Abstract
This study explores the feasibility and clinical value of using 3D-Slicer for preoperative planning in posterior C1–C2 fixation in patients with vertebral artery variations. A total of 118 patients who underwent posterior atlantoaxial fixation from January 2013 to December 2022 were included, with 51 cases utilizing 3D reconstruction and 67 cases not utilizing this approach. We employed four personalized screw placement strategies tailored to the type of vertebral artery variation: (1) Normal vertebral artery: pedicle screws for both C1 and C2; (2) Axis vertebral foramen variation: pedicle screws for C1 and Laminar screws for C2; (3) vertebral artery hypoplasia: pedicle screws for C1 and Laminar screws for C2; and (4) posterior atlas arch variation: lateral mass screws for C1 and pedicle screws for C2. The preoperative demographic and diagnostic data showed no significant differences between the groups. However, the use of 3D reconstruction with 3D-Slicer led to significantly improved outcomes, including 27.97% increase in optimal fixation method selection, reduced operating time, decreased intraoperative blood loss, and a lower risk of vertebral artery injury (VAI). The use of 3D-Slicer for preoperative planning in posterior C1-C2 fixation in patients with vertebral artery variations shows promising potential.
Similar content being viewed by others
Introduction
Atlantoaxial instability caused by traumatic fractures, developmental deformities, tumors, inflammation, and degeneration can easily compromise the spinal cord, leading to serious consequences and posing a direct threat to life1,2,3.
Therefore, atlantoaxial dislocation often requires surgical treatment to relieve its compression of the spinal cord while restoring the sequence and stability of the atlantoaxial spine. Despite this, the duty of carrying out the surgery for atlantoaxial dislocation is a difficult one given its intricate anatomical vicinity to vital centers such as the medulla oblongata, the vertebral artery, as well as the intervertebral venous plexus. Furthermore, extremely severe atlantoaxial dislocations admittedly have a mortality rate of up to 35% due to damages to the medulla oblongata4. Variations in vertebral artery anatomy, in particular, can markedly alter the feasibility of standard screw trajectories. In a case report by Ivetic and colleagues, a high-riding vertebral artery precluded the use of conventional C2 pedicle screws. Instead, translaminar fixation was employed to avoid vascular injury-highlighting the importance of preoperative vascular imaging and individualised planning in high-risk cases5. Beyond anatomical variability, the precision required for safe screw placement has driven the adoption of intraoperative navigation. Harel et al. compared fluoroscopic guidance with O-arm-assisted navigation in posterior C1-C2 fusion. While operative times were slightly longer with navigation, screw placement accuracy improved and revision rates decreased6. This underscores a broader shift in spinal surgery toward technology-assisted accuracy and safety. Taken together, atlantoaxial fixation demands careful navigation of anatomical constraints, high-resolution imaging, and an increasing reliance on intraoperative guidance systems. These challenges continue to shape the evolution of surgical techniques in this critical region of the spine.
In recent years, thanks to the advancements in modern surgical techniques, and especially atlantoaxial anatomy research, the development of atlantoaxial internal fixation techniques, and greater understanding and clinical staging of atlantoaxial subluxation, breakthroughs have been made when it comes to treating this condition, reducing the risks of surgery considerably. The key to effective atlantoaxial internal fixation is the art of accurate screw placement. Due to the unique positioning of the atlantoaxial, personalized screw placement is essential to avoid potential intraoperative accidents and post-operative complications. This necessitates that the surgeon has not only high proficiency in cervical surgery but also a thorough pre-operative comprehension of the patient’s cervical anatomy. Additionally, with traditional posterior atlantoaxial screw placement, considerable soft-tissue dissection is required to identify anatomical features and the C-arm X-ray machine must be used multiple times to confirm the arrangement of the screws7. Therefore, a more thorough preoperative evaluation of the anatomy should be performed by studying imaging, with an especially keen focus on the internal height measurement and the design of the screw path8,9.
Advancements in medical imaging have revolutionized preoperative planning, yet the accessibility and adaptability of imaging software remain critical challenges. Commercial software solutions often provide robust, specialised functionalities but come at a substantial financial cost and with limited flexibility for customization. In contrast, 3D Slicer-a free, open-source platform-offers comprehensive tools for multi-modality image processing, visualization, and analysis across diverse operating systems. Its open architecture facilitates user-driven extension and integration with other computational tools, fostering innovation in both clinical and research settings. This democratization of technology not only reduces barriers to adoption but also accelerates the development of tailored applications, positioning 3D Slicer as a pivotal resource in modern medical imaging. According to Google Scholar and PubMed, 16,200 + and 1684 + respective literature searches and paper citations have been identified concerning the Slicer platform, respectively (data updated on December 5th, 2022).
This study aims to explore a screw placement strategy for C1 and C2 posterior fixation in patients with vertebral artery variations using preoperative 3D reconstruction based on 3D Slicer.
Materials and methods
The study was approved by the Ethics Committee, and written informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations.
Clinical material
Inclusion criteria: 1) Patients who have undergone atlantoaxial internal fixation; 2) Patients with complete imaging data and Computed Tomography Angiography (CTA) before and after surgery.
Exclusion criteria: 1) Patients with severe osteoporosis (T score ≤ -5.0); 2) Patients with uncontrolled hypertension (e.g., SBP ≥ 180 mmHg or DBP ≥ 100 mmHg); 3) Patients with significant cardiovascular disease: recent MI/ACS, unstable angina, CHF, stroke/TIA within recent months; 4) Patients with active bleeding / coagulopathy or platelet dysfunction; 5) Patients with active cancer under treatment; 6) Patients for whom informed consent has not been obtained.
Based on the above inclusion and exclusion criteria, from January 2013 to December 2022, a total of 118 cases of posterior approach atlantoaxial internal fixation were performed in the Department of Spine Surgery at our hospital. The patients had an average age of 58.9 years and consisted of 75 males and 43 females. Patient demographics are collected and shown in (Table 1). Of them, 51 cases underwent a three-dimensional reconstruction of computed tomography angiography (CTA) using the 3D-Slicer before the operation. Finally, 118 cases were divided into two groups, those who received 3D reconstruction with 3D-Slicer (51) before the operation and those who did not use it (67). We systematically analyzed each patient’s preoperative preparation, clinical presentation, visual analogue scale (VAS) score for pain, and neurological function as assessed by the modified Japanese orthopaedic association (mJOA) score. Postoperative outcomes were evaluated through clinical follow-up and serial imaging studies, including CT and CTA where applicable. The incidence of vertebral artery injury (VAI) was included as a predefined outcome measure. Estimated blood loss (EBL) was determined by the cumulative volume from surgical gauze and suction canisters. The data were simultaneously verified and assessed by the operating surgeon, anesthesiologist, and circulating nurse. All surgical procedures were performed by senior spine surgeons from different surgical teams, each adhering to a standardized operative protocol. Preoperative planning was conducted collaboratively by a panel of three experienced spine surgeons, including the lead surgeon responsible for the operation. To ensure the accuracy and reproducibility of clinical data, all records were independently reviewed and cross-validated by two attending physicians.
3D reconstruction with 3D-Slicer
After the patient is admitted to the hospital and completes the CTA scan, the Digital Imaging and Communication in Medicine (DICOM) standard images are retrospectively obtained from our hospital’s Medicine radiology database. DICOM images data are transferred to a standard personal computer (Intel Core i5-8250U CPU 1.80 GHz, 8 GB RAM, Windows 10 × 64) and preprocessed with dcm2nillGUI (MRIcron version 1.0.20190902; https://www.nitrc.org/projects/mricron/). The scanner images in DICOM format are converted to NIfTI format. Subsequently, the CT and CTA data in NIfTI format were imported into 3D Slicer software (version 5.2.1; https://www.slicer.org/) for 3D reconstruction. (Supplementary Figs. 1–4) The following steps were performed within the software: 1) select “Segment Editor” in the function area, 2) add CT and CTA data in sequence in the “Source volume” column, 3) double-click in the “Segment color” column and select “Bone” and “Artery” respectively in the pop-up window “Terminology”, 4) adjust the “Threshold Range” so that the bones and arteries are independently developed and run the “Apply” program, 5) finally run the “Show 3D”. The 3D reconstruction is completed and then to observe the vertebral artery of the atlantoaxial segment and measure the anatomical data required for atlantoaxial screw placement.
Surgical methods
According to the type of vertebral artery variation, personalized screw placement is the optimal method for atlantoaxial internal fixation surgery in this study. Various types of screws including pedicle screws, laminar screws, and lateral mass screws are chosen in accordance with the patient’s anatomical features, which are identified by their vertebral artery variation (Table 2).
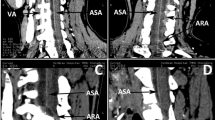
The measurement of value (a, e, h) is shown in (Fig. 1). Value a: the length from the entrance of the vertebral artery of the axis to the inner wall of the spinal canal; Value e: the length from the apex of the bulb of the vertebral artery to the upper articular surface of the axis; Value-h: the height of posterior atlas arch.
Measurement of value (a,e,h). (A–C: Value-a), the length from the entrance of the vertebral artery of the axis to the inner wall of the spinal canal; Value-e, the length from the apex of the bulb of the vertebral artery to the upper articular surface of the axis; (D: Value-h), the height of posterior atlas arch.
Normal vertebral artery: pedicle screws for both C1 and C2. (Fig. 2)
Normal vertebral artery: Pedicle screws for both C1 and C2. A patient with wide and high of axial vertebral artery foramen underwent atlantoaxial pedicle screw fixation due to odontoid fracture (Anderson II). (A) measurement of value-e, e < 4.5 mm; (B) measurement of value-a, a > 4.5 mm; (C,F) preoperative X-ray; (D,G) postoperative X-rays; (E,H) postoperative CT image. (Value a: the length from the entrance of the vertebral artery of the axis to the inner wall of the spinal canal; Value e: the length from the apex of the bulb of the vertebral artery to the upper articular surface of the axis.)
Pedicle screws are used for three types of Axis Vertebral Foramen: Wide and Low, Wide and High, and Narrow and Low. They are also applied for cases where the value h≥3.5 mm in posterior atlas arch variation.
Axis vertebral foramen variation: pedicle screws for C1 and Laminar screws for C2. (Fig. 3)
Axis vertebral foramen variation: pedicle screws for C1 and Laminar screws for C2. Two patients with narrow and high of axial vertebral artery foramen underwent laminar screw fixation due to odontoid fracture. (A) 3D reconstruction; (B) measurement of value-e, e < 4.5 mm; (C,D) measurement of value-a, a ≤ 4.5 mm; (E,F) Anderson II; (G,H) Anderson III. (Value a: the length from the entrance of the vertebral artery of the axis to the inner wall of the spinal canal; Value e: the length from the apex of the bulb of the vertebral artery to the upper articular surface of the axis.).
Laminar screws are utilized for the type of Narrow and High in Axis Vertebral Foramen Variation.
Vertebral Artery Hypoplasia: Pedicle screws for C1 and Laminar screws for C2. (Fig. 4)
Vertebral Artery Hypoplasia: Pedicle screws for C1 and Laminar screws for C2. A patient with left vertebral artery dominant underwent laminar screw fixation due to Jefferson fracture. (A) image in PACS; (B) 3D reconstruction in 3D-Slicer; (C–E) postoperative imaging. (Arrow: right vertebral artery).
Laminar screws are also applied to cases of Vertebral Artery Hypoplasia.
Posterior atlas arch variation: lateral mass screws for C1 and pedicle screws for C2. (Fig. 5)
Lateral mass screws are typically exploited in cases with the value h < 3.5 mm in posterior atlas arch variation.
Statistical methods
All statistical analyses were conducted using SPSS software (version 27.0; SPSS Inc., Chicago, IL, USA). The preoperative general characteristics were analyzed using the Chi-square test, while clinical parameters were compared using the independent samples t-test. To compare the incidence of VAI between the 3D-Slicer group and the control group, Fisher’s exact test was employed due to the presence of expected cell counts less than 5 in the contingency table, rendering the Chi-square test inappropriate. All tests were two-sided, and a p-value < 0.05 was considered statistically significant.
Results
All 118 patients were followed up for at least 3 months. Postoperative X-rays and CT scans of the cervical spine for both groups revealed no abnormal displacement, angular deformity, loosening, breaking, or detachment of internal fixation.
Preoperative general information such as gender, age, and primary diagnosis did not differ significantly between the two groups (P > 0.05). (Table 3) The comparison of clinical indicators with and without 3D-Slicer is presented in (Table 4).
3D slicer improves fixation preference rate
According to the types of vertebral artery variation, personalized screw placement was utilized in the atlantoaxial internal fixation surgery. Among 118 patients, 85 (72.03%) underwent pedicle screws, 28 (23.73%) received lateral mass screws, and 5 (4.24%) had laminar screws.
Optimized surgical duration
The operation time of the patient group using the 3D-Slicer (95 ± 29 min) was shorter than those of the patient group without the 3D-Slicer (135 ± 31 min), and the difference was statistically significant (P<0.001). A shorter operative time implies that patients are exposed to lesser X-ray radiation.
Hemorrhage control
The intraoperative blood loss of the 3D-Slicer group (88 ± 43 ml) was less than those of the patient group without the 3D-Slicer (250 ± 88 ml), and the difference was statistically significant (P<0.001). Decreased intraoperative blood loss indicates fewer vertebral artery injuries and less bleeding from the venous plexus.
Vertebral artery protection
No VAI was observed in the 3D-Slicer group (51 cases), while 4 cases occurred in the common group (67 cases). These findings suggest that utilizing the 3D-Slicer for preoperative three-dimensional reconstruction of patient CTA data can prevent intraoperative complications and decrease the likelihood of VAI.
Discussion
There are various surgical methods for atlantoaxial joint reduction and internal fixation through the posterior approach. The application of the pedicle screw technique for atlantoaxial dislocation was first reported by Goel and Laher et al. in 1994 and modified to a multiaxial screw by Harms and Melcher et al. in 200110,11. Atlantoaxial pedicle screw fixation (Harms technique) is the most commonly used treatment for upper cervical instability due to its superior biomechanical properties and low requirement for anatomical consistency12,13,14. However, given the proximity of the atlantoaxial pedicle to the spinal cord and nerve roots, there is a risk of causing medically-induced injury to the vertebral artery and spinal cord with pedicle screw placement, which cannot be completely avoided15,16. The reported risk of VAI in a clinical series was 4.1–8.2%2. Additionally, Chiapparelli et al. and Chen et al. found that the probability of VAI is much higher than that of spinal cord injury17,18. VAI can lead to intraoperative vertebral artery rupture and profuse bleeding, basilar artery ring insufficiency, cerebellar infarction, and even death. Previous studies have suggested that the type of the vertebral artery foramen, the course of the vertebral artery, and variations in the atlantoaxial vertebral artery all have an impact on pedicle screw placement19. Therefore, we believe that damage to the vertebral artery due to atlantoaxial pedicle screws deserves more attention and that individualized screw placement for patients with large anatomical differences needs to be further studied. In addition to VAI, intraoperative complications which should be avoided include venous bleeding, iatrogenic trauma at the screw entry zones, and surgical disorientation20,21. At present, two methods are most commonly used to preoperatively assess the risk of atlantoaxial pedicle screw placement: first, measuring the width of the pedicle on standard horizontal axial CT, and second, assessing the high-span variability of the vertebral artery. CT multiplanar reconstruction (MPR) is generally used to measure the narrowest part of the pedicle in reported studies, making it suitable for preoperative assessment of the feasibility of pedicle screw placement as well as a gold standard for postoperative evaluation of pedicle screw tract location22,23,24,25.
In this study, we used 3D-Slicer software to 3D-reconstruct preoperative images of patients since 2021, saving the time required for preoperative planning. This technique allowed the operator to better visualize the patient’s anatomy, facilitating the optimal placement and length of screws. Where the axial vertebral artery foramen was narrow and high, the pedicle screw was modified to a laminar screw to minimize the risk of injury. Especially in the case of anatomical variations of the vertebral artery, it helps the operator to effectively avoid intraoperative injury to the vertebral artery and improve the success rate of the operation. Thirteen patients in this study had a slender vertebral artery on one side due to development and there exists one patient whose left vertebral artery shares a common trunk with the left subclavian artery. Moreover, prevention of VAI ensures adequate blood supply to the brain in patients with vertebral artery atherosclerosis. The outcomes of this study suggest a significant reduction in operative time and intraoperative blood loss, as well as the absence of vertebral artery injuries in the 51 patients who underwent preoperative 3D reconstruction using 3D-Slicer. In contrast, four cases of VAI were observed in the group (n = 67) that did not receive preoperative planning with 3D-Slicer. Although this difference did not reach statistical significance, the VAI rate was 0% in the 3D-Slicer group compared to 5.97% (4/67) in the non-3D-Slicer group, indicating a potential protective effect in clinical practice. According to the classification of Minimally Invasive Cervical Pedicle Screw (CPS) placement26,27, four cases of VAI were identified in this study. Among them, three cases were categorized as Grade IIa, indicating a cortical breach of less than 25% without neurovascular contact. One case was classified as Grade IIb, defined by a breach of less than 25% with neurovascular contact. Notably, none of these injuries resulted in significant clinical consequences. These findings highlight the effectiveness of 3D-Slicer-assisted preoperative planning in enhancing surgical safety. Moreover, a shorter operative time reduces patient exposure to intraoperative fluoroscopy, and decreased intraoperative blood loss implies not only less bleeding from the venous plexus but also fewer complications related to vascular injury.
VAI, though infrequent, remains a serious complication of posterior C1-C2 instrumentation due to the close proximity of osseous and vascular structures in the upper cervical spine. Iatrogenic VAIs are most commonly associated with screw malposition involving the pedicle or lateral mass, particularly affecting the V2-V3 segments. Anatomical variations such as high-riding vertebral arteries (HRVAs) or narrow pedicles further elevate the risk, especially in revision surgeries where anatomical landmarks may be obscured. In the present study, no VAI events were observed among the 51 patients who underwent preoperative 3D reconstruction using 3D-Slicer, whereas 4 cases (5.97%) occurred in the 67 patients who did not receive such planning. Although the difference did not reach statistical significance (Fisher’s exact test, p = 0.122), the calculated odds ratio (OR = 0.15; 95% CI: 0.008–2.59) and absolute risk reduction (5.97%; 95% CI: -12.5% to + 0.63%) suggest a potential benefit of preoperative 3D planning. The 95% confidence interval for VAI incidence in the 3D-Slicer group (0.00–7.02%) was narrower and shifted toward zero compared to the non-Slicer group (1.86–14.60%), supporting a clinically meaningful trend. These findings are consistent with prior studies highlighting the utility of computer-assisted planning and intraoperative navigation in improving screw trajectory accuracy and reducing vascular complications. Park et al. demonstrated that 3D simulation aids in avoiding VA encroachment in revision procedures, while Patkar proposed a modified C2 screw trajectory to circumvent the vertebral artery with favorable outcomes and no reported injuries28,29. Furthermore, the occurrence of asymptomatic bilateral VAI with preserved cerebral perfusion, as reported by Jung et al., underscores the variable and sometimes unpredictable clinical consequences of such injuries30. In addition to planning software, alternative fixation techniques-such as intra-articular screw placement or cantilever constructs-have been proposed for patients with HRVAs or challenging bony anatomy31. Collectively, the evidence supports the integration of personalized preoperative imaging, including CT angiography and 3D simulation, into standard practice for C1-C2 fusion, particularly in anatomically complex or high-risk cases.
The image processing software 3D-Slicer used in this study was mainly applied in five areas such as cancer, brain, lung, breast, and prostate rather than bone before this study. Being open-source, it can be employed in various kinds of hospitals and developed beyond what is achievable by similar software like Mimics, all whilst disregarding the cost. Going forward, we intend to use 3D-Slicer in combination with Mimics and CAD-RP (Computer-Aided Design and Rapid Prototyping). The initial step shall be to elect a suitable personalized screw tract on the computer simulation model and simulate the position of the pedicle screws. Subsequently, the application of 3D printing technology will follow to perform pre-surgery on the model. Ultimately, a clinical operation shall be conducted, therefore enhancing the efficacy and safety of the said surgery. Furthermore, the above preoperative preparations should be extended to other orthopedic operations for further research.
Study limitations
This study has inherent limitations. Its retrospective nature and relatively small sample size may restrict the generalizability of the findings and limit causal inference. Future large-scale, prospective randomized trials are needed to validate these results. In subsequent research, we aim to address these limitations and incorporate advances in artificial intelligence to enhance technical support and clinical applicability.
Conclusion
The use of 3D-Slicer for preoperative planning in posterior C1-C2 fixation in patients with vertebral artery variations shows promising potential.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Xu, S. et al. Evaluation of vertebral artery anomaly in basilar invagination and prevention of vascular injury during surgical intervention: CTA features and analysis. Eur. Spine J. 27 (6), 1286–1294. https://doi.org/10.1007/s00586-017-5445-4 (2018).
Wang, Y., Wang, C. & Yan, M. Clinical outcomes of atlantoaxial dislocation combined with high-riding vertebral artery using C2 Translaminar screws. World Neurosurg. 122, e1511–e1518. https://doi.org/10.1016/j.wneu.2018.11.092 (2019).
Vaněk, P. et al. Vertebral artery and osseous anomalies characteristic at the craniocervical junction diagnosed by CT and 3D CT angiography in normal Czech population: analysis of 511 consecutive patients. Neurosurg. Rev. 40 (3), 369–376. https://doi.org/10.1007/s10143-016-0784-x (2017).
Evaniew, N. et al. Atlantoaxial instability in acute odontoid fractures is associated with nonunion and mortality. Spine J. 15 (5), 910–917. https://doi.org/10.1016/j.spinee.2014.03.029 (2015).
Vetic, D., Pavlicevic, D. & Antic, B. C1–C2 screw fixation in the patient with anomalous course of vertebral artery—A case report Article. Vojnosanit. Pregl. 76 (5), 555–558. https://doi.org/10.2298/vsp160622143i (2019).
Harel, R., Nulman, M. & Knoller, N. Intraoperative imaging and navigation for C1–C2 posterior fusion. Surg. Neurol. Int. 10, 149. https://doi.org/10.25259/sni_340_2019 (2019).
Zhan, J. et al. Accuracy and safety of robot-assisted versus fluoroscopy-guided posterior C1 lateral mass and C2 pedicle screw internal fixation for atlantoaxial dislocation: A preliminary study. Biomed. Res. Int. 2022, 8508113. https://doi.org/10.1155/2022/8508113 (2022).
Hou, Z. et al. Application of C2 subfacetal screws for the management of atlantoaxial dislocation in patients with Klippel-Feil syndrome characterized by a narrow C2 pedicle and high-riding vertebral artery. J. Orthop. Surg. Res. 17 (1), 495. https://doi.org/10.1186/s13018-022-03391-z (2022).
Byun, C. W. et al. The association between atlantoaxial instability and anomalies of vertebral artery and axis. Spine J. 22 (2), 249–255. https://doi.org/10.1016/j.spinee.2021.08.014 (2022).
Goel, A. & Laheri, V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir. (Wien). 129 (1–2), 47–53. https://doi.org/10.1007/bf01400872 (1994).
Harms, J. & Melcher, R. P. Posterior C1–C2 fusion with polyaxial screw and rod fixation. Spine (Phila Pa 1976) 26 (22), 2467–2471. https://doi.org/10.1097/00007632-200111150-00014 (2001).
Melcher, R. P. et al. Biomechanical testing of posterior atlantoaxial fixation techniques. Spine (Phila Pa 1976) 27 (22), 2435–2440. https://doi.org/10.1097/00007632-200211150-00004 (2002).
Hott, J. S., Lynch, J. J., Chamberlain, R. H., Sonntag, V. K. & Crawford, N. R. Biomechanical comparison of C1–2 posterior fixation techniques. J. Neurosurg. Spine 2 (2), 175–181. https://doi.org/10.3171/spi.2005.2.2.0175 (2005).
Su, B. W. et al. Comparison of fatigue strength of C2 pedicle screws, C2 pars screws, and a hybrid construct in C1–C2 fixation. Spine (Phila Pa 1976) 39 (1), E12–E19. https://doi.org/10.1097/brs.0000000000000063 (2014).
Shimizu, T. et al. Correlation between osteoarthritis of the atlantoaxial facet joint and a high-riding vertebral artery. BMC Musculoskelet. Disord. 22 (1), 406. https://doi.org/10.1186/s12891-021-04275-9 (2021).
Lang, Z. et al. Posterior atlantoaxial internal fixation using Harms technique assisted by 3D-based navigation robot for treatment of atlantoaxial instability. BMC Surg. 22 (1), 378. https://doi.org/10.1186/s12893-022-01826-2 (2022).
Chiapparelli, E. et al. Spinal cord medial safe zone for C2 pedicle instrumentation: An MRI measurement analysis. Spine (Phila Pa). 47 (3), E101-e106. https://doi.org/10.1097/brs.0000000000004137 (2022).
Chen, Q. et al. Posterior atlantoaxial fusion: a comprehensive review of surgical techniques and relevant vascular anomalies. J. Spine Surg. 6 (1), 164–180. https://doi.org/10.21037/jss.2020.03.05 (2020).
Klepinowski, T., Pala, B., Cembik, J. & Sagan, L. Prevalence of high-riding vertebral artery: A meta-analysis of the anatomical variant affecting choice of craniocervical fusion method and its outcome. World Neurosurg. 143, e474–e481. https://doi.org/10.1016/j.wneu.2020.07.182 (2020).
Seçer, M. et al. Salvage posterior atlantoaxial fixation techniques: A retrospective study. Neurocirugia (Astur. Engl. Ed.) 33 (6), 310–317. https://doi.org/10.1016/j.neucie.2021.08.001 (2022).
Tian, Y. et al. Strategies to avoid internal carotid artery injury in “sandwich” atlantoaxial dislocation patients during surgery. Acta Neurochir. (Wien) https://doi.org/10.1007/s00701-022-05449-7 (2022).
Maki, S. et al. Medially-shifted rather than high-riding vertebral arteries preclude safe pedicle screw insertion. J. Clin. Neurosci. 29, 169–172. https://doi.org/10.1016/j.jocn.2015.11.026 (2016).
Marques, L. M., d’Almeida, G. N. & Cabral, J. “Two-step” technique with OsiriX™ to evaluate feasibility of C2 pedicle for surgical fixation. J. Craniovertebr. Junct. Spine 7 (2), 75–81. https://doi.org/10.4103/0974-8237.181826 (2016).
Simpson, V., Clair, B., Ordway, N. R., Albanese, S. A. & Lavelle, W. F. Are traditional radiographic methods accurate predictors of pedicle morphology?. Spine (Phila Pa 1976) 41 (22), 1740–1746. https://doi.org/10.1097/brs.0000000000001628 (2016).
Davidson, C. T., Bergin, P. F., Varney, E. T., Jones, L. C. & Ward, M. S. Planning C2 pedicle screw placement with multiplanar reformatted cervical spine computed tomography. J. Craniovertebr. Junct. Spine 10 (1), 46–50. https://doi.org/10.4103/jcvjs.JCVJS_116_18 (2019).
Srikantha, U. et al. Minimally invasive subaxial cervical pedicle screw placement with routine fluoroscopy: Cadaveric Feasibility study and report of 6 clinical cases. J. Min. Invasive Spine Surg. Tech. 7 (1), 98–106 (2022).
Lee, J. H. et al. Cervical pedicle screw placement using medial funnel technique. Korean J. Spine 14 (3), 84–88. https://doi.org/10.14245/kjs.2017.14.3.84 (2017).
Park, S. et al. C1–2 fixation using polyaxial screws and rods assisted by computer simulation for revision of failed posterior fusion—A technical report. 컴퓨터 시뮬레이션의 도움을 받아 제1–2 경추간 유합술의 개정술로 시행한나사-강봉 고정술- 증례 보고. J. Korean Orthop. Assoc. 40 (6), 778–781 (2005).
Patkar, S. V. New entry point for C2 screw, in posterior C1–C2 fixation (Goel-Harm’s technique) significantly reducing the possibility of vertebral artery injury. Neurol. Res. 38 (2), 93–97. https://doi.org/10.1080/01616412.2015.1105582 (2016).
Jung, J. H., Lee, J. K., Moon, B. J. & Hong, J. H. Asymptomatic iatrogenic bilateral occlusion of vertebral artery after atlantoaxial fusion: a case report. Acta Orthop. Et Traumatol. Turcica https://doi.org/10.5152/j.aott.2024.23206 (2024).
Wang, L. N. et al. Comparison of two temporary fixation techniques for the treatment of type II odontoid fracture. Acta Orthop. Belgica 84 (1), 108–115 (2018).
Author information
Authors and Affiliations
Contributions
J.Z. and S.W. contributed to data acquisition of this study. Z.K., G.Z., Y.L., D.Z. and Y.W. were responsible for the study design. H.W. and J.Z. performed statistical analysis and completed the first draft of this manuscript. Y.W. provided methodology consultation and critical revision of this manuscript. Figures 2–5 were all originally drawn and created by J.Z. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was reviewed and approved by the Ethics Review Committee of the Second affiliated hospital of Chongqing Medical University. The written informed consent was obtained before study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Jx., Ke, Zy., Zhao, Gs. et al. Application of 3D Slicer for preoperative planning in upper cervical posterior fixation with vertebral artery variations. Sci Rep 15, 27492 (2025). https://doi.org/10.1038/s41598-025-13469-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13469-3