Abstract
The prevalence of alcohol consumption among the younger generation remains alarmingly high. A hangover is a common short-term consequence observed after consuming alcohol. To effectively study alcohol-induced hangovers, reliable and translational animal models, along with appropriate testing methods, are required. While several testing approaches have been used in hangover-induced mice, they often fail to assess innate behaviors comprehensively and are limited by short observation periods. Although existing studies have developed methods to assess hangover-related behaviors in rodents, few have focused on innate behaviors. This study aimed to establish a model for assessing the innate behaviors of hangover-induced mice using automated home-cage-like behavioral monitoring. Mice were intraperitoneally injected with ethanol at doses of 3, 2, or 1 g/kg, followed by behavioral assessments, including exploratory actions and long-term home-cage-like behaviors during both day and night phases. Results showed a significant reduction in mobile behaviors (climbing, locomotion, rearing), speed, and distance traveled, along with increased immobility in both exploratory and long-term home-cage-like assessments. Furthermore, there was a significant decrease in exploratory behaviors and long-term home-cage-like activities, which were linked to hangover symptoms. This study provides a preliminary approach for assessing hangover behaviors in mice using automated behavioral monitoring, ensuring improved animal welfare, optimised timing, and extended assessment durations. Hence, we propose automated home-cage-like behavioral assessment as an exploratory model for evaluating hangover behaviors in mice, which may serve as a useful tool for future research on the therapeutic efficacy of anti-hangover compounds.
Similar content being viewed by others
Introduction
The prevalence of alcohol consumption worldwide remains significantly high, with rates of 43% in individuals aged ≥ 15 years in both 2016 and 20191,2. Alcohol consumption is particularly concerning among younger individuals aged 20–39 years, with a prevalence of 22%, contributing to a substantial number of alcohol-related deaths1. The dark side of alcohol consumption has been associated with 2.6 million deaths and 115.9 million disability-adjusted life years (DALYs) lost in 20191. Beyond mortality, alcohol consumption increases the risk of several chronic diseases, such as dementia, liver disease, diabetes, and cardiovascular diseases2,3. Furthermore, alcohol consumption leads to short-term effects such as impaired motor coordination, vision and balance disturbances, apathy, drowsiness, dysarthria, unconsciousness, and hangovers3.
A hangover is an unpleasant condition that occurs the day after alcohol consumption, characterized by both molecular and behavioral changes. At the molecular level, hangover development is associated with neurochemical alterations, oxidative stress, and neuroinflammatory responses4. It has been linked to mitochondrial respiratory dysfunction and increased production of free radicals in the cerebellum5, modulation of the glutamate system in the dorsal midbrain6 and nitric oxide regulation in the central nervous system (CNS)7. Behaviorally, a hangover is characterized by symptoms such as headache, fatigue, diarrhea, nausea, mood disturbances, cognitive impairment, and disruptions in sleep and biological rhythms8. Despite the high prevalence of alcohol consumption and its significant effects, including hangovers, research on animal models and methodologies for studying hangover-induced behavioral changes in rodents remains limited.
Recently, several methodologies have been applied to measure behavioral changes in mice following ethanol administration. Commonly used approaches include the operant chamber, elevated plus maze, and open field test to assess anxiety-like behaviors associated with ethanol consumption5,9. Stimulus and reflex testing have also been used to assess hangover-related behaviors, such as headache-associated responses10. Other models including the tightrope test, footprint pattern analysis, and hanging wire test, have been used to evaluate motor coordination and balance11. Furthermore, several models have been used for pharmacological testing of anti-hangover agents. For instance, the efficacy of melatonin in alleviating hangover behaviors was assessed using the tightrope test as an indicator of motor coordination12. Ganshuang granules, a Traditional Chinese Medicine formulation, have been evaluated using the righting reflex and climbing tests13, while Ginseng Berry Kombucha has been tested for its anti-hangover effects using the balance beam and elevated plus maze tests14. Although widely used, most existing behavioral models for studying hangover-related behaviors are conducted under controlled and simplified conditions, often overlooking environmental influences and innate behaviors of rodents. Developing a model that more closely mimics human hangover conditions requires incorporating spontaneous behaviors, which could enhance the translational value and reproducibility of experimental settings15.
Automated home-cage behavioral monitoring is a powerful approach for assessing mouse behavior to study human-disease conditions. This system allows mice to live in a home-like environment with free access to food and water, bedding materials, and minimal human intervention16. By enabling natural behavior, automated home-cage monitoring reduces handling-induced stress in mice. This method allows behavioral measurements to be conducted over varying durations ranging from seconds to days, facilitating the study of circadian behaviors across light-dark cycles16. Several automated home-cage systems have been developed for behavioral studies in mice, including PhenoMaster17, Intellicage18, PhenoRack19, PhenoTyper®20, and Laboratory Animal Behaviour Observation, Registration and Analysis System (LABORAS; Fig. 1)21. The LABORAS system has been widely used to study disease models, including osteoarthritis22, inflammatory pain23, Parkinson’s disease24, and Alzheimer’s disease25. Despite extensive research using automated home-cage behavioral analysis in numerous disease models, its application in studying hangover behaviors in rodents remains unexplored. Therefore, in the present study, we characterized the home-cage-like behaviors of mice experiencing ethanol-induced hangovers, using automated behavioral analysis.
Materials and methods
Animals
ICR male mice aged 4–5 weeks were obtained from Nomura Siam and maintained in the Animal Facility of the Faculty of Pharmaceutical Sciences, Chulalongkorn University. The mice were maintained under controlled conditions at a temperature of 22 ± 2 °C and a humidity range of 40–60%, with a 12-hour light/dark cycle. Food and water were provided ad libitum. Based on power analysis, 48 mice were used in this study. For each treatment, animals were randomly selected from different cages. Blinding procedures were not applied, as behavioral readings were conducted automatically by the automated monitoring system. Following behavioral assessments, mice were euthanized using inhalation of carbon dioxide, followed by cervical dislocation. All experimental procedures were conducted with efforts to minimize stress and suffering in the mice. No animals were excluded from the study or analysis. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Pharmaceutical Sciences, Chulalongkorn University (Protocol No. 22-33-023). All experimental methods were performed in accordance with relevant guidelines and regulations. The procedures were in compliance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Ethanol administration
To induce hangover-related behaviors in mice, ethanol was administered intraperitoneally at doses of 1, 2, and 3 g/kg. These doses were selected based on previous studies11,26,27,28. The intraperitoneal (i.p.) route was chosen over oral administration to ensure rapid and reliable absorption, minimize variability associated with gastrointestinal factors29, and maintain consistency with established preclinical models9,11,26.
Exploratory behaviors and wire hanging latency were first assessed following ethanol administration across the different dose groups (Fig. 2a). Mice were divided into four experimental groups: (1) control, (2) ethanol 1 g/kg, (3) ethanol 2 g/kg, and (4) ethanol 3 g/kg. The control group received an intraperitoneal injection of physiological saline (0.9% NaCl) at the same volume (10 mL/kg) as the ethanol-treated groups to ensure equivalent handling and injection conditions.
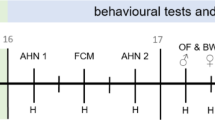
Ethanol administration was performed at 12:00 PM (ZT6), corresponding to the resting (light) phase of the mice. Exploratory behavior assessments were conducted at 6:00 PM (ZT12), marking the onset of the active (dark) phase. Based on these assessments, the optimal ethanol dose was selected for subsequent long-term home-cage-like behavioral monitoring, covering both light and dark cycles. To exclude the influence of exploratory behavior, mice were acclimated to the home-cage-like environment before initiating long-term behavioral assessment. Behavioral data were analyzed based on Zeitgeber Time (ZT), with ZT0 defined as lights on at 6:00 AM and ZT12 as lights off at 6:00 PM, following a 12:12 h light–dark cycle. As mice are nocturnal, ZT0–ZT12 (6:00 AM–6:00 PM) corresponds to the resting (light) phase, while ZT12–ZT24 (6:00 PM–6:00 AM) represents the active (dark) phase (Fig. 2b).
Schematic representation of the experimental design. (a) Timeline of exploratory behavior assessment followed by the hanging wire test. Mice received ethanol at 12:00 PM (ZT6) and were tested for exploratory behavior at 6:00 PM (ZT12), followed immediately by the hanging wire test to assess motor coordination. (b) Long-term home-cage-like behavioral monitoring. Mice received ethanol at 12:00 PM (ZT6), and behavior was continuously monitored for 24 h, covering both the active phase (6:00 PM–6:00 AM, ZT12–ZT24, dark phase) and the resting phase (6:00 AM–6:00 PM, ZT0–ZT12, light phase) under a 12:12 h light–dark cycle, with ZT0 defined as lights on at 6:00 AM and ZT12 as lights off at 6:00 PM.
LABORAS automated home-cage behavioral analysis
To measure spontaneous and innate behaviors of mice after ethanol consumption, behavioral analysis was conducted using the LABORAS automated home-cage-like system (Metris BV, Hoofddorp, The Netherlands). In the present study, behaviors were measured using single mice per cage, as LABORAS is designed for single-animal monitoring. Mice were housed in the LABORAS cages containing bedding material, with free access to food and water throughout the experiment. This system records mouse behavior by detecting vibrations produced by movement on the platform. These vibrations are then analyzed and categorized into various behavioral parameters, such as the duration and frequency of climbing, locomotion (walking and running), rearing, immobility, as well as speed and distance traveled.
Hanging wire test
The hanging wire test is one of the methods used to assess the behavioral changes in ethanol-induced hangover mice30. In the present study, a wire cage lid was used as the apparatus. Each mouse was placed on top of the lid, and the lid was gently shaken three times to allow the mouse to grip the wire. The lid was then flipped, and the latency to fall was recorded as the wire hanging latency.
Data analysis
Data are presented as mean ± SEM. Sample size for each experimental group was determined using G*Power 3.1.9.6, based on an alpha level of 0.05 (two-tailed), statistical power of 0.80 and an expected medium-to-large effect size (Cohen’s d = 0.5–0.8). Statistical analysis and data visualization were performed using GraphPad Prism version 10. Exploratory behavior and hanging wire test data were analyzed using one-way ANOVA, followed by Dunnett’s post hoc test. Correlations between exploratory behavior parameters and wire hanging latency were assessed using Pearson’s correlation and presented in a correlation matrix. Long-term home-cage-like behavioral data were analyzed using two-way repeated measures ANOVA, followed by Sidak’s multiple comparisons test. Differences between daytime and nighttime behaviors were analyzed using Student’s t-test. A p-value < 0.05 was considered statistically significant.
Results
Ethanol reduces exploratory behaviors in automated home-cage-like monitoring
To assess the effects of ethanol on exploratory behaviors, mice were administered ethanol at doses of 1, 2, 3 g/kg (i.p.). The results demonstrated a dose-dependent reduction in exploratory behaviors, including climbing, locomotion, rearing, distance traveled and speed, alongside an increase in immobility (Fig. 3). However, the 1 and 2 g/kg doses did not significantly impair exploratory behaviors compared to the vehicle-treated group. In contrast, the 3 g/kg dose led to a significant reduction in spontaneous locomotor activity, as evidenced by decreased frequency and duration of mobile behaviors (rearing, climbing, and locomotion), increased immobility, and reduced speed and distance traveled (Fig. 3). These findings were further supported by position distribution analysis, which revealed distinct movement patterns. Mice administered 3 g/kg ethanol showed reduced movement traces compared to the control and lower-dose groups, confirming a decline in exploratory activity (Fig. 4).
Effects of ethanol on spontaneous locomotor activity. The behavioral parameters are presented as the duration (a-d) and frequency (e-h) of exploratory behaviors, along with speed (i) and distance traveled (j). Data are presented as means ± SEM (n = 8 mice/group). Differences between groups were analyzed using one-way ANOVA followed by Dunnett’s post-hoc test. Significant differences between the vehicle group (0 g/kg) and ethanol-treated groups (1, 2, and 3 g/kg) are indicated as follows: *p < 0.05, ***p < 0.001.
Position distribution of the mice on the cage. Representative traces showing the position distribution of mice within the cage for the vehicle (0 g/kg) group (a) and ethanol-treated groups (1, 2, and 3 g/kg) (b-d). The color spectrum represents activity levels, progressing from green (low activity) to red, magenta, and white (high activity). A shift toward the right on the color scale denotes a greater frequency or longer duration of activity in specific cage locations.
Ethanol reduces wire hanging latency in hangover mice
Following exploratory behavior assessments, the hangover mice were tested using the wire hanging test. This test determines motor activity of mice by assessing muscle strength and motor coordination. As shown in Fig. 5, administration of ethanol at doses of 2 g/kg and 3 g/kg significantly reduced wire hanging latency compared to the control group, indicating impaired motor activity. In contrast, the 1 g/kg dose did not significantly impair motor activity in the hanging wire test.
The results of the hanging wire test were consistent with the findings from exploratory behavior assessments. Correlation analysis revealed significant relationships between the two tests. Mobile behaviors (climbing, rearing, locomotion, speed, and distance traveled) were positively correlated with wire hanging latency, while immobility was negatively correlated. These findings demonstrate a strong correlation between the two methods and indicate that exploratory behavior assessment in the LABORAS system aligns with established methods of assessing hangover-like behaviors in mice.
Wire hanging latency in hangover mice and its correlation with exploratory behaviors in automated home-cage-like monitoring. (a) Wire hanging latency of mice after treatment with various doses of ethanol (b) Correlation matrix showing the relationships between exploratory behaviors and wire hanging latency. Differences between groups (n = 8 mice/group) were analyzed using one-way ANOVA, followed by Dunnett’s post-hoc test. Significant differences between the vehicle (0 g/kg) and ethanol-treated groups (1, 2, and 3 g/kg) are indicated as follows: ***p < 0.001.
Ethanol reduces long-term home-cage-like behaviors over 24-hour monitoring
Following assessment of the effects of ethanol on exploratory behaviors, we next evaluated its impact on long-term home-cage-like behaviors. The optimal ethanol dose (3 g/kg) was administered at 12:00 PM, with behavioral assessments conducted at 6:00 PM and over the next 24 h. As shown in Figs. 6 and 7, the behavioral data were divided into 2-hour time bins following ethanol or vehicle administration. Significant differences between the ethanol- and vehicle-treated groups were observed at several time points, notably at 2, 4, 6, and 8 h after cage placement (corresponding to 8, 10, 12, and 14 h post-ethanol administration). These findings indicate a clear temporal pattern, with the most pronounced behavioral changes occurring within the first 8 h post-administration.
To examine the influence of circadian rhythms on ethanol-induced behavioral changes, data were further grouped into nighttime (ZT12–24; 6:00 PM–6:00 AM) and daytime (ZT0–12; 6:00 AM–6:00 PM) periods. Significant behavioral differences between the ethanol- and vehicle-treated groups were primarily observed during the nighttime. As expected for nocturnal animals, control mice displayed higher nighttime activity than daytime activity, reinforcing nighttime as the optimal period for behavioral monitoring. Ethanol-treated mice showed marked reductions in all measured nighttime behaviors, including the duration and frequency of climbing, locomotion, and rearing, as well as reductions in speed and distance traveled, and a significant increase in immobility (Figs. 6 and 7).
Furthermore, reduced mobility persisted into the daytime, with significant reductions observed in the duration of locomotion, indicating prolonged effects of ethanol in mice. To capture broader trends, total behavioral scores were also analyzed in 6-hour intervals (ZT0–6, ZT6–12, ZT12–18, ZT18–24). The most substantial ethanol-induced behavioral alterations occurred within the first 6 h (ZT0–6), with notable but diminished effects persisting throughout the 24-hour monitoring period—particularly during the daytime phase of ZT12–18 (Supplementary Figs. 1 and 2). Overall, these findings suggest that ethanol diminishes long-term home-cage-like behaviors in mice, especially during the early post-administration and nighttime phases. This reduction in spontaneous activity may serve as a non-invasive behavioral indicator of hangover-related effects in preclinical models.
The effects of ethanol on the duration of home-cage-like behaviors in mice. Behaviors are presented as the duration of long-term innate behaviors, including climbing (a), locomotion (b), rearing (c), immobility (d), speed (e), and distance traveled (f). Data are presented as means ± SEM (n = 8 mice/group). Statistical analyses were performed using two-way repeated measures ANOVA followed by Šidák’s multiple comparisons test (left panels), and Student’s t-test (right panels). ***, ** and * denote significant differences at p < 0.001, p < 0.01, and p < 0.05, respectively.
The effects of ethanol on the frequency of home-cage-like behaviors in mice. Behaviors are presented as the frequency of long-term innate behaviors, including climbing (a), locomotion (b), rearing (c), and immobility (d). Data are presented as means ± SEM (n = 8 mice/group). Statistical analyses were performed using two-way repeated measures ANOVA followed by Šidák’s multiple comparisons test (left panels), and Student’s t-test (right panels). ***, ** and * denote significant differences at p < 0.001, p < 0.01, and p < 0.05, respectively.
Discussion
The present study demonstrated that ethanol administration induces alterations in home-cage-like activities, affecting both exploratory behaviors and long-term locomotor activity. In the short term, administration of 3 g/kg of ethanol significantly reduced mobile behaviors while increasing immobility. Notably, behavioral assessments conducted during both day and night revealed significant differences between control and ethanol-treated mice, with more pronounced effects observed during the night phase. Furthermore, the results from correlation analysis between exploratory behaviors and the hanging wire test were statistically correlated, indicating that automated home-cage-like monitoring aligns with conventional methods of assessing hangover-like behaviors in mice. These findings suggest that ethanol-induced impairments in locomotion and exploration are closely linked to the development of hangover-like symptoms in mice.
An important aspect of this study was the development of alternative models for assessing hangover-related behaviors in mice. Alcohol-induced behavioral changes were assessed using automated home-cage-like behavioral analysis, a method that offers several advantages over conventional behavioral tests. As highlighted in previous studies, automated home-cage-like analysis allows for long-term behavioral monitoring, which is particularly beneficial when studying hangover-related impairments, as these effects are known to persist for approximately 18.4 h—beyond the duration typically captured by traditional tests. This approach enables the observation of behavioral changes across multiple time points, over extended periods, and throughout circadian cycles31,32.
In the present study, the behaviors of mice were continuously recorded over 24 h, covering both nighttime and daytime phases. This design was particularly relevant because alcohol consumption in humans, typically occurring at night (22:00–02:00 h), has been shown to affect next-morning mood through symptoms such as sleep disturbances, mood instability, fatigue, and anxiety33. As rodents are nocturnal animals, their activity rhythms differ from those of humans, with the daytime in mice corresponding to nighttime in humans34. As such, in the present study, ethanol was administered during the daytime, and behavioral assessments started at nighttime (18:00 h) and were performed for 24 h, capturing subsequent changes over the full circadian cycle.
In addition, automated home-cage monitoring promotes animal welfare by allowing behavioral assessments in a familiar, home-like environment with minimal disruption. This system provides a home-like setting with nesting materials, along with free access to food and water35. By limiting direct interaction between animals and personnel, this approach helps reduce stress induced by human contact and environmental factors such as noise—both known to affect mouse behavior and welfare36. In the present study, interactions between mice and personnel were restricted to ethanol administration and cage transfer, while all behavioral data were collected by automated systems, thereby minimizing experimenter bias.
Hangovers are characterized by behavioral changes manifested through both physical and emotional disturbances. In this study, emotional state indicators were inferred based on exploratory behaviors following ethanol administration. A series of ethanol doses was tested to determine the most effective dose for inducing hangover-related behavioral changes. As previously demonstrated, rodents, including mice, exhibit innate exploratory behaviors in response to novel environments, driven by either motivational states or emotional factors, such as anxiety37,38. These exploratory behaviors are widely used in behavioral pharmacology models to assess anxiety-like behaviors, including the open field test37.
In relation to the emotional aspects of hangover behaviors, previous findings suggest that home-cage-like behavioral assessments can effectively detect emotional aspects, such as anxiety-like behaviors in rodents39. Notably, studies using automated home-cage systems including the Light Spot Test40, the PhenoTyper-based Residual Avoidance test41, and other longitudinal tracking systems42 have demonstrated high reproducibility and sensitivity in detecting anxiety-related behavioral phenotypes. These methods provide ethologically relevant environments, minimize human interference, and allow for extended monitoring of anxiety- and stress-related behaviors. In the present study, short-term behavioral assessments were first used to identify the optimal ethanol dose, which was then applied for long-term assessments using automated home-cage-like behavioral monitoring. The findings demonstrated that ethanol administration significantly diminished exploratory behaviors and locomotor activity, particularly during nighttime, when non-ethanol-exposed mice exhibited greater activity compared to ethanol-exposed mice. These behavioral changes were characterized by reduced durations of mobile behaviors and increased immobility, which may reflect the reduced physical activity observed in humans experiencing hangovers. Consistent with previous reports, heavy alcohol consumption in humans is associated with decreased physical activity and fatigue during hangover periods43.
While this study provides a practical approach for evaluating hangover-related behaviors in a naturalistic setting using an automated home-cage-like monitoring system, several important limitations should be considered. Behavioral testing was conducted six hours post-ethanol administration (3 g/kg, i.p.), a dose well below the toxic threshold (LD₅₀ ≈ 7 g/kg)44, thereby reducing the likelihood that systemic toxicity contributed to the observed reductions in locomotion and increases in immobility. Although measuring blood ethanol concentrations would have provided additional confirmation of ethanol clearance, such measurements were not included in the present study. However, previous studies have consistently demonstrated that ethanol levels return to baseline or near-baseline approximately six hours following administration9,11,26. Accordingly, this time point was selected to minimize the confounding effects of acute intoxication and to better reflect behavioral features associated with the hangover phase.
The observed behavioral changes may also reflect ethanol-induced sedation or affective disturbances such as anxiety, both of which are known components of hangover. However, specific tests for affective states (e.g., the elevated plus maze for anxiety, or the sucrose preference test for anhedonia) were not included. Despite this limitation, extensive literature supports the impact of ethanol on these behavioral domains in rodents9,26. Motor coordination was directly assessed using the hanging wire test, which revealed significant impairment following ethanol exposure. This finding supports the presence of physical symptoms characteristic of hangover and alings with prior reports of ethanol-induced motor dysfunction11,26.
Only male mice were used in this study to minimize variability associated with hormonal fluctuations, which are known to affect locomotor activity45,46, the primary behavioral outcome assessed. While this approach helped reduce within-group variability, it also limits the generalizability of the findings. Future studies should include both sexes to examine potential sex-specific effects and improve translational relevance.
Another limitation of the present study is the absence of cognitive testing, despite the relevance of cognitive symptoms in hangover. This was due to the study’s focus on non-invasive, continuous monitoring, which would have been disrupted by conventional cognitive tests requiring handling and training. Future work should integrate complementary cognitive and affective assessments to provide a more comprehensive understanding of hangover-related impairments. Additionally, the lack of molecular or physiological analyses limits insights into the underlying neurobiological mechanisms. Including such analyses would help differentiate between emotional and physical aspects of the observed behavioral impairments and strengthen mechanistic interpretations.
Another limitation of this study is the lack of direct assessment of sleep architecture. While the LABORAS system captures immobility, which may serve as an indicator of rest or sleep-like states, it does not distinguish between true sleep and quiet wakefulness. The inclusion of electroencephalogram (EEG) recordings in future studies would enable more precise characterization of ethanol-induced alterations in sleep stages and fragmentation, thereby improving the translational relevance of the findings to human hangover conditions.
This model is not intended to replace established behavioral assays but rather to serve as a complementary, non-invasive approach for capturing hangover-like symptoms in a familiar, low-stress environment. Although similar automated systems have been previously used to study the effects of ethanol, their application to hangover-specific behaviors remains limited. The current findings suggest that ethanol at 3 g/kg (i.p.) induces reduced exploratory behavior and long-term changes in spontaneous activity in mice. These effects mirror physical hangover symptoms observed in humans, including motor deficits and decreased activity levels.
In conclusion, automated home-cage-like monitoring may represent a useful exploratory tool for studying hangover-related behaviors in rodents. However, further validation is required, particularly through pharmacological testing, integration of cognitive and affective domains, investigation of underlying molecular mechanisms, and inclusion of both sexes and different rodent species. Such efforts will be essential to establish this model’s utility in advancing our understanding of hangover pathophysiology.
Data availability
All the data of this study are available upon request from the corresponding author.
References
Organization, W. H. Global status report on alcohol and health and treatment of substance use disorders. Geneva World Heal Organ (2024).
Organization, W. H. Global Status Report on Alcohol and Health 2018 (World Health Organization, 2018).
Hendriks, H. F. J. Alcohol and human health: what is the evidence?? Annu. Rev. Food Sci. Technol. 11, 1–21 (2020).
Palmer, E. et al. Alcohol hangover: underlying biochemical, inflammatory and neurochemical mechanisms. Alcohol. Alcohol. 54, 196–203 (2019).
Karadayian, A. G. et al. Alcohol hangover induces mitochondrial dysfunction and free radical production in mouse cerebellum. Neuroscience 304, 47–59 (2015).
Ezequiel Leite, L. & Nobre, M. J. The negative effects of alcohol hangover on high-anxiety phenotype rats are influenced by the glutamate receptors of the dorsal midbrain. Neuroscience 213, 93–105 (2012).
Karadayian, A. G., Bustamante, J. & Lores-Arnaiz, S. Alcohol hangover induces nitric oxide metabolism changes by impairing NMDA receptor-PSD95-nNOS pathway. Nitric Oxide. 113–114, 39–49 (2021).
Swift, R. & Davidson, D. Alcohol hangover: mechanisms and mediators. Alcohol Health Res. World. 22, 54–60 (1998).
Karadayian, A. G., Busso, M. J., Feleder, C. & Cutrera, R. A. Alterations in affective behavior during the time course of alcohol hangover. Behav. Brain Res. 253, 128–138 (2013).
Lu, S. et al. Hangover headache and its behavioral changes in rats. Iran. J. Basic. Med. Sci. 26, 326–334 (2023).
Karadayian, A. G. & Cutrera, R. A. Alcohol hangover: type and time-extension of motor function impairments. Behav. Brain Res. 247, 165–173 (2013).
Karadayian, A. G., Mac Laughlin, M. A. & Cutrera, R. A. Estrogen blocks the protective action of melatonin in a behavioral model of ethanol-induced hangover in mice. Physiol. Behav. 107, 181–186 (2012).
Li, Q. et al. Ganshuang granule plays a Pharmacological role in anti-alcoholic and anti-hangover via regulating alcohol metabolism and affecting neurotransmitters. Int J. Neurosci. 135, 345–357 (2025).
Choi, E. J., Kim, H., Hong, K. B., Suh, H. J. & Ahn, Y. Hangover-Relieving effect of ginseng berry Kombucha fermented by Saccharomyces cerevisiae and Gluconobacter oxydans in Ethanol-Treated cells and mice model. Antioxidants (Basel Switzerland) 12, 774 (2023).
Puścian, A. & Knapska, E. Blueprints for measuring natural behavior. iScience 25, 104635 (2022).
Richardson, C. A. The power of automated behavioural homecage technologies in characterizing disease progression in laboratory mice: A review. Appl. Anim. Behav. Sci. 163, 19–27 (2015).
Robinson, L., Plano, A., Cobb, S. & Riedel, G. Long-term home cage activity scans reveal Lowered exploratory behaviour in symptomatic female Rett mice. Behav. Brain Res. 250, 148–156 (2013).
Galsworthy, M. J. et al. A comparison of wild-caught wood mice and bank voles in the intellicage: assessing exploration, daily activity patterns and place learning paradigms. Behav. Brain Res. 157, 211–217 (2005).
Aniszewska, A., Szymanski, J., Winnicka, M. M. & Turlejski, K. Interleukin 6 deficiency affects spontaneous activity of mice in age- and sex-dependent manner. Acta Neurobiol. Exp. (Wars). 74, 424–432 (2014).
de Visser, L., van den Bos, R., Kuurman, W. W., Kas, M. J. H. & Spruijt, B. M. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes Brain Behav. 5, 458–466 (2006).
Quinn, L. P. et al. LABORAS™: initial Pharmacological validation of a system allowing continuous monitoring of laboratory rodent behaviour. J. Neurosci. Methods. 130, 83–92 (2003).
Piel, M. J., Kroin, J. S. & Im, H. J. Assessment of knee joint pain in experimental rodent models of osteoarthritis. Methods Mol. Biol. 1226, 175–181 (2015).
Hasriadi, D., Wasana, P. W., Vajragupta, O., Rojsitthisak, P. & Towiwat, P. Automated home-cage monitoring as a potential measure of sickness behaviors and pain-like behaviors in LPS-treated mice. PLoS One. 16, e0256706 (2021).
Quinn, L. P. et al. Further validation of LABORAS using various dopaminergic manipulations in mice including MPTP-induced nigro-striatal degeneration. J. Neurosci. Methods. 156, 218–227 (2006).
Pugh, P. L., Richardson, J. C., Bate, S. T., Upton, N. & Sunter, D. Non-cognitive behaviours in an APP/PS1 Transgenic model of alzheimer’s disease. Behav. Brain Res. 178, 18–28 (2007).
Prediger, R. D. S., da Silva, G. E., Batista, L. C., Bittencourt, A. L. & Takahashi, R. N. Activation of adenosine A1 receptors reduces Anxiety-Like behavior during acute ethanol withdrawal (Hangover) in mice. Neuropsychopharmacology 31, 2210–2220 (2006).
Karadayian, A. G., Bustamante, J., Czerniczyniec, A., Cutrera, R. A. & Lores-Arnaiz, S. Effect of melatonin on motor performance and brain cortex mitochondrial function during ethanol hangover. Neuroscience 269, 281–289 (2014).
Hori, H., Fujii, W., Hatanaka, Y. & Suwa, Y. Effects of fusel oil on animal hangover models. Alcohol Clin. Exp. Res. 27, 37S–41S (2003).
Al Shoyaib, A., Archie, S. R. & Karamyan, V. T. Intraperitoneal route of drug administration: should it be used in experimental animal studies?? Pharm. Res. 37, 12 (2019).
Asorey, L. G., Carbone, S., Gonzalez, B. J. & Cutrera, R. A. Behavioral effects of the combined use of alcohol and energy drinks on alcohol hangover in an experimental mice model. Neurosci. Lett. 670, 1–7 (2018).
Jhuang, H. et al. Automated home-cage behavioural phenotyping of mice. Nat. Commun. 1, 68 (2010).
Iannello, F. Non-intrusive high throughput automated data collection from the home cage. Heliyon 5, e01454 (2019).
Mc Kinney, A. & Coyle, K. Alcohol hangover effects on measures of affect the morning after a normal night’s drinking. Alcohol. Alcohol.. 41, 54–60 (2006).
Acosta, J. et al. Circadian modulation of motivation in mice. Behav. Brain Res. 382, 112471 (2020).
Spangenberg, E. M. F. & Keeling, L. J. Assessing the welfare of laboratory mice in their home environment using animal-based measures – a benchmarking tool. Lab. Anim. 50, 30–38 (2015).
Castelhano-Carlos, M. J. & Baumans, V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab. Anim. 43, 311–327 (2009).
Crawley, J. N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav Rev. 9, 37–44 (1985).
Mackay, M. K. & Pillay, N. Environmental correlates of exploratory behavior and anxiety in three African striped mouse (Rhabdomys) taxa occurring in different habitats and contexts. J. Comp. Psychol. 135, 304–314 (2021).
Eraslan, E. et al. Home-cage behavior is impacted by stress exposure in rats. Front. Behav. Neurosci. 17, 1195011 (2023).
Aarts, E. et al. The light spot test: measuring anxiety in mice in an automated home-cage environment. Behav. Brain Res. 294, 123–130 (2015).
Prevot, T. D. et al. Residual avoidance: A new, consistent and repeatable readout of chronic stress-induced conflict anxiety reversible by antidepressant treatment. Neuropharmacology 153, 98–110 (2019).
Grieco, F. et al. Measuring behavior in the home cage: study design, applications, challenges, and perspectives. Front. Behav. Neurosci. 15, 735387 (2021).
Devenney, L. E., Coyle, K. B., Roth, T. & Verster, J. C. Sleep after heavy alcohol consumption and physical activity levels during alcohol hangover. Journal Clin. Medicine 8, 752 (2019).
Tsibulsky, V. L. & Amit, Z. Tolerance to effects of high doses of ethanol: 1. Lethal effects in mice. Pharmacol. Biochem. Behav. 45, 465–472 (1993).
Ogawa, S., Chan, J., Gustafsson, J. A., Korach, K. S. & Pfaff, D. W. Estrogen increases locomotor activity in mice through Estrogen receptor alpha: specificity for the type of activity. Endocrinology 144, 230–239 (2003).
Coop, A. D., Stavarache, M. A., Pfaff, D. W. & Reeke, G. N. Mathematical analysis of locomotor behavior by mice in a radial maze. Proc. Natl. Acad. Sci. 103, 15710–15715 (2006).
Acknowledgements
This research was supported by the Second Century Fund (C2F), Chulalongkorn University (H.). The authors express their gratitude to the Ratchadaphiseksomphot Endowment Fund for the Center of Excellence in Natural Products for Ageing and Chronic Diseases, Chulalongkorn University (GCE 3330170003).
Author information
Authors and Affiliations
Contributions
H. H: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draftPWDW: Data curation, Formal analysis, Investigation, Writing – original draftSN: Data curation, InvestigationSS: Resources, Supervision, Writing – review & editingPP: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hasriadi, H., Dasuni Wasana, P.W., Nwe, S.Y. et al. Automated home-cage-like monitoring for assessing innate behaviors in a murine hangover model. Sci Rep 15, 29489 (2025). https://doi.org/10.1038/s41598-025-13334-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13334-3