Abstract
Metal oxide nanoparticles (NPs) have attracted great attention for their potential to control pathogens. This study assessed the effects of silver oxide (Ag2O) NPs, titanium dioxide (TiO2) NPs, and their bulk counterparts on soybean development and the control of Meloidogyne javanica. Additionally, the effect of different doses of TiO2 NPs and bulk TiO2 was determined. Two greenhouse experiments were conducted, each comprising two trials (repetitions). In Experiment 1, seed treatment with Ag2O NPs had no effect on nematodes. By contrast, TiO2 NPs provided a mean reduction in nematode population density (number of nematodes per gram of root) of 52.97% in both trials, whereas their bulk counterpart achieved a 36.33% reduction. Soybean development was not influenced by treatment. In Experiment 2, five doses of bulk TiO2 and TiO2 NPs were tested (0, 125, 250, 500, and 1000 mg L−1). No effect was observed for particle size, but dose effects were significant. In Trial 1, seed treatment led to a linear reduction in nematode number. In Trial 2, a maximum reduction of 69.90% was estimated to be achieved with 581.21 mg L−1 TiO2. Treatment positively influenced shoot height and dry weight in at least one of the trials, with the maximum values estimated to be achieved with doses of 545 and 533.33 mg L−1, respectively. It is concluded that Ag2O NPs have no effect on M. javanica in soybean, whereas adequate doses of TiO2 NPs contribute to parasite control and soybean growth.
Similar content being viewed by others
Introduction
Metal oxide nanoparticles (NPs) have attracted great attention in agriculture for their potential as nanofertilizers and nanopesticides1,2. Metal-based fertilizers and pesticides are not commonly available in NP form, being subject to leaching, precipitation induced by soil components, and volatilization3. Given these losses, large amounts of product need to be applied to achieve effective plant fertilization and pathogen control. NP formulations are promising solutions to enhance fertilizer effectiveness and ensure targeted delivery of active ingredients to plant pests. Thus, it becomes possible to maintain or even improve crop yields with lower application rates, while minimizing the negative impacts of agriculture on ecosystems and human health4.
Some metal oxides, such as silver oxide (Ag2O) and titanium dioxide (TiO2), are well-known for their antimicrobial properties, with studies showing their effectiveness against bacteria, fungi, and nematodes5,6,7. An in vitro study reported that Ag2O NPs at doses of 200, 400, and 800 mg mL−1 caused 100% mortality of Meloidogyne incognita after 24 h8. A similar result was observed for Meloidogyne graminicola after 12 h of exposure to 0.3 µg mL−1 Ag2O NPs9. In the same study, the authors observed that lower doses (0.1 µg mL−1) of NPs applied via seed treatment resulted in a significant reduction in nematode numbers. Of note, most studies addressing the effects of NPs on plant-parasitic nematodes have focused on Ag2O and M. incognita8,10,11. Less attention has been paid to TiO2 NPs and other nematode species.
TiO2 NPs represent a new generation of nanomaterials with photocatalytic and antimicrobial properties12. In a recent study, the parental generation (P0) and offspring (F1) of the model organism Caenorhabditis elegans were exposed to various concentrations of TiO2 NPs13. The results showed that TiO2 NPs impaired reproduction, survival, and growth in P0 and exerted even more pronounced toxic effects in F1. Additionally, the authors found that parental exposure to TiO2 NPs induced toxicity in unexposed progeny.
Members of the genus Meloidogyne have a broad range of hosts and are among the most important species of plant-parasitic nematodes worldwide. Meloidogyne javanica and M. incognita are notably damaging in tropical regions14. In line with the search for more sustainable management methods, organic antimicrobial agents have gained momentum in recent years. Nevertheless, these nematodes continue to pose a significant challenge due to their rapid reproduction and high potential for damage15. Therefore, there is a need for alternative methods that enable effective control of plant-parasitic nematodes, such as metal oxides, which offer greater stability compared to organic antimicrobial agents16.
The solution to successfully applying metal oxides for pathogen control and yield enhancement may lie in the difference in particle size between bulk and nano formulations. An advantageous characteristic of NPs is their larger surface area. However, to date, few studies have compared plant exposure to NPs and their bulk counterparts. In view of the foregoing, this study aimed to compare the effect of Ag2O and TiO2 NPs and their bulk forms and determine the optimal dose of TiO2 for controlling M. javanica and promoting soybean development.
Materials and methods
Synthesis and characterization of Ag2O and TiO2 NPs
Ag2O NPs (≤ 20 nm) and TiO2 NPs (≤ 50 nm) used in this work were synthesized and characterized as reported in detail by Almeida Junior et al.17,18. Ag2O and TiO2 NPs were synthesized by coprecipitation and sol–gel methods, respectively. Their bulk counterparts were purchased from Merck Chemicals Ltd. (Merck KGaA, Darmstadt, Germany).
General experimental procedures
Two experiments were conducted in a greenhouse (23° 47′ 28.4′′ S 53° 15′ 24.0′′ W, 379 m a.s.l.) according to a completely randomized design. Experiment 1 (Trials 1 and 2) was performed to determine the effect of seed treatment with Ag2O and TiO2 NPs and their bulk forms on M. javanica reproduction and soybean development. Experiment 2 (Trials 1 and 2) was performed to investigate the effect of different doses of TiO2 NPs compared with their bulk counterpart.
NP and bulk suspensions were prepared at the desired concentrations in distilled water and sonicated for 1 min using an ultrasonic homogenizer (Bandelin Electronic GmbH & Co., Berlin, Germany). Shortly after, suspensions were applied to seeds of soybean M6410 IPRO (Bela sementes, Tamarana, Brazil) at a dose of 600 mL 100 kg−1 seed.
Experimental units consisted of polystyrene cups containing 0.5 L of a 1:1 (v/v) mixture of clay soil and sand, previously autoclaved at 120 °C. Each experimental unit (cup) was planted with a seed according to the respective treatment.
The M. javanica inoculum was obtained from a pure population maintained on tomato (Solanum lycopersicum) ‘Santa Clara’ in a greenhouse. Species identification was confirmed by perineal pattern analysis. Nematode extraction followed the method proposed by Hussey and Barker19 as adapted by Boneti and Ferraz20. The inoculum suspension was counted in a Peters chamber under an optical microscope (Motic B1-252) and adjusted to 1000 eggs and eventual second-stage juveniles (J2) of M. javanica mL−1.
Experiment 1: M. javanica reproduction and soybean development after seed treatment with Ag2O and TiO2 NPs and their bulk counterparts
Trial 1 of Experiment 1 was conducted between October and December 2022, with mean minimum, average, and maximum temperatures of 16.9, 22.9, and 26.6 °C, respectively. Trial 2 lasted from November 2022 to January 2023, with mean minimum, average, and maximum temperatures of 18.7, 23.5, and 26.9 °C, respectively. Five treatments were tested: Ag2O NPs, bulk Ag2O, TiO2 NPs, bulk TiO2, and untreated control. The metal oxides were applied via seed treatment at a fixed dose of 500 mg L−1. Each treatment comprised eight replications.
At 7 days after sowing, a pipette was used to inoculate seedlings with 2 mL of a suspension containing 2000 eggs and eventual J2 of M. javanica. The nematode inoculum was deposited in two 2 cm deep holes made in the soil close to the base of the plant. Plants were maintained in a greenhouse. Manual irrigation was performed daily.
At 60 days after inoculation, the plants were removed from the pots and separated into shoots and roots. The root system was thoroughly washed, placed on paper towels to remove excess water, and weighed on a semi-analytical scale to obtain the root fresh weight (g). Then, roots were subjected to nematode extraction according to the previously mentioned method. The total number of nematodes was determined using a Peters chamber and an optical microscope. This variable was divided by the root fresh weight to obtain the population density (number of nematodes per gram of root).
Shoots were evaluated for height (cm), measured using a ruler. For determination of shoot dry weight, shoots were placed in paper bags, dried in a forced-air oven at 65 °C until constant weight was reached (72 h), and weighed on a semi-analytical scale.
Experiment 2: effects of TiO2 NP and bulk TiO2 doses on M. javanica reproduction
Trial 1 of Experiment 2 was carried out between February and April 2023, with mean minimum, average, and maximum temperatures of 20.3, 23.7, and 27.3 °C, respectively. Trial 2 lasted from March to May 2023, with mean minimum, average, and maximum temperatures of 18.4, 22.9, and 26.5 °C, respectively. Treatments followed a 2 × 5 factorial design, where the first factor was TiO2 source (NPs and bulk oxide) and the second factor was dose (0, 125, 250, 500, and 1000 mg L−1). Eight replications were included per treatment. The experiment was conducted and analyzed as described for Experiment 1.
Statistical analysis
Data were evaluated for normality and homogeneity of variance by the Shapiro–Wilk and Levene tests, respectively. One-way analysis of variance (ANOVA) was used to examine treatment effects on nematode and vegetative variables (p < 0.05). Two-way factorial ANOVA was performed to assess the effects of TiO2 sources, doses, and their interaction on nematode and vegetative variables (p < 0.05). In Experiment 1, differences between treatments were compared using the Scott–Knott test (p < 0.05). In Experiment 2, linear and quadratic regression models were used to examine the effect of TiO2 doses within each source, and Tukey’s test was used to assess the effect of sources within each dose, (p < 0.05). Analyses were performed using SISVAR software21.
Results
Experiment 1: M. javanica reproduction and soybean development after seed treatment with Ag2O and TiO2 NPs and their bulk counterparts
In Trials 1 and 2 of Experiment 1, treatment had a significant effect on nematode variables (Table 1). Ag2O treatments did not improve nematode control in Trial 1. In Trial 2, bulk Ag2O reduced nematode number and population density by 54.46% and 48.83%, respectively, compared with the control. Unlike Ag2O, TiO2 controlled M. javanica in both trials. TiO2 NP treatment decreased nematode number by 54.97% and 42.09% in Trials 1 and 2, respectively. Bulk TiO2 treatment reduced nematode number by 45.57% and 30.69% in Trials 1 and 2, respectively. For population density, the reduction was 59.93% and 45.81% for TiO2 NPs and 42.65% and 29.87% for bulk TiO2 in Trials 1 and 2, respectively. No significant differences were observed between TiO2 NPs and bulk TiO2.
Treatments did not influence soybean development (Table 2). The mean plant height ranged from 36.75 to 39.11 cm in Trial 1 and from 44.75 to 50.08 cm in Trial 2. The range of shoot dry weight was 2.00 to 2.36 g in Trial 1 and 4.25 to 5.30 g in Trial 2. Root fresh weight ranged from 9.00 to 10.56 g and from 20.63 to 25.60 g in Trials 1 and 2, respectively.
In Experiment 1, it was observed that TiO2 NPs provided superior performance in nematode control compared with Ag2O NPs. Therefore, the former was chosen for dose–response analyses in Experiment 2.
Experiment 2: effects of TiO2 NP and bulk TiO2 doses on M. javanica reproduction
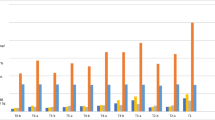
The interaction effects of factors (TiO2 source and dose) on nematode number were not significant in Experiment 2, Trial 1 (Table S1, Supplementary Material). However, the variable was influenced by the main effect of TiO2 dose. M. javanica number decreased with increasing TiO2 dose (Fig. 1a). In Trial 2, the interaction effect on nematode number was significant (Table S2, Supplementary Material). The relationship between factors was explained by a quadratic model, and the maximum reduction in nematode number (48.10%) was estimated to be achieved by using 665.52 mg L−1 bulk TiO2 (Fig. 1b) The regression model did not fit well to the data for TiO2 NPs. In Trial 2, TiO2 NPs afforded a lower nematode number than the bulk treatment at a dose of 125 mg L−1. TiO2 sources did not differ from each other at the other doses.
There were no significant interaction effects on nematode population density, but the variable was influenced by the main effect of dose in both trials (Tables S1 and S2, Supplementary Material). In Trial 1, nematode population density was explained by a linear model. Thus, the higher the TiO2 doses, the lower the population density (Fig. 1c). In Trial 2, the variable was explained by a quadratic model. Maximum control efficiency (69.90%) was estimated to be achieved using 581.21 mg L−1 TiO2 (Fig. 1d).
Regarding soybean development, the interaction effect of factors on vegetative parameters was not significant (Tables S1 and S2, Supplementary Material). However, plant height was influenced by the main effect of dose in Trial 1. Maximum gains in plant height were observed at a dose of 545 mg L−1, representing a 48.40% increase compared with the control (Fig. 2a). Plant height, by contrast, was not influenced by the main effects in Trial 2; the means of treatments ranged from 21.71 to 26.45 cm (data not shown). In Trial 1, factors did not exert significant main effects on shoot dry weight, with means of 1.44 to 1.69 g (data not shown). In Trial 2, shoot dry weight was influenced by the main effect of dose. The highest shoot dry weight was estimated to be achieved using 533.33 mg L−1, representing an increase of 62.33% compared with the control (Fig. 2b). No main effects were exerted on root fresh weight in either trial, with means ranging from 2.69 to 3.61 g in Trial 1 and from 3.81 to 5.56 g in Trial 2 (data not shown).
Discussion
Ag2O NPs did not exert nematicidal effects against M. javanica in soybean. In a study by Taha and Abo-Shady22, only high concentrations of Ag2O NPs were toxic to entomopathogenic nematodes. The toxic effect of Ag2O is due to the binding of Ag ions to cysteine-containing proteins in plasma membrane, affecting membrane integrity23. The penetration of Ag ions into the cytoplasm inactivates respiratory enzymes and induces the formation of reactive oxygen species (ROS)24. The generated ROS then induce oxidative stress in nematode cells25,26, ultimately leading to cell death. However, this mechanism did not affect M. javanica when Ag2O was used in the form of NPs. This result is likely due to other factors influencing the effectiveness of NPs against microorganisms, such as target species, type of NP, concentration, exposure time, and soil physical and chemical properties27,28,29,30.
Further studies are needed to evaluate the performance of improved NP formulations. For instance, surface modifications, such as coating with biocompatible materials, may enable the controlled release of active substances. These strategies can help modulate toxicity and efficacy while reducing exposure to non-target organisms and mitigating adverse environmental impacts31,32.
TiO2 applied as seed treatment was effective in controlling M. javanica, regardless of particle size; however, TiO2 NPs exhibited higher efficacy at lower doses (125 mg L−1). This enhanced performance may be attributed to the small size, increased surface area, and higher chemical reactivity of NPs compared to their bulk counterpart33. These properties improve interactions with cellular structures, facilitate penetration into target organisms, and stimulate ROS generation, which may contribute to the observed nematicidal effects32.
In a previous study, electron microscope images showed that TiO2 NPs at 200 mg L−1 caused damage to the cuticle of M. incognita J25. One of the hypotheses for this result was that NPs affected glycogen, lipids, and mucopolysaccharides, leading to changes in membrane permeability and function. Both bulk and NP forms of TiO2 were found to accumulate in the intestine of C. elegans following ingestion, as evidenced by light microscopy34. In the cited study, the impact on nematode reproduction was dose-dependent, with greater effects observed at higher doses. In vitro experiments showed that 0.02% (v/v soil) TiO2 NPs provided 100% control of M. incognita reproduction on tomato8. Overall, the literature suggests that TiO2 can exert positive effects on nematode control in a dose-dependent manner.
Here, it was found that M. javanica populations decreased linearly with increasing doses of TiO2 in Trial 1 of Experiment 2. In Trial 2, nematode population density was estimated to decrease by 69.90% with the application of 581.21 mg L−1 TiO2, with a reduction in control at higher doses. This behavior may be correlated with the toxicity of TiO2 toward soybean, owing to the overproduction of ROS35. ROS-mediated toxicity causes DNA damage and micronucleus formation35,36.
In addition to the direct toxic effect generated by ROS, plants activate defense mechanisms to mitigate the damage caused by these molecules. These mechanisms include enzymatic antioxidants such as superoxide dismutase, peroxidases, and catalases, which play a crucial role in scavenging activated oxygen within plant cells37,38. The enzymes also contribute to plant defense against nematodes by preventing the lipid peroxidation of cell membranes39,40.
The findings of the current study suggest that the effect of TiO2 on soybean development is dose-dependent, not being influenced by particle size. Seed treatment using 545 mg L−1 TiO2, regardless of particle size, may increase soybean plant height by 48.40% compared with the control. Similarly, 530 mg L−1 TiO2 can increase shoot dry weight by 62.33%. Such findings agree with those of previous studies. For instance, TiO2 NPs promoted an increase in shoot dry weight and chlorophyll content in eggplant (Solanum melongena)5, as well as in root and shoot length, phosphorus uptake, and chlorophyll content in wheat (Triticum aestivum), when applied at doses of 20 to 60 mg kg−1 soil35. The authors found that wheat does not tolerate concentrations higher than 60 mg kg−1, owing to the hyperproduction of ROS, such as H2O2, and micronucleus formation. Of note, the cited studies did not compare TiO2 NPs with their bulk counterpart.
Overall, Ag2O NPs did not exhibit nematicidal activity against M. javanica under the conditions evaluated in this study. In contrast, TiO2 nanoparticles significantly suppressed nematode reproduction and promoted vegetative growth in soybean. The positive effects of TiO2 NPs were dose-dependent, emphasizing the importance of identifying an optimal concentration that maximizes nematode control while avoiding phytotoxic effects. A dose of about 540 mg L−1 proved to be both effective and safe, resulting in up to 46.25% reduction in total nematode number (Experiment 2, Trial 1) and 69.75% reduction in population density (Experiment 2, Trial 2), with no adverse effects on plant development.
These findings underscore the potential of TiO2 NPs as a promising tool for integrated nematode management. Their high efficacy at low doses compared with conventional materials aligns with the principles of sustainable agriculture by minimizing the input of active substances into the environment. However, the use of NPs in agriculture raises important concerns regarding their persistence in soil and water, potential effects on non-target organisms, and risk of bioaccumulation. Thus, long-term studies, particularly under field conditions, are necessary to assess their ecotoxicological safety.
Advancing the use of NP-based nematicides in a sustainable and responsible manner will require strong interdisciplinary collaboration. Nanotechnology experts are vital for improving particle synthesis and functionalization, aiming to enhance stability and specificity. Toxicologists play a critical role in assessing potential risks to human health and non-target species. Soil scientists contribute to understanding the fate and mobility of NPs in agroecosystems, while agricultural engineers are key to optimizing application technologies. Additionally, methods such as ICP-MS and SEM-EDX, applied in collaboration with plant scientists, are needed to clarify NP uptake and localization in plant tissues and root zones, thereby supporting the mechanistic basis of nematode suppression. Collectively, these multidisciplinary efforts are crucial for ensuring that nanotechnological innovations are effectively and safely integrated into modern crop protection strategies.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Parisi, C., Vigani, M. & Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today. 10 (2), 124–127. https://doi.org/10.1016/j.nantod.2014.09.009 (2015).
Usman, M. et al. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 721, 1–16. https://doi.org/10.1016/j.scitotenv.2020.137778 (2020).
Servin, A. et al. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 17, 1–21. https://doi.org/10.1007/s11051-015-2907-7 (2015).
Kah, M., Kookana, R. S., Gogos, A. & Bucheli, T. D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 13 (8), 677–684. https://doi.org/10.1038/s41565-018-0131-1 (2018).
Khan, M. et al. Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita. Nanotechnol. Rev. 11 (1), 1606–1619. https://doi.org/10.1515/ntrev-2022-0097 (2022).
Khan, M., Khan, A. U., Bogdanchikova, N. & Garibo, D. Antibacterial and antifungal studies of biosynthesized silver nanoparticles against plant parasitic nematode Meloidogyne incognita, plant pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 26 (9), 2462. https://doi.org/10.3390/molecules26092462 (2021).
Nassar, A. M. K. Effectiveness of silver nano-particles of extracts of Urtica urens (Urticaceae) against root-knot nematode Meloidogyne incognita. Asian J. Nematol. 5, 14–19 (2016).
Ardakani, A. S. Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology 15 (6), 671–677. https://doi.org/10.1163/15685411-00002710 (2013).
Baronia, R., Kumar, P., Singh, S. P. & Walia, R. K. Silver nanoparticles as a potential nematicide against Meloidogyne graminocola. J. Nematol. 52 (1), 1–9. https://doi.org/10.21307/jofnem-2020-002 (2020).
El-Deen, A. H. N. & El-Deeb, B. A. Effectiveness of silver nanoparticles against root-knot nematode, Meloidogyne incognita infecting tomato under greenhouse condition. J. Agric. Sci. 10 (2), 148–156. https://doi.org/10.5539/jas.v10n2p1487 (2018).
Taha, E. H. Nematicidal effects of silver nanoparticles on root-knot nematodes (Meloidogyne incognita) in laboratory and screenhouse. J. Plant. Prot. Pathol. 7 (5), 333–337. https://doi.org/10.21608/jppp.2016.50566 (2016).
Silva, S., Dias, M. C. & Silva, A. M. Titanium and zinc based nanomaterials in agriculture: A promising approach to deal with (a)biotic stresses? Toxics 10 (4), 172. https://doi.org/10.3390/toxics10040172 (2022).
Yen, H., Huang, C. W., Wu, C. H. & Liao, V. H. C. Life cycle exposure to titanium dioxide nanoparticles (TiO2-NPs) induces filial toxicity and population decline in the nematode Caenorhabditis elegans. Environ. Sci. Pollut Res. 31, 31467–31478. https://doi.org/10.1007/s11356-024-33159-3 (2024).
Goode, K. & Mitchum, M. G. Pattern-triggered immunity against root-knot nematode infection: A minireview. Physiol. Plant. 174, 1–9. https://doi.org/10.1111/ppl.13680 (2022).
Machado, A. C. Bionematicides in Brazil: An emerging and challenging market. Rev. Anual Patol. Plantas. 28, 35–49. https://doi.org/10.31976/0104-038321v270002 (2022).
Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods. 54 (2), 177–182. https://doi.org/10.1016/S0167-7012(03)00037-X (2003).
de Almeida Junior, J. H. V. et al. Synthesis of silver and cobalt nanoparticles and assessment of their effects on germination and biometric parameters in maize (Zea mays L). Ecotoxicol. Environ. Saf. 287, 117257. https://doi.org/10.1016/j.ecoenv.2024.117257 (2024).
de Almeida Junior, J. H. V. et al. Synthesis, characterization and application of silicon and titanium nanoparticles to enhance the early development of maize (Zea mays L). Arch. Agron. Soil. Sci. 70 (1), 1–21. https://doi.org/10.1080/03650340.2024.2423649 (2024).
Hussey, R. S. & Barker, K. R. A. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant. Dis. Rep. 57, 1025–1028 (1973).
Boneti, J. I. S. & Ferraz, S. Modificação do Método de Hussey & Barker Para Extração de Ovos de Meloidogyne exigua de Raízes de Cafeeiro. Fitopatol Bras. 6, 553 (1981).
Ferreira, D. F. Sisvar: A computer statistical analysis system. Cienc. Agrotec. 38, 109–112 (2014). TEM DOI.
Taha, E. H. & Abo-Shady, N. M. Effect of silver nanoparticles on the mortality, pathogenicity, and reproductivity of entomopathogenic nematodes. Int. J. Zool. Res. 12 (3–4), 47–50. https://doi.org/10.3923/ijzr.2016.47.50 (2016).
Ocsoy, I. et al. Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano. 7 (10), 8972–8980. https://doi.org/10.1021/nn4034794 (2013).
Prabhu, S. & Poulose, E. K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2, 1–10. https://doi.org/10.1186/2228-5326-2-32 (2012).
Lim, D. et al. Oxidative stress-related PMK‐1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 31 (3), 585–592. https://doi.org/10.1002/etc.1706 (2012).
Roh, J. Y., Eom, H. J. & Choi, J. Involvement of Caenorhabditis elegans MAPK signaling pathways in oxidative stress response induced by silver nanoparticles exposure. Toxicol. Res. 28, 19–24. https://doi.org/10.5487/TR.2012.28.1.019 (2012).
Arumugam, V., Bhat, A. H., Savarirayan, I. K. S., Ataya, F. S. & Fouad, D. Root-knot nematode suppression through biogenic silver nanoparticles: A promising path for sustainable agriculture. J. Nanopart. Res. 26, 249. https://doi.org/10.1007/s11051-024-06160-7 (2024).
Dzięgielewska, M. et al. Effects of silver, gold, and platinum nanoparticles on selected nematode trophic groups. J. Hortic. Res. 31 (2), 23–34. https://doi.org/10.2478/johr-2023-0035 (2023).
Grün, A. L. et al. Impact of silver nanoparticles (AgNP) on soil microbial community depending on functionalization, concentration, exposure time, and soil texture. Environ. Sci. Eur. 31 (15), 1–22. https://doi.org/10.1186/s12302-019-0196-y (2019).
Rahmatpour, S., Shirvani, M., Mosaddeghi, M. R., Nourbakhsh, F. & Bazarganipour, M. Dose-response effects of silver nanoparticles and silver nitrate on microbial and enzyme activities in calcareous soils. Geoderma 285, 313–322. https://doi.org/10.1016/j.geoderma.2016.10.006 (2017).
Kora, A. J. Copper-based nanopesticides. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems (ed Abd-Elsalam, K. A.) 133–153 https://doi.org/10.1016/B978-0-12-823833-2.00019-2 (Elsevier, 2022).
Rodriguez-Seijo, A., Santas-Miguel, V., Arenas-Lago, D., Arias-Estevez, M. & Perez-Rodriguez, P. Use of nanotechnology for safe agriculture and food production: Challenges and limitations. Pedosphere 35, 20–32. https://doi.org/10.1016/j.pedsph.2024.09.005 (2025).
Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 15, 15–22. https://doi.org/10.1007/s10311-016-0600-4 (2017).
Ma, H., Lenz, K. A., Gao, X., Li, S. & Wallis, L. K. Comparative toxicity of a food additive TiO2, a bulk TiO2, and a nano-sized P25 to a model organism Caenorhabditis elegans. Environ. Sci. Pollut Res. 26, 3556–3568. https://doi.org/10.1007/s11356-018-3810-4 (2019).
Rafique, R. et al. Dose-dependent physiological responses of Triticum aestivum L. to soil applied tio₂ nanoparticles: Alterations in chlorophyll content, H2O2 production, and genotoxicity. Agric. Ecosyst. Environ. 255, 95–101. https://doi.org/10.1016/j.agee.2017.12.010 (2018).
Shahid, M. et al. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. In: Reviews of Environmental Contamination and Toxicology (ed Whitacre, D.) Volume 232, 1–44. https://doi.org/10.1007/978-3-319-06746-9_1 (Springer, 2014).
Kuniak, E. & Sklodowska, M. Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant. Sci. 160, 723–731. https://doi.org/10.1016/S0168-9452(00)00457-X (2001).
Pnueli, L., Liang, H., Rozenberg, M. & Mittler, R. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant. J. 34 (2), 187–203. https://doi.org/10.1046/j.1365-313X.2003.01715.x (2003).
Sharma, I. P. & Sharma, A. K. Physiological and biochemical changes in tomato cultivar PT-3 with dual inoculation of mycorrhiza and PGPR against root-knot nematode. Symbiosis 71, 175–183. https://doi.org/10.1007/s13199-016-0423-x (2017).
Singh, H. B., Singh, B. N., Singh, S. P. & Nautiyal, C. S. Solid-state cultivation of Trichoderma Harzianum NBRI-1055 for modulating natural antioxidants in soybean seed matrix. Bioresour Technol. 101, 6444–6453. https://doi.org/10.1016/j.biortech.2010.03.057 (2010).
Acknowledgements
The authors thank the Brazilian National Council for Scientific and Technological Development (CNPq) for the doctoral fellowship awarded to MTRS (grant No. 141287/2021-7), as well as for the research and productivity grants awarded to CRDA (grant No. 303269/2020-0) and MAB (grants Nos. 131.641/2021-2 and 312.764/2023-5). We are also grateful to the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for the doctoral fellowship awarded to other authors.
Author information
Authors and Affiliations
Contributions
Conceptualization, MTRS and CRDA; Data curation, MTRS and CRDA; Formal analysis, MTRS, ETS, LZA, JHVAJ, MAB and CRDA; Funding acquisition, CRDA; Investigation, MTRS; Methodology, MTRS and CRDA; Project administration, CRDA; Supervision, CRDA; Writing—original draft MTRS; Writing—review and editing, MTRS, and CRDA. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Human and animal participants
This article does not contain any studies with human and/or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
e Silva, M.T.R., Sonda, E.T., Alves, L.Z. et al. Comparison between the effects of silver and titanium nanoparticles and their bulk counterparts on Meloidogyne Javanica. Sci Rep 15, 31279 (2025). https://doi.org/10.1038/s41598-025-13162-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13162-5