Horto Co, Herrestadsvagen 24, Sweden).
Seedling inoculation
Single spore-derived colonies were transferred into a liquid medium composed of 1% (w/v) yeast extract powder + 1% (w/v) sucrose, and cultures were maintained in an orbital shaker at 130 rpm for 2 weeks for spore multiplication. Spore pellets were then recovered by centrifugation at 10,000 rpm for 5 min. The pellets were suspended in distilled sterilized water, and the spore concentration adjusted to 107 spore/ml using hemacytometer. The solution was supplemented with 0.15% Tween 20 (polyoxyethylene – sorbitan monolaurate; Sigma-Aldrich, Poznan, Poland), and 10 µl (107 spore/ml) of mono-spore suspensions of the individual isolates were hand sprayed until run-off32. To avoid cross-contamination, inoculated plantlets were covered with polyethylene plastic bags.
Data collection and disease evaluation
Wheat differential lines carrying distinct Septoria tritici blotch (STB) resistance genes (Stb2–Stb11, excluding Stb8 and Stb9) were used to analyze the virulence variability of the Ethiopian Z. tritici isolates. The susceptible wheat variety Taichung 29 was used as a baseline for the virulence spectra of the isolates and the efficacy of the genes. The responses of the wheat genotypes were evaluated at the seedling stage under greenhouse conditions as described by31, with minor modifications, and the plants were monitored for symptom development for a period of 3 weeks. Based on extensive research performed on interactions between Z. tritici isolates and host cultivars29, two parameters were used to assess disease severity: the percentage of necrotic leaf area (NLA) and the percentage of pycnidia coverage (PC). Disease severity scoring was carried out at 21 days post-inoculation (dpi) on the second leaf of 15 plants per isolate–genotype combination, by visual estimation of the %NLA and %PC. The values were averaged per pot for further analysis. The percentage data was then converted into a scale of 0–553, where 0 (Immune - Imm) (0%): No pycnidial formation, with no symptoms or only occasional hypersensitive flecks, 1 (Highly Resistant - HR) (5–10%): No or very few isolated pycnidia, mainly in older leaf tissue, with hypersensitive flecking in younger leaves, 2 (Resistant - R) (11–20%): Very light pycnidial formation, 3 (Intermediate - I) (21–29%): Light pycnidial formation with noticeable lesion coalescence, especially towards the leaf tip and in older leaf tissue, 4 (Susceptible - S) (30–50%): Moderate pycnidial formation with significant lesion coalescence and 5 (Very Susceptible - VS) (51% and above): Large, abundant pycnidia with extensive lesion coalescence 16,54.
Data analysis
In studies on the interaction between various Z. tritici isolates and host cultivars, disease severity was estimated using the percentage of leaf area with necrosis, pycnidia coverage, and their combinations16. The percentage data were transformed using the arcsine method and the generalized linear model was used to examine the normalized data in order to determine the source of variance (ANOVA) using SAS software version 9.4 (SAS Institute, Cary, NC, USA). The effects of isolate, wheat cultivar, and their two-way interactions, were considered to be fixed effects, and the block effect as random effect. Significant means were separated using the Tukey procedure at the α = 5% significance level55. Specific interactions between wheat genotypes and pathogen isolates were determined by computing the least significant differences (LSD) of means of wheat genotype-by-isolate interactions at α = 1% and 5% significance levels33,34. The interaction means values lower than the LSD values at α = 1% and 5% significant levels were considered as resistant and highly resistant genotypes, respectively. Mean disease severity values of the genotypes (differential lines)-by-isolate were subjected to a hierarchical cluster analysis. The analyses were performed using a hierarchical clustering method56, and a dissimilarity matrix was measured using Ward’s method implemented in JMP pro17 (SAS Institute).
Data availability
The data that support the study are in the article and supplementary materials. Sequence data has been deposited at the National Centre for Biotechnology Information (NCBI) under the accession SUB14926029: PQ755050 - PQ755210.
References
CSA. 2017/2018: I–Report on area and production of major crops (Private peasant holdings, Meher Season). Stat. Bull. 586, 53 (2018).
Latta, G. S., Sjølie, H. K. & Solberg, B. A review of recent developments and applications of partial equilibrium models of the forest sector. J. For. Econ. 19 (4), 350–360 (2013).
Eyob, B., Haregewoin, T. & Dejene, H. F. Daniel and belay baye, change and growth rate analysis in area, yield and production of wheat in Ethiopia. Int. J. Dev. Res. 4 (10), 1994–1995 (2014).
Dinsa, G. F. & Bogale, M. Potential yield and gap analysis (PYGA) of wheat in Ethiopia. AgriRxiv 2023, 20230515976 (2023).
Dadrasi, A. et al. Global insight into Understanding wheat yield and production through Agro-Ecological zoning. Sci. Rep. 13 (1) (2023).
Gemechu, T. & Tadese, F. Participatory evaluation and demonstration of bread wheat (Triticum aestivum L) varieties at Dugda and lume districts, oromia regional state, Ethiopia. Int. J. Res. Stud. Agricultural Sci. (IJRSAS). 4 (7), 26–30 (2018).
Senbeta, A. F. & Worku, W. Ethiopia’s wheat production pathways to self-sufficiency through land area expansion, irrigation advance, and yield gap closure. Heliyon 9 (10), e20720 (2023).
Bekele, E. A review of research on diseases of barley, tef and wheat in Ethiopia. In A Review of Crop Protection Research in Ethiopia, 79–107. (Institute of Agricultural Research (IAR), 1985).
Getaneh Woldeab, A. H., Endale, W. D. & Hailu Distribution of wheat stem rust (Puccinia graminis F. Sp. Tritici) in West and Southwest Shewa zones and identification of its phsiological races. Adv. Crop Sci. Technol. 03 (04) (2015).
Mann, M. L. & Warner, J. M. Ethiopian wheat yield and yield gap estimation: A spatially explicit small area integrated data approach. Field Crops Res. 201, 60–74 (2017).
Olivera, P. et al. Phenotypic and genotypic characterization of race TKTTF of puccinia Graminis f. Sp. tritici that caused a wheat stem rust epidemic in Southern Ethiopia in 2013-14. Phytopathology 105 (7), 917–928 (2015).
Mekonnen, T. et al. Molecular screening of zymoseptoria tritici resistance genes in wheat (Triticum aestivum L.) using tightly linked simple sequence repeat markers. Eur. J. Plant Pathol. 155 (2), 593–614 (2019).
Mekonnen, T. et al. Genetic diversity and population structure of zymoseptoria tritici in Ethiopia as revealed by microsatellite markers. Fungal Genet. Biol. 141, 103413 (2020).
Simón, M. R. et al. Population structure of Mycosphaerella graminicola and location of genes for resistance to the pathogen: Recent advances in Argentina. Int. J. Agron. 2012, 1–7. (2012).
Ponomarenko, A., Goodwin, S. & Kema, G. Septoria tritici blotch (STB). Plant. Health Inst. 10 (2011).
Eyal, Z. & Levy, E. Variations In pathogenicity patterns ofmycosphaerella graminicola withintriticum spp. In Israel. Euphytica 36 (1), 237–250 (1987).
Makhdoomi, A. et al. Efficacy of wheat genotypes and Stb resistance genes against Iranian isolates of zymoseptoria tritici. J. Gen. Plant Pathol. 81 (1), 5–14 (2014).
Adhikari, T. B. & Goodwin, A. J. Identification and molecular mapping of a gene in wheat conferring resistance toMycosphaerella graminicola. Phytopathology 93, 1158–1164 (2003).
Brown, J. K. et al. Genetics of resistance to zymoseptoria tritici and applications to wheat breeding. Fungal Genet. Biol. 79, 33–41 (2015).
Odilbekov, F. et al. GWAS-assisted genomic prediction to predict resistance to septoria tritici blotch in nordic winter wheat at seedling stage. Front. Genet. 10 (2019).
Tabib Ghaffary, S. M. et al. Genetic analysis of resistance to septoria tritici blotch in the French winter wheat cultivars balance and Apache. Theor. Appl. Genet. 123 (5), 741–754 (2011).
Saintenac, C. et al. Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen zymoseptoria tritici. Nat. Genet. 50 (3), 368–374 (2018).
Zhong, Z. et al. A small secreted protein in Zymoseptoria tritici is responsible for avirulence on wheat cultivars carrying the Stb6 resistance gene. New Phytol. 214 (2), 619–631 (2017).
McDonald, B. A. et al. The population genetics of septoria tritici (teleomorph mycosphaerella graminicola). Can. J. Bot. 73 (S1), 292–301 (1995).
Ye, X. et al. Genome-wide association study of resistance to Stripe rust (Puccinia striiformis f. Sp. tritici) in Sichuan wheat. BMC Plant Biol. 19 (1), 147 (2019).
Sidhu, G. S. & Webster, J. M. The genetics of plant-nematode parasitic systems. Bot. Rev. 47 (3), 387–419 (1981).
Czembor, P. C., Radecka-Janusik, M. & Mańkowski, D. R. Virulence spectrum of mycosphaerella graminicola isolates on wheat genotypes carrying known resistance genes to septoria tritici blotch. J. Phytopathol. 159, 146–154 (2011).
Mahboubi, M. et al. Resistance and virulence variability in wheat–Zymoseptoria tritici interactions. Crop Pasture Sci. 71 (7). (2020).
Kema, G. et al. Genetic variation for virulence and resistance in the wheat-Mycosphaerella graminicola pathosystem. I. Interactions between pathogen isolates and host cultivars. Phytopathology 86 (1996).
Ababa, G. & Mekonnen, T. Virulence variation and pathotypes of zymoseptoria tritici isolates causing wheat leaf blotch in oromia. Ethiopia Fungal Biology. 128 (7), 2167–2176 (2024).
Mekonnen, T. et al. Virulence variability of Ethiopian zymoseptoria tritici isolates and efficacy of wheat genotypes and Stb resistance genes against the isolates. Eur. J. Plant Pathol. 158 (4), 895–910 (2020).
Perello, A. et al. Variation in virulence of septoria tritici Rob ex Desm isolates on wheat. Agronomie 11 (7), 571–579 (1991).
Hosseinnezhad, A. et al. Effectiveness determination of wheat genotypes and Stb resistance genes against Iranian mycosphaerella graminicola isolates. Archives Phytopathol. Plant. Prot. 47 (17), 2051–2069 (2014).
Ghaneie, A. et al. Genetic variation for resistance to septoria tritici blotch in Iranian tetraploid wheat landraces. Eur. J. Plant Pathol. 132, 191–202 (2012).
Chartrain, L. et al. Genetics of resistance to septoria tritici blotch in the Portuguese wheat breeding line TE 9111. Theor. Appl. Genet. 110, 1138–1144 (2005).
Chartrain, L. et al. Sources of resistance to septoria tritici blotch and implications for wheat breeding. Plant. Pathol. 53 (4), 454–460 (2004).
Tidd, H. et al. A large bioassay identifies Stb resistance genes that provide broad resistance against septoria tritici blotch disease in the UK. Front. Plant. Sci. 13, 1070986 (2022).
Chartrain, L., Brading, P. A. & Brown, J. K. M. Presence of the Stb6 gene for resistance to septoria tritici blotch (Mycosphaerella graminicola) in cultivars used in wheat-breeding programmes worldwide. Plant. Pathol. 54 (2), 134–143 (2005).
Badebo, A. et al. Review of Two Decades of Research on Diseases of Small Cereal Crops. Increasing Crop Production through Improved Plant Protection, Vol. I, 375. (2006).
Takele, A. et al. Estimated Yield Loss Assessment of Bread Wheat (Triticum aestivum L.) due to Septoria Leaf Blotch Septariatritici (Roberge in Desmaz) on Wheat in Holeta Agricultural Research Center, West Shewa, Ethiopia. (2015).
Tadesse, Y. Survey of septoria tritici blotch (Septoria tritici) of bread wheat (Triticum aestivum L.) in the central highlands of Ethiopia. Am. J. Bioscience Bioeng. 6 (5), 36 (2018).
McDonald, B. A. & Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 40 (1), 349–379 (2002).
Cowger, C., Hoffer, M. & Mundt, C. Specific adaptation by mycosphaerella graminicola to a resistant wheat cultivar. Plant. Pathol. 49 (4), 445–451 (2000).
Deng, Y. et al. Molecular basis of disease resistance and perspectives on breeding strategies for resistance improvement in crops. Mol. Plant. 13 (10), 1402–1419 (2020).
Tucho, A. et al. Genetic Diversity of Zymoseptoria Tritici Populations in Central and South-eastern Ethiopia (Cold Spring Harbor Laboratory, 2024).
White, T. J. et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 18 (1), 315–322 (1990).
Adhikari, T. B., Wallwork, H. & Goodwin, S. B. Microsatellite markers linked to theStb2andStb3Genes for resistance to septoria tritici blotch in wheat. Crop Sci. 44 (4), 1403–1411 (2004b).
Adhikari, T. B., Dubcovsky, C. J., Gieco, J., Schlatter, J. O. & Goodwin A. R. Molecular mapping of the stb4 gene for resistance to septoria tritici blotch in wheat. Phytopathology 94 (2004).
Arraiano, L. S. et al. Chromosomal location of a gene for resistance to septoria tritici blotch (Mycosphaerella graminicola)in the hexaploid wheat ’synthetic 6x’. Theor. Appl. Genet. 103 (5), 758–764 (2001).
Brading, P. A. et al. A Gene-for-Gene relationship between wheat and mycosphaerella graminicola, the septoria tritici blotch pathogen. Phytopathology® 92 (4), 439–445 (2002).
McCartney, C. A. et al. Chromosomal location of a race-specific resistance gene to mycosphaerella graminicola in the spring wheat ST6. Theor. Appl. Genet. 107 (7), 1181–1186 (2003).
Chartrain, L. et al. Genetics of resistance to septoria tritici blotch in the Portuguese wheat breeding line TE 9111. Theor. Appl. Genet. 110 (6), 1138–1144 (2005).
Rosielle, A. A. Sources of resistance in wheat to speckled leaf blotch caused by septoria tritici. Euphytica 21 (1), 152–161 (1972).
Turgay, E. B. et al. Pathotype diversity of zymoseptoria tritici (Desm. Quaedvlieg & Crous) isolates collected from central Anatolia. Turk. J. Phytopathol. 170 (9), 588–597 (2022).
Wilson, J. et al. Pennisetum glaucum subsp. Monodii accessions with striga resistance in West Africa. Crop Prot. 23 (9), 865–870 (2004).
Tilahun, M. et al. Virulence variability of Ethiopian zymoseptoria tritici isolates and efficacy of wheat genotypes and Stb resistance genes against the isolates. Eur. J. Plant Pathol. 158 (4), 895–910 (2020).
Acknowledgements
The authors extend their heartfelt gratitude to the wheat-farming community in the study areas for their kind permission to assess their fields and sample STB-symptomatic wheat leaves. We are also deeply thankful to the National Agricultural Biotechnology Research Center (NABRIC) in Holeta, Ethiopia, for providing the laboratory space and facilities essential for this research. Our appreciation also goes to the Institute of Biotechnology at Addis Ababa University for their technical support throughout the study. Furthermore, we sincerely thank the Swedish University of Agricultural Sciences in Alnarp, Sweden, for supplying the laboratory space and chemicals necessary for the molecular study conducted by the first author.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
A.T. led the conceptualization, execution of experiments, data curation, software, formal data analysis, validation, initial drafting of the manuscript, writing review & editing the manuscript; F.G. assisted with the experiments and edited the manuscript; A.C. provided the key planting material used in the study and edited the manuscript; D.M. and T.A. contributed to editing the manuscript; R.R.V. provided the laboratory facilities and resources, supervised the work, and edited the manuscript; K.T. and T.M. supervised the overall project, provided guidance, planned the experiment, and took responsibility for the final draft and approval.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
We declare that this research does not involve any species that are endangered or at risk of extinction. All plant experiments were conducted according to relevant institutional, national and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tucho, A., Mekonnen, T., Tesfaye, K. et al. Efficacy of Stb resistance genes and pathotype diversity in Zymoseptoria tritici from Ethiopia. Sci Rep 15, 28030 (2025). https://doi.org/10.1038/s41598-025-13035-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13035-x