Abstract
Schistosomiasis is earmarked for elimination as a public health problem, while morbidity control is a priority for soil-transmitted helminthiasis (STH) by 2030. To develop targeted interventions for the two diseases, micro-mapping and an understanding of disease transmission dynamics across different age groups are necessary. This study determined the age-stratified and ward-level prevalence of schistosomiasis and STH in Chevakadzi ward, Shamva district, Mashonaland Central province, Zimbabwe. A community-wide cross-sectional pilot study was conducted in June and July 2023 in all the 14 villages in Chevakadzi ward, targeting 1680 participants aged ≥ 1 year. Thirty households per village were selected using simple random selection method. Urine and stool samples were collected to diagnose urinary, intestinal schistosomiasis and STH using the urine filtration and Kato Katz techniques respectively. Snail surveys were conducted at identified water contact sites in each village. Intermediate host snails were assessed for human schistosome cercaria. The data were analysed using descriptive statistics, spatial autocorrelation and logistic regression. The overall ward-level prevalence of combined schistosomiasis and STH was 15.6% (95%CI 13.7–17.7) and 2.5%, (95% CI 1.7–3.7) respectively. Schistosoma mansoni prevalence was 13.3% (95% CI 11.4–15.6) while S. haematobium was 6.8% (95% CI 5.5–8.3). Adults had the highest prevalence of both forms of schistosomiasis with an overall of, 20.4% (95%CI 17.6–23.5) compared to school-aged children (SAC) [10.5%, 95%CI 7.7–14.0] and preschool-aged children (PSAC) [4.7%, 95%CI 2.1–10.1], p < 0.001. Combined schistosomiasis and single species prevalence significantly differed among villages and age groups (p < 0.05), while no significant difference was observed in the intensity of infections. The risk of infection was highest in adults (AOR: 5.1, 95%CI 2.5–14.1) and 2.5 times higher in SAC (AOR: 2.5, 95%CI 1.0–6.1) compared to PSAC. Odds of infections were high in Gonyora (AOR: 2.1, 95% CI 1.0–4.2) and Peter (AOR: 2.6, 95%CI 1.3–5.2) villages. Schistosomiasis infection was clustered at the household level (Moran’s I = 0.0573, p < 0.001). None of the snails collected in the water contact sites were infected. The study has shown that schistosomiasis is focalised at a micro-geographical scale with spatial clustering among households in the study ward. Heterogenous infection rates were noted among age groups, with adults being at high risk, emphasising the need for universal coverage of interventions as opposed to the current school-based mass drug administrations (MDA). Targeted, inclusive, and micro-level-centred approaches to effectively combat schistosomiasis are necessary. Despite the low level of STH infections in the ward, interventions are necessary to interrupt their transmission.
Similar content being viewed by others
Introduction
Schistosomiasis and soil-transmitted helminthiasis (STH) are neglected tropical diseases (NTDs). They are poverty-related diseases predominantly distributed in tropical and subtropical areas of Africa, Asia, the Caribbean and South America where they impose a devastating human, social and economic burden to the most vulnerable, marginalized populations1,2,3. Human schistosomiasis is acquired when the parasitic cercariae penetrates the skin of people exposed to infested freshwater. The use of open water sources has been identified as the main predisposing factor of infection4,5,6,7,8. Soil-transmitted helminths are found in soil in areas where sanitation is poor and infection occurs through ingestion of food and fruits contaminated with STH ova and for hookworms primarily through walking barefooted in contaminated soil9.
In 2022, 897.9 million children, 108 million adolescent girls and 138.8 lactating and pregnant women required treatment for STH. The number of people requiring schistosomiasis preventive chemotherapy increased by 11.2 million from 2021 to 2022 in 50 endemic countries10. According to WHO, the increase in treatment needs for schistosomiasis is attributed to population growth in endemic areas, a limited number of impact assessment surveys conducted, delayed implementation of treatment strategies after surveys, and the persistent use of district-level instead of lower administrative units as implementation units2. Like other endemic countries, Zimbabwe has mapped the distribution of schistosomiasis and STH at the national level, providing prevalence estimates at the district level11. The mapping followed a design recommended by WHO which involved selecting a sample of five schools and a minimum of fifty children per school for parasitological surveys in each implementation unit/ district12. This strategy has been used for determining treatment strategies at the district level in different endemic countries11,13. However, notable among different studies conducted in Zimbabwe and other endemic countries is the heterogeneous distribution of schistosomiasis and STH among sites or at sub-district levels8,11,13,14,15,16. Thus, many endemic foci might have been previously mis-classified following the strategy of selecting five schools to represent a district subsequently leading to over-treatment in some areas or under-treatment or no treatment in areas in need of treatment.
Differences in prevalence of schistosomiasis have been noted among different age groups in the same area6,14,17,18,19,20,21, and population age groups which are not included in the school-based mass drug administrations have been shown to harbour infection6,14,19,22. Thus, the control strategy of targeting SAC only leaving out the younger and older population may leave a significant reservoir of infection, and infection-associated morbidity6,14,22,23,24. Among the critical actions for the elimination of schistosomiasis as a public health problem are extending preventive chemotherapy to all populations in need, and micro-mapping of the disease for targeted interventions including targeted snail control and preventive chemotherapy25. Thus, the exclusion of other population age groups from treatment, coupled with the mis-classification of sites for intervention may hinder the achievement of national, regional and global elimination targets.
Zimbabwe is endemic to both forms of schistosomiasis; urogenital schistosomiasis caused by Schistosoma haematobium and intestinal schistosomiasis caused by Schistosoma mansoni11,26. Schistosoma haematobium is transmitted by Bulinus globosus while Schistosoma mansoni is transmitted by Biomphalaria pfeifferi27,28,29. The country is also endemic to STHs. However, the two NTDs have varying transmission levels in the districts and at schools within the same district while schistosomiasis is more prevalent than STH11. From 2012 to 2023, the annual mass drug administration (MDA) in Zimbabwe was based on district-level prevalence. The MDA was implemented in school-aged children, excluding preschool-aged children and the adult population. Thus, to enhance schistosomiasis and STH control efforts in Zimbabwe and shift the focus from morbidity control toward interruption of transmission, there is a need to provide fine-grained geographical level and age-specific evidence on the distribution of the disease to inform targeted interventions. This study aimed to determine the age-stratified and village/community level prevalence of schistosomiasis and STH among residents of Chevakadzi ward in Shamva district, Mashonaland Central Province. Shamva district is considered to be one of the highly endemic districts for schistosomiasis in Zimbabwe, based on the 2010 national survey11. The results will assist in refining control measures and complementing preventive chemotherapy with specific information about the dynamics of infection at the community level and among age groups which could be used to enhance tailor-made interventions.
Methods
Study area and design
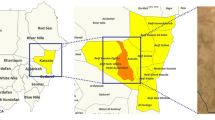
This was a cross-sectional pilot study, conducted in June and July 2023 to test the feasibility of conducting a large-scale China- Zimbabwe schistosomiasis control program planned to be implemented in Shamva and Mt Darwin districts in the Mashonaland Central Province. Shamva district was selected for the pilot study because it is one of the districts with high endemicity for schistosomiasis in Zimbabwe and is endemic to both urogenital and intestinal schistosomiasis11. The district has an average yearly temperature of 24.6 °C (range: 15.79—29.27 °C,) and it receives approximately 126.17 mm of rainfall annually. It is located at an elevation of 952.92 m above sea level30. Chevakadzi ward was purposively selected among the twenty-nine wards in Shamva based on the vast water bodies running through it. Figure 1 shows the location of Chevakadzi ward in the Shamva district. The ward has 14 villages, with an estimated population size of 8248 residents31, in an area covering approximately 315 km2. The residents are primarily of the Shona tribe, and most are subsistence farmers. The ward population is served by one rural clinic.
Study population and sample size
The study targeted all ward residents aged ≥ 1 year for screening for schistosomiasis. The household sample size (n) was calculated using the following equation for standard error (SE):
where σ2 is the between-village, τ2 is the between-household within-village components of variance in prevalence, m is the number of villages which is 14 for Shamva, and k is the number of participants that can be sampled per household. In this study, we assumed that m = 14, k = 4, τ = 0.01 over a range of values of n and σ gives the different SE values32.
Table 1 shows that the more important determinant of precision is σ; increasing n shows diminishing returns beyond n = 30. It is therefore recommended to randomly sample an average of 30 households (HHs) per village (cluster). Thus, based on the above argument, the sample size being the number of households, n, for Chevakadzi ward = number of villages in the ward x number of HHs selected per village = 14 villages × 30 HHs per village = 420 households.
On average, a household in Zimbabwe has 4 people33. Thus, the total number of participants expected to be sampled in this study was 14 villages × 30 HHs/village × 4 persons/household = 1680 participants.
Household sampling and participant recruitment
Households included in the pilot study were selected by simple random sampling. Initially, the Environmental Health Technician (EHT) was requested to list all HHs in Chevakadzi ward by village. For each of the 14 villages, the HH names were then written on pieces of paper, and thoroughly mixed in a hat per village, after which the EHT was asked to pick one HH per time until 30 HHs per village were selected. The EHT sensitized the selected HHs through the village health workers and they were informed in advance about the dates when the research team would visit them. The research team was accompanied by the village health worker to the selected HHs for recruitment and sample collection.
Data and sample collection
Before the commencement of field sampling, all data collection teams were trained. At each participating household, all available members aged ≥ 1 year were recruited into the study. Demographic data such as age, gender, village name and HH location coordinates were collected for each participant. The HH locations were mapped using a geographical positioning system (GPS) device. Following this, participants were requested to provide approximately 50 ml of urine and 1 g of stool into separate clean and dry 50 ml screw-capped specimen bottles. The samples were collected between 10:00 AM and 2:00PM, a period when peak egg excretion is expected34. At HHs with preschool-aged children, their caregivers were provided with urine bags (Hollister 7511 U-Bag Urine Specimen Collector, Hollister Inc., Chicago, IL, USA) and pampers labelled with the child’s identification number and instructed on how to collect the samples from the children. The collected samples were then transported to the local laboratory for processing and examination for Schistosoma eggs. Specimens were collected for a single day only.
Intermediate host snail collection and infection testing
In each village, water contact sites were identified with the assistance of the EHT. The identified water contact sites were mapped using a GPS device. Other information collected per site were the water contact activity and the description of the site substratum. Snail collection was performed as described previously32. At each site, two trained snail collectors performed snail collection using long-handled, flat-mesh scoops. A maximum of 100 scoops were performed at each site, focusing along the edges and central parts of the water body, vegetation, sticks, decaying leaves and discarded plastics. If the water contact site was rocky, snails were handpicked for 30 min. To standardize the sampling, the same collectors were responsible for scooping in all identified water contact sites. The snails were placed in trays, wrapped with cotton wool soaked with water from the collection site and placed in site-labelled plastic bags. The snails were transported to the laboratory, where intermediate host snails for schistosomes were morphologically identified as previously described35, and screened for schistosome infection. To check for patent infection, the snails were individually placed in 10 ml flat–bottomed glass tubes with dechlorinated water and exposed to artificial light for a maximum time of 4 h. After 3 h of exposure snails were checked after every 30 min for the emergence of cercariae28. Cercariae were identified based on morphology under a dissecting microscope as described by Frandsen & Christensen36. After undergoing the shedding process, the snails were further checked for sporocysts infection by placing each snail between two thick, clean slides and applying pressure to the slides to crush the snail. The snail was then de-shelled before examination of the soft tissue for cercariae and /or sporocysts under the dissecting microscope.
Laboratory analysis of urine and stool samples
Urine samples were processed using the urine filtration technique as described previously37, to check for S. haematobium eggs. From the single stool sample collected per participant, two thick smear slides were prepared for the diagnosis of S. mansoni and soil-transmitted helminths using the Kato Katz technique as described previously38. The slides were examined for hookworm ova within 1 h of preparation before the ova hatch. The slides were then left to clear for at least 24 h after which they were examined for S. mansoni and other STH ova.
Data analysis
The data were double-entered into SPSS version 27, cleaned, and exported to Stata version 15 (Stata Statistical Software 2017; Stata Corp, College Station, TX). Descriptive statistics were used to calculate the proportion of participants with S. haematobium, S. mansoni, combined schistosomiasis and STHs. The proportion of infected snails was also calculated using descriptive statistics. All inferential analyses were conducted at a 95% confidence level (95% CI) and 5% significance level (p < 0.05). Chi-square or Fisher’s exact tests were used to compare proportions/categorical variables (sex, age group, and village). Logistic regression analysis was performed to identify significant predictors of schistosomiasis and STH infection, with univariate models assessing crude associations and multivariate models identifying independent risk factors after adjusting for potential confounders. The variables assessed included the demographic factors such as age, sex and village of origin. Age was categorized into the following three age groups, PSAC (< 5 years), SAC (5–14 years) and adults (≥ 15 yrs). S. haematobium egg intensity was expressed as the number of eggs per 10 ml of urine. For urine samples, which were less than 10 ml, the volumes were measured before filtration and the egg count was adjusted proportionally. For S. mansoni and STH eggs, arithmetic mean egg counts were calculated from the counts of two Kato Katz slide smears and multiplied by 24 to obtain the number of eggs per gram of faeces per individual. The intensity of infection was classified using WHO criteria39 as low, moderate or heavy for S. mansoni, 1–99 eggs per gram of faeces (epg), 100–399 epg and ≥ 400 epg, respectively; for hookworm, 1–1999, 2000–3999 and ≥ 4000 epg, respectively; for T. trichiura, 1–999, 1000–9999 and ≥ 10,000, respectively; for A. lumbricoides, 1–4999, 5000–49,999 and ≥ 50,000, respectively and for S. haematobium: light infection (< 50 eggs/10 ml of urine) and heavy infection (≥ 50 eggs/10 ml of urine). The geometric mean egg count for schistosomes was estimated based on infected participants only, overall, by gender and age group. A GPS was employed to map spatial patterns of infections among the households and the presence of intermediate host snails among the water contact sites. Maps were generated using ArcGIS Pro version 3.0. The Moran’s Index statistic was used to compute spatial autocorrelation among the households. The results were visualized on Moran scatter plots.
Ethical approval and consent to participate
Before the commencement of the study, the research team obtained approval from the Ministry of Health and Child Care through the Permanent Secretary, provincial and district directorate. The study was conducted in accordance with the Declaration of Helsinki. Ethical clearance was provided by the National Ethical Review Board, Medical Research Council of Zimbabwe (MRCZ/A/3049). Approval was also sought from the community leaders who assisted in the mobilization and sensitization of the community in the local language. Household members willing to participate were requested to sign informed consent forms or assent forms. For children between the ages of 7–18 years, parental informed consent was sought first. For children aged 1 to 7 years, only parental informed consent was sought. Participation was entirely voluntary and only participants with signed informed consent and or assent forms were enrolled into the study. All participants who tested positive were treated following standard guidelines for treatment of schistosomiasis and STH.
Consent for publication
Participants agreed to share their anonymous data through publication during the consent form signing.
Results
Study participant demographics
Overall, 1387/1680 (82.6%) participants were recruited into the study from all 14 villages in the ward. The total number of households sampled was 397/420 (94.5%). Amongst the participants, 665 (48.0%) were males and 722 (52.1%) were females (Fig. 2). The age ranged from 1 to 99 years [mean (M) = 24.8, standard deviation (SD) = 19.9].
Of the 1387 recruited participants, 1239 (89.3%) submitted at least one sample of either urine or stool for the diagnosis of schistosomiasis. The 1239 participants included 573 (46.3%) males and 666 (53.7%) females (Fig. 2). The median age of all the participants was 20 years [Interquartile range (IQR) = 9–39 years]. Males had a median age of 15 years (IQR = 8–39 years) while females’ median age was 24 years (IQR = 9–39 years).
Prevalence and distribution of combined Schistosomiasis
The overall prevalence of combined schistosomiasis in Chevakadzi ward was 15.6% (193/1239), (95% CI 13.7–17.7) (Table 2). The distribution of schistosomiasis among the households is shown in Fig. 3. Of the 397 households sampled, 147 (37.03%) had one or more individuals infected with either S. mansoni, S. haematobium or both while 27 (6.8%) households had one or more individuals coinfected with intestinal and urinary schistosomiasis. Out of the 193 schistosomiasis-positive participants, 24 (12.4%) had co-infection with S. mansoni and S. haematobium. Among the villages schistosomiasis prevalence was significantly different (χ2 = 70.35, p < 0.001). Peters village recorded the highest prevalence of 30.8% (95% CI 22.5–40.4), closely followed by Gonyora; 30.0% (95% CI 20.8–41.1) while Chevakadzi 1 village registered the lowest prevalence; 3.4% (95% CI 0.8–13.0). Schistosomiasis infection was also significantly different among the age groups (χ2 = 31.69, p < 0.001), with adults exhibiting the highest prevalence at 20.4% (95% CI 17.6–23.5) (Table 2).
Spatial autocorrelation analysis on infection across the households in the ward was performed. When all households tested were included, a Moran’s I of 0.0564 (p < 0.001) was yielded. Four data collection points were identified as outliers (Fig. 4). When the outliers were excluded, Moran’s I slightly changed to 0.0573 (p < 0.001), showing that despite the influence of outliers on auto-correlation magnitude, there is spatial clustering of schistosomiasis infection at household level in the ward (Fig. 5).
Prevalence and distribution of Schistosoma mansoni
Among the 397 households, 111 (27.99%) had one or more individuals infected with S. mansoni. Out of the 1387 recruited participants, 1027(70.0%) provided stool samples. Of these 137 (13.3%) (95% CI 11.4–15.6) had S. mansoni infection. Schistosoma mansoni prevalence did not differ by gender; females, (14.2%) versus males (12.4%), p = 0.394 (Table 3). However, prevalence significantly differed among age groups (χ2 = 32.41, p < 0.001) and was highest among the adults; (18.6%) while PSAC had the lowest prevalence rate (6.2%). Similarly, prevalence differed significantly among the 14 villages (χ2 = 77.05, p < 0.001), with the highest prevalence being recorded in Peter village; 29.0% (95% CI 20.8–38.8) and the lowest in Chevakadzi 1; 2.1% (95% CI 0.3–14.5).
Schistosoma mansoni infection intensity according to WHO classification
There were 99(72.3%) light, 33 (24.1%) moderate and 5(3.7%) heavy infections among the S. mansoni positive participants. There were no significant differences in the intensity of infections by gender (p = 0.208), age group (p = 0.464) and village (p = 0.283). Only light infections were recorded in Chiororo (n = 3), Pindukai (n = 3) and Chevakadzi 1 (n = 1) villages (Table 4).
Prevalence and distribution of S. haematobium
Of the 397 households, 63 (15.87%) had one or more individuals infected with urinary schistosomiasis. Overall, 1186/1387 (85.3%) participants submitted urine samples. Of these 6.8% (80/1186), (95% CI 5.5–8.3) had S. haematobium infection (Table 5). Prevalence was not significantly different between females, (7.3%) and males, (6.1%), p = 0.391. However, it was significantly different between age groups (χ2 = 9.12, p = 0.010), with the highest prevalence recorded among adults; 7.8%, followed by SAC (6.8%) while the PSAC had 0%. S. haematobium prevalence was also significantly different among the villages (χ2 = 48.90, p < 0.001), with highest value in Gonyora; (17.7%, 95% CI 10.7–28.0), while Chevakadzi 1 village had the lowest (1.8%, 95% CI 0.2–12.5).
Schistosoma haematobium infection intensity according to WHO classification
Among the positive participants, 70(87.5%) and 10 (12.5%) had light and heavy infections respectively (Table 6). All the heavy infections observed were in adults and the intensity of infections among the villages was not significantly different (χ2 = 15.96, p = 0.251).
Geometric mean egg counts of S. mansoni and S. haematobium infection
The overall geometric mean number of S. mansoni eggs per gram (epg) of faeces was 46.4 epg 95%CI 38.2–56.4, with no difference between males and females [39.4 epg (95%: 29.7–52.3)] versus 52.6 epg (95%CI 40.2–68.9) (Table 7). The overall geometric mean number of S. haematobium eggs/10 ml of urine was 8.5 eggs/10 ml (95%CI 6.0–12.0) with no difference between males and females [7.8 eggs/10 ml (95%CI 4.7–12.9) versus 9.0 eggs/10 ml (95%CI 5.5–14.7) (Table 7).
Distribution of water contact sites and intermediate host snails
A total of 35 water contact sites were identified for the villagers in the ward. The highest number of water contact sites were identified in Chidumbwe village 1 (8 sites) and Gonyora village (7 sites). Most villages had one water contact site only. Out of 834 intermediate host snails collected, 342 (41.0%) were Bulinus species [317 (92.7%) B. globosus and 25 (7.3%) B. truncatus] whilst 492 (59.0%) were Biomphalaria pfeifferi (Table 8). Out of the 35 water contact sites, 14 (40%) had no intermediate host snails. Sporocyts or cercariae were not detected in any of the intermediate host snails collected.
Soil-transmitted helminths infection
Out of 397 sampled households, 24 (6.05%) had one or more individuals with STH infections (Fig. 6). The ward-level prevalence of STHs was 2.5% (95% CI 1.7–3.7). Of the STH infections, 2.1% (95% CI 1.4–3.2) were of Ascaris lumbricoides and 0.4% (95% CI 0.2–1.0) were hookworms. Prevalence was not significantly different between genders and among age groups but was significantly different among the villages (χ2 = 41.73, P value < 0.001), with the highest prevalence in Gonyora 7(11.7%). Four villages, namely, Chiororo, Peter, Ben, and Chevakadzi 1 did not record any STH infection (Table 9).
Risk factor analysis of schistosomiasis and STH infection
Univariate analysis showed that combined schistosomiasis infection was associated with adults and being a resident of Gonyora and Peter villages. After controlling for confounders, multivariate analysis showed that the risk of infection was 6 times higher in adults (AOR: 5.1, 95% CI 2.5–14.1) while that of SAC was 2.5 times higher (AOR: 2.5, 95% CI 1.0–6.1) compared to that of PSAC. Gonyora and Peter village residents were at high odds of being infected; (AOR: 2.1, 95% CI 1.0–4.2) and (AOR: 2.6, 95% CI 1.3–5.2) respectively. Chidumbwe 3, Pindukai and Chevakadzi 1 residents were less likely to be infected with schistosomiasis (Table 10).
Both Univariate and multivariate analyses showed that the risk of STH infection was high in the Gonyora village. Chibondo village had borderline risk of infection (AOR: 9.5, 95% CI 1.01–89.8). Age group and gender did not emerge as predictors of STH infection in both univariate and multivariate models (Table 10).
Discussion
This study reports the ward-level distribution of schistosomiasis and STHs in Chevakadzi, Shamva district, Mashonaland Central Province, Zimbabwe. Both forms of schistosomiasis (S. haematobium and S. mansoni) occurred in the ward with an overall prevalence of 15.6% but with varying levels of transmission, among the villages and age groups. In line with WHO recommendations, annual treatment with a single dose of praziquantel may be necessary for the ward population from the age of 2 years to control morbidity and advance towards elimination of schistosomiasis as a public health problem1. Soil-transmitted helminthiasis was also observed although with scanty distribution among the population. The heterogeneity in disease distribution is probably due to a combination of environmental factors such as availability and proximity to water bodies, socioeconomic factors and behavioural factors40,41.
The overall prevalence observed confirms previous findings showing that the Shamva district is endemic for schistosomiasis11. Of note is that our current study included all household members at a selected household while the national study sampling was conducted in schools selected across the district. However, the district-level prevalence of schistosomiasis recorded previously during a national survey of ≥ 50% substantially surpasses the ward-level prevalence of schistosomiasis recorded in this study. Point prevalence among schools within the same district in the national survey were different11, confirming that disease distribution is heterogeneous at micro-geographical scale level in Zimbabwe. Focal distribution of schistosomiasis is known to be affected by water contact behaviour, transmission heterogeneity, socio-demographic characteristics and water sanitation and hygiene coverage14,40,41. As shown in our study, heterogeneity can be at village level. Villages fall in different categories of WHO recommended interventions. Gonyora and Peter village residents are at high risk of infection while others like Pindukai and Chevakadzi 1 and Chidumbwe 3 village residents were less likely to get schistosomiasis infection. This focality of transmission at a micro-geographical scale in the same district has been confirmed in our previous study14 and in Côte d’Ivoire42. This highlights the importance of micro-planning to target interventions based on knowledge of transmission dynamics. Significant clustering of infections was observed among the households as shown by spatial autocorrelation analysis. This suggests that infected individuals are not randomly distributed but adjoining household individuals are likely to share similar infection status. Spatial clusters of schistosomiasis at the household level have also been observed in other settings43,44, supporting the known focal nature of schistosomiasis. The World Health Organisation encourages community mapping of schistosomiasis to implement targeted treatment to at risk populations and reduce the needs of praziquantel1. Therefore, for the elimination of schistosomiasis, the next step in Zimbabwe and elsewhere should be determining disease epidemiology in micro-geographical settings to inform and optimize targeted interventions, focusing resources on areas with higher endemicity for improved impact of interventions on disease burden.
Schistosomiasis prevalence, overall and single species infection was highest in adults compared to SAC and PSAC with the PSAC showing the lowest level of infection. For both S. haematobium and S. mansoni, heavy infections were only recorded in the adults. The increase in prevalence with an increase in age proves that schistosomiasis infection is a function of the duration of exposure45. Adults have had or have more water contact exposure and, thus are likely to have a higher prevalence compared to PSAC. In rural settings like Chevakadzi ward, adults engage in domestic and occupational activities such as gardening, laundry, bathing, fishing and farming, which increase their direct contact with water. If there are no protected water sources or if they prefer to use unprotected water source, chances of being infected are high from potentially infested water bodies. This behaviour is different in PSAC who are limited in their mobility and they depend on their caregivers for water contact activities. This may result in limited exposure with infested water bodies. We have previously reported a similar trend between caregivers and their PSAC in another area in the same district6. These findings also corroborate observations made in Zanzibar17. Preventive chemotherapy for schistosomiasis in Zimbabwe has been school-based, mainly focusing on SAC, leaving out the adult population and PSAC in line with the WHO strategic plan 2012–202025. Thus, a lower prevalence in the SAC compared to adults, while SAC has been previously regarded as the heavily burdened population age group in the community46 is not surprising in this scenario. The heavy infections in adults are probably a result of repeated infections without treatment in contrast to SAC who have been receiving treatment yearly since 2012 except for disruptions from 2018 to 2021. On the other hand, despite being few, the infections observed in PSAC are an indicator of early exposure to contaminated water bodies when they accompany their mothers/ caregivers for different water contact activities in open-infested water sources6,17,18. Thus, to achieve universal health coverage for schistosomiasis and hence elimination of this NTD as a public health problem by 2030, equity for treatment access should be promoted by ensuring that treatment strategies are expanded beyond school-based programs. The results observed also add to evidence that besides SAC, adults and PSAC are also a significant reservoir of infection and can contribute to ongoing transmission within endemic communities6,17,18,22. Comprehensive community-wide interventions targeting all age groups are necessary to break schistosomiasis transmission, consequently leading to disease elimination.
S. mansoni was more prevalent in the ward compared to S. haematobium. The distribution of schistosome species is dependent on the availability and abundance of their intermediate host snails, B. globosus and B. pfeifferi, respectively29. Despite that none of the intermediate host snails collected were infected, there were more B. pfeifferi snails than B. globosus snails in the ward. The survey was conducted during the cold season in Zimbabwe, thus, the absence of patent infection is expected due to low temperatures47. However, assessment infections in intermediate hosts provide insights into transmission dynamics of schistosomiasis. While cercarial shedding provide information on patent infection which shows active transmission of the disease, it cannot detect pre-patent infections48,49, which can extend for many weeks in field snails50. On the other hand snail crushing and searching for sporocyst is also limited by delayed development of sporocysts during the cold periods51, leading to more pre-patent than patent infection over time48. Thus, to understand the transmission status of water contact sites in endemic areas, it is paramount to use more sensitive methods like molecular techniques such as the polymerase chain reaction (PCR) method or loop mediated isothermal amplification (LAMP) method48,49,52. Schistosomiasis infection was noted in all villages, including those villages where the intermediate host snails were not observed. Notably schistosomiasis prevalence was highest in Peter village, but no intermediate host snails were identified during the snail survey. The absence of intermediate host snails in the water contact sites at the time of the survey could be due to fluctuations in environmental factors affecting the snail life cycle and reproduction rates leading to temporary absence. The survey was conducted during the cold season from June–July 2023 in Zimbabwe. Seasonal variability in intermediate host snail distribution has been noted previously29,53.
In addition to schistosomiasis, there were also STH infections in the ward. Despite that the STH infections were low, their presence show that the community is at risk of further spread of the disease. Farming, drinking contaminated water, and poor hygiene and sanitation expose individuals to infection15. Thus, to avoid future surges in infection, there is a need for clean water supplies, sanitation, hygiene, and public health education that aims to change the community risk behaviour.
Whilst our study has provided important insights into the epidemiology of schistosomiasis in the Chevakadzi ward, the generalizability of the results is limited to the specific population, thus further studies in other wards are necessary to allow extrapolation of the results in a broader context. Due to day to day variability in helminths egg excretion34, the collection and analysis of one stool and urine sample per participant may have resulted in underestimating the true prevalence of these helminths infections, thus it is a limitation of our study. Another limitation of our study was that it was a cross-sectional study conducted in winter, yet some studies have noted significant seasonal variations in the prevalence of patent infections in snails29,53. Thus, future research should aim to conduct seasonal snail studies to understand the dynamics of schistosomiasis transmission in the ward. Furthermore, our study was limited because we only conducted cercarial shedding and snail crushing which are not ideal for early pre-patency identification. In addition, we did not measure other water-related parameters affecting water quality for parasite viability such as pH and turbidity.
Conclusion
The study showed substantial variation in the prevalence of schistosomiasis among different villages within a single ward, with significant clustering of infection across households, and the highest prevalence among adults. To address the observed varying endemicity, there is a need for targeting and tailoring intervention strategies with considerations of inclusive treatment to reduce parasite burden and prevent reinfections. This, contributes to sustainable disease control and health equity while highlighting the importance of micro-planning, to understand localized schistosomiasis transmission dynamics which will inform resource allocation for reducing disease burden and improving health outcomes.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- EHT:
-
Environmental Health Technician
- HH:
-
Households
- GPS:
-
Geographical positioning system
- NTDs:
-
Neglected tropical diseases
- PSAC:
-
Preschool-aged children
- SAC:
-
School-aged children
- STH:
-
Soil-transmitted helminthiasis
- WHO:
-
World Health Organization
References
World Health Organization. WHO Guideline on Control and Elimination of Human Schistosomiasis. World Health Organization (2022).
World Health Organization. Global Report on Neglected Tropical Diseases 2024. World Health Organization (2024).
Steinmann, P., Keiser, J., Bos, R., Tanner, M. & Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6, 411–425 (2006).
Mwai, J., Omogi, J. O. & Abdi, M. H. Environmental factors influencing prevention and control of schistosomiasis infection in Mwea, Kirinyaga County Kenya: A cross sectional study. East Afr. Health Res. J. 5, 99 (2021).
Nyati-Jokomo, Z. & Chimbari, M. J. Risk factors for schistosomiasis transmission among school children in Gwanda district Zimbabwe. Acta Trop. 175, 84–90 (2017).
Mutsaka-Makuvaza, M. J. et al. Urogenital schistosomiasis and risk factors of infection in mothers and preschool children in an endemic district in Zimbabwe. Parasites Vectors 12, 1–5 (2019).
Angora, E. K. et al. Prevalence and risk factors for schistosomiasis among schoolchildren in two settings of Côte d’Ivoire. Trop. Med. Infect. Dis. 4, 110 (2019).
Cha, S. et al. Epidemiological findings and policy implications from the nationwide schistosomiasis and intestinal helminthiasis survey in Sudan. Parasites Vectors 12, 1–4 (2019).
World Health Organization. Soil-Transmitted Helminth Infections. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections. Accessed 24 Feb 2025.
World Health Organization. Schistosomiasis and soil-transmitted helminthiases: Progress report 2023. Wkly. Epidemiol. Rec. 99, 707–717 (2024).
Midzi, N. et al. Distribution of schistosomiasis and soil transmitted helminthiasis in Zimbabwe: Towards a national plan of action for control and elimination. PLoS Negl. Trop. Dis. 8, e3014 (2014).
World Health Organization. Guide for Mapping Neglected Tropical Diseases Targeted by Preventive Chemotherapy in the African Region. World Health Organization-Regional Office for Africa (2014).
Rujeni, N., Morona, D., Ruberanziza, E. & Mazigo, H. D. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: An update on their epidemiology and control. Infect. Dis. Poverty 6, 1–1 (2017).
Mutsaka-Makuvaza, M. J. et al. Reinfection of urogenital schistosomiasis in pre-school children in a highly endemic district in Northern Zimbabwe: A 12 months compliance study. Infect. Dis. Poverty 7, 1–6 (2018).
Mazigo, H. D. et al. Precision and geographical prevalence mapping of schistosomiasis and soil-transmitted helminthiasis among school-aged children in selected districts of north-western Tanzania. Parasites Vectors 15, 492 (2022).
Afifi, A., Ahmed, A. A., Sulieman, Y. & Pengsakul, T. Epidemiology of schistosomiasis among villagers of the New Halfa agricultural scheme Sudan. Iran J. Parasitol. 11, 110 (2016).
Sousa-Figueiredo, J. C. et al. A parasitological survey, in rural Zanzibar, of pre-school children and their mothers for urinary schistosomiasis, soil-transmitted helminthiases and malaria, with observations on the prevalence of anaemia. Ann. Trop. Med. Parasitol. 102, 679–692 (2008).
Betson, M. et al. Intestinal schistosomiasis in mothers and young children in Uganda: Investigation of field-applicable markers of bowel morbidity. Am. J. Trop. Med. Hyg. 83, 1048 (2010).
El-Khoby, T. et al. The epidemiology of schistosomiasis in Egypt: Summary findings in nine governorates. Am. J. Trop. Med. Hyg. 62, 88–99 (2000).
Exum, N. G. et al. The prevalence of schistosomiasis in Uganda: A nationally representative population estimate to inform control programs and water and sanitation interventions. PLoS Negl. Trop. Dis. 13, e0007617 (2019).
Knopp, S. et al. Praziquantel coverage in schools and communities targeted for the elimination of urogenital schistosomiasis in Zanzibar: A cross-sectional survey. Parasites Vectors 9, 1–4 (2016).
Njenga, S. M. et al. Adult population as potential reservoir of NTD infections in rural villages of Kwale district, Coastal Kenya: Implications for preventive chemotherapy interventions policy. Parasites Vectors 4, 1–6 (2011).
Toor, J. et al. Are we on our way to achieving the 2020 goals for schistosomiasis morbidity control using current World Health Organization guidelines?. Clin. Infect. Dis. 66, S245–S252 (2018).
Anderson, R. M. et al. What is required in terms of mass drug administration to interrupt the transmission of schistosome parasites in regions of endemic infection?. Parasites Vectors 8, 1–1 (2015).
World Health Organization. Schistosomiasis: Progress Report 2001–2011, strategic plan 2012–2020. https://iris.who.int/handle/10665/78074 (2013).
Taylor, P. & Makura, O. Prevalence and distribution of schistosomiasis in Zimbabwe. Ann. Trop. Med. Parasitol. 79, 287–299 (1985).
Mutsaka-Makuvaza, M. J. et al. Genetic diversity of Biomphalaria pfeifferi, the intermediate host of Schistosoma mansoni in Shamva district, Zimbabwe: Role on intestinal schistosomiasis transmission. Mol. Biol. Rep. 47, 4975–4987 (2020).
Mutsaka-Makuvaza, M. J. et al. Molecular diversity of Bulinus species in Madziwa area, Shamva district in Zimbabwe: Implications for urogenital schistosomiasis transmission. Parasites Vectors 13, 1–3 (2020).
Woolhouse, M. E. & Chandiwana, S. Spatial and temporal heterogeneity in the population dynamics of Bulinus globosus and Biomphalaria pfeifferi and in the epidemiology of their infection with schistosomes. Parasitology 98, 21–34 (1989).
Weather and Climate. The Global Historical Weather and Climate Data. https://weatherandclimate.com/zimbabwe/mashonaland-central/shamva (2024).
Zimbabwe 2022 Population and Housing Census Report, vol. 2. https://zimbabwe-geoportal-asc.hub.arcgis.com/documents/asc::zimbabwe-population-and-housing-census-2022-volume-2-by-district-and-ward/about (2022).
Qian, Y. et al. Understanding the feasibility to implement schistosomiasis elimination project under China-Zimbabwe cooperation: A pilot study protocol. J. Epidemiol. Glob. Health 15, 75 (2025).
Zimbabwe 2022 Population and Housing Census Report, Vol. 1. https://zimbabwe-geoportal-asc.hub.arcgis.com/documents/asc::zimbabwe-population-and-housing-census-2022-volume-1/about (2022).
Doehring, E., Feldmeier, H. & Daffalla, A. A. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann. Trop. Med. Parasitol. 77, 587–594 (1983).
Brown, D. S. Freshwater Snails of Africa and Their Medical Importance (CRC Press, London, 1994).
Frandsen, F. & Christensen, N. Ø. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 41, 181–202 (1984).
Mott, K. E., Baltes, R., Bambagha, J. & Baldassini, B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Trop. Med. Parasitol. 33, 227–228 (1982).
Katz, N., Chaves, A. & Pellegrino, J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo 14, 397–400 (1972).
WHO Expert Committee on the Control of Schistosomiasis. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis: Report of a WHO Expert Committee (2002).
Zerdo, Z. et al. Prevalence, intensity and endemicity of intestinal schistosomiasis and soil-transmitted helminthiasis and its associated factors among school-aged children in Southern Ethiopia. Sci. Rep. 12, 4833. https://doi.org/10.1038/s41598-022-08333-7 (2022).
Bartlett, A. W. et al. Burden and factors associated with schistosomiasis and soil-transmitted helminth infections among school-age children in Huambo, Uige and Zaire provinces. Angola. Infect. Dis. Poverty 11, 74. https://doi.org/10.1186/s40249-022-00975-z (2022).
N’goran, E. K. et al. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Côte d’Ivoire. Trop. Med. Int. Health 6, 817–825 (2001).
Peng, W. X. et al. Identifying high-risk areas of schistosomiasis and associated risk factors in the Poyang Lake region China. Parasitology 137, 1099–1107. https://doi.org/10.1017/S003118200999206X (2010).
Zhou, Y. B. et al. Spatial-temporal variations of Schistosoma japonicum distribution after an integrated national control strategy: A cohort in a marshland area of China. BMC Public Health 13, 297. https://doi.org/10.1186/1471-2458-13-297 (2013).
Dalton, P. R. & Pole, D. Water-contact patterns in relation to Schistosoma haematobium infection. Bull. World Health Organ. 56, 417–426 (1978).
Montresor, A. Helminth Control in School-age Children: A Guide for Managers of Control Programmes (World Health Organization, 2011).
Woolhouse, M. E. & Chandiwana, S. K. Temporal patterns in the epidemiology of schistosome infections of snails: A model for field data. Parasitology 100, 247–253. https://doi.org/10.1017/S0031182000061254 (1990).
Hamburger, J. et al. Large-scale, polymerase chain reaction-based surveillance of Schistosoma haematobium DNA in snails from transmission sites in coastal Kenya: A new tool for studying the dynamics of snail infection. Am. J. Trop. Med. Hyg. 71, 765–773 (2004).
Joof, E. et al. Comparing PCR techniques against conventional cercarial shedding methods for detecting Schistosoma mansoni infection in Biomphalaria snails. Acta Trop. 212, 105716. https://doi.org/10.1016/j.actatropica.2020.105716 (2020).
Joubert, P. H., Pretorius, S. J. & Kruger, F. J. Further studies on the susceptibility of Bulinus africanus to infection with Schistosoma haematobium. Ann. Trop. Med. Parasitol. 85, 253–258 (1991).
Shiff, C. J., Evans, A., Yiannakis, C. & Eardley, M. Seasonal influence on the production of Schistosoma haematobium and S. mansoni cercariae in Rhodesia. Int. J. Parasitol. 5, 119–123 (1975).
Qin, Z. Q. et al. Field evaluation of a loop-mediated isothermal amplification (LAMP) platform for the detection of Schistosoma japonicum infection in Oncomelania hupensis snails. Trop. Med. Infect. Dis. 3, 124. https://doi.org/10.3390/tropicalmed3040124 (2018).
Manyangadze, T., Chimbari, M. J., Rubaba, O., Soko, W. & Mukaratirwa, S. Spatial and seasonal distribution of Bulinus globosus and Biomphalaria pfeifferi in Ingwavuma, uMkhanyakude district, KwaZulu-Natal, South Africa: implications for schistosomiasis transmission at micro-geographical scale. Parasites Vectors 14, 482. https://doi.org/10.1186/s13071-021-04720-7 (2021).
Acknowledgements
We highly appreciate the support from the Ministry of Health and Child Care, Zimbabwe and the National Health Commission of the Peoples’ Republic of China. The authors are grateful to the people of Chevakadzi ward for attending to the study requirements. We are grateful to the Provincial and District Medical Directorate and community leaders for support in sensitization and mobilization of participants. We also appreciate the technical team from National Institute of Health Research, Shamva District Hospital, Madziwa Health Centre, Chevakadzi Clinic for fieldwork support.
Funding
The study was funded by the National Health Commission of the People’s Republic of China (No. 2020-C4-0001-2) to foster the China-Zimbabwe Cooperation Project for Schistosomiasis elimination, and the Research on the Distribution of Major Infectious Diseases and Comprehensive Prevention and Control Technologies in Key Areas of Africa (No. 2024YFC2310902). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
NM, XNZ, SZL, MJMM, YJQ conceptualized the study and wrote the proposal. NM, MJMM, YJQ, ZQQ, HML, LT, YXL, CLL, WS, TM trained the data collectors. NM, MJMM, Y JQ, TM, WS, ZQQ, HML, LT, YXL, CLL, SL collected the data. NM, MJMM, TM, analysed the data, NM, MJMM drafted the manuscript. All the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Midzi, N., Mutsaka-Makuvaza, M.J., Lv, S. et al. Village and age based precision mapping of schistosomiasis and soil-transmitted helminths in Chevakadzi ward of Shamva district in Zimbabwe. Sci Rep 15, 28136 (2025). https://doi.org/10.1038/s41598-025-13202-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13202-0