Abstract
The objective of this research was to identify the factors contributing to the decline in handgrip strength among middle-aged and elderly individuals with this condition. In addition, an algorithmic model for the detection of probable sarcopenia will be developed. This research encompassed the collection and evaluation of fundamental data, laboratory indicators, body composition metrics, and lifestyle factors. Patients were diagnosed with handgrip strength loss according to the diagnostic criteria established by the Asian Working Group for Sarcopenia in 2019, specifically for “probable sarcopenia”. A multifactorial logistic regression model was employed to discern the independent variables that significantly influence the occurrence of handgrip strength reduction among patients suffering from coronary artery disease. An internal validation of this model was conducted using the bootstrap repetitive sampling technique. The predictive efficacy of the model was assessed through comparisons of the area under the receiver operating characteristic curve, the calibration curve, and the decision curve for the subjects. High gait speed (OR 0.015; 95%CI 0.001–0.232), high calf circumference (OR 0.650; 95%CI 0.503–0.839), and high albumin level (OR 0.714; 95%CI 0.572–0.891) were significantly and negatively associated with reduced handgrip strength, which were protective factors for the development of probable sarcopenia. (all p < 0.05). Gait speed, calf circumference, and serum albumin levels were independent factors that influenced the likelihood of developing probable sarcopenia. The nomogram model based on these factors has a certain predictive value of probable sarcopenia, which can guide the development of disease prevention strategies.
Similar content being viewed by others
Introduction
Sarcopenia is a systemic muscle disorder defined by a decline in muscle mass, reduced muscle strength, and diminished physical performance.1 Studies indicate that the projected number of individuals suffering from sarcopenia is anticipated to rise to approximately 500 million by 2050.2 The concept of probable sarcopenia was first introduced by the European Working Group on Sarcopenia in Older People (EWGSOP2) through the identification of low muscle strength.3 However, given the specific context of this study, which focused on a Chinese population, the diagnostic criteria for muscle strength loss established by the 2019 Asian Working Group for Sarcopenia (AWGS) were adopted, with cutoff values of < 28 kg for men and < 18 kg for women.4 The primary description of sarcopenia centered on muscle bulk, but current international guidelines now place greater emphasis on muscle function.5 Sarcopenia diagnosis often centers on muscle strength, typically assessed through handgrip strength (HGS). The prevalence of sarcopenia in patients with cardiovascular disease ranges from 10.1 to 68.9%, indicating a notable presence, especially at 43% in those with coronary artery disease (CAD).6 However, most studies on coronary artery disease and sarcopenia have concentrated on assessing muscle mass and analyzing related risk factors, with comparatively few examining handgrip strength in isolation. Previous studies have indicated that lower handgrip strength is associated with a higher likelihood of death from cardiovascular causes.7 Analyses from a genetic risk perspective also found a negative correlation between coronary heart disease and handgrip strength.8 Thus, a simple measurement such as handgrip strength appears to have a strong ability to predict outcomes in cardiovascular disease, leading to benefits in terms of improved disease prognosis and reduced economic burden.
Sarcopenia is the most prevalent health issue among older adults and is a leading cause of falls, fractures, and declines in cardiorespiratory function in this population.9 Sarcopenia typically manifests insidiously, with subtle or no discernible symptoms during its early stages. Consequently, many patients are not diagnosed or treated proactively.10 In comparison, measuring handgrip strength is a convenient method for risk stratification of cardiovascular mortality and cardiovascular disease.11 However, there is currently no validated predictive model for probable sarcopenia based on simple clinical and functional parameters such as HGS. Although generic screening tools (e.g. SARC-F)3 are available, they lack specificity for cardiovascular populations and fail to account for CAD-specific confounders, such as problems with cut-off point settings.12 To address this critical gap, we developed and validated the first clinical prediction model integrating general characteristics and CAD-specific medical history data. This model facilitates the early detection of sarcopenia and prompt treatment, which contributes to improved healthcare results for elderly CAD patients.13.
The prevalence of sarcopenia increases with age, but it is not exclusively an elderly condition. Several studies have reported that handgrip strength declines from middle age onward.14 Therefore, we focused our study on the middle-aged and elderly population with coronary heart disease. By identifying individuals with decreased handgrip strength, we aimed to uncover more cases of probable sarcopenia. The inclusion of both groups enables the development of a predictive model with broader applicability and provides a reference for sarcopenia screening and management in patients across different age groups. This is in line with EWGSOP2’s call to action for healthcare professionals treating patients at risk of sarcopenia to promote early detection and treatment. We believe that detecting “probable sarcopenia” may hold greater interventional value and clinical significance than identifying sarcopenia itself. Therefore, the availability of easily accessible indicators with strong correlations is an important topic for clinicians, as it will influence the extent to which this predictive model can be generalized and adapted. Therefore, the current study investigated the risk factors for probable sarcopenia in middle-aged and elderly patients with coronary heart disease, categorizing participants based on their handgrip strength levels. This analysis aimed to lay a theoretical groundwork for the development of more effective predictive and intervention strategies.
Materials and methods
Study subjects
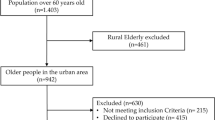
This hospital-based cross-sectional analytical study was conducted at the Hospital of Chengdu University of Traditional Chinese Medicine between October 2022 and September 2024 to investigate the predictive value of general information and clinical parameters for probable sarcopenia in consecutively enrolled CAD patients aged ≥ 45 years. The study adhered to STROBE guidelines for observational studies.
Inclusion criteria: (i) Age ≥ 45 years. (ii) Coronary angiography identifies a stenosis of more than 50% of the diameter of one or more major coronary arteries.15
Exclusion criteria: (i) Patients with aphasia, delirium, and severe cognitive impairment resulting in an inability to communicate. (ii) Those with incomplete clinical information.
Data collection
-
(1)
The following information was collected by inspecting the digital medical records:
-
(i)
Demographic information: gender, age, height, weight, smoking history, and alcohol consumption history (Smoking history: Defined as consecutive or cumulative smoking for ≥ 6 months during a lifetime.16 Alcohol consumption history: Defined as consuming > 40 g of alcohol per day for ≥ 12 months.17)
-
(ii)
Co-morbidities: including hypertension, type 2 diabetes, chronic obstructive pulmonary disease (COPD), and the number of long-term medications. Diagnostic criteria: Hypertension is defined as follows18: when measuring blood pressure on three non-consecutive days without the use of antihypertensive medication, a systolic blood pressure (SBP) ≥ 140 mmHg and/or a diastolic blood pressure (DBP) ≥ 90 mmHg. A patient with a prior history of hypertension who is currently on antihypertensive medication is still diagnosed with hypertension even if their blood pressure is below 140/90 mmHg. Diabetes is defined as follows19: a clear prior history of diabetes with the use of hypoglycemic agents or insulin, or a fasting blood glucose level ≥ 7.0 mmol/L on two consecutive occasions. COPD was defined as FEV1/FVC < 70% with bronchodilators.20
-
(iii)
Laboratory parameters: red blood cells (RBC), white blood cells (WBC), lymphocytes (LYM), neutrophils (NEU), hemoglobin (Hb), albumin (ALB), uric acid (UA), serum creatinine (Scr), γ-glutamyl transferase (GGT), choline esterase (ChE), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
-
(2)
Subjects’ dietary habits, permanent exercise, etc. were collected through in-person questioning.
(Permanent exercise: Defined as engaging in at least 150 min of moderate-intensity aerobic exercise per week.21)
-
(3)
The Simple Five-item Scoring Scale for Sarcopenia (SARC-F)22: The survey includes five sections: muscle power, walking aid, chair rise, stair climbing, and fall history (Table 1).
Body composition analysis, measurement of handgrip strength, five-repetition sit-to-stand test, and gait speed
-
(1)
Body composition analysis: All subjects ate a normal diet, emptied their bowels, and wore light clothing on the day of testing. Body composition was assessed using bioelectrical impedance analysis (DBA-510) without strenuous exercise. The appendicular skeletal muscle mass (ASM) was recorded, and the appendicular skeletal muscle index (ASMI) was computed.
-
(2)
Measurement of HGS: This was done by an electronic handgrip strength device (EH101). Subjects were asked to maintain their elbows in 90° flexion while holding the device at maximum force for 3 seconds in a seated position. The highest measurement obtained from two trials using their dominant hand was recorded. As outlined in the Asian Working Group for Sarcopenia (AWGS) 2019, low HGS was defined as < 28 kg for men and < 18 kg for women.4 The measurement process was guided and supervised by trained professionals.
-
(3)
Five-repetition sit-to-stand test (FTSST): Subjects crossed their arms over their shoulders, held this position, and quickly rose from their chairs and sat down again. The time taken for five consecutive times was recorded.
-
(4)
Gait speed (GS): Subjects walked 6 meters at a normal speed on a flat surface. The duration was noted, and gait speed was calculated.
Statistical analysis
The SPSS Statistics version 25.0 (IBM) and R 4.4.1 software package were used for statistical analysis. Normally distributed data were presented as mean ± SD, with comparisons between the two groups conducted using the t-test. Non-normally distributed data were expressed as median (interquartile range), and the Mann–Whitney U test was employed for group comparisons. Categorical data were reported as frequency (percentage), and group comparisons were performed using the chi-square test.
To assess potential multicollinearity among predictor variables, we calculated Variance Inflation Factors (VIF), which quantify the inflation of regression coefficient variances due to collinear relationships among predictors. Following O’Brien’s 23 methodological review, we used VIF > 10 as the diagnostic threshold for problematic multicollinearity that requires remediation, as this level of inflation has been shown to substantially distort parameter estimates and their inferential statistics.
LASSO regression was employed to reduce the dimensionality of the included variables and identify the significant influencing factors. Multivariate logistic regression analysis was conducted to assess risk factors, with odds ratios (OR) and 95% confidence intervals (CI) used to express the results. Statistical significance was determined with a p-value threshold of less than 0.05.
The R 4.4.1 software package was employed to build a nomogram model for predicting the likelihood of sarcopenia. Internal validation was carried out through bootstrap resampling, involving 1000 iterations. Receiver operating characteristic (ROC) curves were utilized to evaluate the predictive capability of the nomogram model. Calibration curves were applied to gauge the model’s accuracy in data representation, and a decision curve was constructed to assess the clinical utility of the nomogram model.
Results
General characteristics of the study population
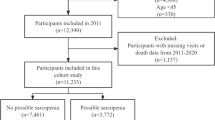
Based on the criteria for participation, 81 individuals with coronary artery disease were enrolled in the research. Participants’ ages ranged from 48 to 92 years (mean ± SD = 69.36 ± 9.59). According to the diagnostic criteria of the AWGS 2019, 45 were categorized in the low HGS group (probable sarcopenia), and 36 fell into the normal HGS category. Of these, 18 participants were confirmed sarcopenia. (Fig. 1).
Comparison of data in patients with CAD in the low HGS group and the normal HGS group
Comparison of general data in patients with CAD in the low HGS group and the normal HGS group
Participants in the low handgrip strength group were significantly older than those in the normal group (72.67 ± 9.04 vs. 65.22 ± 8.70 years, p < 0.001; Table 2). Furthermore, both height and weight measurements were significantly lower (p < 0.05) in comparison to the normal HGS group. It’s also worth noting that there were no statistically substantial variations between the two groups concerning gender, BMI, smoking history, alcohol consumption history, or permanent exercise (all p-values > 0.05).
Comparison of clinical data in patients with CAD in the low HGS group and the normal HGS group
As shown in Table 3, the low HGS group showed significantly lower levels of erythrocytes, hemoglobin, and albumin (all p < 0.05), along with poorer performance on sarcopenia-related measures (FTSST time, gait speed, calf circumference, and SARC-F score) compared to the normal HGS group. Body composition analysis revealed significantly lower ASM and ASMI in the low HGS group (p < 0.05). No significant differences were found in other metabolic parameters or clinical characteristics between groups.
Independent risk factors for probable sarcopenia in middle-aged and elderly patients with CAD
Covariance diagnostics were run for 12 statistically significant variables such as age, height, weight, erythrocytes, hemoglobin, etc. in Tables 2 and 3. Initial analysis revealed high multicollinearity, with variance inflation factor (VIF) values of 156.171 for ASM and 63.205 for ASMI. After excluding ASM, all remaining variables exhibited acceptable collinearity (VIF < 10). Subsequently, the 11 retained variables were subjected to LASSO regression with tenfold cross-validation. The optimal model (lambda = 0.026) identified six predictors with non-zero coefficients: age, height, FTSST, GS, CC, and ALB, all of which demonstrated low multicollinearity (VIF < 5) (Fig. 2).
A multifactorial logistic regression analysis was performed using whether the subjects experienced a reduction in HGS (no = 0, yes = 1) as the outcome variable. The above statistically significant indicators were included as the independent variables, and the regression process was performed using the forward LR method. The inclusion criterion was p < 0.05 (Table 4). The results showed that high GS (OR 0.015; 95% CI 0.001–0.232), high CC (OR 0.650; 95% CI 0.503–0.839) and high ALB level (OR 0.714; 95% CI 0.572–0.891) were protective factors for the development of probable sarcopenia. The Hosmer–Lemeshow test for the regression model resulted in a p-value exceeding 0.05, suggesting a satisfactory model fit.
Development and evaluation of the prediction model
The three independent factors identified from the multifactorial logistic regression analysis were utilized to create a nomogram model for forecasting the incidence of probable sarcopenia (Fig. 3). Internal validation was conducted through bootstrap resampling, consisting of 1000 iterations. The evaluation outcomes indicated the consistency index of the model was 0.89. Analysis of the ROC curve, as depicted in Fig. 4, revealed an Area Under the Curve (AUC) for the nomogram model to be 0.888, with a 95% confidence interval spanning from 0.815 to 0.96. As shown in Fig. 5, both the apparent line and the bias-corrected line of the calibration curve showed slight fluctuations near the ideal line. The model’s predictions, characterized by a mean absolute error of 0.015, generally aligned with the observed variables in the dataset, indicating robust predictive capacity. After bias correction, the predictive results of the model were able to reflect the actual risk quite well, particularly performing ideally within the moderate risk range. The decision curve analysis underscored that the red curve outperformed both the “none” and “all” lines, affirming that the model delivered superior net benefits across various high-risk thresholds (Fig. 6).
Discussion
The univariate analysis of this research showed that the low HGS group experienced notably reduced values in height, weight, Hb, RBC, ALB, GS, CC, ASM, and ASMI, as well as higher scores for age, FTSST, and SARC-F compared to the normal HGS group. Based on the outcomes of the univariate variable screening, a multivariate logistic regression model was developed to identify three distinct diagnostic markers. Among them, gait speed, calf circumference, and ALB were protective factors for the development of probable sarcopenia in patients in middle age and beyond with coronary artery disease. The AWGS defined probable sarcopenia as a lack of muscle strength or poor physical performance, especially concerning primary care and community-based health promotion for early lifestyle interventions.4 The three independent predictors derived from this study are conveniently accessible in the clinic and are applicable and actionable in many clinical settings. Furthermore, this research showed that the nomogram model we developed demonstrated strong discriminative and calibration capabilities. It will be a promising method to identify the risk of sarcopenia in middle-aged and older individuals with CAD, as demonstrated by the ROC curve and the calibration curve in this study.
As reported by the World Health Organization, coronary artery disease, the leading form of cardiovascular disease, has caused a serious socioeconomic burden.24 A prospective study including 345 elderly patients with CAD indicated a prevalence of 22.6% for sarcopenia.25 The two often interact and influence each other, having a significant negative effect on the health and general life quality of elderly individuals.
Among the various risk factors associated with sarcopenia, advanced age is likely the most significant.4 Compared to measuring muscle strength, assessing muscle mass requires specific equipment and specialized personnel, which makes it challenging and limited in clinical practice. Therefore, we focused our study on the middle-aged and elderly population with coronary heart disease. By identifying individuals with decreased handgrip strength, we aimed to uncover more cases of probable sarcopenia. The results indicated a higher prevalence of “probable sarcopenia” at 55.56% in contrast to the prevalence of sarcopenia, which was 22.22%. It suggests that our study is an important guide for identifying probable sarcopenia, exploring independent influencing factors, and developing a nomogram prediction model. Therefore, it can be a potent method for the preliminary identification of sarcopenia and improve the outlook for coronary heart disease.
The prevalence of malnutrition tends to rise in middle-aged and elderly individuals with coronary artery disease, attributed to the gradual impairment of cardiopulmonary function, a decline in digestive and absorptive capabilities, and limited physical activity. Complications, including osteoporosis, muscle wasting, and cognitive decline, may arise. When the body ages or experiences a chronic disease state, a series of changes in skeletal muscle may lead to decreased protein synthesis and increased degradation. Proteins have a significant impact on changes to muscle structure and function.26
Malnutrition elevated the likelihood of having low handgrip strength by nearly twice as much (p < 0.001).27 Chronic malnutrition may lead to anemia. Previous studies have found that low hemoglobin level served as an autonomous risk factor for sarcopenia in patients with Crohn’s disease, cirrhosis of the liver, and Alzheimer’s disease in women.28,29,30 Malnutrition is also positively associated with handgrip strength.31,32 The findings from this research indicated that the hemoglobin level in the low HGS group (x = 133.00 g/L) was substantially below that of the normal HGS group (x = 144.50 g/L). Nevertheless, low hemoglobin level was not identified as a standalone contributing element for the onset of probable sarcopenia in patients with CAD.
For individuals in middle age and later life, ensuring sufficient protein intake is crucial. According to a meta-analysis, protein supplements could augment the effectiveness of resistance training in boosting muscle mass, handgrip strength, and gait speed in older people.33 Albumin is a nonspecific protein transporter for acute-phase reactants as well as for various hormones.34 In the absence of a disease state affecting albumin production, hypoalbuminemia is thought to result from poor nutritional status and chronic inflammation.35 Nonetheless, the assessment related to sarcopenia in middle-aged and elderly patients with CAD does not necessarily need to be conducted in the presence of reduced albumin levels to indicate risk. A recent prospective cohort study found that even serum albumin levels within the normal spectrum could serve as a potential warning sign for reduced muscle performance.36 Whereas calf circumference and gait speed measurements reflect muscle mass and function respectively.37 Therefore, in conjunction with the results of this study, healthcare professionals need to consider the patient’s calf circumference and gait speed at different albumin levels. Our findings underscore the multifactorial nature of sarcopenia and its potential impact on health outcomes in the CAD population. These will complement handgrip strength measurements and enrich the screening criteria for “probable sarcopenia”. Using this nomogram model, a comprehensive assessment of probable sarcopenia can be made.
Previous studies have demonstrated the prognostic value of sarcopenia components (e.g., gait speed, and calf circumference). Xiao et al. reported a significant association between slow gait speed and cardiometabolic disease risk,38 while Canonico et al. identified calf circumference as a predictor of mortality in frail elderly patients.39 These findings support our selection of core indicators (gait speed and calf circumference).
However, existing sarcopenia screening tools show limited applicability in specific populations. For instance, in COPD patients, the SARC-F questionnaire exhibits moderate specificity (74.47%) but poor sensitivity (55.56%), potentially leading to underdiagnosis. Although the Ishii test demonstrates better sensitivity/specificity (71.59%/90.48%),40 its performance in cardiovascular diseases remains unvalidated. Critically, no validated screening model exists for middle-aged and elderly coronary artery disease patients, a high-risk population with elevated comorbidity burden and accelerated muscle loss.25
To address this gap, we developed and validated the first clinical model for probable sarcopenia in CAD patients, incorporating gait speed, calf circumference, and serum albumin levels. Compared to prior tools, our model provided a tailored solution for early sarcopenia detection in this population.
While this study provides novel insights into sarcopenia screening in CAD patients, several important limitations should be considered. First, as a single-center study conducted at Chengdu University of Traditional Chinese Medicine Hospital, our cohort may not fully represent broader CAD populations due to regional healthcare disparities and local socioeconomic factors. Second, we acknowledge potential confounding from unmeasured variables including dietary patterns, protein intake, and physical activity levels, which could influence the sarcopenia-CAD relationship. Third, the cross-sectional design limits our ability to establish causal relationships between sarcopenia components and long-term CAD prognosis. Additionally, while our sample size (n = 81) was adequate for initial model development, it restricts more detailed subgroup analyses by CAD severity or age stratification. To address these limitations, future multi-center studies should incorporate: (1) standardized nutritional assessments, (2) longitudinal follow-up for mortality outcomes, and (3) ethnicity-specific calibration of anthropometric measures like calf circumference to validate and refine our model.
Conclusion
This research involved utilizing three parameters—walking speed, calf muscle thickness, and albumin levels—to construct a nomogram that forecasts the likelihood of probable sarcopenia development. Our model exhibited robust predictive capabilities and practical clinical value, and it can be used to assist clinicians with screening recommendations. Identifying individuals at risk promptly and initiating early treatment can slow the decline in muscle power and mass, thereby preventing future functional impairments and adverse cardiovascular events.
Data availability
The dataset generated and analyzed during the current study is available from the corresponding author upon reasonable request.
Abbreviations
- ALB:
-
Albumin
- ASM:
-
Appendicular skeletal muscle mass
- ASMI:
-
Appendicular skeletal muscle index
- AUC:
-
Area under the curve
- AWGS:
-
Asian working group for sarcopenia
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CC:
-
Calf circumference
- ChE:
-
Choline esterase
- CI:
-
Confidence intervals
- COPD:
-
Chronic obstructive pulmonary disease
- DBP:
-
Diastolic blood pressure
- EWGSOP:
-
European working group on sarcopenia in older people
- FTSST:
-
Five-repetition sit-to-stand test
- GGT:
-
γ-Glutamyl transferase
- GS:
-
Gait speed
- Hb:
-
Hemoglobin
- HDL-C:
-
High-density lipoprotein cholesterol
- HGS:
-
Handgrip strength
- LDL-C:
-
Low-density lipoprotein cholesterol
- LYM:
-
Lymphocytes
- NEU:
-
Neutrophils
- OR:
-
Odds ratios
- RBC:
-
Red blood cells
- ROC:
-
Receiver operating characteristic
- SARC-F:
-
The simple five-item scoring scale for sarcopenia
- SBP:
-
Systolic blood pressure
- Scr:
-
Serum creatinine
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- UA:
-
Uric acid
- VIF:
-
Variance inflation factor
- WBC:
-
White blood cells
- WHR:
-
Waist-to-hip ratio
References
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet Lond Engl. https://doi.org/10.1016/S0140-6736(19)31138-9 (2019).
Hida, T., Harada, A., Imagama, S. & Ishiguro, N. Managing sarcopenia and its related features to improve quality of life in geriatric populations. Aging Dis. 5(4), 226–237. https://doi.org/10.14336/AD.2014.0500226 (2014).
Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 48(1), 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 21(3), 300-307.e2. https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Sayer, A. A. & Cruz-Jentoft, A. Sarcopenia definition, diagnosis, and treatment: consensus is growing. Age Ageing. 51(10), afac220. https://doi.org/10.1093/ageing/afac220 (2022).
Zuo, X. et al. Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 14(3), 1183–1198. https://doi.org/10.1002/jcsm.13221 (2023).
Strand, B. H. et al. The association of grip strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromsø study. J Epidemiol Commun Health. https://doi.org/10.1136/jech-2015-206776 (2016).
Tikkanen, E., Gustafsson, S. & Ingelsson, E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: Longitudinal analyses in the UK biobank study. Circulation https://doi.org/10.1161/CIRCULATIONAHA.117.032432 (2018).
Inoue, T. et al. Undernutrition, sarcopenia, and frailty in fragility hip fracture: Advanced strategies for improving clinical outcomes. Nutrients 12(12), 3743. https://doi.org/10.3390/nu12123743 (2020).
Petermann-Rocha, F. et al. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 13(1), 86–99. https://doi.org/10.1002/jcsm.12783 (2022).
Li, D. et al. Relative handgrip strength is inversely associated with metabolic profile and metabolic disease in the general population in China. Front Physiol. 9, 59. https://doi.org/10.3389/fphys.2018.00059 (2018).
Noda, T. et al. Screening for sarcopenia with SARC-F in older patients hospitalized with cardiovascular disease. Eur J Cardiovasc Nurs. 23(6), 675–684. https://doi.org/10.1093/eurjcn/zvae017 (2024).
Hurst, C. et al. Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age Ageing. 51(2), afac003. https://doi.org/10.1093/ageing/afac003 (2022).
Strand, B. H. et al. The association of grip strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromsø study. J Epidemiol Commun Health. 70(12), 1214–1221. https://doi.org/10.1136/jech-2015-206776 (2016).
Section of interventional cardiology of Chinese society of cardiology, section of atherosclerosis and coronary artery disease of Chinese society of cardiology, specialty committee on prevention and treatment of thrombosis of Chinese college of cardiovascular physicians, editorial board of Chinese Journal of Cardiology. Guideline on the diagnosis and treatment of stable coronary artery disease. Chinese Journal of Cardiovascular Medicine.2018;46(9):680–694. https://doi.org/10.3760/cma.j.issn.0253-3758.2018.09.004.
World Health Organization. global adult tobacco survey (GATS): Core questionnaire with optional questions, version 3.0. World health organization, Geneva (2021). Available at: https://www.who.int/tobacco/surveillance/survey/gats/en/.
World Health Organization. global status report on alcohol and health 2018. World Health Organization, Geneva (2018). Available at: https://www.who.int/publications/i/item/9789241565639.
Writing Group of 2018 chinese guidelines for the management of hypertension, Chinese hypertension league, chinese society of cardiology, Chinese medical doctor association hypertension committee, hypertension branch of China international exchange and promotive association for medical and health care, hypertension branch of Chinese geriatric medical association. 2018 chinese guidelines for the management of hypertension. Chinese Journal of Cardiovascular Medicine. 2019;24(1):24–56. https://doi.org/10.3969/j.issn.1007-5410.2019.01.002.
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chinese Journal of Diabetes Mellitus. 2021;13(04):315–409. https://doi.org/10.3760/cma.j.cn115791-20210221-00095.
Chronic Obstructive Pulmonary Disease Group of Chinese Thoracic Society, Chronic Obstructive Pulmonary Disease Committee of Chinese Association of Chest Physician. Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021). Chinese Journal of Tuberculosis and Respiratory Diseases. 2021;44(03):170–205. https://doi.org/10.3760/cma.j.cn112147-20210109-00031.
World Health Organization. Global recommendations on physical activity for health. world health organization, Geneva (2010). Available at: https://www.who.int/publications/i/item/9789241599979.
Malmstrom, T. K. & Morley, J. E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 14(8), 531–532. https://doi.org/10.1016/j.jamda.2013.05.018 (2013).
O’brien, R. M. A. Caution regarding rules of thumb for variance inflation factors. Qual Quant 41, 673–690. https://doi.org/10.1007/s11135-006-9018-6 (2007).
Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet Lond Engl. 2018;392(10159):1736–1788. https://doi.org/10.1016/S0140-6736(18)32203-7.
Zhang, N. et al. Prevalence and prognostic implications of sarcopenia in older patients with coronary heart disease. J Geriatr Cardiol JGC. 16(10), 756–763. https://doi.org/10.11909/j.issn.1671-5411.2019.10.002 (2019).
Meng, S. J. & Yu, L. J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 11(4), 1509–1526. https://doi.org/10.3390/ijms11041509 (2010).
Riviati, N., Setiati, S., Laksmi, P. W. & Abdullah, M. Factors related with handgrip strength in elderly patients. Acta Med Indones. 49(3), 215–219 (2017).
Hu, N. et al. The association between hemoglobin level and sarcopenia in Chinese patients with Crohn’s disease. BMC Gastroenterol. 24(1), 95. https://doi.org/10.1186/s12876-024-03182-2 (2024).
Shibamoto, A. et al. Hemoglobin levels as a surrogate marker of sarcopenia in patients with liver cirrhosis. Hepatol Res Off J Jpn Soc Hepatol. 53(8), 713–722. https://doi.org/10.1111/hepr.13904 (2023).
Lee, H. N., Chang, Y. S., Wu, Y. H., Wu, C. H. & Wang, C. J. Sarcopenia in female patients with Alzheimer’s disease are more likely to have lower levels of hemoglobin and 25-hydroxyvitamin D. Psychogeriatr Off J Jpn Psychogeriatr Soc. 20(6), 858–864. https://doi.org/10.1111/psyg.12593 (2020).
Yoshimura, Y. et al. Low hemoglobin levels are associated with compromised muscle health: Insights from a post-stroke rehabilitation cohort. Geriatr Gerontol Int. 24(3), 305–311. https://doi.org/10.1111/ggi.14834 (2024).
Öztürk, E., Çiğiloğlu, A., Efendioğlu, E. M. & Öztürk, Z. A. A different outlook to consequences of anemia in older adults. Postgrad Med. 135(5), 486–492. https://doi.org/10.1080/00325481.2023.2200124 (2023).
Liao, C. D. et al. Comparative efficacy of different protein supplements on muscle mass, strength, and physical indices of sarcopenia among community-dwelling, hospitalized or institutionalized older adults undergoing resistance training: A network meta-analysis of randomized controlled trials. Nutrients 16(7), 941. https://doi.org/10.3390/nu16070941 (2024).
Rothschild, M. A., Oratz, M. & Schreiber, S. S. Serum albumin. Hepatol Baltim Md. 8(2), 385–401. https://doi.org/10.1002/hep.1840080234 (1988).
Sullivan, D. H. What do the serum proteins tell us about our elderly patients?. J Gerontol A Biol Sci Med Sci. 56(2), M71-74. https://doi.org/10.1093/gerona/56.2.m71 (2001).
Snyder, C. K. et al. Serum albumin in relation to change in muscle mass, muscle strength, and muscle power in older men. J Am Geriatr Soc. 60(9), 1663–1672. https://doi.org/10.1111/j.1532-5415.2012.04115.x (2012).
Şenoymak, İ, Egici, M. T. & Şenoymak, M. C. Sarcopenia and associated factors in adults aged 40 and above: A study conducted in primary healthcare. Cureus. 16(8), e67618. https://doi.org/10.7759/cureus.67618 (2024).
Xiao, Y. et al. Role of sarcopenia in temporal progression trajectory of cardiometabolic diseases: A prospective study in UK biobank. BMC Public Health 25(1), 1294. https://doi.org/10.1186/s12889-025-22500-1 (2025).
Canonico, S. et al. Measuring calf circumference in frail hospitalized older adults and prediction of in-hospital complications and post-discharge mortality. Front Med 11, 1439353. https://doi.org/10.3389/fmed.2024.1439353 (2024).
Dilektaşlı, A. G. et al. Diagnostic performance of sarcopenia screening tests in chronic lung disease patients. Eurasian J Med. 57(1), 1–7. https://doi.org/10.5152/eurasianjmed.2025.25806 (2025).
Acknowledgements
The authors wish to express gratitude to all respondents for their enthusiastic participation in this study.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by Xiaolan Sun, Jia Xu, Feier Chen, Haiyan Lei, Wei Chen, and Fang Ding. The first draft of the manuscript was written by Xiaolan Sun and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Ethics approval
This study was conducted at the Hospital of Chengdu University of Traditional Chinese Medicine and has been approved by the Ethics Committee of our hospital by the STROBE criteria and the Declaration of Helsinki. As all included data were anonymized and retrospective, informed consent from patients was waived by the ethical committee.
Consent to participate
Due to the retrospective nature of the study, the institutional review board of the Hospital of Chengdu University of Traditional Chinese Medicine waived the need of obtaining informed consent. Patient confidential data was removed from the entire dataset before analysis. The study was conducted by the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, X., Xu, J., Chen, F. et al. Predicting the occurrence of probable sarcopenia in middle-aged and elderly patients with coronary artery disease: development and validation of a clinical model. Sci Rep 15, 28830 (2025). https://doi.org/10.1038/s41598-025-13712-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13712-x