Abstract
Dieback episodes in Juniperus procera, driven by climate and soil characteristics, are documented across forest ecosystems worldwide. However, these phenomena remain underexplored in Saudi Arabia, especially concerning elevation gradients. We conducted an analysis of the environmental characteristics that led to dieback events impacting the Juniperus procera scrublands in the southwestern mountainous region. This study was carried out across three sites in Saudi Arabia: Taif (L1–L4), Al-Baha (L5–L7), and Aseer (L8–L12). Our approach included efforts toward biological restoration by applying NPK fertilizer at concentrations of 10 and 15 g/L to the threatened J. procera trees. The data revealed that the peak concentrations of nitrogen, potassium, phosphorus, calcium, and organic matter, alongside the lowest pH value, were observed at an elevation of 3238 m (L10) in the Aseer region. In contrast, the minimum levels of nitrogen, potassium, phosphorus, calcium, and organic matter, alongside the highest pH value, were observed at an elevation of 2672 m in L1 of the Taif region. Interestingly, Juniper populations presented the most favorable health status, with a dieback rate of only 5% at an elevation of 2966 m in the Taif region. In contrast, the most significant dieback, reaching 72%, was observed at an elevation of 2780 m in the same region. A notable negative correlation was identified between juniper dieback and elevation, as well as between soil variables, including N, K+, Na+, P, Ca+ 2, and OM (r = −0.48, −0.63, −0.46, −0.48, −0.63, −0.41, and −0.48, respectively). Furthermore, notable relationships were identified between elevated terrains and the concentrations of nitrogen (N), potassium (K+), sodium (Na+), phosphorus (P), and calcium (Ca+ 2) and magnesium (Mg+ 2) in the soil, alongside a significant association with the pH. Thus, this research revealed that variations in climate and soil adversely affect juniper dieback.
Similar content being viewed by others
Introduction
The genus Juniperus L. represents the second most prevalent category of conifers globally, encompassing coniferous evergreen trees and shrubs that are part of the Cupressaceae family. As a result, it is located in the Enemas region of southern Saudi Arabia1,2 (Mujwah et al., 2010; Salih et al., 2021). This species is referred to as “Arar” in Arabic and is indigenous to the mountainous regions of eastern Africa, extending from eastern Sudan to Zimbabwe and the southwestern part of the Arabian Peninsula. It is widely found in southern Saudi Arabia, typically at altitudes ranging from 1750 to 2500 m above sea level2,3. Juniper forests are crucial for establishing habitats, as they prevent soil erosion, regulate hydrographic conditions, and delay the development of debris-mud rivers4.
The primary applications of Juniperus procera Hochst. ex Endl. wood encompass residential building, fence posts, poles, floors, and outdoor wooden structures necessitating toughness, such as beehives and pencils.The wood of the Juniperus procera tree possesses a distinctive yet nonirritating aromatic scent, which becomes more pronounced when freshly cut.The wood of J procera is highly fragrant, possessing a distinctive and enduring aromatic cedar scent5. Essential oil mostly extracted from the sawdust of J procera wood (Cedarwood oil) and utilised in the cosmetic industry for soaps and perfumes6.
Juniper resurgence faces dioecy-related reproductive loss, population decrease, and tree dieback. Conversely, climate influences have caused forest dieback and mortality worldwide. However, climate change has caused forest dieback and mortality worldwide7,8. Given their greater height and biomass accumulation than shrubs do, trees are viewed as more resilient to drought conditions, which may explain this observed bias9. Moreover, as noted by Gazol and Camarero10, the growth of shrubs is influenced more by the specific conditions of their microsites than by tree meristems, which are more closely associated with air temperatures. While drought-resistant shrubs may face dieback during extreme water shortages11,12, the precise mechanisms behind drought-induced mortality in these plants and the potential for recognizing early indicators of dieback from growth patterns are still unclear. In this context, Camarero et al.7 characterized dieback as the slow yet continuous decline of individual branches or shoots, starting from the tips and extending to the main stem.
The concepts seek to rehabilitate river dynamics, facilitate direct restoration, and establish process regimes rather than static states. Conditions ought to differ instead of being uniform13. Restoration involves removing human-induced structures such as levees that impede channel migration or dams that obstruct the movement of water, sediment, and biota, so allowing natural variability to govern habitat formation and ecosystem functionality. Partial process restoration reinstates natural system characteristics while retaining human impacts. Restoring environmental flows in regulated rivers emulates natural hydrologic patterns by reinstating floods to low flow conditions. The causes and consequences of salt marsh-to-mudflat dieback events that resulted in prolonged plant loss and their susceptibility to sea level rise. Salt marsh trajectories have been employed to forecast and evaluate marsh persistence in the context of climate change and other ecological disturbances, as well as to investigate ecosystem recovery following restoration or marsh building14. We investigated the impact of vegetation dieback on the resilience of salt marshes to sea level rise utilising mechanistic models. Immediate restoration of marsh dieback is essential to avert additional damage. Neglecting the heightened frequency and severity of catastrophic climatic events that cause irreversible marsh diebacks underappreciates the susceptibility of salt marshes to climate change15.
The landscape of the Kingdom of Saudi Arabia features a variety of natural features, including mountains, plateaus, plains, valleys, and dunes. Its predominantly arid characteristics profoundly influence the physical environment, as well as the diversity of plant life, agricultural practices, and ecosystems16. The elevated terrains in the Kingdom of Saudi Arabia are found within the Sarawat Mountain range, stretching 1550 km from the Taif region in northern China to Yemen in southern China, with elevations varying between 800 and 3000 m17. Highlands are composed of igneous and metamorphic rocks, which are known for their durability and adaptability to erosive processes18,19. Moreover, dieback is particularly noticeable in the northern mountainous region of Aseer adjacent to a recently built road. The majority of juniper populations, with their roots nestled in delicate soils, either perish or show clear signs of distress. Consequently, numerous dead or weakened juniper populations are observable in these areas20.
Nonetheless, a defining feature of mountain ecosystems is the sudden change in vegetation and temperature that takes place from the base of a mountain to its peak17. A variety of interconnected biological, environmental, and historical elements influence how species are distributed across elevation gradients21. A multitude of environmental factors fluctuate concurrently across a complex gradient: altitude22. Variations in altitude create distinct climates, which in turn contribute to soil diversity and promote the richness of plant species. The relationships between species distributions and altitude ranges provide insights into the potential consequences of environmental changes. For example, by utilizing fundamental data, we can assess the impacts of human activities and climate change on plant cover23. Simultaneously, in mountainous regions, factors such as elevation, slope side, and slope angle contribute to primary topographical features, which affect soil patterns and properties by altering the local climate, water flow patterns, local drainage capacity, movement of soil materials along hillside slopes, and distribution and decline of plant communities16,24. In a similar vein, soils are fundamental to the operation of terrestrial ecosystems. The production of biomass is essential, but it also plays a crucial role in regulating environmental interactions. This includes the transformation of substances, the purification and supply of water, and the accumulation of carbon. Furthermore, it acts as a biological habitat and genetic reservoir for a diverse array of organisms16. This research focused on examining how variations in elevation and soil properties influence the decline in Juniperus procera populations in the mountainous areas of southwestern Saudi Arabia.
Materials and methods
Study area and climate indices
The mountainous highlands of Saudi Arabia are divided into three sections: the Sarawat, Hijaz, and Midian Mountains. The Sarawat Mountains are distinguished by high altitudes, which rise approximately 3,700 m above sea level near Abha (Aseer) and decrease gradually northward. This mountainous area is associated with heavy rains, high relative humidity, and low temperatures. However, the slopes of the highlands and foothills of the Sarawat Mountains are distinguished by coarse pink granite mixed with gray diorite and granodiorite; it has largely exposed rock and a steep frontage with almost no soil cover, sparsely dotted with vegetation primarily confined to small crevices or depressions, where fine sediments accumulate in pockets. However, it is also found on large boulders, small stones, gravel, and steep streams25.
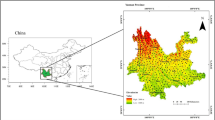
Moreover, the study was conducted at 12 locations in 3 districts (Taif, Al-Baha, and Aseer) along the Al-Sarawat Mountain chain to characterize the environmental changes associated with the presence of Juniperus procera plants as illustrated in map (Fig. 1). However, the meteorological station (Al-Sooda Station) located inside the study area provided the rainfall data. In contrast, the meteorological station is situated approximately 20 km to the southwest in terms of temperature data. (Station Al-Baha). The temperature, rainfall, and relative humidity statistics were obtained and maintained by the Saudi Arabian Presidency of Meteorology and Environment (PME) and the Ministry of Environment, Water, and Agriculture. The climatic elevation at different locations is summarized in (Fig. 2).
Data collection
For the present study, three main districts (Taif, Al-Baha, and Aseer) in the Sarawat Mountains were chosen as the locations to estimate the impacts of climatic and edaphic factors, as well as the impact of the elevation gradient, on the survival of juniper trees (Fig. 1). In total, there are 12 locations in the three districts, with four sites in Taif, 3 in Al-Baha, and 5 in Aseer (Table 1; Fig. 1). A geographical position system (GPS) determines each site’s coordinates and elevation. The percentage of dieback of juniper trees was determined visually for each recorded tree as a cover percentage of the withered area compared with the total crown cover. The total average of all trees recorded at each site was subsequently calculated. Dieback symptoms included foliage being lost from outside the crown and moving toward the center.
Soil sampling and analysis
Six soil samples from a 0–50 cm soil profile were collected from each of the 12 sites selected in the Sarawat Mountains. The soils were air-dried and sieved (2 mm sieve). Soil–water extracts (1:5 w/v) were prepared to measure the pH via a pH meter (JENWAY Model 3510)26. In accordance with Jackson27 method, the organic matter in the soil (OM) was assessed via oxidation with potassium dichromate and titration with ferrous sulfate28. The total nitrogen (N) content was assessed via the Kjeldahl method29. In contrast, available phosphorus (P) was extracted with a sodium bicarbonate solution (0.5 M) at pH 8.5 and then measured with a spectrophotometer (Unico 2000UV, Unico photometers, and spectrophotometers, Ontario, Canada) at 660 nm (26 Burt, 2004). The soluble Na, Ca, K, and Mg were detected via inductively coupled plasma‒optical emission spectrometry (ICP − OES, Thermo iCAP 6000 series, Germany). The methods mentioned above for cation analysis are described by Allen et al.30. The different heavy metals as zinc, lead, cupper, manganese, cadmium and iron (Zn, Pb, Cu, Mn, Cd & Fe) were determined by using GSC on the ECA flow 150 GLP device. Samples of hydro-soil in addition to water were analyzed using the same method according to Allen et al.30.
Statistical analysis
After testing for data heterogeneity, significant variance in the investigated soil variables and the percentage of dieback of J. procera populations among the studied sites was estimated via one-way analysis of variance (ANOVA) via SPSS software (SPSS 2012). In addition, the interrelationships among the soil variables, elevation, and dieback percentage were estimated by calculating the simple linear correlation coefficient (r). Principal component analysis (PCA) and the color Pearson correlation among various soil parameters effectively illustrated the degree of similarity among different soil properties and elements on the basis of the Dice coefficient via the R Studio interface and R software (R Studio Team31; R Core Team32.
Results
Soil properties
The findings of the present study demonstrated substantial variations (p < 0.05) in every soil variable examined across several sites (Fig. 3). Notably, L10 had the highest soil contents of N, K+, P, and Ca+ 2 (0.16 ± 0.03, 645.23 ± 5.20, 44.77 ± 2.06, and 545.53 ± 17.37 mg kg− 1, respectively), with the highest organic matter (OM) content. The soils in the study area were mostly alkaline, with pH values ranging from 7.03 ± 0.15 to 7.97 ± 0.21, with elevations of 3670 and 2742 m a.s.l. for L10 and L1, respectively. Similarly, the highest value of OM (8.03%) was recorded at L10, and the lowest value (3.02%) was observed at lower elevations at L2.
In parallel, L8 had the highest Na+ content (367.13 ± 29.56 mg kg− 1), whereas L7 had the highest Mg+ 2 content (77.40 ± 8.50 mg kg− 1). However, L1 had the lowest N, Na+, P, and Ca+ 2 contents (0.05 ± 0.01, 100.00 ± 5.40, 15.27 ± 1.12, and 100.67 ± 2.66 mg kg− 1, respectively). Moreover, the lowest K+ (100.13 ± 3.70 mg kg− 1) was recorded in the soils of L3, whereas the lowest Mg+ 2 (23.63 ± 6.50 mg kg− 1) was recorded along L8.
For heavy metals zinc, lead, manganese, cadmium, cupper and iron; Fig. 4 illustrated that, L1 had the highest cupper concentrations (0.76 ± 0.01 ppm), while the L7 showed the highest concentrations from Mn, Pb and Zn (0.065 ± 0.001, 0.686 ± 0.2, and 0.566 ± 0.01ppm respectively). In parallel, L8 had the highest contents from Fe (0.736 ± 0.11 ppm) and Cd (0.093 ± 0.001 ppm).
Plant population dieback
Figure 5 shows a significant decline in the J. procera population below 2800 m a.s.l., characterized by unhealthy trees, relatively low production of berries and male cones, and instances of tree mortality. In contrast, the plant populations exhibited robust health effects at elevated altitudes. The dieback data of J. procera populations revealed notable differences across various elevations in the western highlands of Saudi Arabia. The populations exhibiting the most significant decline, with a dieback percentage of 72%, were observed in L3 at an elevation of 2780 m a.s.l. In contrast, the populations that displayed the least decline, at just 5%, were documented in L6 at 2966 m a.s.l. Analyzing the differences between healthy and unhealthy soils revealed that the former are enriched with essential nutrients (N, P, Na+, Ca+ 2, Mg+ 2, and OM) and possess greater water retention than their unhealthy counterparts (Fig. 6).
Environment–plant dieback relationships
The Pearson simple linear relationship between the environmental variables and the dieback percentage of J. procera revealed a noteworthy negative correlation (r = -0.48) between elevation and plant dieback (Table 2). Additionally, notable negative correlations were observed between the percentage of dieback and various soil variables, including nitrogen (N), potassium (K+), sodium (Na+), phosphorus (P), calcium (Ca+ 2), and organic matter (OM), with correlation coefficients of r = -0.63, -0.46, -0.48, -0.63, -0.41, and − 0.48, respectively. Additionally, notable increases were observed in the soil contents of nitrogen, potassium, sodium, phosphorus, and calcium (r = 0.71, 0.93, 0.45, 0.55, and 0.47), whereas a significant decline in soil pH (r = -0.57) was recorded with increasing elevation. Conversely, certain soil variables, including organic matter, nitrogen, phosphorus, calcium, and magnesium (Mg+ 2), presented positive correlations (r = 0.75, 0.77, 0.74, and 0.51, respectively). Furthermore, elevation was strongly correlated (r = 0.71, 0.93, 0.45, 0.55, and 0.47) with N, K+, Na+, P, and Ca+ 2 respectively.
The statistical analysis employing color Pearson correlations for the soil components, illustrated in Fig. 7, revealed the highest positive correlation (0.98) between zinc (Zn) and manganese (Mn), similar to the correlation observed between lead (Pb) and manganese, with a subsequent correlation of 0.96 between cadmium (Cd) and iron (Fe). The most significant positive correlation observed was 0.95 between Zn and Pb. The most notable negative correlation observed was − 0.73 between pH and cupper (Cu), closely followed by -0.7 between Zn and K. In parallel, the lowest negative correlation was − 0.02 between Na and Mn and also between Mn and Ca.
Figure 8 illustrates the outcomes of principal component analysis (PCA), where PCA1 represents 31.1% of the variance and PCA2 accounts for 25.4%. Principal component analysis (PCA) revealed that twelve sites from three regions (Taif, Al-Baha, and Aseer) were systematically organized and classified into three distinct categories. The initial cluster in the left quadrant of the PCA consists of three locations in Al-Baha, distinguished by the key identifying metrics rainfall, zn, and pb. The size of the arrow indicates the strength of the variable, whereas the orientation of the arrow represents the peak value of the variable. The second group included five Asser sites situated in the upper quadrant of the PCA, employing soil metrics such as humidity, temperature, Ca, Mg, and pH. The third group included four sites from Taif that were completely intermingled with the Asser group, utilizing soil elements such as Cd, Mn, and Cu.
Discussion
Soil properties
The differences in soil characteristics across altitudinal gradients in the mountainous regions of Saudi Arabia remain underresearched because of the challenges associated with accessing these locations17,33. The findings of the current study highlighted notable variations in all the examined soil indices across the various locations, likely attributed to the buildup of organic compounds and the extent of vegetation damage34. In a similar vein, the documented values for soil pH, K, Na, P, Ca, Mg, and OM align with those noted in the research conducted by El-juhany and Aref35 and Yasir et al.36 in the southwestern highlands of Saudi Arabia.
The pH of soil is a crucial indicator that significantly influences the dissolution of various nutrients and their accessibility for plants, thereby impacting overall plant growth18. The findings indicated that the soils in the study area were predominantly alkaline, likely due to the beneficial influence of the significant accumulation of organic matter at the surface36. The observed increase in soil organic matter may be attributed to the ongoing release of basic cations, which results from the gradual decomposition of the accumulated organic matter in a cool, moist environment36. In this context, Al-Ghamdi et al.18 highlighted that the soils of southwestern highlands presented low organic matter and nutrient levels. Nonetheless, the soils exhibited slight alkalinity and were nonsaline, with the elevated area at Al-Souda showing the highest organic matter content and the lowest pH value. Thus, the reduction in pH at elevated altitudes can be linked to the buildup and gradual breakdown of organic matter, along with the increased precipitation observed in those regions37. The accumulation of runoff from the upper regions might be linked to the leaching of vital cations from the solum soil38. Additionally, there was a decrease in soil phosphorus with increasing altitude, whereas sodium and potassium increased, with no distinct pattern noted for calcium and magnesium. The influence of soil organic matter on the chemical, physical, and biological properties of soil is substantial, as noted by Kumar et al.37. Additionally, organic matter plays a crucial role in replenishing what is lost from the soil due to the thick vegetation cover, which could eventually result in soil degradation39.
Plant population dieback
The occurrence of juniper dieback has been extensively documented across different mountain forest regions and is linked to changes in altitude and soil characteristics that can influence the composition and biomass of vegetation, especially in populations of J. procera, due to their broad distribution in the southwestern mountainous zone20. Furthermore, deficiencies in soil nutrients play a crucial role in influencing the growth of trees40. The J. procera population significantly decreased below 2800 m a.s.l., characterized by unhealthy trees, whereas the plant populations thrived at relatively high altitudes20,41. The latest assessment indicates that the optimal elevations for the growth and thriving of the examined populations are above 3000 m a.s.l., which is correlated with the highest levels of rainfall and cooler temperatures. The decline in population was significant at lower elevations characterized by reduced rainfall and elevated temperatures. Nonetheless, the boundary between a thriving and deteriorating forest, aligned with a reduced altitudinal threshold, indicates that the decline in the forest could be linked to climatic factors. These findings align with those of Negash42, who noted that natural regeneration beneath trees and at lower elevations tends to be somewhat limited, likely because of factors such as animal grazing, human activities, challenges in seed growth, shifts in land use, and adverse climatic conditions. Moreover, Allen et al.43 reported that a variety of elements, including diseases and pathogens, insect infestations, and challenging climate conditions, typically lead to dieback.
The primary factors contributing to dieback appear to be environmental stressors and a global decline; drought stands out as a significant cause affecting rural areas and forests7. In this context, the data revealed that optimal conditions for the growth and thriving of J. procera occur at elevations exceeding 3000 m a.s.l., which is correlated with the highest levels of rainfall and cooler temperatures44. When comparing the soils of healthy and unhealthy populations, it was observed that healthy soils contained higher levels of common nutrient elements (N, P, Na, Ca, Mg, and OM) and greater water content than their unhealthy counterparts did45. Jurskis46 demonstrated that in response to water scarcity, trees undergo temporary physiological changes to increase their survival, which can inadvertently increase their susceptibility to various biotic and abiotic stresses, including pests, diseases, and other environmental factors. Furthermore, the diminished resilience of landscapes caused by climate alterations could lead to extensive dieback41. The existing tree population might be on the brink of decline due to factors such as aging, drought, or disease; in this context, climate change could serve as the ultimate catalyst for extensive mortality43. Additionally, owing to their lower vulnerability to xylem cavitation, evergreen needle leaves, such as those of junipers, might experience increased dieback during prolonged droughts in dry regions47. Furthermore, regardless of rainfall, rising temperatures can intensify forest drought and considerably accelerate the mortality rate attributed to drought.
The greatest risk of dieback was observed at the lower elevations in the Taif dry region, with this risk diminishing as one ascended to higher elevations in the Aseer wet area. Allen et al.43 documented various cases of mortality due to drought that can affect trees across multiple forest ecosystems. Previous dieback events during periods of drought have been extensively recorded across the globe. Examples of significant mortality events can be observed in various regions: the extensive loss of Callitris, Eucalyptus, and Acacia species in northeastern Australia48; the decline of Nothofagus in New Zealand49; the mortality of Picea meyeri in northern China50; and the die-off of multiple pine species in the southwestern United States51,52.
Environment–plant dieback relationships
Although “high-altitude dieback” is characterized as a natural progression of rainforests53,54, it occurs in areas that are unsuitable for rainforest growth55. The alteration of tree root systems resulting from shifting soil conditions56 hinders the growth of trees alongside understory vegetation as the sapwood area diminishes. With an abundance of water in the soil, trees struggle to utilize it effectively, given the overly saturated conditions46. The Pearson simple linear relationship between the environmental variables and the dieback percentage of J. procera revealed a noteworthy negative correlation between elevation and plant dieback. Furthermore, notable negative correlations were observed between the percentage of dieback and various soil variables, including nitrogen, potassium, sodium, phosphorus, calcium, and organic matter. Additionally, notable increases in the soil contents of nitrogen, potassium, sodium, phosphorus, and calcium were detected, whereas a significant decrease in the soil pH was detected with increasing elevation. Elevated nitrogen levels in soils coupled with diminished phosphorus availability in trees could heighten the likelihood of dieback57,58. Tree sap and leaves gain increased nutrient density, making them more appealing to pests, parasites, and diseases59. These alterations also hinder beneficial fungi and encourage detrimental soil microorganisms60. Additionally, Wardle et al.58 noted that elevated N/P ratios in degraded forest soils globally lead to prolonged tree degradation. Furthermore, the potassium content in the soil observed in this study was found to be highest in the region with the greatest elevation, which is correlated with a reduced incidence of dieback. This phenomenon may be attributed to the role of potassium in influencing tree growth and enhancing tissue degradation60.
The present investigation revealed favorable relationships among soil characteristics, including organic matter and essential nutrients such as nitrogen, phosphorus, calcium, and magnesium. Comparable findings were reported by Agbeshie and Abugre38, Mukhopadhyay et al.61, and Sidari et al.62, who reported notable relationships between soil organic matter and essential nutrients such as nitrogen, phosphorus, and potassium, highlighting the importance of these factors for nutrient accessibility and the subsequent absorption of these crucial soil elements by plants. Furthermore, elevation was strongly correlated with N, K, Na, P, and Ca. Huang et al.63 noted that the important relationships between elevation and soil physicochemical characteristics significantly influence tree growth outcomes. In a similar vein, Paoli et al.64reported that enhanced tree growth and traits are found in nutrient-dense soils, corroborating the findings of the current study.
Soil studies exemplify a field where multivariate analysis techniques, including principal component analysis (PCA) and Pearson color correlation, are widely utilized. These methods are employed for identifying, classifying, and modeling data65,66. Principal component analysis (PCA) is a statistical technique that adeptly uncovers the most crucial principal components responsible for the bulk of information within a dataset, thereby reducing the number of features present in the dataset67,68. The current study classified the examined sites from three locations into three groups; the Al-Baha location stood out from the other two locations because of differences in soil pH and specific soil elements (Pb and Mn), whereas the Taif location was situated between Aseer locations on the basis of climatic data and various soil elements (K, Mg, and Ca).
The findings provide light on the environmental variables causing J. procera scrubland dieback, notably in the southwestern mountainous region. The researchers found strong relationships between juniper dieback rates and environmental variables by analyzing elevation gradients’ effects on soil properties and nutrient levels. For biological restoration, NPK fertilizer at varying doses could be applied to vulnerable J. procera trees. The study’s extensive investigation of soil characteristics and juniper health status at different elevations improves our understanding of forest ecosystem dieback episodes. This research illuminates the mechanisms of juniper dieback in Saudi Arabia and emphasizes the relevance of climatic and soil conditions in conservation and restoration. This work could improve future conservation measures and help preserve regional juniper populations.
Conclusion
The highest concentrations of nitrogen, potassium, phosphorus, calcium, and organic matter, alongside the lowest pH levels, were observed in elevated areas of the Aseer region, which also presented the lowest risk of dieback. In contrast, the minimum levels of nitrogen, potassium, phosphorus, calcium, and organic matter, along with the lowest pH values, were observed at the lower elevations of the Taif region, which corresponded with a higher percentage of dieback. The substantial negative correlation observed between juniper dieback and various factors, such as elevation and soil components, including nitrogen, potassium, sodium, phosphorus, calcium, and organic matter, supports this finding. These findings indicate that variations in climate and soil adversely affect juniper dieback. The primary causes of juniper dieback are highlighted, which can aid in the conservation of these vital highland species. Nonetheless, the insights gained from the early warning metrics established at that location could be applicable to other regions within Saudi Arabia. Also, recommend this Saudi Arabian Juniperus procera dieback study to forestry and environmental researchers, policymakers, and conservationists.
Data availability
Data of the present article are available in the paper.
References
Mujwah, A. A., Mohammed, M. A. & Ahmed, M. H. First isolation of a flavonoid from Juniperus procera using Ethyl acetate extract. Arab. J. Chem. 3 (2), 85–88. https://doi.org/10.1016/j.arabjc.2010.02.003 (2010).
Salih, A. M. et al. Mass propagation of Juniperus procera hoechst. Ex endl. From seedling and screening of bioactive compounds in shoot and callus extract. BMC Plant Biol. 21, 1–13. https://doi.org/10.1186/s12870-021-02946-2 (2021).
Hazubska-Przybył, T. Propagation of Juniper species by plant tissue culture: A mini-review. Forests 10 (11), 1028. https://doi.org/10.3390/f10111028 (2019).
Rahmonov, O. et al. The human impact on the transformation of Juniper forest landscape in the Western part of the Pamir-Alay range (Tajikistan). Environ. Earth Sci. 76, 1–17. https://doi.org/10.1007/s12665-017-6643-4 (2017).
Fern, K. Useful Tropical Plants Database. http://tropical.theferns.info/viewtropical.php?id=Juniperus+procera (2014).
Oteng Amoako, A. A. & Brink, M. Plant Resources of Tropical Africa 7 (1). Timbers 1. Prota Fundation, Wageningen, Netherlands/ Baekhuys Publishers, Leiden, Netherlands/ CTA Wageningen, Netherlands,319–324 (2008).
Camarero, J. J. et al. Dieback and mortality of junipers caused by drought: dissimilar growth and wood isotope patterns preceding shrub death. Agric. Meteorol. 291, 108078. https://doi.org/10.1016/j.agrformet.2020.108078 (2020).
Camarero, J. J., Valeriano, C., Gazol, A., Colangelo, M. & Sanchez-Salguero, R. Climate differently impacts the growth of coexisting trees and shrubs under semiarid mediterranean conditions. Forests 12 (3), 381. https://doi.org/10.3390/f12030381 (2021).
Fajardo, A., McIntire, E. J. & Olson, M. E. When short stature is an asset in trees. Trends Ecol. Evol. 34 (3), 193–199. https://doi.org/10.1016/j.tree.2018.10.011 (2019).
Gazol, A. & Camarero, J. J. Mediterranean Dwarf shrubs and coexisting trees present different radial-growth synchronies and responses to climate. Plant. Ecol. 213, 1687–1698. https://doi.org/10.1007/s11258-012-0124-3 (2012).
Gazol, A., Sangüesa-Barreda, G., Granda, E. & Camarero, J. J. Tracking the impact of drought on functionally different Woody plants in a mediterranean scrubland ecosystem. Plant. Ecol. 218, 1009–1020. https://doi.org/10.1007/s11258-017-0749-3 (2017).
Krutovsky, K. V., Popova, A. A., Yakovlev, I. A., Yanbaev, Y. A. & Matveev, S. M. Response of pedunculate oak (Quercus Robur L.) to adverse environmental conditions in genetic and dendrochronological studies. Plants 14, 109. https://doi.org/10.3390/plants14010109 (2025).
Beechie, T. J. et al. Process based principles for restoring river ecosystems. BioScience 60, 209–222 (2010).
Wu, F. et al. Tong. Disturbance is complicated: Headward-Eroding saltmarsh creeks produce multiple responses and recovery trajectories. Limnol. Oceanogr. 67, S86–S100 (2022).
Marsh, A., Blum, L. K., Christian, R. R., Ramsey, E. & Rangoonwala, A. Response and resilience of spartina alterniflora to sudden dieback. J. Coast Conserv. 20, 335–350 (2016).
Griffiths, R. P., Madritch, M. D. & Swanson, A. K. The effects of topography on forest soil characteristics in the Oregon cascade mountains (USA): implications for the effects of climate change on soil properties. Ecol. Manag. 257 (1), 1–7. https://doi.org/10.1016/j.foreco.2008.08.010 (2009).
Al-Yasi, H. M., Alotaibi, S. S., Al-Sodany, Y. M. & Galal, T. M. Plant distribution and diversity along altitudinal gradient of Sarrawat mountains at Taif province, Saudi Arabia. Biosci. Res. 16 (2), 1198–1213 (2019).
Al-Ghamdi, A. A., Tadesse, Y., Adgaba, N. & Alghamdi, A. G. Soil degradation and restoration in Southwestern Saudi Arabia through investigation of soil physiochemical characteristics and nutrient status as indicators. Sustainability 13 (16), 9169. https://doi.org/10.3390/su13169169 (2021).
Contreras, A. et al. Land-Use impacts on soil erosion: geochemical insights from an urban drinking catchment, South-Central Chile. Water 16, 3246. https://doi.org/10.3390/w16223246 (2024).
Al-Yasi, H. M. Floristic diversity and dynamics in Sarrawat Mountains with special emphasis to Juniper forests, Taif, KSA. MSc. 211 (Taif University, 2011)
Colwell, R. K. & Lees, D. C. The mid-domain effect: geometric constraints on the geography of species richness. Trends Ecol. Evol. 15 (2), 70–76. https://doi.org/10.1016/S0169-5347(99)01767-X (2000).
Austin, A. T. et al. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141, 221–235. https://doi.org/10.1007/s00442-004-1519-1 (2004).
Lomolino, M. V. Elevation gradients of species-density: historical and prospective views. Glob Ecol. Biogeogr. 10 (1), 3–13. https://doi.org/10.1046/j.1466-822x.2001.00229.x (2001).
Fisk, M. C., Schmidt, S. K. & Seastedt, T. R. Topographic patterns of above-and belowground production and nitrogen cycling in alpine tundra. Ecology 79 (7), 2253–2266. https://doi.org/10.1890/0012-9658( (1998). 1998)079[2253:TPOAAB]2.0.CO;2.
Galal, T. M., Al-Yasi, H. M. & Fadl, M. A. Vegetation zonation along the desert-wetland ecosystem of Taif highland, Saudi Arabia. Saudi J. Biol. Sci. 28 (6), 3374–3383. https://doi.org/10.1016/j.sjbs.2021.02.086 (2021).
Burt, R. Soil survey laboratory methods manual (2004).
Jackson, M. L. Soil Chemical Analysis (Constable and Co. LTD, 1962).
Black, C. A. Methods of soil analysis. ASA 2, 771–1572 (1979).
Chibnall, A. C., Rees, M. W. & Williams, E. F. The total nitrogen content of egg albumin and other proteins. Biochem. J. 37 (3), 354–359 (1943).
Allen, S. E. Chemical Analysis of Ecological Materials (Blackwell Scientific, 1989).
R Studio Team. RStudio: Integrated Development for R. RStudio (Inc., 2015).
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R.
Kutby, A. M., Asiri, N. A., Alsherif, E. A. & Fadl, M. A. Floristic composition of Shamansir mountain, West Saudi arabia, as sustainable economic resources. Sci. Rep. 15, 16800. https://doi.org/10.1038/s41598-025-00441-4 (2025).
Ruiz-Sinoga, J. D. & Diaz, A. R. Soil degradation factors along a mediterranean pluviometric gradient in Southern Spain. Geomorphology 118 (3–4), 359–368. https://doi.org/10.1016/j.geomorph.2010.02.003 (2010).
El-Juhany, L. I. & Aref, I. M. The present status of the natural forests in the Southwestern Saudi arabia: 3-Asir and East Jazan forests. World Appl. Sci. J. 21 (5), 710–726 (2013).
Yasir, M. et al. Composition of soil Microbiome along elevation gradients in Southwestern highlands of Saudi Arabia. BMC Microbiol. 15, 1–9. https://doi.org/10.1186/s12866-015-0398-4 (2015).
Kumar, S., Suyal, D. C., Yadav, A., Shouche, Y. & Goel, R. Microbial diversity and soil physiochemical characteristic of higher altitude. PLoS One 14 (3). https://doi.org/10.1371/journal.pone.0213844 (2019). e0213844.
Agbeshie, A. A. & Abugre, S. Soil properties and tree growth performance along a slope of a reclaimed land in the rainforest agroecological zone of Ghana. Sci. Afr. 13, e00951. https://doi.org/10.1016/j.sciaf.2021.e00951 (2021).
Obalum, S. E., Okpara, I. M., Obi, M. E. & Wakatsuki, T. Short term effects of tillage-mulch practices under sorghum and soybean on organic carbon and eutrophic status of a degraded ultisol in southeastern Nigeria. Trop. Subtrop Agroecosystems. 14 (2), 393–403 (2011).
Reza, M. N. et al. Trends of soil and solution nutrient sensing for open field and hydroponic cultivation in facilitated smart agriculture. Sensors 25 (2), 453. https://doi.org/10.3390/s25020453 (2025).
Jurskis, V. & Turner, J. Eucalypt dieback in Eastern australia: a simple model. Australian Forestry. 65 (2), 87–98. https://doi.org/10.1080/00049158.2002.10674859 (2002).
Negash, L. Successful vegetative propagation techniques for the threatened African pencil Cedar (Juniperus procera hoechst. Ex Endl). Ecol. Manag. 161 (1–3), 53–64. https://doi.org/10.1016/S0378-1127(01)00501-1 (2002).
Allen, C. D. et al. Cobb, N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Ecol. Manag. 259 (4), 660–684. https://doi.org/10.1016/j.foreco.2009.09.001 (2010).
Neyland, M. Tree decline in tasmania: A review of the factors implicated in tree decline and management recommendations for its control. Land. Water Manag. (1996).
Sánchez-Navarro, A., Salas-Sanjuan, M. C., Blanco-Bernardeau, M. A., Sánchez-Romero, J. A. & Delgado-Iniesta, M. J. Medium-Term effect of organic amendments on the chemical properties of a soil used for vegetable cultivation with cereal and legume rotation in a semiarid climate. Land 12 (4), 897. https://doi.org/10.3390/land12040897 (2023).
Jurskis, V. Eucalypt decline in australia, and a general concept of tree decline and dieback. Ecol. Manag. 215 (1–3), 1–20. https://doi.org/10.1016/j.foreco.2005.04.026 (2005).
Maherali, H., Pockman, W. T. & Jackson, R. B. Adaptive variation in the vulnerability of Woody plants to xylem cavitation. Ecology 85 (8), 2184–2199. https://doi.org/10.1890/02-0538 (2004).
Fensham, R. J. & Holman, J. E. Temporal and Spatial patterns in drought-related tree dieback in Australian savanna. J Appl. Ecol., 1035–1050 https://www.jstor.org/stable/2655878 (1999).
Grant, P. J. Drought effect on high-altitude forests, Ruahine range, North island, new zealand. N. Z. J. Bot. 22 (1), 15–27. https://doi.org/10.1080/0028825X.1984.10425231 (1984).
Liang, E., Shao, X., Kong, Z. & Lin, J. The extreme drought in the 1920s and its effect on tree growth deduced from tree ring analysis: a case study in North China. Ann. Sci. 60 (2), 145–152 (2003).
Allen, C. D. & Breshears, D. D. Drought-induced shift of a forest–woodland ecotone: rapid landscape response to climate variation. Proc. Natl. Acad. Sci. USA 95 (25), 14839–14842. https://doi.org/10.1073/pnas.95.25.14839 (1998).
Swetnam, T. W. & Betancourt, J. L. Mesoscale disturbance and ecological response to decadal Climatic variability in the American Southwest. J. Clim. 11 (12), 3128–3147 (1998).
Stone, C. & Bacon, P. E. Leaf dynamics and insect herbivory in a Eucalyptus camaldulensis forest under moisture stress. Austral Ecol. 20 (4), 473–481. https://doi.org/10.1111/j.1442-9993.1995.tb00566.x (1995).
Old, K. M. Eucalypt Diseases of Complex Etiology. Diseases and Pathogens of Eucalypts 411–425 (CSIRO, 2000).
Jurskis, V. Overview of forest decline in coastal New South Wales. In Fundamental causes of eucalypt forest decline and possible management solutions. Proceedings of a colloquium at Batemans Bay, 18, 4–7 (2004).
Ellis, R. C. & Pennington, P. I. Factors affecting the growth of Eucalyptus delegatensis seedlings in inhibitory forest and grassland soils. Plant. Soil. 145, 93–105. https://doi.org/10.1007/BF00009545 (1992).
Guinto, D. F., Xu, Z. H., House, A. P. N. & Saffigna, P. G. Soil chemical properties and forest floor nutrients under repeated prescribed-burning in Eucalypt forests of south–east queensland, Australia. N Z. J. Sci. 31 (2), 170–187 (2001).
Wardle, D. A., Walker, L. R. & Bardgett, R. D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305 (5683), 509–513 (2004).
Shearer, B. L. & Smith, I. W. Diseases of eucalypts caused by soilborne species of phytophthora and pythium. Dis. Pathogens Eucalypts, 259–291 (2000).
Turner, J. Aspects of nutrient cycling and plant physiology in forest stands. In Fundamental Causes of Eucalypt Forest Decline and Possible Management Solutions. Proceedings of a Colloquium at Batemans Bay, 18, 15–17) (2004).
Mukhopadhyay, S., Masto, R. E., Cerdà, A. & Ram, L. C. Rhizosphere soil indicators for carbon sequestration in a reclaimed coal mine spoil. Catena 141, 100–108. https://doi.org/10.1016/j.catena.2016.02.023 (2016).
Sidari, M., Ronzello, G., Vecchio, G. & Muscolo, A. Influence of slope aspects on soil chemical and biochemical properties in a Pinus Laricio forest ecosystem of Aspromonte (Southern Italy). Eur. J. Soil. Biol. 44 (4), 364–372. https://doi.org/10.1016/j.ejsobi.2008.05.001 (2008).
Huang, Y. M., Liu, D. & An, S. S. Effects of slope aspect on soil nitrogen and microbial properties in the Chinese loess region. Catena 125, 135–145. https://doi.org/10.1016/j.catena.2014.09.010 (2015).
Paoli, G. D., Curran, L. M. & Slik, J. W. F. Soil nutrients affect Spatial patterns of aboveground biomass and emergent tree density in Southwestern Borneo. Oecologia 155, 287–299. https://doi.org/10.1007/s00442-007-0906-9 (2008).
Csomós, E., Simon-Sarkadi, L. & Héberger, K.& Principal component analysis of biogenic amines and polyphenols in Hungarian wines. J. Agric. Food Chem. 50, 3768–3774 (2002).
Badr, A. et al. Ameliorative impacts of gamma-aminobutyric acid (GABA) on seedling growth, physiological biomarkers, and gene expression in eight wheat (Triticum aestivum L.) cultivars under salt stress. BMC Plant. Biol. 24, 605. https://doi.org/10.1186/s12870-024-05264-5 (2024).
Jolliffe, I. T. & Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R Soc. Math. Phys. Eng. Sci. 374, 20150202 (2016).
Essa, N. M., Ibrahim, A. A. & Soliman, M. I. Association study between some cultivated species and their wild relatives from apiaceae, Asteraceae and brassicaceae families based on molecular and DNA barcoding in Egypt. Genet. Resour. Crop Evol. 71, 1125–1143. https://doi.org/10.1007/s10722-023-01681-x (2024).
Acknowledgements
The author extends his appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-163).
Funding
This research was funded by Taif University, Taif, Saudi Arabia (TU-DSPP-2024-163).
Author information
Authors and Affiliations
Contributions
H.M.Al. Conceptualization the paper, methodology, software, validation, formal analysis, investigation, resources , data curation, writing—original draft preparation, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Al-Yasi, H.M. Elevation, climate, and soil characteristics influence Juniperus procera dieback and restoration efforts in Southwestern Saudi Arabia. Sci Rep 15, 28825 (2025). https://doi.org/10.1038/s41598-025-13677-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13677-x