Abstract
Enhancing treatment outcomes for drug-resistant tuberculosis is a major global priority for tuberculosis control programs. India has the highest number of Multidrug-resistant Tuberculosis cases worldwide, yet no longitudinal studies have assessed the factors affecting treatment outcomes in public sector conditions. This study aimed to evaluate factors associated with ineffective treatment outcomes in patients with Multidrug-resistant Tuberculosis receiving outpatient care under the National Tuberculosis Elimination Programme in Puducherry, India, from January 2020 to December 2023. We employed multivariate regression methods to calculate odds ratios with 95% confidence intervals to identify factors linked to unsuccessful treatment outcomes. Clinical data from patients with Multidrug-resistant Tuberculosis revealed an overall treatment success rate of 60.42%. The findings showed that patients undergoing retreatment were more likely to experience unsuccessful outcomes. Additionally, co-infection with HIV, as well as the use of alcohol or tobacco, increased the odds of treatment failure. Patients with heteroresistant patterns had 2.72 times higher odds of unsuccessful treatment outcomes compared to those with inferred and true-resistant patterns. Furthermore, patients living in rural areas typically experienced worse treatment outcomes than those in urban areas, with higher rates of loss to follow-up. Patients on longer treatment regimens were also more likely to be lost to follow-up compared to those on shorter regimens. Notably, true resistance due to rpoB gene mutations accounted for 65.9% (29 out of 44) of total deaths, with mutations at codon S450L contributing to 47.7% of these fatalities, a finding that has not been reported elsewhere. The study highlighted a strong association between heteroresistance in the rpoB gene and poor treatment outcomes. These results emphasize the need for detailed molecular-level studies to improve treatment outcomes by ensuring appropriate drug selection for MDR/RR Tuberculosis. Additionally, further research is necessary to determine the impact of heteroresistance on treatment outcomes in individual patients.
Similar content being viewed by others
Introduction
Drug-Resistant Tuberculosis (DR-TB) remains the leading infectious disease globally. Multidrug-resistant TB (MDR-TB), caused by Mycobacterium tuberculosis resistant to rifampicin and isoniazid, poses a significant threat to Tuberculosis (TB) control. In 2023, there were approximately 450,000 cases of MDR-TB, marking a 3.1% increase from the previous year, with around 160,000 deaths associated with MDR/RR-TB1. MDR-TB is highly contagious and can be transmitted similarly to Drug-Susceptible Tuberculosis (DS-TB). Delays in recognizing drug resistance can lead to increased transmission. In India, about 135,000 MDR/RR-TB cases were reported, with only 32% of new cases officially notified and 82% of previously treated patients testing positive for Rifampicin resistance2. Recent advances in diagnosing and treating MDR/RR-TB could improve the current global treatment success rate of 63%, while the death rate stands at 15%. India (27%) is among the countries most affected by MDR-TB, followed by China (14%) and Russia (8%)3.
Managing MDR-TB treatment is complex and places significant strain on countries and their national health systems. High-quality management of the disease is essential for improving treatment outcomes for tuberculosis. Research conducted in various countries has revealed a wide range of factors influencing the treatment outcomes of MDR-TB. However, in India, there is limited reporting on these treatment outcomes and the factors associated with unfavourable results for Drug-Resistant Tuberculosis. The increasing incidence of DR-TB complicates efforts to achieve the goals set forth in the End TB Strategy by 20351. MDR-TB is particularly concerning in countries heavily affected by tuberculosis, leading to poor treatment outcomes. The global treatment success rate for MDR/RR-TB is 63%, with a death rate of 15%4. Effective treatment not only cures patients but also reduces disease transmission and prevents the emergence of drug-resistant strains. The factors influencing Tuberculosis treatment outcomes serve as vital indicators of the success of TB control programs5. Prompt initiation and adherence to national TB treatment standards are crucial for effective therapy. Understanding TB treatment outcomes is essential, and categories such as cure rates, treatment completion, treatment failure, death during treatment, and loss to follow-up are used to evaluate the effectiveness of these approaches. Cases classified as cured or having completed treatment are considered successful, while the other outcomes are deemed unsuccessful6. Moreover, managing drug-resistant tuberculosis can be complicated by comorbidities related to other communicable and non-communicable diseases. MDR-TB is often associated with poor outcomes due to treatment failures and relapses, which impede progress against Tuberculosis7– 8. However, there is a lack of comprehensive information regarding successful treatment outcomes and the major risk factors associated with unsuccessful outcomes among patients with Rrifampicin-Resistant (RR) or MDR-Tuberculosis in India. In this study, we retrospectively analyzed the clinical data of MDR-TB patients treated exclusively as outpatients in India to assess treatment outcomes and identify predictors of treatment success. Understanding the factors that contribute to successful treatment outcomes will help develop strategies and facilitate informed decision-making regarding MDR-TB management in the region, ultimately promoting more efficient and effective treatment across the country.
Materials and methods
Study settings
All suspected TB patients were initially screened at Nucleic Acid Amplification Test (NAAT) sites connected to each district’s Drug-Resistant Tuberculosis centre. Those diagnosed with rifampicin resistance through the Xpert MTB/RIF / TrueNat assays were referred to Programmatic Management of Drug-Resistant Tuberculosis (PMDT) sites, and their samples were sent to the Intermediate Reference Laboratory (IRL) in Puducherry for drug susceptibility testing. Patients with Rifampicin-resistant or Multidrug-resistant tuberculosis received treatment according to the National Tuberculosis Elimination Programme guidelines. This study followed WHO methodology and included patients with Rifampicin monoresistance and Multidrug-resistant Tuberculosis from January 2020 to December 2023 in southern India.
Genotype MTBDRplus assay -version 2
Samples collected from NAAT sites were tested using the Genotype MTBDRplus assay to identify genetic factors associated with Rifampicin resistance in tuberculosis. The GenoType MTBDRplus assay was performed on sputum specimens using the manufacturer’s instructions. The process involves three main steps: DNA extraction from the sputum specimens, Amplification of the target region using multiplex polymerase chain reaction (PCR), and Hybridization of the PCR product to specific oligonucleotide probes that are immobilized on the strip9– 10. Drug resistance is indicated by the absence of a wild-type band and/or the presence of a mutation band. Once visible bands appear, the strips should be rinsed twice with distilled water to halt the reaction. Finally, using tweezers, remove the strips from the tray and adhere them to the evaluation sheet provided in the kit11,12.
Clinical data
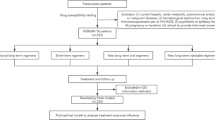
All patients with rifampicin and Multidrug resistance initiated shorter and longer MDR regimen, following the intensive and continuation phases outlined in the PMDT guidelines13. Treatment outcomes are classified according to standardized international consensus14. Successful outcomes include cure and treatment completion, while unsuccessful outcomes encompass death, treatment failure, and interruption or loss to follow-up.
Ethical consideration
The Ethics and Scientific Review Committee at the General Hospital Institute, which is part of the Directorate of Health and Family Welfare Services in Puducherry, approved this study (study (Ref. No/GHIEC/2020/243; June 2020) and granted a waiver for informed consent. All methods were conducted in accordance with the guidelines and regulations established by the WHO and the NTEP, and the Declaration of Helsinki.
Statistical analysis
Data analysis was conducted using MedCalc Software Ltd. (Odds Ratio Calculator, Version 23.1.6)15 and online tools for meta-analysis16. Categorical variables were reported as percentages and counts. A logistic regression analysis was performed to assess the relationship between the unfavourable treatment outcome (dependent variable) and independent variables. Significant factors were identified using multivariate binary logistic regression. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for each variable, with statistical significance set at p < 0.05.
Results
From 2020 to 2023, 192 patients with Multidrug-resistant and Rifampicin-resistant Tuberculosis were enrolled in an ambulatory care program. Of these, 61 (31.8%) had RR-TB and 131 (68.2%) had MDR-TB. In 150 new cases, 45 (30%) had RR-TB, while 105 (70%) had MDR-TB. Among 42 previously treated patients, 16 (38.1%) had RR-TB, and 26 (61.9%) had MDR-TB. Of those with MDR/RR-TB, 148 adults (77.1%) received shorter regimens, achieving a 63.2% treatment success rate, compared to 56.8% for the 44 adults (22.9%) on longer regimens (Fig. 1).
Detection of mutations predicting M. tuberculosis drug resistance
Of the 150 new cases, 62.7% were classified as true resistance, with one or more wild-type (WT) probes failing to develop while one or more mutant (MUT) probes did. Additionally, 28.6% were categorized as inferred resistance (WT probes absent, no MUT probes detected), and 8.7% were identified as heteroresistant tuberculosis (all WT probes present alongside one MUT probe). Of the 42 treated cases, 66.7% were classified as true resistant, 19.0% as inferred resistant, and 14.3% as heteroresistant, as shown in Table 1. In the 94 true resistant new cases, 75 (50%) had a mutation at codon S450L; mutations at codons H445Y, H445D, and D435V were found in 9 (6%), 5 (3.3%), and 5 (3.4%) cases, respectively. For the 28 true resistant previously treated cases, 21 (50%) had mutations at codon S450L, while 2 (4.8%) had mutations at codons H445Y and H445D, and 3 (7.1%) at codon D435V. Among 13 heteroresistant new cases, mutations at codon S450L were in 3 (2%), while 6 (4%), 2 (1.4%), and 2 (1.4%) had mutations at codons H445Y, H445D, and D435V, respectively. In the treated heteroresistant group, 1 (2.4%) had a mutation at codon S450L, and mutations at codons H445Y and H445D were found in 3 (7.1%) and 2 (4.8%) cases, respectively (Fig. 2). The majority of inferred resistance in new cases was in the WT8 region (13 cases), followed by WT2 (9 cases) and WT7 (5 cases). For treated cases, inferred resistance was mainly found in the WT3 region (3 cases) and WT2 (2 cases). In a study of 192 cases of RR/MDR tuberculosis, 60.42% of the treatments were successful, while 39.58% were unsuccessful. The success rate for new cases was 61.3%, and for previously treated cases, it was 57.1%, as shown in Table 2. In a subset of 150 new cases, 50% had mutations at the S450L codon, with 32.7% successful outcomes. Among 94 true resistant cases, the success rate was 68.1%, while 53.5% success was seen in 43 inferred resistant cases. Heteroresistant cases had a success rate of 38.5%. In a group of 42 treated cases with 50% showing mutations, success rates varied: 33.3% overall, 60.7% for true resistance, and 50% for both inferred and hetero-resistant cases. Overall success for true resistant cases was 42.2%, 14.1% for inferred resistance, and 4.2% for heteroresistant cases, with unsuccessful rates of 21.3%, 12.5%, and 5.7%, respectively.
Treatment outcomes among patients who had received MDR/RR-TB treatment
In a study of 192 patients, 81 (42.2%) were cured, 35 (18.2%) completed treatment, 8 (4.1%) had treatment failures, 19 (9.9%) were lost to follow-up, and 44 (22.9%) died, resulting in an overall treatment success rate of 60.4% from 2020 to 2023. Among 61 patients with Rifampicin-resistant Tuberculosis, the success rate was 52.5%, with a death rate of 26.2%. For 131 patients with MDR-TB, the success rate was 64.1%, and the death rate was 21.4%. New RR-TB cases were 1.61 times more likely to have unsuccessful treatment outcomes than previously treated patients [OR, 1.61; 95% CI (0.51–5.07)]. Among the 192 patients, who sputum culture conversion (SCC), 116 (60.4%) achieved a positive outcome, while 44 (22.9%) died, 19 (9.9%) were lost to follow-up, 8 (4.2%) experienced treatment failure, and 5 (2.6%) did not receive treatment. In total, 76 (39.6%) had negative outcomes, as detailed in Table 3. Of the 192 cases that underwent a second-line drug susceptibility test using the MTBDRsl assay, 151 patients (78.7%) were found to be sensitive to treatment, 40 patients (20.8%) were classified as pre-extensively drug-resistant (pre-XDR), and 1 patient (0.5%) was identified as extensively drug-resistant (XDR), as shown in Table 4.
Factors associated with RR/MDR-Tuberculosis treatment outcomes
Figure 3 presents a forest plot analysis of thirteen variables related to Multidrug-resistant and Rifampicin-resistant Tuberculosis treatment outcomes. A summarized odds ratio (OR) of 1.01 (95% CI: 0.80 to 1.29) was found, with no significant difference or heterogeneity, indicating consistent effect sizes across studies. Retreating patients had 1.18 times the odds of unsuccessful treatment (OR, 1.19). Patients on HIV treatment had slightly higher odds (OR, 1.03), while those with a history of alcohol use (OR, 1.42) and tobacco use (OR, 1.95) faced greater risks of unsuccessful outcomes. Furthermore, patients with heteroresistant patterns had notably higher odds (OR, 2.71). Finally, longer treatment regimens were associated with a higher likelihood of unsuccessful outcomes, with an odds ratio of 1.31.Our study found that MDR-TB patients with diabetes had a lower odds ratio (OR 0.96, 95% CI: 0.52–1.79) for poor treatment outcomes.
Factors associated with death- as unsuccessful outcomes
Figure 4 shows a forest plot analysis of thirteen variables related to increased death, using multivariate regression with a random effects model. The summarized odds ratio (OR) is 0.88, with a 95% confidence interval of 0.69–1.13, indicating no statistical difference. The overall effect is not significant, and there is low heterogeneity across cohorts, suggesting consistent effect sizes. Multivariate binary logistic regression (MVBLR) showed that males were 1.81 times more likely to die than females (OR: 1.81; 95% CI: 0.87–3.75). Unsuccessful outcomes were more prevalent in rural patients (OR: 1.01; 95% CI: 0.44–2.33) and those with a history of alcohol use (OR: 1.40; 95% CI: 0.57–3.44). Patients with MDR tuberculosis had a 1.08 times higher death likelihood than those with RR tuberculosis (OR: 1.08; 95% CI: 0.55–2.11). Among MDR patients, high-dose isoniazid users faced increased mortality risk, and longer treatment regimens were associated with higher odds of death (OR: 1.08; 95% CI: 0.55–2.11). Overall mortality was 22.9%, with 16.7% of deaths occurring among new MDR/RR patients; however, MVBLR found no significant association between independent variables and treatment failure.
Factors associated with LTFU- as unsuccessful outcomes
Figure 5 shows a forest plot of eleven variables associated with increased loss to follow-up using a random effects model. The summarized odds ratio (OR) was 1.13 (95% CI: 0.78–1.64), indicating no statistical difference. The overall effect test was not significant, suggesting uniform effect sizes across studies. In a study of 76 patients with unsuccessful treatment outcomes, 19 (25.0%) were lost to follow-up, including 15 (9.9%) who discontinued treatment during the intensive phase. Multivariable logistic regression showed that patients aged 45 and older had higher odds of unfavourable outcomes (OR: 2.52), and rural patients also faced increased odds (OR: 1.05). Married patients were significantly more likely to have unsuccessful outcomes (OR: 3.76). Retreated patients had slightly higher odds (OR: 1.05) compared to those receiving initial treatment. Patients with MDR-TB had 1.28 times higher odds of poor outcomes than those with rifampicin mono-resistance, and among MDR-TB patients, those on high doses of isoniazid faced a 1.64 times greater likelihood of poor outcomes. Notably, 8.9% of those lost to follow-up were new male patients with MDR/RR-Tuberculosis.
Discussion
The study assessed treatment outcomes in patients with Multidrug-resistant and Rifampicin-resistant tuberculosis receiving outpatient care in southern India. After 24 months, the overall treatment success rate was 60.4%, slightly below the WHO target of 63%17, with 39.6% experiencing unsuccessful outcomes. MDR tuberculosis had a higher success rate of 64.1%, compared to 52.5% for RR Tuberculosis. While this rate is higher than those reported in India (53.8%)18 and Pakistan (32.1%)19it is lower than rates in high-burden countries like Ghana (71.4%) and Ethiopia (69%, 77.1%)20– 21. Past Indian studies reported even lower success rates of 51.7%22 and 47%23 for MDR TB. The sputum culture conversion rate (SCC) is a crucial initial target for treating patients with multidrug-resistant tuberculosis (MDR-TB) and serves as the most reliable indicator of treatment outcomes. Prolonged infectivity increases the risk of spreading both drug-resistant and drug-sensitive TB (Khan et al.)24. In our study, we found the SCC to be 60.4%, which is lower than the 84% reported by Igbal et al.25 Additionally, the rate of pre-extensively drug-resistant TB (Pre-XDR-TB) among our MDR-TB cases was 20.8%. In contrast, a study in India by Utpat et al. (2023)26 reported a higher rate of 29.7%. Furthermore, studies by Nwachukwu et al. (2023)27 in Nigeria and Diriba et al. (2025)28 in Ethiopia reported rates of only 3.1% and 2.35%, respectively, both of which are lower than our findings. The high rate of Extensively Drug-Resistant (XDR) TB, especially resistance to fluoroquinolones (FQs), is mainly attributed to several factors. These include the widespread and inappropriate use of FQs for various infections unrelated to TB, as well as inadequate management of TB, which encompasses issues such as treatment adherence and the use of appropriate drug regimens.
Factors influencing treatment success include patient condition at admission, program organization, and care setting. Our study found that individuals with a history of drug-resistant tuberculosis or previous first-line anti-TB treatment are more likely to experience treatment failure than newly diagnosed patients. This aligns with research from Ethiopia and Sudan, where many MDR-TB patients had prior TB treatment29. Similarly, findings from Brazil indicated that previous anti-TB treatment increases the risk of developing MDR-TB30. Drug resistance often stems from incomplete or inappropriate treatment, typically lasting less than a month, which allows resistant strains to flourish31. Thus, patients with MDR-TB may have had inadequate prior treatment, compounded by poor adherence and premature therapy discontinuation, further increasing the risk of recurrence and resistance.MDR/RR Tuberculosis patients co-infected with HIV have lower chances of successful treatment outcomes. Kajogoo et al.32 reported that these patients were twice as likely to interrupt TB treatment, leading to poor results. Kumar et al.33 highlighted HIV as a key factor in treatment failure. HIV often causes intestinal dysfunction and malabsorption, suggesting many MDR Tuberculosis patients are malnourished, making diet crucial for treatment. Additionally, the need for multiple medications increases the risk of drug interactions, reduced adherence, and side effects34. Nair et al.23 found worse outcomes for HIV-positive patients, and Johnson et al.2 identified HIV co-infection as significantly linked to unsuccessful treatment outcomes.
Our study found that alcohol consumption increased the odds of unsuccessful treatment outcomes for tuberculosis, with individuals having 1.42–2.86 times greater odds compared to non-drinkers. Song et al.35 reported similar findings, noting a 1.3–1.8 times increase in poor outcomes for MDR-Tuberculosis patients who abused alcohol. Ragan et al.36 also found a 1.5-2.0 fold higher risk of poor outcomes in those with alcohol use. Karthickeyan et al.37 noted alcohol as the most significant risk factor for unsuccessful MDR-Tuberculosis treatment. The relationship between alcohol consumption and treatment effectiveness is complex; alcohol can suppress immune functions and hinder drug metabolism, leading to missed doses and unsuccessful outcomes. Additionally, alcohol negatively impacts general health and immune responses to M. tuberculosis, potentially causing treatment failures. Studies show alcohol enhances mycobacterial survival in macrophages and inhibits T-cell activation, while chronic exposure can suppress essential cytokine production. Overall, alcohol significantly contributes to poor treatment outcomes in MDR-Tuberculosis patients due to increased noncompliance and relapse rates. Research indicates that tobacco use negatively impacts treatment outcomes for MDR Tuberculosis, with users facing higher rates of treatment failure. In India, home to about 266.8 million tobacco users33the health risks are significant, as tobacco weakens lung defences and raises infection susceptibility. It reduces Natural Killer (NK) cell activity and mucociliary clearance, inhibits nitric oxide synthase essential for macrophage function against M. tuberculosis, and increases iron availability in the lungs, disrupting macrophage and cytokine functions. Older smokers also experience diminished antioxidant defences in alveolar macrophages, damaging lung tissue. The emergence of multidrug-resistant tuberculosis (MDR-TB) in patients with diabetes mellitus (DM) raises concerns regarding treatment outcomes and financial burdens, and it may potentially lead to extensively drug-resistant tuberculosis (XDR-TB). However, our study found that MDR-TB patients with diabetes had a lower odds ratio (OR 0.96, 95% CI: 0.52–1.79) for poor treatment outcomes. This reduced likelihood of treatment failure may be influenced by factors such as the limited number of patients studied, geographical variations, and unadjusted covariates. These factors could explain the discrepancies in results and should be explored further.
Our study found that 9.9% of participants exhibited heteroresistance in the rpoB gene, showing all eight wild-type bands and at least one mutant band. This is higher than the 5.4% reported by Desikan et al.38 in India (2022) and similar to the 8.3% found by Crowder et al.39 in the Philippines. Individuals with heteroresistance had odds of unsuccessful treatment outcomes 2.71–7.28 times greater than those with true resistance. Shin et al.40 noted that mixed-strain M. tuberculosis infections are linked to poorer treatment outcomes, and various case series have shown that heteroresistant strains negatively impact treatment results. Research indicates that longer treatment regimens increase the odds of unsuccessful outcomes by 1.31–2.60 times. Munir et al.41 suggested that shorter regimens are as effective as longer ones but have fewer side effects. Similarly, Abidi et al.42 found better outcomes with shorter treatments. Our study reported a 61.5% success rate among 148 DR-Tuberculosis patients on a shorter regimen, and Mleoh et al.43 noted similar findings in Tanzania. Implementing shorter regimens at decentralized sites may enhance success rates. Additionally, improving nutritional status at treatment onset and introducing new, shorter regimens could further benefit outcomes. A study by Soeroto et al.34 revealed a 64.5% success rate among 315 MDR/RR-Tuberculosis patients in Indonesia, highlighting that patients over 45 tend to have lower success rates due to factors like delayed treatment and age-related diseases30.
The study reported a 22.9% mortality rate and 9.9% loss to follow-up among MDR/RR-Tuberculosis patients. Factors linked to mortality included male gender, residing in rural areas, alcoholic, Multidrug resistance, and katG resistant. Weiangkham et al. (2022)44 reported that alcohol consumption in patients with multidrug-resistant tuberculosis (MDR-TB) significantly increases the risk of death, with an odds ratio (OR) of 1.53 (95% CI: 1.12–2.09, p < 0.001). Our study supports this, showing an OR of 1.40 (95% CI: 0.57–3.44). Alcohol negatively impacts MDR-TB treatment outcomes, heightening the risk of death, treatment failure, and loss to follow-up. It may also impair the metabolism of TB medications, leading to lower drug concentrations. Additionally, alcohol affects immune responses, lung function, and may hinder the absorption of both TB and HIV drugs, increasing the risk of relapse and mortality among TB patients. Most deaths (59.1%) occurred within the first six months of treatment, while 40.9% occurred in the following 18 months. This emphasizes the need for intensive care during initial treatment phases. The observed mortality rate was higher than 13.4% in Ethiopia45 and 15.2% in India46. The higher number of TB-related deaths observed in this study may be attributed to a greater mortality rate among males compared to females, as indicated by the multivariate logistic regression analysis. Additionally, the study found that patients living in rural areas experienced higher death rates, which further contributes to this disparity. More research is needed to understand the underlying reasons for the increased TB-related deaths in the study area. A significant factor contributing to poor outcomes in the ambulatory treatment of MDR/RR-Tuberculosis in this study was loss to follow-up (LTFU), which was 9.9%. This is higher than the 8% found in other studies but lower than the 49.5% reported by Soeroto et al.26 in Indonesia. The WHO target for LTFU is below 6%47, emphasizing that our rate poses a severe threat to validity. LTFU was notably higher among individuals aged 35–60 years, with 78.9% of cases occurring within the first six months of treatment. In India, high illiteracy rates, lack of health awareness, and poverty contribute to LTFU, along with the easy access to unqualified practitioners and alternative medicine, highlighting the need for better integration of healthcare systems48– 49.
Several studies have explored the link between gene mutations and MDR/RR Tuberculosis drug resistance. The impact of common mutations on treatment outcomes remains unclear. Understanding how genotypic resistance affects treatment could improve drug selection. Our study found that true resistance (21.4%) linked to poor outcomes was more significant than heteroresistant (5.7%) and inferred resistance (12.5%). Notably, true resistance due to rpoB gene mutations accounted for 65.9% (29 out of 44) of total deaths, with mutations at codon S450L contributing to 47.7% of these fatalities, a finding that has not been reported elsewhere. Among 100 patients with this mutation, 36% experienced unsuccessful treatment outcomes. Similarly, 38.7% of 31 patients with the H445Y/D mutation had unsuccessful outcomes. Changes at codon S450L affected resistance levels, with small residues like serine or leucine and larger residues like tyrosine or histidine increasing resistance50. Future molecular testing should distinguish between mutants and wild-type strains and identify relevant mutations for improved MDR/RR Tuberculosis treatment. Enhancing treatment strategies for MDR/RR-Tuberculosis is essential to meeting the global goal of ending the Tuberculosis epidemic by 2030. Addressing identified risk factors could improve treatment success rates. This study has limitations due to reliance on secondary data, which restricts our understanding of factors affecting outcomes. The lack of specific information on co-morbidities and BMI may have influenced our analysis. Our study found that patients with multidrug-resistant tuberculosis (MDR-TB) who also have diabetes exhibited a lower odds ratio for poor treatment outcomes. This decreased likelihood of successful treatment may be influenced by several factors, including the small sample size, geographical variations, and unadjusted covariates. These factors might explain the discrepancies in our results and should be investigated further. Additionally, as a retrospective study, we couldn’t obtain follow-up details for all patients.
Conclusion
In summary, the treatment success rate for Multidrug-resistant tuberculosis in India is 60.4%, below the World Health Organization’s target, highlighting the need for strategies to improve outcomes. This study reveals insights into unfavourable outcomes linked to heteroresistance, which can influence policies for managing Multidrug-resistant Tuberculosis. Notably, mutations in the rpoB gene caused 65.9% of deaths, with one specific mutation at codon S450L responsible for 47.7% of fatalities. While many studies focus on drug-susceptible Tuberculosis, more research is urgently needed on MDR/RR Tuberculosis to fill existing knowledge gaps related to treatment outcomes in India.
Foot notes
-
The Tuberculosis treatment success rate is currently 60.4%, below the World Health Organization’s target but still notable given the treatment challenges. Notably, true resistance due to rpoB gene mutations accounted for 65.9% (29 out of 44) of total deaths, with mutations at codon S450L contributing to 47.7% of these fatalities, Amino acid changes at codon S450L affected resistance levels Among 100 patients with this mutation, 36% faced unsuccessful treatment outcomes, and 38.7% of 31 patients with the H445Y/D mutation had similar results.
-
Notably, 40 of the 44 patients who died did so within the first year of treatment. MVBLR analysis showed that male patients were 1.81 times more likely to die than females (OR, 1.81; 95% CI: 0.87–3.75). Moreover, patients with a history of alcohol use had higher odds of unsuccessful treatment outcomes (OR, 1.40; 95% CI: 0.57–3.44) than non-alcoholics.
-
Unsuccessful treatment outcomes were more common in rural patients (OR: 1.01; 95% CI: 0.44–2.33) compared to urban patients.
-
Individuals with heteroresistance had 2.71 to 7.28 time’s greater odds of unsuccessful outcomes than those with true resistance.
-
Success rates for shorter bedaquiline regimens were 63.2%, versus 56.8% for longer regimens. Additionally, MDR/RR-TB patients on longer treatments had increased odds of unsuccessful outcomes (OR: 1.31; 95% CI: 0.66–2.60) compared to those receiving shorter treatments.
Data availability
All primary and secondary data are available with the corresponding author and in the Nikshay portal, Government of India. Permission is granted to the corresponding author to access the data through login credentials. The datasets generated and analyzed during the current study are part of the first author’s Ph.D. thesis and are not publicly available. The datasets are available from the corresponding author upon reasonable request. Contact no: +91 9944737597Email.ID: drmuthurajm@gmail.com.
Abbreviations
- WHO:
-
World Health Organization
- MDR:
-
Multidrug-resistant
- RR:
-
Rifampicin-resistant
- LTFU:
-
Loss-to-follow-up
- TB:
-
Tuberculosis
- HIV:
-
Human immunodeficiency virus
- LZD:
-
Linezolid
- BDQ:
-
Bedaquiline
- FQ:
-
Fluoroquinolone
- XDR:
-
Extensively drug-resistant
- DR:
-
Drug-resistant
- NAAT:
-
Nucleic acid amplification test
- PMDT:
-
Programmatic management of drug-resistant tuberculosis
- IRL:
-
Intermediate reference laboratory
- NALC:
-
N-Acetyl L-Cysteine
- MTB:
-
Mycobacterium tuberculosis
- IP:
-
Initial phase
- CP:
-
Continuation phase
- OR:
-
Odds ratio
- WT:
-
Wild type
- MVBLR:
-
Multivariate binary logistic regression analysis
References
Global & World Health Organization. Geneva: ; 2023 (2023). https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023
Johnson, J. M., Mohapatra, A. K., Velladath, S. U. & Shettigar, K. S. Predictors of treatment outcomes in drug resistant tuberculosis observational retrospective study. Int. J. Mycobacteriol. 11, 38–46 (2022).
Bayowa, J. R. et al. Mortality rate and associated factors among patients co-infected with drug resistant tuberculosis/hiv at Mulago National referral hospital, uganda, a retrospective cohort study. PLOS Glob Public. Health. 3, e0001020 (2023).
Alemu, A. et al. Poor treatment outcome and associated risk factors among patients with Isoniazid mono-resistant tuberculosis: A systematic review and meta-analysis. PLoS ONE. 18, e0286194 (2023).
Chen, Y., Jiang, Q., Peierdun, M., Takiff, H. E. & Gao, Q. The mutational signatures of poor treatment outcomes on the drug-susceptible Mycobacterium tuberculosis genome. Elife 12, e84815 (2023).
Jose Vadakunnel, M. et al. Impact of RpoB gene mutations and Rifampicin-resistance levels on treatment outcomes in Rifampicin-resistant tuberculosis. BMC Infect. Dis. 25, 284 (2025).
Silva, D. R. et al. Isoniazid-resistant TB: treatment outcomes and impact of regimens with fluoroquinolones. Int. J. Tuberc Lung Dis. 27, 638–640 (2023).
Sulis, G. & Pai, M. Isoniazid-resistant tuberculosis: A problem we can no longer ignore. PLoS Med. 17, e1003023 (2023).
Kang, J. Y. et al. Clinical implications of discrepant results between genotypic MTBDRplus and phenotypic Löwenstein-Jensen method for Isoniazid or rifampicin drug susceptibility tests in tuberculosis patients. J. Thorac. Dis. 11, 400–409 (2019).
Javed, H. et al. Evaluation of genotype MTBDRplus and MTBDRsl assays for rapid detection of drug resistance in extensively drug-Resistant Mycobacterium tuberculosis isolates in Pakistan. Front. Microbiol. 9, 2265 (2018).
Bokop, C., Faye, L. M. & Apalata, T. Analysis of discordance between genotypic and phenotypic assays for rifampicin-resistant mycobacterium tuberculosis Isolated from healthcare facilities in Mthatha. Pathogens.12, 909 (2023).
Smita, S. S. et al. Prevalence and factors associated with multidrug-resistant tuberculosis in South India. Sci. Rep. 10, 17552 (2020).
India 2021,National TB Elimination Programme, Guidelines for Programmatic Management of Drug-resistant TB in, Central, T. B. & Division Ministry of Health and Family Welfare, Government of India, New Delhi, India.
Linh, N. N. et al. World health organization treatment outcome definitions for tuberculosis: 2021 update. Eur. Respir J. 58, 2100804 (2021).
MedCalc: MedCalc’s Relative risk calculator. MedCalc Software Ltd. (2024).
Fekete, J. T. & Gyorffy, B. MetaAnalysisOnline.com: an online tool for the rapid meta-analysis of clinical and epidemiological studies. J Med Internet Res, PMID, 39928123 (2025).
Tuberculosis Multidrug-resistant (MDR-TB) or rifampicin-resistant TB (RR-TB), Global Tuberculosis Programme, World Health Organiztion, 20 May 2024).
Sharma., Khanna, A. et al. Treatment outcomes of multidrug-resistant tuberculosis in delhi, India (2009–2014): A retrospective record-based study. Indian J. Med. Res. 151, 598–603 (2020).
Muhammad, A. et al. Drug resistance patterns, treatment outcomes and factors affecting unfavourable treatment outcomes among extensively drug resistant tuberculosis patients in pakistan; a multicentre record review. Saudi Pharm. J. 30, 462–469 (2022).
Panford, V. et al. Treatment outcomes and associated factors among patients with multidrug-resistant tuberculosis in Ashanti region,ghana: a retrospective, cross-sectional study. BMJ Open. 12, e062857 (2022).
Wakjira, M. K., Sandy, P. T. & Mavhandu-Mudzusi, A. H. Treatment outcomes of patients with MDR-TB and its determinants at referral hospitals in Ethiopia. PLoS ONE. 17, e0262318 (2022).
Giri, V. P. et al. The characteristics and patterns of Drug-Resistant pulmonary tuberculosis in Eastern India. Trop. Med. Infect. Dis. 7, 244 (2022).
Nair, D. et al. Predictors of unfavourable treatment outcome in patients with multidrug-resistant tuberculosis in India. PHA. 7, 32–38 (2017).
Melese, A. & Zeleke, B. Factors associated with poor treatment outcome of tuberculosis in Debre tabor, Northwest Ethiopia. BMC Res. Notes. 11, 1–6 (2018).
Khan, S., Choudhary, S., Aurangabadkar, G. & Bankar, N. Sputum culture conversion among patients with drugresistant tuberculosis: A study of various predictors. J. Datta Meghe Inst. Med. Sci. Univ. 18, 255–262 (2018).
Iqbal, Z., Khan, M. A., Aziz, A. & Nasir, S. M. Time for culture conversion and its associated factors in multidrugresistant tuberculosis patients at a tertiary level hospital in peshawar, Pakistan. Pak J. Med. Sci. 38, 1009–1015 (2022).
Utpat, K. V., Rajpurohit, R. & Desai, U. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among extra pulmonary (EP) multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai in pre bedaquiline (BDQ) era. Lung India. 40 (1), 19–23 (2023).
Nwachukwu, N. O., Ulasi, A. E., Okoronkwo, C. U. & Unegbu, V. N. Pre–extensively drug–resistant tuberculosis among pulmonary multidrug–resistant tuberculosis patients in Eastern Nigeria. Lung India. 40, 492–495 (2023).
Diriba, G. et al. Second-line drug resistance among multidrug-resistant tuberculosis patients in ethiopia: A laboratory-based surveillance. J. Glob Antimicrob. Resist. 42, 167–174 (2025).
lduma, A. H. et al. Assessment of the risk factors associated with multidrug-resistant tuberculosis in sudan: a case-control study. Epidemiol. Health. 41, e2019014 (2019).
Soares, V. M. et al. Factors associated with tuberculosis and multidrug-resistant tuberculosis in patients treated at a tertiary referral hospital in the state of Minas gerais, Brazil. J. Bras. Pneumol. 46, e20180386 (2020).
Kajogoo, V. D. et al. Treatment outcomes of multi-drug resistant tuberculosis patients with or without human immunodeficiency virus co-infection in Africa and asia: systematic review and meta-analysis. Ann. Med. Sur. 78, 103753 (2022).
Kumar, A., Harakuni, S., Paranjape, R., Korabu, A. S. & Prasad, J. B. Factors determining successful treatment outcome among notified tuberculosis patients in Belagavi district of North karnataka, India. CEGH 25,101505 (2024).
Soeroto, A. Y. et al. Factors associated with treatment outcome of MDR/RR-TB patients treated with shorter injectable based regimen in West Java Indonesia. PLoS ONE. 17, e0263304 (2022).
Song, W. et al. Impact of alcohol drinking and tobacco smoking on the drug-resistance of newly diagnosed tuberculosis: a retrospective cohort study in shandong, china, during 2004–2020. BMJ Open. 12, e059149 (2022).
Ragan, E. J. et al. The impact of alcohol use on tuberculosis treatment outcomes: a systematic review and meta-analysis. Int. J. Tuberc Lung Dis. 24, 73–82 (2020).
Karthickeyan, D. et al. Does alcohol consumption during Multidrug-resistant tuberculosis treatment affect outcome?? A Population-based study in kerala, India. Annals ATS. 11, 712–718 (2014).
Desikan, P. et al. Heteroresistance to rifampicin & Isoniazid in clinical samples of patients with presumptive drug-resistant tuberculosis in central India. Indian J. Med. Res. 157, 174–182 (2023).
Crowder, R. et al. Impact of heteroresistance on treatment outcomes of people with drug-resistant TB. IJTLD Open. 1, 466–472 (2024).
Shin, S. S. et al. Mixed Mycobacterium tuberculosis-Strain infections are associated with poor treatment outcomes among patients with newly diagnosed tuberculosis, independent of pretreatment heteroresistance. J. Infect. Dis. 218, 1974–1982 (2018).
Munir, M. K., Saeed, M. S., Haider, S. Z. & Shamim, S. Comparison of short term and long term multidrug resistant tuberculosis treatment outcomes in tertiary care settings. J King Saud Univ. Sci 36,103133 (2024).
Abidi, S. et al. Standardised shorter regimens versus individualised longer regimens for Rifampin or multidrug-resistant tuberculosis. Eur. Respir J. 55, 1901467 (2020).
Mleoh, L. et al. Shorter regimens improved treatment outcomes of multidrug-resistant tuberculosis patients in Tanzania in 2018 cohort. Trop. Med. Int. Health. 28, 357–366 (2023).
Weiangkham, D., Umnuaypornlert, A., Saokaew, S., Prommongkol, S. & Ponmark, J. Effect of alcohol consumption on relapse outcomes among tuberculosis patients: A systematic review and meta-analysis. Front. Public. Health. 3, 10, 962809 (2022).
Getahun, G. K. et al. Survival status and risk factors for mortality among multidrug-resistant tuberculosis patients in addis ababa, ethiopia: A retrospective follow-up study. J. Clin. Tuberc Other Mycobact. Dis. 33, 100398 (2023).
Vaman, R. S., Kalyanasundaram, M., Amina, T. P. & Murhekar, M. V. Epidemiology and outcomes of drug-resistant tuberculosis cases notified in a low-resource district in kerala, India 2017–2021 – A 5-year retrospective analysis. Indian J. Med. Sci. 76, 110–116 (2024).
Jiang, Y. et al. Factors associated with loss to follow-up before and after treatment initiation among patients with tuberculosis: A 5-year observation in China. Front. Med. (Lausanne). 10, 1136094 (2023).
Lecai, J. et al. Treatment outcomes of multidrug-resistant tuberculosis patients receiving ambulatory treatment in shenzhen, china: a retrospective cohort study. Front Public. Health 11,1134938 (2023).
Sandeep, P., Vishal, K., Shahnawaz, K. & Amit, S. Loss to Follow-up: A deceptive enigma. Ann. Natl. Acad. Med. Sci. (India). 57, 120–122 (2021).
Rigouts, L. et al. Specific GyrA gene mutations predict poor treatment outcome in MDR-TB. J. Antimicrob Chemother. 71, 314–323 (2016).
Acknowledgements
We would like to extend our gratitude to all the staff working in the Intermediate Reference Laboratory at the Government Hospital for Chest Diseases in Puducherry for their unwavering support. Additionally, we want to express our thanks to the medical officers and supporting staff working at the TB treatment and primary health centres for their support during the data collection period.
Author information
Authors and Affiliations
Contributions
MJV and VJN--prepared manuscriptUB, VR, SP --prepared figuresGP, AM and BRM--prepared tablesSSR and MM--prepared manuscript, KV and MM--statistical analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Before starting the study, we received ethical clearance from the General Hospital Institution Ethics Committee at the Indira Gandhi Government General Hospital and Postgraduate Institute (Ref. No/GHIEC/2020/243; June 2020). Before collecting data, we informed the hospital director and laboratory personnel about the study objectives. Since we used secondary data, we did not need the patients’ informed consent. We did not include personal identifiers on the data collection sheet to protect the confidentiality of the participant’s records. Additionally, access to secured data from participant records was restricted to the investigators only.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vadakunnel, M.J., Nehru, V.J., Brammacharry, U. et al. Factors associated with unfavourable treatment outcomes among patients with Multidrug-resistant Tuberculosis receiving outpatients care. Sci Rep 15, 28335 (2025). https://doi.org/10.1038/s41598-025-13227-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13227-5