Abstract
Traumatic Brain Injury (TBI) affects millions of people each year, causing loss of workforce, a significant burden on the healthcare system. Research into the biological effects of TBI has focused on cellular and tissue responses in the order of hours to days after exposure. Unfortunately, treatments that address these secondary processes after TBI have not been effective. Addressing earlier events and identifying first responders is necessary to prevent or limit irreversible damage. Here, we analyze molecular and cellular changes that occur during and immediately after injury in the somatosensory cortex of mice after an airblast TBI using various techniques. Early changes recorded within 5 min of TBI injury show persistent calcium activation, followed by unique phosphorylation patterns activating specific protein binding and calcium-activated pathways. Imaging mass lipidomics and Raman microspectroscopy identified conformational changes of lipid membranes, pointing towards membrane damage as the first response to mechanical pressure. Real-time monitoring of blood vessels shows rapid constriction followed by reduced blood flow within minutes of injury. The present study uncovers the early sequence of events in TBI: ionic calcium imbalance occurs within seconds, activating subcellular phosphorylation patterns, which in turn trigger downstream pathways and a parallel destabilization of membrane phospholipids.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is a complex and multifaceted disease that can cause significant damage to the brain, leading to cognitive, behavioral, and motor deficits1,2. Nearly one-third of U.S. Service Members deployed in Iraq and Afghanistan sustained mild to moderate TBI3. A recent report shows that sports-associated TBI due to football in-game accelerations/decelerations poses a greater risk of developing mTBI-associated severe concussion4. It is a major public health issue, affecting 50 million people internationally every year, with significant societal and economic burdens5. Understanding the underlying mechanisms of TBI and developing effective therapies and tools to monitor the progression of the impact is an important research focus. There is an urgent need for field management along with clinical guidelines to reduce the burden of long-term/sustained functional deficits. TBI can be caused by blunt force or blast-mediated mechanical impact; here, we focus on blast-induced TBI. The injuries caused by blast exposure or blunt force include primary injury resulting from immediate physical damage to the brain from the mechanical force, which occurs at the time of injury; this includes contusion6laceration, and diffuse axonal injury7. The rapid pressure transients and/or shock waves affect brain structure and function, transmitting massive energy directly to the tissue8. The transferred kinetic energy causes low-frequency stress waves that result in rapid deformations, acceleration-deceleration, and torsional effects on the brain9. Characterizing the molecular changes that occur minutes after injury and drive secondary neurodegeneration after blast induced TBI is the subject of this study. An appropriate understanding of the physics of blast waves and the features of the underlying molecular environment created by blast injuries can help monitor and manage the aftereffects of these exposures. The mammalian brain consists of a complex interweaving and interaction between neurons, astrocytes, microglia, and vasculature that respond to blasts in a multifaceted manner. From a tissue perspective, blast-damaged portions of the brain can undergo inflammation, ischemia, edema, and vasoconstriction10.
Following an injury, chronic inflammation, along with motor and cognitive disorders, persists for days or even years11,12. A change in expression of neuronal proteins, including NeuN, parvalbumin (PV), and Ca2+/calmodulin independent protein kinase II (CamKII) only to the areas with blood-brain disruption13. Neutrophils were reported to infiltrate at the site of injury 24 h after TBI; this coincides with the activation of microglia and macrophages, exhibiting a higher phagocytic activity to remove necrotic and apoptotic cells, astrocytes were noted to accelerate the gliosis process to form a scar barrier surrounding the brain lesion14. Although there is no consensus in the existing literature regarding the precise duration following injury within which a patient is evacuated for treatment, the current presumption is 15–30 min. In the present study, we adopt the definition of the initial injury phase to encompass the period within 5 min following the injury.
Mechanical force-induced TBI is commonly mimicked in preclinical models using the weight-drop and controlled cortical impact (CCI) setups. However, the weight drop approach has drawbacks such as poor accuracy and reproducibility, and CCI TBI is considered a more reliable method that causes a penetrative injury in the field15. Blast-induced TBI causes drastic increases and decreases in pressures passing through the brain as a blast wave propagates through space and time16. Craniocerebral blast injuries caused by blast waves have a direct relevance to war field incidence17and the outcomes of this model have a direct relevance to pathophysiology and the energy dissipated from a penetrating projectile. CCI TBI model does not mimic the blast injuries caused by high-pressure waves encountered in combat zones. In contrast, the airblast TBI model offers the advantage of controlling pressure outcomes and is suitable for investigating impacts that do not result in penetrating injuries to brain tissue, a characteristic feature of CCI TBI. The main goal of this study is to conduct real-time imaging of the cortical region to capture immediate changes after a TBI. However, using the blast tube setup to mimic the Friedlander waveform will be challenging because positioning the animal and angling the microscope objective within a blast tube is arduous. As a result, the study was designed to capture the immediate changes in a smaller area with the barrel set up of an air blast gun (Stahl et al., 2024)18. Battlefield injuries often involve penetrating and blunt injuries that result in skull fractures and exposure of brain tissue caused by high-velocity projectiles such as shrapnel or direct concussive forces19(Young et al., 2015). The open-skull model replicates these injury mechanisms more accurately than closed-skull models, which may not account for the specific pathophysiological effects of direct cranial damage, such as localized hemorrhage, skull bone fragments, and exposure of brain tissue to the external environment. Complex injury cascades involving multiple biomechanical and biological responses are challenging to study in closed-skull models with direct imaging modalities. The open skull approach allows for observing more detailed interactions between external forces and brain tissue, including the effects on intracranial pressure, focal lesions, and the potential exacerbation of injury due to direct cranial exposure. These characteristics make this model highly relevant for simulating battlefield conditions.
The current study focuses on identifying early/first respondents of a TBI in a murine airblast model relevant to modern warfare-associated injuries. We used multimodal evaluation techniques, including microscopy tools, omics analysis, and intravital Laser Speckle Contrast Imaging (LSCI), to examine ionic, subcellular, and macrostructural changes within 5 min after TBI. The results fill an important knowledge gap in TBI by revealing the initial dynamics of calcium, which activate key phosphorylation pathways and weaken membrane bilayer phospholipids, ultimately progressing toward a secondary inflammatory response.
Materials and methods
Airblast system
The airblast gun utilized to deliver pneumatic pressure transients was a modified version of the previously described setup20. Briefly, the pneumatic mechanism of the marker (Empire Mini GS, MI, USA) was left intact. A custom barrel was designed and fabricated to both accommodate the distance to the imaged field (barrel length: 19.05 cm), as well as constrain the exit aperture at the tip of the barrel (continuous internal taper to exit aperture of ø=6.5 mm). The marker was additionally adapted to accept ultrapure N2, rather than mixed air via a custom-adapted stainless steel braided [high-pressure] hose (A-L Compressed Gas, Inc., TN, USA), dual-stage N2 regulator, and ultrapure N2 floor standing cylinder (A-L Compressed Gasses, Inc., TN, USA). To position the marker reproducibly, a custom bracket was fabricated to bolt its main body to a manual precision rotation stage (UTR-120, Newport Corporation/MKS, CA, USA). This setup was given an additional two degrees of freedom via pivot support (XT95P4, ThorLabs, NJ, USA) that could be vertically positioned along a 95 mm optical construction rail (XT95B, ThorLabs, NJ, USA) in combination with a rail clamp (CX95A, Newport, Inc., CA, USA). The entire assembly could either be directly bolted to the optical table or bolted to a small breadboard to confer portability and positioning flexibility. Blast pressure and temporal profile were empirically characterized at a range of distances and angles from the barrel tip using a pressure transducer (Sensotec model-STJE Honeywell, Morristown, NJ, USA). Data acquisition was performed using a custom Labview (National Instruments, TX, USA) script. For each blast, the N2 tank’s regulator (at the output stage) was set to 400 psi, with the marker’s internal regulator at 200 psi, resulting in 15–25 psi pressures at the transducer.
Animal Preparation and TBI exposure
Adult male C57Bl/6 or C57BL/6J-Tg (Thy1-GCaMP6f)GP5.17Dkim/J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA), and before blast exposure, mice were anesthetized with urethane. The mouse was positioned on a stereotaxic frame, and the skull flap was cut open to expose the skull. All animal experiments were performed on the right somatosensory cortex. A craniotomy window was made between the midline, lambda, and bregma, ranging in diameter from 3 to 5 mm, leaving the dura intact on the right somatosensory cortex region. The craniotomy window was positioned at 1 cm, angled about 45 degrees to the barrel of the airblast gun. Real-time imaging experiments (calcium and vascular imaging) were performed only with 15 psi blast exposure to minimize motion artifacts during imaging; ex-vivo experiments were performed on output pressures of 15, 20, and 25 psi. The experimental timeline and microscope mount for imaging is depicted in Figure 1. Pressures were measured before and after each blast exposure to validate the applied blast pressure. Animals were euthanized at the end of each experiment by an overdose of isoflurane inhalation. This study was conducted in accordance with ARRIVE guidelines.
Two-photon GCamp-6f imaging of calcium dynamics
A high-performance genetically encoded Ca2+ sensor, GCaMP6f mice, was used to record real-time calcium transients with 2-photon fluorescence microscopy. GCaMP6f imaging was performed on n = 5 mice in vivo underneath the non-linear imaging arm of the Multimodal Advanced Non-linear and Thermal Imaging System (MANTIS)21. A 200-fs laser pulsed at 80 MHz (Insight DS+, Spectra-Physics, CA, USA) was tuned to 920 nm to excite GCaMP6f fluorescence. The laser power at the sample was measured to be 20 mW. Imaging was performed in layer I of the cortex. Fluorescence emission was detected after filtering with a 593 nm long pass dichroic mirror and 525/50 nm bandpass filter (Semrock, NY, USA) using a photomultiplier tube (PMT) (Thorlabs, NJ, USA) biased with a gain of 0.663 V. Image acquisition was performed using ThorImage 4.2 (Thorlabs, NJ, USA). 512 × 512-pixel images were acquired through a 20 × 1.0 NA water dipping objective (Olympus XLUMPLN, Thorlabs, NJ, USA), providing a spatial resolution of 1.266 μm/pixel. Pixel dwell time was set to 2 µs with a 3x frame average, yielding a 0.9 Hz frame rate. We developed a custom 3D-printed head plate with a window diameter of 5 mm for cortical imaging over the right somatosensory cortex to reduce motion artifacts. The transgenic expression was homogenous and readily observed in the superficial cortical layers. Images were taken before and after a 15-psi air blast in vivo. Pre-blast images were used as the baseline of the GCaMP6f signal. Post-blast images were acquired every minute for 5 min.
Raw data acquired during imaging were processed using a custom FIJI script22. Image processing pipelines differed slightly for pre- and post-blast images because they were taken from different fields of view (FOV). Pre- and post-blast images were used to generate a mask to isolate the GCaMP6f signal to regions in the tissue, excluding blood vessels. The noise2noise plugin (Mannam 2022) was used to denoise the images for pre-blast images. Next, a 5-pixel Gaussian blur was applied to smooth the images. The grayscale fills holes function was subsequently used to group pixel values. Next, the images were segmented by applying a threshold that excluded large blood vessels where no GCaMP6f signal was present. A single region of interest (ROI) was extracted from the segmented images and then applied to the original raw image. Post-blast time-series images were concatenated into a time series image stack. A minimum intensity image was projected from the stack and subjected to the same processing pipeline as the pre-blast images. The resulting ROI was then applied to the raw image stack. The standard deviation of gray values within the ROI of the raw images was measured and used as a contrast metric, independent of imaging depth. Standard deviation (σ) was measured within the ROI and plotted as ROI s. Statistical analysis was performed in MATLAB using a Student’s two-sided t-test.
Phosphoproteomics
C57B6 wild-type mice (n = 3 each group) were subjected to craniotomy on the right side of the skull centered on the somatosensory cortex. The exposed brain was then subjected to 15 psi airblast, and cortices were extracted at a 5-minute time point and immediately frozen on dry ice. Each group had n = 3 mice. If a protein was not detected in at least one of the TBI samples or one sham sample, it was excluded from the analysis. Tissues were homogenized with 50 mM ammonium bicarbonate in 5% SDS with 1X protease inhibitor cocktail and 1X phosphatase inhibitor cocktail, and the lysate concentrations were measured by a Bradford assay and normalized to a final concentration of 2 mg/mL. Sample loading to the column, TiO2 enrichment of phosphopeptides, and LC-MS/MS analysis were done following the previous publication23. Following database searching and peptide quantitation using Maxquant (Max-Planck-Institute of Biochemistry, Germany), the frequency distribution of log2 H/L peptide ratios was plotted in GraphPad Prism 6.0 (La Jolla, CA). Log2 ratios followed a normal distribution and were fit using least squares regression. The mean and standard deviation derived from the Gaussian fit were used to calculate p-values using Z-score statistics. Calculated p-values were subsequently corrected for multiple comparisons by the Benjamini-Hochberg (BH) method.
Ingenuity Pathway Analysis software (IPA®, QIAGEN, www.quiagen.com/ingenuity Toronto, Ontario, Canada) was used to identify the most significantly downregulated pathways in response to compound treatment. The Ingenuity Knowledge Base (genes only) was used as the reference set, and relationships considered for affected networks included direct and indirect relationships. The IPA package generates a p-value for each pathway using Fisher’s exact test, which corresponds to the probability that a given pathway is affected by random chance, and the p-value is then converted to a pathway score. In brief, the calculated p-value of a given pathway is converted to the negative log of the p-value, such that the higher the score (-log(p-value)), the more significant the affected pathway. The proteins with an enrichment ratio of greater than 2-fold were selected for further analysis to represent upregulation or downregulation. The identified proteins were analyzed with DAVID online platform (https://david.ncifcrf.gov/)24 to derive KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways (MAPK signaling pathway (map04010), Calcium signaling pathway (map04020), cAMP signaling pathway (map04024), Endocytosis (map04144)25. The final list of identified phospho-enriched proteins was analyzed with Search Tool for the Retrieval of Interacting Proteins (STRING), which displays interactions between protein products of the genes.
Cytokine array
The quantification of pro-inflammatory cytokine concentrations and intracellular pathway protein concentrations was made via multiplex analysis to detect multiple cytokines in a single sample at the same time The right somatosensory cortex tissue at the site of injury spanning about 5 mm x 5 mm cube was excised within 5 min of TBI from Sham, 15, 20 and 25 psi exposed animals (n = 5 each group). The tissue was weighed and immediately frozen in liquid nitrogen. The tissue was homogenized with 1 ml of lysis buffer (Cell Signaling Technology Cell Lysis buffer (#9803) supplemented with protease and phosphatase inhibitor cocktail in a chilled mortar and pestle. The lysate was incubated on a rocker at 4 °C for 15 min. Subsequently, the samples were centrifuged at 10,000 g for 10 min at 4 °C, and the supernatants were collected. Protein quantification was performed with BCA assay (Pierce BCA Protein Assay, Thermo Scientific). The tissue lysates were then adjusted to 1 mg/ml total protein using the same lysis buffer. Pro-inflammatory cytokines such as TNFα, IL-1β, IL-1α, and IL-6 were quantified using Milliplex Panel RECYTMAG-65 K (Millipore Sigma, Burlington, MA, USA) by the Vanderbilt Hormone Assay & Analytical Services Core.
In situ imaging mass spectrometry analysis
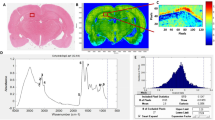
Five minutes after TBI, the ipsilateral right brain tissue was collected immediately and snap-frozen on dry ice. The fresh frozen brain tissue was fixed on a cryostat chuck (Leica CM 3050 S, Leica Biosystems, CA, USA) with optimal cutting temperature polymer (OCT), and 12 μm thin sections were obtained. Two serial sections were processed for positive and negative modes of imaging mass spectrometry, and a third serial section was reserved for H&E staining to validate physical damage to the tissue (Supp. Figure 2). The two sections used for mass spectroscopy analysis were mounted on the indium-tin-oxide (ITO coated slide (Delta Technologies, Ltd., Loveland, CO, USA)). The collected tissue section was saturated with nitrogen gas and stored at 180 °C until analysis. The slides were allowed to stabilize at room temperature and sublimate on a sealed benchtop desiccator. 1,5-Diaminonaphthalene (Sigma-Aldrich, Burlington, MA, USA) was used as the matrix for MALDI imaging. Before applying the matrix, the tissue sections were rinsed with cold ammonium formate (50mM) thrice for 5 s. Approximately 100 mg of the matrix was applied to the bottom of a custom sublimation apparatus and allowed to sublimate onto the tissues at 130 °C and 25 mTorr for 4 min. Lipid images were acquired on a Solarix 15 T FT-ICR MS (Bruker Daltonics, Billerica, MA, USA) equipped with Smartbeam II Nd: YAG laser run at 2 kHz and 355 nm. The serial sections were analyzed in positive (protonation) and negative (deprotonation) mode at 75 mm spatial resolution. Each slide consisted of four sections: one section from the sham and one each from the airblast exposed group − 15, 20, and 25 psi, respectively. The negative ion mode charged the analyte through deprotonation, while the positive ion mode charged through protonation. The tissue sections were sectioned, prepared, and analyzed in parallel to avoid intergroup variation. Five hundred shots were acquired per spot, with the stage moving in a random smart walk at each pixel. Data were acquired over the mass range from 345 to 2,000 m/z. Each run was externally calibrated using red phosphorous in positive and negative ion modes. After data acquisition, data were recalibrated internally using known abundant lipid species, resulting in mass errors of generally 5 ppm. The signals were curated by eliminating isotropic and low-intensity signals, resulting in 8 differentially expressed signals in the positive and 4 in the negative modes. These signals were searched against the LIPID MAPS database 926 with a mass window of 60.01 amu (autonomic mass unit) (13 ppm mass error) to provide tentative identifications of relevant lipid components.
Imaging mass spectroscopy reveals a unique lipid signature in positive modes. Positive MALDI-TOF images of mouse brain sections in each row display lipid signatures as indicated at a specific m/z value. The mass spectrum of individual groups of animals, Sham Vs TBI, is shown in sequence. Each image reveals the spatial distribution of distinct lipid molecular ions, utilizing a color scheme to denote the relative abundance of these specific lipids across various X-Y coordinates within the sample. The last panel to the right indicates the intensity of each m/z among the groups.
Small molecule identification of lipids from injured tissues
To obtain more accurate identification of lipids with varying abundances, tissues (n = 3 from each group) from the injury site, specifically the exact craniotomy site (5 mm3, were excised, homogenized, and subjected to lipid extraction and analysis by LC-MS/MS. The total lipids identified from the LC-MS/MS analysis were correlated with the species identified by in situ mass spectrometry. These studies revealed numerous lipids that were immediately localized and identifiable following damage. Briefly, 10% sample homogenates were prepared in 1xPBS using a bead mill, and lipids were extracted using the Bligh and Dyer procedure27; the chloroform layer was dried, reconstituted in MeOH, and analyzed by QExactive high-resolution mass spectrometer coupled with a high-performance LC system (Thermo Scientific, San Jose, CA, USA). Raw data were processed using MS-Dial software28 in lipidomic mode.
Histological validation
Immediately after TBI, the mouse was removed from the stereotaxic frame, and the tissue was collected for analysis at 5 min post-blast after euthanasia (n = 5 in each group). The sham mice were subjected to craniotomy and timed appropriately before organ collection. Brain tissue slices were obtained 5 min after the blast injury, and these sections were subjected to immunohistochemistry to detect changes in cytoarchitecture and assess the health of astrocytes and neurons. and perfused intracardially with approximately 25 mL of saline. Brains were dissected through the cranium and then postfixed overnight in formalin at 4 °C. Tissues were treated in 10% sucrose overnight at 4 °C, then placed in 30% sucrose at 4 °C to await further processing. Following sucrose infiltration, tissues were embedded in optimal cutting temperature (OCT, Tissue-Tek®, Sakura Finetek, USA) compound. A Microm HM 550 Cryostat collected 12 μm thick coronal sections containing the injured site. Sections were immediately transferred to SuperFrost-coated glass microscope slides and stored at 4 °C.
Frozen sections were thawed at room temperature for 15 min, then washed briefly with PBS before blocking in 5% bovine serum albumin, 0.5% Triton-X, and 5% normal donkey serum for 30 min. Primary antibodies (mentioned in Table 1) include rabbit anti-GFAP (1:500) (an indicator of astrocyte cellular integrity), rabbit anti-NeuN (1:1000) (a marker of neuronal damage), or rabbit anti-S100 beta (1:700) (a marker of acute brain damage). The sections were incubated in primary antibody overnight at 4 °C, then washed in PBS with 0.5% Triton-X for 3 × 5 min. Slides were incubated in secondary Alexa Fluor antibodies (Thermo Fisher Scientific, MA, USA, 1:1000 or 1:500) at room temperature for 2 h, then washed in PBS with 0.5% Triton-X for 3 × 5 min. Following washes, slides were mounted with Fluoromount-G with DAPI. Negative control slides were processed identically apart from excluding the primary antibody. Slides were imaged on a Nikon Eclipse Ni-E microscope (Nikon Instruments Inc., Melville, NY, USA) using a 20x objective. For purposes of comparative quantification, images of the right somatosensory cortex were taken approximately 1.1 mm distal to the central sulcus initially and then in spatial succession across the injured site, covering in total approximately 2.8 mm of the somatosensory cortical surface. The immunoreactivity of individual astrocytes, identified by their classic morphology, was quantified across the sham, 15, 20, and 25 psi blasted groups. Positive immunostaining was quantified using ImageJ.
Raman mapping
High-resolution Raman images were generated for brain sections obtained from sham, 15 psi, and 20 psi exposed groups of animals; the imaging area was restricted to the 5 mm injury site on the right somatosensory cortex. Frozen brain sections about 10 μm thickness were collected on calcium fluoride (CaF2) slides and briefly fixed for 2 min with 4% PFA and washed three times with 1X PBS. The lingering salts were washed carefully using ultra-pure water (Thermo Scientific). The TBI injured region on the cortical region was visualized using a Renishaw inVia™ Raman microscope (model number- 428K42, Gloucestershire, UK). Brightfield images were captured with a 100X objective, and a 5mmX5mm region of interest within the TBI zone was selected for Raman mapping. Raman maps were collected with a 785 nm laser (10 mW) and 1200 lines per mm grating, using a step size of 5 μm and a 10 s acquisition time per spot. Raman spectra were collected within the fingerprint region of 600–1725 cm−1. The acquired spectra were pre-processed with methods described in the literature29. First, cosmic ray removal was performed with nearest neighbor method using the Renishaw WiRE 3.4 (Renishaw, IL, USA). Next, a custom R-Studio software code was used to perform noise smoothing, fluorescence background correction, and normalization30. Smoothing was performed to reduce the noise in the Raman spectra, using a Savitzky-Golay filter31 with a fifth-order polynomial within a seven-point moving window. Next, fluorescence background subtraction was performed using asymmetric least squares method (lambda = 4; p = 0.0005), followed by spectral mean normalization of the individual spectra acquired.
Laser speckle contrast imaging
The LSCI system employs a 785 nm diode laser from Innovative Photonics Solutions (Monmouth Junction, NJ), coupled through a 400 μm fiber optic patch cord (Thorlabs in Newton, NJ, USA), and directed through a lens tube that contains a 75 mm focal length biconvex lens from Thorlabs. The lens diverges the laser light to a spot approximately 3 cm in diameter at 20 cm from the lens. The surface irradiance was determined to be approximately 0.6 mW/cm2. An infrared-compatible camera (C14440-20UP, Hamamatsu Photonics in Bridgewater Township, NJ, USA) is used to capture images, and the camera is arranged vertically with the angle between the illumination beam and the camera’s line of sight fixed at approximately 30° to reduce specular reflection. An objective lens and corresponding tube lens (UPLFLN 10X, Center Valley, PA, USA) are attached to the front end of the lens system. This technique generates a spatial contrast map that correlates to the speed of flow. The spatial contrast, Ks,
Note here, σs represents the spatial standard deviation of the speckle intensity, and < I > denotes the average of the speckle intensity. The speckle image was convolved with a 5 × 5 or 7 × 7 pixel box to generate a spatial contrast map using the equation above. After craniotomy, animals were imaged before the blast injury (n = 5) as baseline blood flow with LSCI imaging and reimaged after inflicting a 15 psi airblast at the same field of view to record the cerebral blood flow for 120 s.
Vascular diameter measurements
Fluorescein isothiocyanate 0.1 ml was injected (2,000 kDa) through the tail vein to visualize the cerebral vasculature (n = 5). After 15 min of intravenous delivery, animals were anesthetized and subjected to pre and post-injury imaging on a wide-field microscope. Time series videos capturing the prominent vessels, including pial and parenchymal vessels in the right somatosensory cortical region, were recorded at a rate of 1 frame per second pre and post-injury. The microcirculation and vascular diameter changes were estimated by monitoring the blood vessels under the microscope. Representative time traces from 3 min of continuous recording and imaging at one location show vasculature was identified with FITC fluorescence and imaged continuously without delay at a frame rate of 50 ms. Immediately after the blast (approximately 15 s), the water immersion objective was placed on the tissue and continued to image for 3 min. Images were processed with NIS elements (Melville, NY, USA), denoised, and aligned to minimize motion artifacts. An intensity profile was drawn across a major vessel identified as an artery (featuring straight morphology). The branching points were taken as a reference for measuring the diameter changes pre- and post-blast. Full-width half-max intensity was measured every 15 s of pre- and post-stimulus. N = 6 animals were included in this experiment.
Statistical analysis
Differences between the two groups were analyzed by pairwise comparison, and multigroup comparison of physiological background between groups was assessed by one-way ANOVA followed by Bonferroni post hoc analysis to correct for multiple comparisons. A p values below 0.05 were considered significant. Data was summarized as mean ± standard error of the means (SE). Phosphoproteomics statistical analysis was performed using R software.
Results
Calcium dynamics evoked by airblast TBI (5–10 min post TBI)
The GCaMP6f expression was homogenous and readily observed in the superficial layers of the somatosensory sensory cortex (n = 4). Baseline fluorescence before TBI exposure was low and homogenous (Fig. 3A). All four independent trials exhibit a consistent trend characterized by a sharp rise in the mean fluorescence within the ROI from 1720 ± 201 A.U. before TBI to 2658 ± 502 A.U., five min after injury (Fig. 3B). Likewise, the standard deviation of the fluorescence within the ROI increases before injury from σ = 58 ± 9.9 to σ = 382 ± 157 5 min after injury as seen in Fig. 3C. The GCaMP6f fluorescence plateaus remain elevated for 5 to 10 min after TBI exposure. The active Ca2+ fluorescence does not return to pre-blast levels during the entire imaging period, which lasts up to 10 min post-TBI. Among the four trials shown in Fig. 3C, trial 1 shows an abnormal background Ca2+ fluorescence compared to trials 2, 3, and 4: this unusually higher fluorescence was observed in the one case (trial 1) where bleeding was observed at the site of TBI.
Increase in calcium levels in superficial layers of the somatosensory cortex triggered by the airblast TBI. (A-B) Contrast normalized images of in vivo GCaMP6f signal before and t = 5 min after TBI, respectively, from a representative mouse. The gray value’s standard deviation (σ) was measured within the yellow ROI. Measurements excluded regions where vasculature occluded GCaMP6f fluorescence. (C) Measurements from 4 trials show statistically significant changes in the GCaMP6f signal after TBI (p > 0.05 for t = 5, 6, 7, 8, 9 min; not significant at t = 10 min).
Immunohistology evidence of classical blast injury markers
Visible signs of hemorrhage or hematoma were observed in the ipsilateral somatosensory cortex of animals exposed to the highest peak pressure of 25 psi. However, the groups subjected to pressures of 15 psi and 20 psi did not exhibit any visible changes. We observed a slight increase in GFAP-positive area in blast-injured brain sections from all three groups compared to the sham group (Fig. 4A &B). A significant decrease in the intensity of S100B immunoreactivity was noted within each astrocytic cell (Fig. 4C &D). Two types of NeuN-positive neurons were detected: “filled” cells (immunoreactivity to the entire nuclear region) and “edged” cells (immunoreactivity pertaining to the periphery of the nucleus). All the TBI groups showed a significant shift (p < 0.001) increase in the number of edged cells NeuN (Fig. 4E &F).
Immunohistochemical evidence of cellular changes after TBI. (A) Individual astrocytes labeled with GFAP showed increased immunoreactivity. (B) Mean fluorescent intensity was measured per field of view. (C) Immunohistology of S100B marker in astrocytic cell bodies, (E) a decreased S100B localization was noted in all three TBI groups. (D) NeuN localization in mature neurons tends to have uniform localization of the antigen throughout the nucleus (normal cell); however, after TBI, this neuronal marker segregates to the nuclear membrane (faint cells), as shown by yellow arrows. (E, F) Faint cells in a field of view were significantly increased in the injured areas within 5 min of injury.
Combined phosphoproteomics and proteomics analysis captured the molecular signaling profile of airblast TBI
The relative abundance of phosphorylated and dephosphorylated proteins in the sham and 15 psi blasted somatosensory cortical tissue was determined by LC-MS/MS From the whole proteome samples of 977 phospho-enrichment (Table 2.), 277 proteins showed a 2-fold change in the 15 psi group compared with respective to the sham group; of these, sixty-two were dephosphorylated, and sixty were phosphorylated, accounting for rapid signaling events (Fig. 5A, B). The prominent proteins are encircled with green on the volcano plot in Fig. 5A. The proteins displaying a significant fold change greater than 2 (equivalent to a twofold increased/decreased in expression), and a statistical significance indicated by a p-value less than 0.05 (n = 3 each group) were shortlisted, this highlights the differences in the levels of protein phosphoenrichment between the sham and the15 psi group. Specific phospho-sites are indicated in Table 2.. Enrichment analysis was performed using STRING analysis (Fig. 5C), which revealed a pattern of activation of canonical pathways relating the protein phosphorylation to downstream pathways. The top molecular functions, including protein binding, ATP binding, macromolecular complex binding, actin binding, and calmodulin binding, are listed in Figure. 4D. Serine residues were more prone to phosphorylation changes, accounting for 77.61%, followed by threonine residues, which accounted for 22.39% (Fig. 5E, Supp. Figure 1, 3 &4).
The molecular signaling profile of airblast TBI was comprehensively captured by phosphoproteomics analysis. (A) Volcano plot showing the magnitude and significance of differential phosphopeptide abundance between sham and 15 psi exposed somatosensory cortex at 5 min post-exposure. Significant protein phosphorylation patterns are encircled in green. (B) The degree of phosphorylation and dephosphorylation for each identified protein is indicated in fold change. Red bars represent phosphorylation, while green represents dephosphorylation. (C) STRING analysis reveals intuitive bioinformatics visualization of the connectome between different phosphorylation patterns identified after the 15 psi blast Vs. Sham group. The network generated by STRING omitted the isolated genes; each node’s color is irrelevant, but the lines connecting each node have significance. Blue lines indicate the interactions in the database, whereas pink lines indicate the interaction was experimentally proven. (D) Proteins are sorted based on biological processes using Gene Ontology (GO) enrichment analysis, which is altered immediately in an airblast TBI. (E) Serine residues were prone to most phosphorylation changes, followed by threonine.
The highest order of dephosphorylation was observed with Ser/Thr protein phosphatase 1G, a negative regulator of the cell stress response pathway, which was significantly dephosphorylated by 7.26-fold (Table 2.). A gene set enrichment analysis was carried out from the protein abundance data, identifying changes in pathways associated with calcium, phospholipid, and actin regulation. Five significant dephosphorylated proteins with the most remarkable fold change in TBI (15psi) Vs Sham included cell cycle exit and neuronal differentiation protein (Cend1), Protein phosphatase 1G (Ppm1g), microtubule-associated protein (Mapt), Ankyrin repeat domain-containing protein 63 (Ankrd63), Aster-B (Gramd1b), Hepatocyte cell adhesion molecule (Hepacam) fold and L1 cell adhesion molecule (L1cam) (as seen in Table 2.). It is also to be noted that no changes were observed in the cytokine profile (Supp. Figure 6).
Raman maps on the injured ipsilateral cerebral cortices. (A) Mean spectra measured the corresponding spectra of the injured somatosensory cortical region of groups sham, 15 psi, and 20 psi. The averaged spectra show distinct regions of differences indicated with dotted lines and shaded areas. For clarity of display, spectra were offset from the baseline. The peak assignments were determined based on findings from the literature. Figures B, C, D, E, F and G display the analysis of peak ratios in Raman spectroscopy, where the peaks are normalized with the phenylalanine peak at 1004 cm−1. We identified specific peaks that exhibited distinct changes and compared them across the three groups.: viz. 1269 cm−1, 1665 cm−1, 1299 cm−1, 1660 cm−1, 1271 cm−1, and 1552 cm−1, respectively. Data from the 25 psi group were excluded from Fig. 6 due to variability across replicates, demonstrating poor repeatability. Therefore, only groups meeting quality control criteria are shown.
Mass lipidomics/imaging mass spectroscopy reveals unique lipid signatures
Sagittal sections of brain tissue were analyzed using Matrix-Assisted Laser Desorption/Ionization (MALDI) Imaging Mass Spectrometry (IMS) paired with microscopy to evaluate the relative abundance and spatial distribution of lipids in cortical tissue post-TBI (Region of Interest analysis). The spatial distribution of modified lipids in the right cortical region is shown in Fig. 2 in sham and TBI groups. Reconstructed ion images of m/z values show differential expression of m/z 856.585 and 896.488. However, there is no trend in intensity across sham and all three TBI groups. The most prominent lipid feature at m/z 918.466 indicates Hexosylceramide (Hex2Cer) 16:1;2O/22:0, decreasing from 25 to 15 psi (maximum pressure tested). The small molecule lipidomic analysis (Table 3) showed the increased presence of Hex2Cer in the injured somatosensory cortex of the 25 psi group. Tissue pixel intensities of m/z 753.5 (PE), 756.5, 793.4 (SM), 804.5 (PC), 868.4 (BMP), 912.4 (DGDG), and 963.4 show a unique pattern of either decreased/increased intensities in TBI groups compared to sham groups. While the negative mode lipidomics did not show statistically significant changes, this result is still impactful. In negative-ion mode, the dominant ionization event is typically the loss of fatty acyl substituents. The lack of observable changes in this mode suggests that the fatty acyl chains remain relatively unaffected by the injury. This absence of deprotonation events contrasts with the protonation dominance (Fig. 2) observed in the positive-ion mode, providing important insights into the stability of fatty acyl components post-injury. Supp Fig. 5 compares the negative mode lipids in TBI Vs. Sham by visualizing five lipid features related to m/z 744.555 (Cer), 747.498, 774.546 (PE), 790.540(PE), and 857.521(PG). Interestingly, the regional distribution of negative ions was relatively abundant in the sham somatosensory cortex compared to the TBI groups. In other words, these molecular species were substantially reduced in the cortical tissue after a mechanical TBI.
Ex-vivo Raman map highlights vibrational features of blast injury
The average normalized Raman spectra (Fig. 6A) show a difference in peak shapes at 1440–1445 cm−1, corresponding to CH2-CH3 bending of collagen or phospholipids. Likewise, the spectral region between 1660 and 1665 cm−1 corresponding to lipids/proteins (Amide I in proteins, c = c groups of unsaturated fatty acids, and ceramide backbone) shows a decrease in the FWHM of the peak. The phenylalanine peak at 1004 cm−1 when comparing the sham and 20 psi exposed group also displays a reduction in the peak intensity. Figure 6B-G corresponds to Raman peak ratio of collagen (1296 cm−1), collagen (1665 cm−1), lipid (1299 cm−1), C = C groups in unsaturated fatty acids/Amide I band (1660 cm−1), Amide III (1271 cm−1) and Amide I/Tyrosine, Tryptophan C = C (1552 cm−1) respectively with respective to phenylalanine 1004 cm−1 peak. All Raman peak ratios indicate significant biomolecular bond changes at 20 psi (p < 0.001) compared to the Sham group. Among the Raman peak ratio listed above, only the Amide I/Tyrosine, Tryptophan C = C (1552 cm−1)/Phenylalanine 1004 cm−1 showed a significant change (p < 0.001) at 15 psi exposure.
Vascular changes
Cerebral Blood Flow (CBF) was evaluated with LSCI and FITC-tagged fluorophore imaging to monitor cortical perfusion in vivo following a 15-psi blast injury in Fig. 7. The raw speckle image illustrates the color-coded distribution of speckle contrast. Pre-blast images show good perfusion of the primary vessel of interest, as reduced speckle contrast indicates. Post-blast acquisitions show an increased contrast, indicating reduced blood perfusion. A drop-in perfusion rate was observed until 120 s after the blast injury (Fig. 7A, B). Increased contrast in the speckle pattern confirmed the immediate decrease in blood flow.
Vascular changes followed by LSCI and FITC injected imaging- (A) Pre-blast and post-blast images are color-coded based on perfusion levels. Reduction in blood flow after TBI is evident in contrast images. Substantial reduction in blood perfusion in background tissue areas is also apparent. (B) Time course speckle contrast data shows reduced blood flow. Reduction in blood flow is evident seconds after blast injury. (C) Vascular images after FITC BSA labeling pre- and post-blast. Cross-sectional profiles were extracted across each major vessel of interest at every time. (D) Full-width half-max values of vascular diameter followed over 180 s. Pre-blast data points are indicated in black, and post-blast data points are in red.
Pre- and post-blast vascular changes in the cortical window under study were continuously imaged for FITC fluorescence for 10 min under the wide field 10X air objective, and a major artery was counted as a fiducial for field registration. The full-width half maximum of each vessel’s diameter was measured with NIS Elements software, and a Gaussian function was fitted for each frame. The distribution over time was extracted to measure the vessel diameter. A full-field image (Fig. 7C, D) of a selected vasculature pre- and post-blast injury, 30 s apart from impact, briefly shows a constriction in blood vessel diameter before returning to baseline. A constriction in vessel diameter was observed with time, with a decrease in the vessel diameter occurring until an average of 45 s post-blast and then recovering to a normal size/diameter later (Fig. 7C, D). Two out of six animals showed embolism in the arteries post-injury at 15 psi of blast injury.
Discussion
This study presents the immediate molecular responses to blast-induced injury within minutes, addressing previous limitations in understanding immediate TBI-associated damages. Calcium ions are known to be essential second messengers that regulate the activities of many proteins and are associated with neuronal death and other forms of brain injury, including stroke and status epilepticus. In fact, XX et al. have shown that CA3 hippocampal neurons isolated after fluid percussion injury exhibit significantly elevated calcium levels from day 1 to day 30 following injury32. However, our results indicate that this increase in Ca2+ occurs within 5 min of injury in cortical neurons. This early increase in Ca2 + portends to long-term changes in membrane excitability, synaptic remodeling, and structural plasticity33,34. Our data suggests that this increased Ca2+ triggers a series of downstream events, including the phosphorylation of signaling molecules and changes in membrane lipids. Given the importance of calcium in post-injury process, the application of calcium channel blockers provides an appropriate avenue for therapeutic intervention as indicated by the clinical trials on calcium channel blockers. However, its effectiveness remain uncertain35. We hypothesize that the treatment in the reported clinical trials was delivered too far down the injury path, missing the right window to halt the calcium-related downstream pathways. The rapid activation of several degradative enzymes, such as proteases and endonucleases in the present study implies that the modulation of these enzymatic kinase and phosphorylase activities is through an increase in phosphorylation or decrease in dephosphorylation. The phosphoprotein dynamics reflect the transient/instantaneous nature of protein phosphorylation post-TBI and define the importance of timely intervention before these pathological responses are triggered, which might be a potential therapeutic opportunity.
Our data in injured brain slices allude to alterations in lipids as a first response to TBI. In particular, a disturbance in membrane lipids is demonstrated by changes in Hex2Cer, PE, PG, PC, and PI-Cer. Charged phospholipids such as PE are known to be localized at the inner leaflet of the membrane36. The detection of a rapid conformational reorganization of lipid signatures fits with the understanding that membrane disruption is a classical and well-established mechanism of TBI37. The specific fatty acid chains can influence the properties and functions of the molecules within cellular membranes and affect lipid metabolism pathways. Recent studies have categorized the TBI plasma lipidome 30 min to 24 h after injury, uncovering the presence of distinct sphingolipids and glycerophospholipids in the plasma. However, the lipid signatures identified in plasma38 were different from the tissue lipidomics presented in our study. The lipid signatures we identified using small molecule lipidomics following blast trauma compared to the sham likely indicates that the first response to a TBI emerges from the lipid bi-layer of cell membranes (Table 3). Neuronal membrane disruption was also observed in the biphasic pattern of NeuN protein labeling. A similar observation was made on a weight drop impact acceleration-induced mild TBI model, where NeuN expression in mature neurons was significantly reduced in less than 5 min after TBI. Thus, our data indicate rapid alterations in membrane properties and demonstrate that trauma induces both mechanical and biochemical changes to the lipid bilayer. This fits with prior literature showing that mechanical injury of TBI changes the biophysical properties of cell membranes and individual lipids, which contributes to membrane rigidity or fluidity39. Cell membranes can quickly repair themselves by remodeling and resealing when injured. However, this healing process may face challenges due to an overwhelming secondary response that occurs after TBI. These changes observed in the injured brain provide evidence that immediate membrane damage to the lipid bilayer accumulates over time, compounded by secondary damage, resulting in massive neuroinflammation and subsequent leaching of these lipid molecules in the bloodstream hours after injury. Exploring and developing acute methods for more effectively sealing the damaged membranes early holds significant potential in providing a promising management strategy.
We also detected decreased cytoplasmic localization of astrocytic S100B. Elevation of S100B in peripheral body fluids is considered a promising biomarker of TBI40. Changes in S100B shown here, therefore, justify the use of this protein as a biomarker for TBI and for clinical decision-making41. Finally, we detected an increase in GFAP immunoreactivity in 5 min; this increase is too fast to be attributed to increased gene expression or protein translation but could be due to an immediate influx of Ca2+. These findings align with research by Lee et al.42where astrocyte monoculture showed increased immunoreactivity of GFAP after exposure to Ca2+ inhibitors due to extracellular acidosis and exposure to antigenic sites.
TBI is known to be a heterogeneous condition with neuronal loss and damage to the vascular components and extended neovascular unit. We detected changes in the rate of perfusion and vessel diameter immediately after blast injury. However, the macrostructural vascular changes observed in this study were transient, in a minor subset, offering limited insight into classifying ischemia-related damage in TBI. Relative cortical blood flow was significantly dampened, which may lead to compromised vascular components that could affect local neuronal activity. This matches changes in cerebral blood flow reported by others at regional and global levels during acute post-traumatic periods from 0 h to 2 weeks43,44,45,46. A decrease in cerebral blood flow reflects the severity of the primary brain injury and can be linked to an altered metabolism, which could translate into ischemia or poor perfusion in the surrounding region. Although embolism has been previously reported, rapid vascular constriction does not always signify pathology47. Initial injury can cause dynamic alterations in the parenchyma and blood vessels, with repeated stretch and strain possibly adding to the observed effects. The exact mechanism for vessel constriction and reduced perfusion is unknown, a quick onset of edema rapidly increases intracranial pressure; this increase in pressure could be one of the reasons for impaired blood flow after TBI48.
Developing therapeutic interventions that arrest membrane disruption and lipid modification, could potentially slow or eliminate secondary effects. While significant knowledge gaps remain, these results highlight the need to re-evaluate current clinical treatments. Multiple Phase II clinical trials involving treatments such as progesterone, Tirilazad mesylate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, Selfotel, Traxoprodil, magnesium sulfate, synthetic cannabinoids, monoaminergic substances, and cyclosporine have failed to show efficacy in treating TBI49. Directly treating the membrane changes and associated pathophysiological events that occur within the first few minutes after injury may result in better therapeutic outcomes based on the current understanding of the critical window of opportunity50.
Conclusion
Our results reveal that within 5 min of a TBI, there is (1) an increase in intracellular calcium ions, (2) spontaneous phosphorylation of intracellular signaling pathways, (3) stretching and flexing of lipid moieties in the cell membrane especially at the site of injury, (4) mislocalization of classical TBI markers such as neuronal NeuN and S100B, and (5) a significant constriction in blood vessel size. Thus, TBI elicits a complex multimodal response within five minutes of the injury. A simultaneous pleiotropic/multi-tiered therapeutic strategy may be the solution for TBI intervention, potentially providing the best therapeutic window to halt further progression. Currently, no single pharmacological compound has made it to clinical use, indicating that a key piece of the puzzle is missing. This missing element could relate to a morphological perspective, adequate dosing, the kinetic component of the mechanical force being translated into a biological response, or perhaps the appropriate timing of treatment.
The study has a few limitations: all the experiments were conducted using male subjects, which restricts the generalizability of the findings across sexes. Additionally, our histological, lipidomics, and phosphoproteomic analysis was restricted to the cortical region (5 mm cubic volume) without investigating deeper brain structures, which will be a focus of future studies. Another limitation is the inability to perform real-time imaging within the shock tube setup, so we opted to use the airblast setup for this study.
Our results push us to consider that given the complexity and variability of TBI pathology, there may be other treatment options than identifying a single therapeutic target. A more comprehensive approach would be to employ a multi-pronged/tiered strategy, addressing primary and secondary aspects of the injury and its consequences and translating these interventions to clinically relevant timeframes. This multifaceted approach could be the next logical step in developing effective TBI treatments.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Chen, Y. et al. WTAP participates in neuronal damage by protein translation of NLRP3 in an m6A-YTHDF1-dependent manner after traumatic brain injury. Int. J. Surg. 110, 5396–5408 (2024).
Deng, H. et al. Time to follow commands in severe traumatic brain injury survivors with favorable recovery at 2 years. Neurosurgery 91, 633–640 (2022).
Le, T. D. et al. Trends in traumatic brain injury among U.S. Service members deployed in Iraq and afghanistan, 2002–2016. Am. J. Prev. Med. https://doi.org/10.1016/j.amepre.2023.01.043 (2023).
Daneshvar, D. H. et al. Leveraging football accelerometer data to quantify associations between repetitive head impacts and chronic traumatic encephalopathy in males. Nat. Commun. 14, 3470 (2023).
Maas, A. I. R. et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048 (2017).
Pellot, J. E. & De Jesus, O. Cerebral Contusion. In StatPearls (StatPearls Publishing, Treasure Island (FL), 2023). PMID: 32965818.
Mesfin, F. B., Gupta, N., Hays Shapshak, A. & Taylor, R. S. Diffuse Axonal Injury. In StatPearls (StatPearls Publishing, Treasure Island (FL), 2023). PMID: 28846342.
Ling, G. S. F. & Ecklund, J. M. Traumatic brain injury in modern war. Curr. Opin. Anaesthesiol. 24, 124–130 (2011).
Su E, Bell M. Diffuse Axonal Injury. In Translational Research in Traumatic Brain Injury (eds Laskowitz, D. & Grant, G.) (CRC Press/Taylor and Francis Group, Boca Raton (FL), 2016). Chapter 3. PMID: 26583181.
Jha, R. M., Kochanek, P. M. & Simard, J. M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 145, 230–246 (2019).
Rasiah, P. K. et al. A Long-Term safety and efficacy report on intravitreal delivery of adipose stem cells and secretome on visual deficits after traumatic brain injury. Translational Vis. Sci. Technol. 11, 1 (2022).
Vázquez-Rosa, E. et al. P7C3-A20 treatment one year after TBI in mice repairs the blood-brain barrier, arrests chronic neurodegeneration, and restores cognition. Proc. Natl. Acad. Sci. U S A. 117, 27667–27675 (2020).
Munoz-Ballester C, Mahmutovic D, Rafiqzad Y, Korot A, Robel S. Mild Traumatic Brain Injury-Induced Disruption of the Blood-Brain Barrier Triggers an Atypical Neuronal Response. Front Cell Neurosci. 2022 Feb 18;16:821885. PMID: 35250487; PMCID: PMC8894613.
Karve, I. P., Taylor, J. M. & Crack, P. J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 173, 692–702 (2016).
Baucom, M. R. et al. Murine traumatic brain injury model comparison: closed head injury Versus controlled cortical impact. J. Surg. Res. 296, 230–238 (2024).
Cernak, I. Understanding blast-induced neurotrauma: how far have we come? Concussion 2, CNC42 (2017).
Zhao, Q., Zhang, J., Li, H., Li, H. & Xie, F. Models of traumatic brain injury-highlights and drawbacks. Front. Neurol. 14, 1151660 (2023).
Stahl, A. N., Artis, E., Ghose, P. & Rex, T. S. System for focal, Closed-System central nervous system injury. J. Visualized Experiments (JoVE). e66948. https://doi.org/10.3791/66948 (2024).
Young L, Rule GT, Bocchieri RT, Walilko TJ, Burns JM, Ling G. When physics meets biology: low and high-velocity penetration, blunt impact, and blast injuries to the brain. Front Neurol. 2015 May 7;6:89. PMID: 25999910; PMCID: PMC4423508.
Hines-Beard, J. et al. A mouse model of ocular blast injury that induces closed Globe anterior and posterior pole damage. Exp. Eye Res. 99, 63–70 (2012).
Adams, W. R. et al. Multi-modal nonlinear optical and thermal imaging platform for label-free characterization of biological tissue. Sci. Rep. 11, 8067 (2021).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9, 676–682 (2012).
Amara, S. et al. Critical role of SIK3 in mediating high salt and IL-17 synergy leading to breast cancer cell proliferation. PLOS ONE. 12, e0180097 (2017).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Fahy, E. et al. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 50, S9–S14 (2009).
Sündermann, A., Eggers, L. F. & Schwudke, D. Liquid Extraction: Bligh and Dyer. in Encyclopedia of Lipidomics (ed. Wenk, M. R.) 1–4Springer Netherlands, Dordrecht, (2016). https://doi.org/10.1007/978-94-007-7864-1_88-1
Tsugawa, H. et al. MS-DIAL: data-independent MS/MS Deconvolution for comprehensive metabolome analysis. Nat. Methods. 12, 523–526 (2015).
Vargis, E., Byrd, T., Logan, Q., Khabele, D. & Mahadevan-Jansen, A. Sensitivity of Raman spectroscopy to normal patient variability. J. Biomed. Opt. 16, 117004 (2011).
Majumder, S. K., Keller, M. D., Boulos, F. I. & Kelley, M. C. Mahadevan-Jansen, A. Comparison of autofluorescence, diffuse reflectance, and Raman spectroscopy for breast tissue discrimination. JBO 13, 054009 (2008).
Chad, A. & Lieber Mahadevan-Jansen, A. Automated method for Subtraction of fluorescence from biological Raman spectra. Appl. Spectrosc. 57, 1363–1367 (2003).
Sun, D. A. et al. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2 + homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 27, 1659 (2008).
Atkins, C. M., Chen, S., Alonso, O. F., Dietrich, W. D. & Hu, B. R. Activation of Calcium/Calmodulin-Dependent protein kinases after traumatic brain injury. J. Cereb. Blood Flow. Metab. 26, 1507–1518 (2006).
Carteri, R. B. Mitochondria as a therapeutic target: focusing on traumatic brain injury. J. Integr. Neurosci. 24, 25292 (2025).
Langham, J., Goldfrad, C., Teasdale, G., Shaw, D. & Rowan, K. Calcium channel blockers for acute traumatic brain injury. Cochrane Database Syst Rev CD000565 (2003). (2003).
Nicolson, G. L. The Fluid-Mosaic model of membrane structure: still relevant to Understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta. 1838, 1451–1466 (2014).
Farkas, O., Lifshitz, J. & Povlishock, J. T. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury? J. Neurosci. 26, 3130–3140 (2006).
Gier, E. C. et al. Lipidome alterations following mild traumatic brain injury in the rat. Metabolites 12, 150 (2022).
Horn, A. & Jaiswal, J. K. Structural and signaling role of lipids in plasma membrane repair. Curr. Top. Membr. 84, 67–98 (2019).
Mondello, S. et al. Blood-Based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: A living systematic review and Meta-Analysis. J. Neurotrauma. 38, 1086–1106 (2021).
Janigro, D. et al. Peripheral blood and salivary biomarkers of blood-Brain barrier permeability and neuronal damage: clinical and applied concepts. Front. Neurol. 11, 577312 (2020).
Lee, Y. B. et al. Rapid increase in immunoreactivity to GFAP in astrocytes in vitro induced by acidic pH is mediated by calcium influx and Calpain I. Brain Res. 864, 220–229 (2000).
Gaggi, N. L. et al. Temporal dynamics of cerebral blood flow during the first year after moderate-severe traumatic brain injury: A longitudinal perfusion MRI study. Neuroimage Clin. 37, 103344 (2023).
Meyer, S., Hummel, R., Neulen, A., Hirnet, T. & Thal, S. C. Influence of traumatic brain injury on ipsilateral and contralateral cortical perfusion in mice. Neurosci. Lett. 795, 137047 (2023).
Wu, Y. et al. Mild traumatic brain injury induces microvascular injury and accelerates Alzheimer-like pathogenesis in mice. Acta Neuropathol. Commun. 9, 74 (2021).
Liu, H. et al. Evolution of cerebral perfusion in the peri-contusional cortex in mice revealed by in vivo laser speckle imaging after traumatic brain injury. Brain Res. 1700, 118–125 (2018).
Hubbard, W. B., Dong, J., Cruz, M. A. & Rumbaut, R. E. Links between thrombosis and inflammation in traumatic brain injury. Thromb. Res. 198, 62–71 (2021).
Greve, M. W. & Zink, B. J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. 76, 97–104 (2009).
Beauchamp, K., Mutlak, H., Smith, W. R., Shohami, E. & Stahel, P. F. Pharmacology of traumatic brain injury: where is the golden bullet?? Mol. Med. 14, 731 (2008).
Chen, S. H., Sun, J. M. & Fang, W. K. The impact of time from injury to surgery in functional recovery of traumatic acute subdural hematoma. BMC Neurol. 20, 226 (2020).
Acknowledgements
This research was developed with funding from the Defense Advanced Research Projects Agency (DARPA). The authors express their views, opinions, and findings. They should not be interpreted as representing the official views of the Department of Defense or the U.S. Government. Imaging mass spectrometry and phosphoproteomics analysis were performed at the Vanderbilt Mass Spectrometry Core, Vanderbilt University. Graphics generated using Servier Medical Art is licensed under CC BY 4.0. Copyright permissions and an open-access license have been obtained for using KEGG data.
Author information
Authors and Affiliations
Contributions
PR: Investigation, Methodology, Data curation, Writing- Original draft preparation. JH: Methodology, Data curation; HD: Methodology, Data curation; RH: Investigation; AL: Investigation, Validation, Review and editing, JLJ: Investigation, EA; Resources, Project Management, CC- Conceptualization, Funding acquisition; BM- Methodology, Data Curation, Validation, Conceptualization, EDJ- Methodology, Validation, Conceptualization, Writing, Funding acquisition, TR- Methodology, Validation, Conceptualization, Writing, Funding acquisition, AMJ- Validation, Conceptualization, Funding acquisition, Writing and review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experiments using mice were carried out in accordance with the recommendations of Vanderbilt IACUC (Institutional Animal Care and Use Committee). The animal protocol was approved by Vanderbilt IACUC (IACUC number- M2100052-00) and all procedures were performed in an AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) accredited facility.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rasiah, P.K., Hardenburger, J., Dong, H. et al. Subcellular and macrostructural immediate responders to airblast traumatic brain injury. Sci Rep 15, 28454 (2025). https://doi.org/10.1038/s41598-025-13288-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13288-6