Abstract
Patients diagnosed with microsatellite stable (MSS) metastatic colorectal cancer (mCRC) typically have an immunosuppressive tumor microenvironment, which leads to a low response rate when treated with immunotherapy. Some studies indicate that chemotherapy and anti-angiogenic therapy could potentially improve the responsiveness of these patients to immunotherapy. Therefore, this study is designed to assess the effectiveness and safety of combining chemotherapy with bevacizumab and anti-PD-1 immunotherapy as a second-line treatment option for MSS mCRC. A retrospective analysis was conducted on patients diagnosed with MSS mCRC at Peking University First Hospital and Jilin Cancer Hospital from January 2020 to December 2024. Patients received second-line chemotherapy in combination with bevacizumab and anti-PD-1 immunotherapy. Progression-free survival (PFS), overall survival (OS), disease control rate (DCR), objective response rate (ORR), and treatment-related adverse reactions were collected. Biomarker analysis was performed to identify potential predictors of a favorable treatment response. Between January 2021 and December 2024, 29 patients were enrolled. Five patients (17.2%) achieved a partial response (PR), and 18 patients (62.1%) had stable disease. The median follow-up period was 13.3 months. The ORR was 17.2%, and the DCR was 79.3%. The median PFS was 7.8 months, and the median OS was 28.8 months. The most common treatment-related adverse events (TRAEs) of all grades were anemia (18/29, 62.1%), leukopenia (12/29, 41.4%), and hand-foot syndrome (10/29, 34.5%). The most frequent grade 3 or 4 TRAEs were anemia (2/29, 6.9%) and elevated triglycerides (1/29, 3.4%). No grade 5 adverse events occurred. Dynamic changes in the Lymphocyte-to-Monocyte Ratio (LMR), Systemic Immune-Inflammation Index (SII), and Platelet-to-Inflammatory Index (PIV) before and after treatment could predict the efficacy of immune combination therapy. In the biomarker exploration, multiple immunohistochemical analyses indicated better tumor immune microenvironment cell infiltration in the PFS-long (≥ 16 weeks) group compared to the PFS-short group. Chemotherapy combined with bevacizumab and anti-PD-1 immunotherapy has demonstrated promising efficacy in the treatment of MSS mCRC, with manageable adverse reactions. Exploratory biomarker assessment analysis showed that hematological dynamic changes and tumor immune microenvironment cell infiltration may predict the efficacy of immune combination therapy.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most prevalent malignant tumor globally and ranks second in terms of mortality rate1, serving as a leading cause of cancer-related deaths. Notably, approximately 25% of CRC patients are diagnosed with metastatic disease, with nearly half of them progressing to metastatic colorectal cancer (mCRC)2. The survival rate after mCRC diagnosis indicates that less than 20% of patients survive for 5 years3. Immune checkpoint blockade (ICB) therapy represents a pivotal area in cancer immunotherapy, focusing on immune checkpoint molecules such as programmed death-1 (PD-1) or programmed cell death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). These molecules are crucial for regulating immune responses, overcoming tumor-induced immunosuppression, and promoting more effective tumor suppression4,5. The benefits of immune checkpoint inhibitors (ICIs) are particularly pronounced in patients with immunoinflammatory tumors, such as melanoma, non-small cell lung cancer, and renal cell carcinoma, as well as in tumors with an increased neoantigen load and high mutation burden6,7. In contrast, ICIs exhibit limited efficacy in patients with immunologically “cold” tumors and those with a low mutation burden 8. Microsatellite instability-high (MSI-H) CRC was among the first tumors to demonstrate the benefits of ICIs9, and ICIs have since become the standard treatment for MSI-H CRC.
However, the efficacy of ICIs in microsatellite stable (MSS) CRC is limited. Compared to MSI-H tumors, MSS CRC exhibits distinct molecular mechanisms and a tumor microenvironment (TME) characterized by significantly lower tumor mutation burden and more pronounced immunosuppression and immune exhaustion 10. Nevertheless, a series of explorations have been conducted on ICIs in MSS CRC, and combination strategies with tyrosine kinase inhibitors (TKIs), vascular endothelial growth factor inhibitors (anti-VEGF agents), and chemotherapy are being tested in clinical trials. Previous studies have demonstrated that anti-angiogenic therapy can reverse the immunosuppressive tumor microenvironment by normalizing blood vessels and inducing T-cell infiltration and activation11. The phase II CheckMate 9X8 study compared the efficacy of nivolumab combined with mFOLFOX6 plus bevacizumab versus standard treatment (mFOLFOX6 plus bevacizumab) in the first-line treatment of mCRC patients12.Subgroup analysis revealed that patients with RAS-mutant/MSS (CMS3) mCRC could benefit from the addition of nivolumab to mFOLFOX6 plus bevacizumab. The KEYNOTE-651 study evaluated the long-term safety and efficacy of pembrolizumab combined with oxaliplatin, leucovorin, and fluorouracil (mFOLFOX7 regimen) for first-line treatment or pembrolizumab combined with irinotecan, leucovorin, and fluorouracil (FOLFIRI regimen) for second-line treatment of MSS/pMMR mCRC. The results suggested that pembrolizumab combined with chemotherapy is safe and effective for both first-line and second-line treatment of MSS/pMMR mCRC13.
Based on these findings, chemotherapy combined with bevacizumab and anti-PD-1 immunotherapy has a solid theoretical foundation and has demonstrated preliminary efficacy and safety in patients with MSS mCRC. Given the current lack of clinical data on the second-line treatment of advanced colorectal cancer with the combination of immunotherapy, anti-angiogenic drugs, and anti-PD-1 immunotherapy, we conducted a multicenter retrospective cohort clinical study to explore the safety and efficacy of this triplet therapy in the second-line treatment of advanced colorectal cancer patients.
Methods
Study design and participants
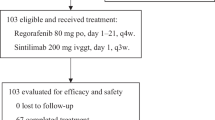
This study was a dual-center retrospective cohort study conducted at Peking University First Hospital and Jilin Cancer Hospital.Inclusion Criteria: Histologically or cytologically confirmed unresectable metastatic colorectal cancer (Stage IV according to the AJCC 8th edition) with measurable lesions based on the RECIST criteria. Progression after prior first-line standard two-drug chemotherapy regimen with or without targeted therapy. Proficient mismatch repair (pMMR) or MSS, BRAF wild-type. Patients receiving chemotherapy combined with bevacizumab and anti-PD-1 immunotherapy or chemotherapy combined with bevacizumab. Not having undergone radiotherapy or having completed radiotherapy more than 4 weeks ago. ECOG score ≤ 2. Exclusion Criteria: Patients with deficient mismatch repair (dMMR) or microsatellite MSI-H, or BRAF mutations. Presence of symptomatic brain metastases. Uncontrolled active infection. Dysphagia, intractable vomiting, or known drug absorption disorders. Patients with symptomatic or high-risk obstruction, bleeding, or perforation, or those who have undergone intestinal stent placement to relieve intestinal obstruction. In most cases, the chemotherapy regimen comprised an oxaliplatin-based doublet (FOLFOX: folinic acid, 5-fluorouracil, oxaliplatin, or CAPEOX: capecitabine and oxaliplatin) or a topoisomerase inhibitor (FOLFIRI: folinic acid, 5-fluorouracil, irinotecan). Anti-PD-1 immunotherapy included penpulimab, pembrolizumab, sintilimab, tislelizumab, and toripalimab. The anti-angiogenic agent was bevacizumab.
This study was conducted in compliance with the postulates of the Declaration of Helsinki and approved by the Ethics Committee of Peking University First Hospital and Jilin Cancer Hospital. The requirement for patient approval or informed consent was waived by the Human Ethics Committee of Peking University First Hospital and Jilin Cancer Hospital, owing to the retrospective nature of the study and because the analysis used anonymous clinical data.
Follow-up
Follow-up data were collected through hospital records, telephone interviews, outpatient visits, and rehospitalizations. The data included age, sex, ECOG status, primary tumor location, number of metastatic sites, percentage reduction in tumor volume, PFS, overall survival (OS), follow-up duration, and survival status. The last follow-up date was in December 2024. PFS was the primary outcome, defined as the time from enrollment to the first documented disease progression according to RECIST version 1.1, or death from any cause, whichever occurred first. Secondary outcomes included OS, objective response rate (ORR), disease control rate (DCR), and safety evaluation. OS was calculated from the date of enrollment to the date of death from any cause, with censored cases defined by the last available follow-up. ORR was defined as the proportion of patients with a best objective response of complete response (CR) or partial response (PR) according to RECIST criteria (version 1.1). DCR was defined as the proportion of patients with CR, PR, or stable disease (SD) according to RECIST criteria (version 1.1). Treatment-related adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
Biomarker analysis
Pre- and on-treatment blood samples were collected from all patients to measure neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), systemic immune-inflammation index (SII), and platelet-to-inflammatory index (PIV). Tumor samples, including both fresh and archival biopsies and resections, were obtained at baseline and subjected to multiple immunohistochemical staining, including CD3, CD4_FOXP3, CD8_PD1, CD20, CD56, CD68, PanCK, PD1, PDL1, and CD68_iNOS. Exploratory analysis was conducted to compare these markers in the PFS-long (≥ 16 weeks) group versus the PFS-short group.
Statistical analysis
The log-rank test was used as the primary analysis for comparison between metastasis subgroups. Differences between efficacy response subgroups were analyzed using the (nonparametric) Wilcoxon’s rank sum test (Mann–Whitney U-test), whereas differences between pre-treatment and post-treatment were analyzed using Wilcoxon’s signed-rank test. 95% confidence intervals (CIs) for the response rate were calculated using the Clopper-Pearson method. All reported P values are two-sided, and a P value < 0.05 was considered statistically significant. All statistical analyses in our study were performed using R software (version 4.4.2).
Results
Patients
Between January 2021 and December 2024, 29 patients were enrolled, including 21 males (72.4%) and 15 patients aged over 60 years (51.7%). There were 9 patients with right-sided colon cancer (31%). The vast majority of patients had undergone primary tumor surgery (26/29, 89.7%). Nineteen patients had more than two metastatic lesions (65.5%), among whom 19 had liver metastases (65.5%) and 18 had lung metastases (62.1%). Twenty patients had RAS mutations (69%). Please refer to Table 1 for details.
Effectiveness
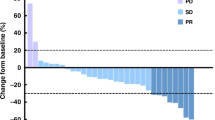
As of December 2024, the median follow-up period was 13.3 months. The median PFS was 7.8 months, and the median OS was 28.8 months (Fig. 1). Based on RECIST version 1.1, the ORR was 17.2%, and the DCR was 79.3%. Among them, 5 patients achieved PR, 18 patients SD, and 6 patients experienced PD. The best percent change in the target lesion diameter from baseline for all 25 patients is shown in Fig. 2A. Six patients were still receiving maintenance therapy at the data cut-off day (Fig. 2B). The median PFS was 6.77 months (95% CI 2.87–10.5) in patients with liver metastasis and 14.7 months (6.9–NA) in patients with non-liver metastasis (HR 3.684, log-rank test P = 0.0118, Fig. 3A). The median PFS was 8.03 months (95% CI 5.7–NA) in patients with lung metastasis and 6.9 months (4.17–NA) in patients with non-lung metastasis (HR 0.6865, log-rank test P = 0.387, Fig. 3B).
Safety
Treatment was generally well tolerated. Treatment-related adverse events (TRAEs) and immune-related adverse events are summarized in Table 2. At the data cut-off date (December 2024), the most common TRAEs of all grades were anemia (18/29, 62.1%), leukopenia (12/29, 41.4%), and hand-foot syndrome (10/29, 34.5%). The most frequent grade 3 or 4 TRAEs were anemia (2/29, 6.9%) and increased triglycerides (1/29, 3.4%). No grade 5 adverse events occurred during the study.
Biomarker analysis
Dynamic changes in the LMR (p = 0.049), SII (p = 0.0045), and PIV (p = 0.0045) before and after treatment were statistically significant. However, there were no statistically significant differences in the Neutrophil-to-Lymphocyte Ratio (NLR) (p = 0.06) and Platelet-to-Lymphocyte Ratio (PLR) (p = 0.44) between the two groups (Fig. 4A–E).
Baseline biopsy or operative specimens were obtained as required from patients for exploratory biomarker assessment. There were no statistically significant differences in CD3, CD8_PD1, CD20, CD56, CD68, PanCK, PD1, PDL1, and CD68_iNOS between the PFS-long (≥ 16 weeks) group and the PFS-short group. However, there were statistical differences in CD4_FOXP3 and CD68_CD163 between the two groups, with the group having better efficacy showing fewer CD4_FOXP3 (p = 0.0033) and CD68_CD163 (p = 0.00007) (Figs. 5, 6).
Discussion
Late-stage microsatellite stability (MSS) colorectal cancer accounts for approximately 95% of all colorectal cancers14. Traditional immune checkpoint inhibitors as monotherapy have shown poor efficacy, and in recent years, there have been explorations into various combination therapies. The main research directions for immune-based combination therapies in the later lines of treatment for late-stage MSS colorectal cancer are as follows: 1. Anti-angiogenic agents combined with immunotherapy (e.g., regorafenib + PD-1 inhibitors) have been shown to enhance efficacy, but the benefit is limited to a very small subset of patients. Subgroups such as those without liver metastases, Asian patients, and those with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 tend to benefit more, suggesting that this combination strategy requires precise patient stratification15. 2. Other strategies to enhance the efficacy of PD-1 inhibitors include combining them with other immune checkpoint inhibitors (CTLA-4 antibodies16, LAG-3 antibodies17, epigenetic modulation (HDAC inhibitors to remodel the immune microenvironment)18 and adenosine pathway blockade (dual antagonists of A2aR and A2bR adenosine receptors)19. 3. For patients with BRAF V600E mutations, the combination of targeted therapy against EGFR-BRAF-MEK with PD-1 antibodies has shown promising efficacy and warrants further exploration with larger sample sizes20.
The focal points for optimizing and exploring first-line immunotherapy in late-stage MSS colorectal cancer are as follows:1. High-intensity chemotherapy (e.g., FOLFOXIRI) combined with bevacizumab and PD-1/PD-L1 inhibitors (as in the AtezoTRIBE study) can prolong PFS, but the absolute benefit is small. The question of whether "more chemotherapy drugs are better" remains controversial, and the risks of toxicity and immune cell damage need to be weighed21. 2. Precise patient selection: For example, patients with high T-cell infiltration may benefit more22; POLE/POLD mutations (hypermutated phenotypes), although rare, are sensitive to immunotherapy 23. 3. Building on the "chemotherapy + anti-angiogenic" regimen, adding CTLA-4 antibodies16 (to enhance T-cell priming), CD47 antibodies24,25 (to block the "don’t eat me" signal and activate macrophages), or other immune checkpoint inhibitors (e.g., TIM-3)26 may break through the current efficacy ceiling. Our study shows that chemotherapy plus bevacizumab and anti-PD-1 immunotherapy has demonstrated promising efficacy in the treatment of MSS mCRC, with manageable adverse reactions.
Studies have shown that the LMR is associated with the infiltration of immune cells in the primary tumor microenvironment of gastric cancer27. Li et al.28 demonstrated that neoadjuvant chemotherapy can significantly reduce some inflammatory biomarkers in patients with locally advanced gastric cancer. When combined with factors such as age, gender, tumor location, and clinical stage in different clinical scenarios, high NLR before neoadjuvant chemotherapy, preoperative anemia, and significant changes in LMR often indicate a poor prognosis. Therefore, LMR and its changes before and after chemotherapy are important indicators for evaluating the efficacy and prognosis of neoadjuvant chemotherapy in gastric cancer. Some studies have shown a close association between higher SII levels and poorer prognosis in bladder cancer patients, indicating that SII is an important prognostic predictor for bladder cancer patients29. A retrospective study by Fucà et al. found that the PIV can guide the treatment decision-making process and the development of new first-line treatment strategies for melanoma. Compared with other biomarkers based on complete blood count (CBC), PIV has significant advantages in predicting the prognosis of melanoma patients30. Studies have also found that PIV has irreplaceable value in the prognosis of small cell lung cancer and breast cancer patients, guiding treatment decisions and helping clinicians select advantageous populations for treatment, thereby further improving the individualization and precision of cancer treatment31,32,33. Our study found that dynamic changes in the LMR, SII, and PIV before and after treatment can predict the efficacy of immune combination therapy.
Currently, immunotherapy for MSS colorectal cancer has transitioned from the era of “ineffective monotherapy” to that of "enhanced combination therapy." In the future, it will be necessary to drive innovative combinations through precise subtyping, achieve the transformation of “cold tumors” into “hot tumors” through cross-mechanism synergies (targeted therapy + immunotherapy + microenvironment modulation) and frontier technology-assisted stratification. The role of regulatory T cells (Tregs) in colorectal cancer (CRC) is controversial. In some studies, FOXP3 + T-cell infiltration indicates a poorer prognosis34. Other studies have shown that there are non-Treg cells expressing Foxp3 in the immune microenvironment of CRC, which have stronger infiltration ability than the former, exert pro-inflammatory effects, and do not express the naive Treg cell marker CD45RA, with unstable FOXP3 expression35. High baseline mRNA expression of macrophages (CD68 +, CD163 +) is significantly associated with partial response (PR) or stable disease (SD) at 16 weeks of immune combination therapy in colorectal cancer15. This study shows that patients with low baseline expression of CD4FOXP3 and CD68CD163 before immune combination therapy have a better prognosis.
We acknowledge that our multicenter retrospective cohort study has several limitations.
First, it had a single-arm design without a control group, which may induce some selection bias. Second, this trial had a limited sample size and the results need to be validated in larger studies. The maturity of PFS and OS data in the retrospective analysis was relatively low, and patient management was also relatively poor, introducing certain biases.
In summary
In the second-line treatment of MSS mCRC, chemotherapy combined with bevacizumab and anti-PD-1 immunotherapy demonstrated a high objective response rate (ORR), disease control rate (DCR), and a manageable safety profile in MSS mCRC. This suggests that chemotherapy combined with bevacizumab and anti-PD-1 immunotherapy is a promising combination strategy that is expected to provide more clinical benefits. Exploratory biomarker assessment analysis revealed that patients with reduced LMR, SII, and PIV after immune combination therapy have a better prognosis. Patients with fewer CD4FOXP3 and CD68CD163 in tumor tissue before treatment have a better prognosis.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263 (2024).
Dekker, E. et al. Colorectal cancer. Lancet 394(10207), 1467–1480 (2019).
Biller, L. H. & Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 325(7), 669–685 (2021).
Carbone, F. R. & Mackay, L. K. Functional T cell tolerance by peripheral tissue-based checkpoint control. Nat. Immunol. 24(8), 1224–1225 (2023).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8(9), 1069–1086 (2018).
Motzer, R. J. et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): Long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 23(7), 888–898 (2022).
Maio, M. et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 33(9), 929–938 (2022).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52(1), 17–35 (2020).
Casak, S. J. et al. FDA approval summary: Pembrolizumab for the first-line treatment of patients with MSI-H/dMMR advanced unresectable or metastatic colorectal carcinoma. Clin. Cancer Res. 27(17), 4680–4684 (2021).
Yuan, S., Almagro, J. & Fuchs, E. Beyond genetics: Driving cancer with the tumour microenvironment behind the wheel. Nat. Rev. Cancer 24(4), 274–286 (2024).
Qian, C. et al. Targeting vascular normalization: A promising strategy to improve immune-vascular crosstalk in cancer immunotherapy. Front. Immunol. 14, 1291530 (2023).
Lenz, H. J., Parikh, A., Spigel, D. R., et al. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: Phase 2 results from the CheckMate 9X8 randomized clinical trial. J. Immunother. Cancer, 12(3), (2024).
Chen, E. X. et al. Pembrolizumab plus binimetinib with or without chemotherapy for MSS/pMMR metastatic colorectal cancer: Outcomes from KEYNOTE-651 cohorts A, C, and E. Clin. Colorectal Cancer 23(2), 183–193 (2024).
Eng, C. et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 20(6), 849–861 (2019).
Fakih, M. et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: A single-arm, open-label, multicentre phase 2 study. EClinicalMedicine 58, 101917 (2023).
Fakih, M. et al. Preliminary results from a randomized, open-label, phase 2 study of botensilimab (BOT) with or without balstilimab (BAL) in refractory microsatellite stable metastatic colorectal cancer with no liver metastases (MSS mCRC NLM). J. Clin. Oncol. 43(4_suppl), 23 (2025).
Segal, N. H., Passhak, M., Köse, F., et al. Co-formulated favezelimab plus pembrolizumab versus standard-of-care in previously treated, PD-L1-positive metastatic colorectal cancer: The phase 3, randomized KEYFORM-007 study. J. Clin. Oncol. 43(4_suppl): LBA248-LBA (2025).
Wang, F. et al. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat Med 30(4), 1035–1043 (2024).
Wang, H. et al. Subtle structural changes across the boundary between A(2A)R/A(2B)R dual antagonism and A(2B)R antagonism: A novel class of 2-aminopyrimidine-based derivatives. J. Med. Chem. 67(6), 5075–5092 (2024).
Tian, J. et al. Combined PD-1, BRAF and MEK inhibition in BRAF(V600E) colorectal cancer: A phase 2 trial. Nat. Med. 29(2), 458–466 (2023).
Antoniotti C. et al. Upfront Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Bevacizumab With or Without Atezolizumab for Patients With Metastatic Colorectal Cancer: Updated and Overall Survival Results of the ATEZOTRIBE Study[J]. J Clinic Oncol: Official J Amer Soc Clinic Oncol. 42(22), 2637–2644. https://doi.org/10.1200/JCO.23.02728.
Chen, Y. et al. Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell 42(7), 1268–85.e7 (2024).
Bae, J. M. et al. Immune landscape and biomarkers for immuno-oncology in colorectal cancers. J. Pathol. Transl. Med. 54(5), 351–360 (2020).
Sugimura-Nagata, A., Koshino, A., Inoue, S., et al. Expression and prognostic significance of CD47-SIRPA macrophage checkpoint molecules in colorectal cancer. Int. J. Mol. Sci., 22(5), (2021).
Fujiwara-Tani, R. et al. Concurrent expression of CD47 and CD44 in colorectal cancer promotes malignancy. Pathobiology 86(4), 182–189 (2019).
Rahimi, A. et al. Combination therapy with immune checkpoint inhibitors in colorectal cancer: Challenges, resistance mechanisms, and the role of microbiota. Biomed. Pharmacother. 186, 118014 (2025).
Yuan J, Zhao X, Li Y, et al. The Association between blood indexes and immune cell concentrations in the primary tumor microenvironment predicting survival of immunotherapy in gastric cancer. Cancers (Basel), 14(15), (2022).
Li, Z. et al. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy. Gastric Cancer 23(3), 540–549 (2020).
Cao, W. et al. Prognostic significance of systemic immune-inflammation index in patients with bladder cancer: A systematic review and meta-analysis. Medicine (Baltimore) 101(36), e30380 (2022).
Fucà, G. et al. The pan-immune-inflammation value in patients with metastatic melanoma receiving first-line therapy. Target Oncol. 16(4), 529–36 (2021).
Zeng, R. et al. PIV and PILE score at baseline predict clinical outcome of anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung cancer patients. Front. Immunol. 12, 724443 (2021).
Şahin, A. B. et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 11(1), 14662 (2021).
Ligorio F, Fucà G, Zattarin E, et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line Taxane-Trastuzumab-Pertuzumab. Cancers (Basel), 13(8) (2021).
Fong, W. et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis. Gut 72(12), 2272–2285 (2023).
Saito, T. et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 22(6), 679–684 (2016).
Acknowledgements
We are grateful to the patients and their families for supporting the study.
Funding
The study was funded by Special project for clinical research on domestic multi-center central high-level hospitals of Peking University First Hospital, No. 2022CR65.
Author information
Authors and Affiliations
Contributions
Zhao Gao and Xiaoyan Wang wrote the manuscript. Xuan Jin and Shikai Wu conceived of the review and edited the manuscript. Zhao Gao collected and analyzed the data. Zhao Gao, Xiaoyan Wang, Tao Song and Shikai Wu analyzed the data and drafted the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study has been approved by the Ethics Committee of the Peking University First Hospital and Jilin Cancer Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, Z., Wang, X., Song, T. et al. Real world effectiveness of chemotherapy plus bevacizumab with immunotherapy in colorectal cancer. Sci Rep 15, 29170 (2025). https://doi.org/10.1038/s41598-025-13701-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13701-0